Encapsulated Allografts Preclude Host Sensitization and Promote Ovarian Endocrine Function in Ovariectomized Young Rhesus Monkeys and Sensitized Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.2.1. Murine Model
2.2.2. Collection of Donor Murine Ovaries
2.2.3. Hydrogel Preparation and Murine Ovarian Tissue Encapsulation
2.2.4. Subcutaneous Implantation in Mice
2.2.5. Vaginal Cytology in Mice
2.2.6. Flow Cytometry of Mouse Serum
2.2.7. Histological Analysis of the Murine Ovarian Allografts and Encapsulated Ovarian Allografts
2.2.8. Immunohistochemistry (IHC) of Mouse Ovarian Allografts
2.3. Rhesus Monkey Model
2.3.1. Ovariectomies and Subcutaneous Implantation in Recipient Rhesus Monkeys
2.3.2. Primate Ovarian Tissue Encapsulation
2.3.3. Primate Urinary Estrone Conjugate (E1C) and Pregnanediol Glucuronide (PdG) Analysis
2.3.4. Mixed Primate Lymphocyte Culture
2.3.5. Histological Analysis of the Encapsulated Ovarian Primate Allografts
2.4. Statistics
3. Results
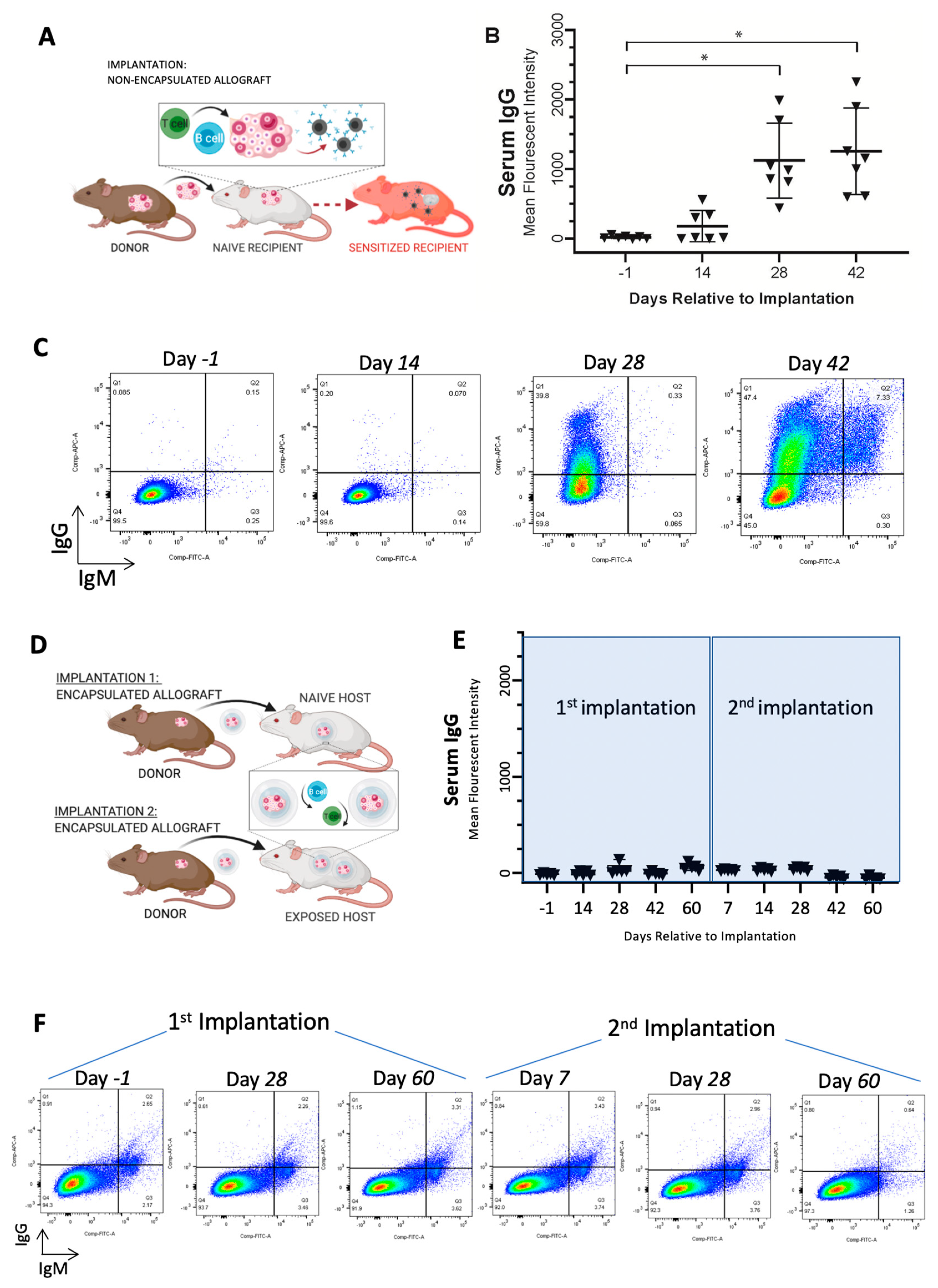
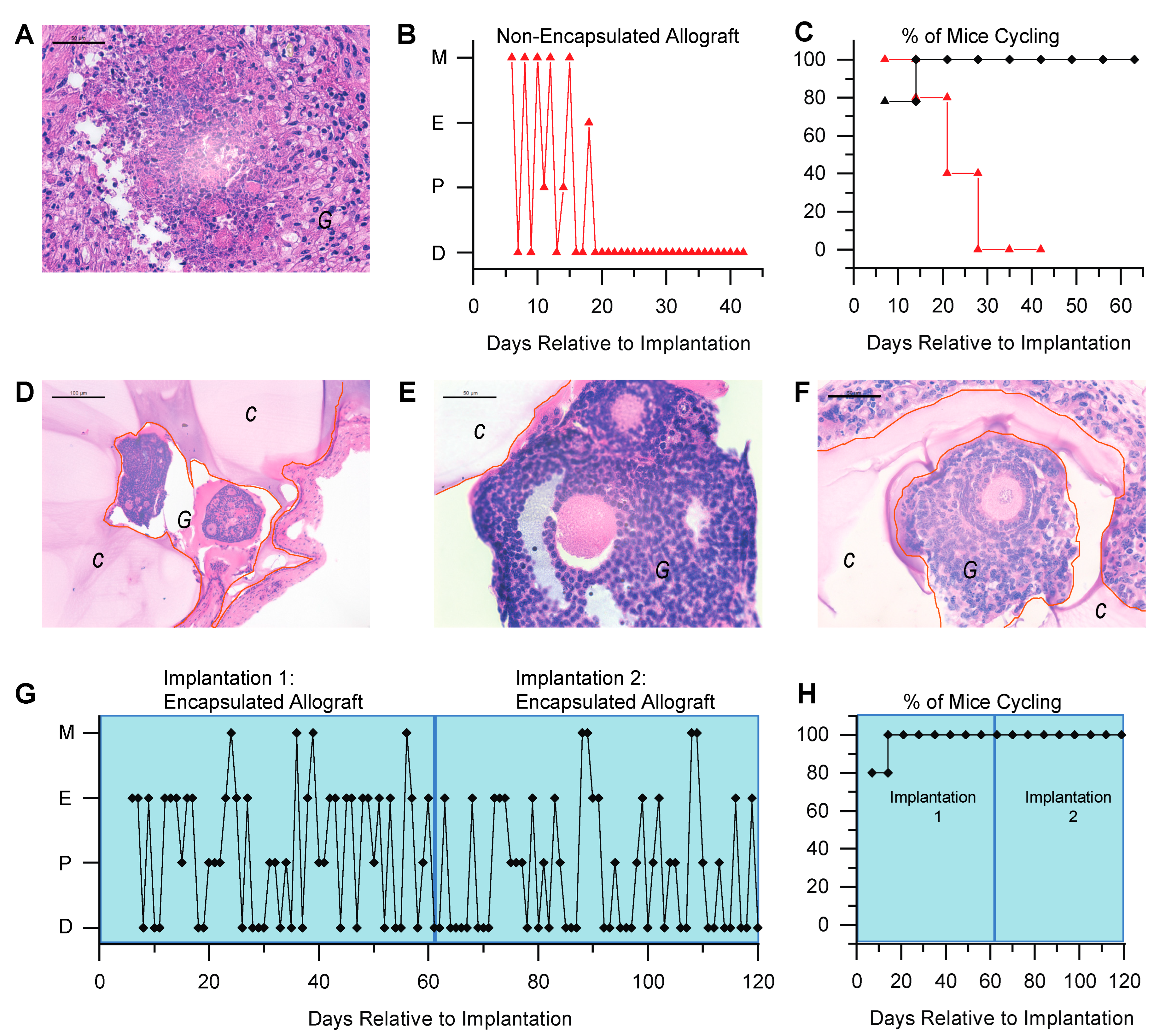
3.1. Immunoisolating Capsule Prevents Sensitization of the Host: Studies in a Murine Model
3.2. Immunoisolating Capsule Prevents Rejection of Allogeneic Ovarian Tissue and Supports Endocrine Function in a Murine Model
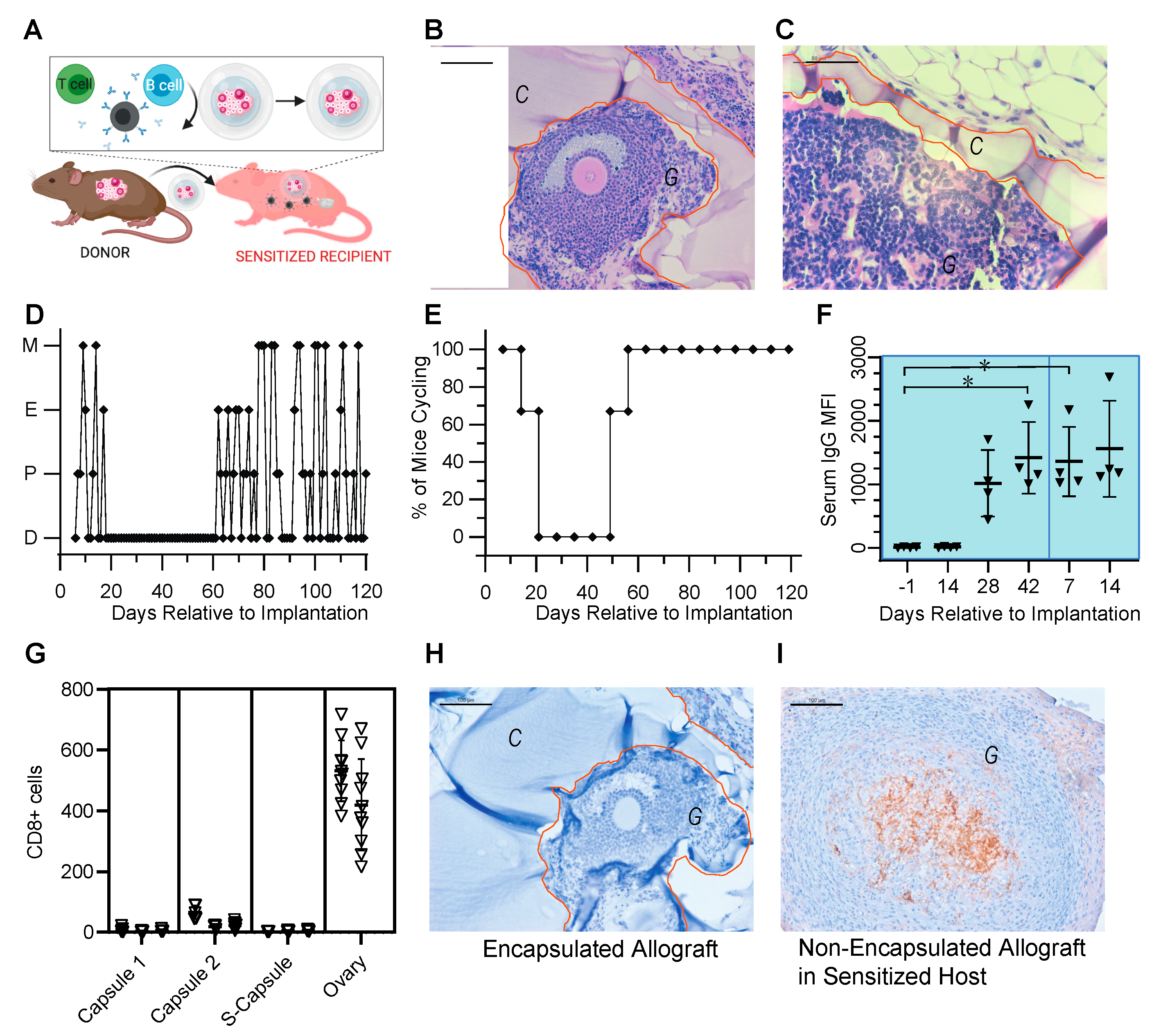
3.3. Encapsulated Allografts Restored Ovarian Endocrine Function and Were Shielded from Rejection in Prior Sensitized Murine Hosts
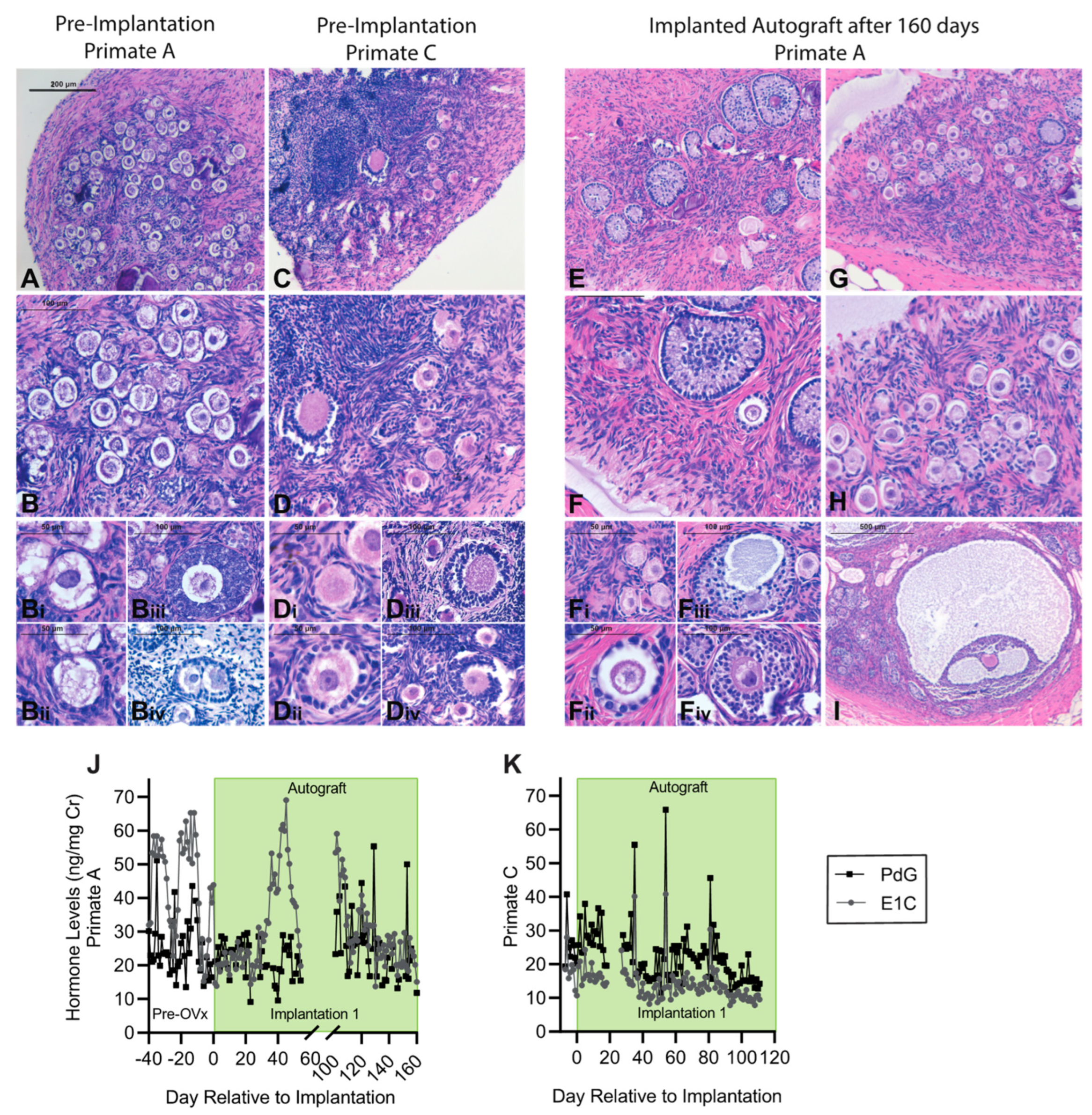
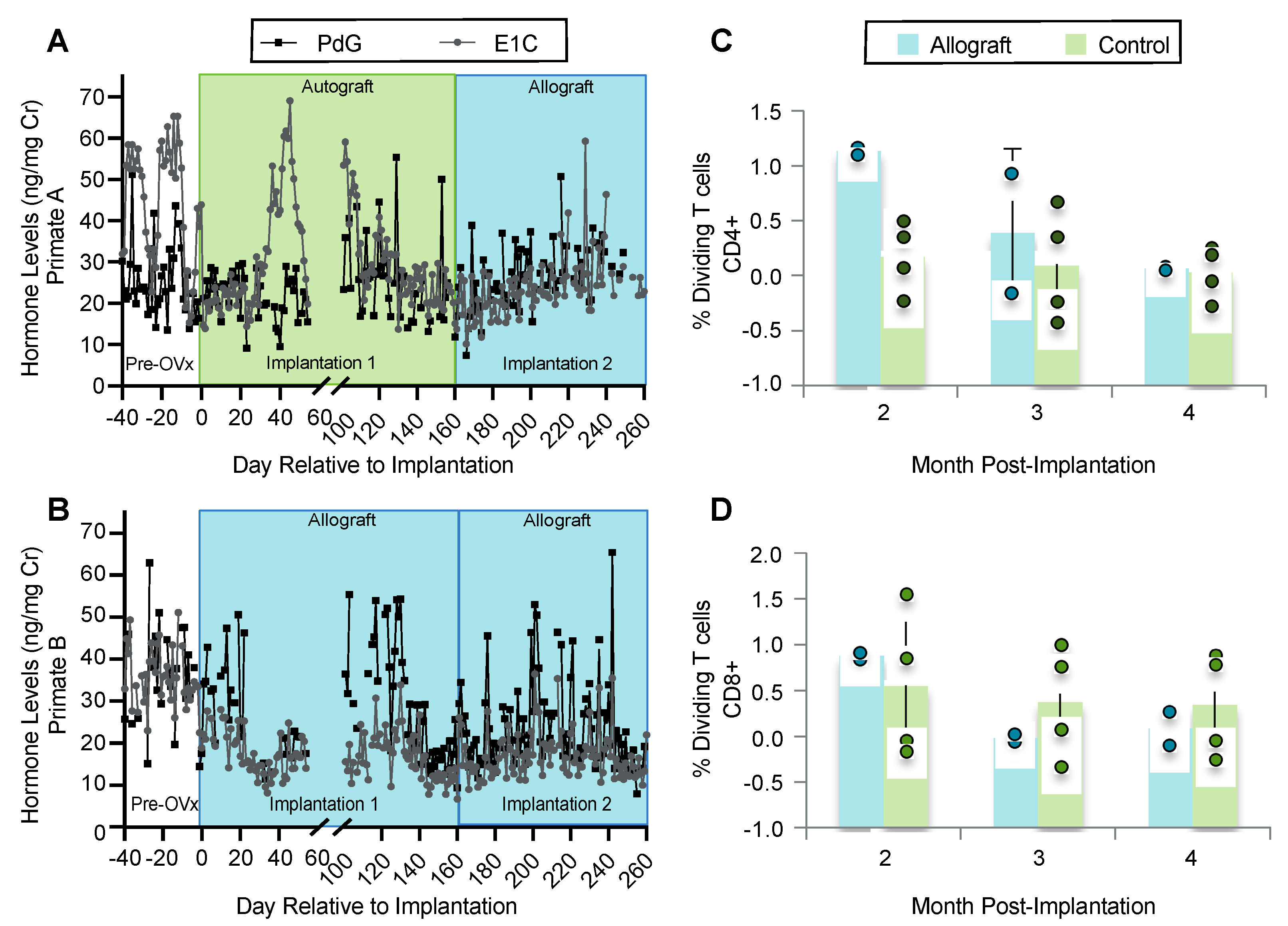
3.4. Encapsulation and Implantation of Nonhuman Primate Ovarian Tissue in Ovariectomized Adolescent Rhesus Monkeys
3.5. Implanted Encapsulated Ovarian Allografts Secrete E1C and PdG and Elicit Minimal Immune Responses
3.6. Ovarian Tissue Encapsulated in PEG Capsules Did Not Elicit an Immune Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glazer, T.S.; Schulte, F. Barriers to Oncofertility Care among Female Adolescent Cancer Patients in Canada. Curr. Oncol. 2022, 29, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Algarroba, G.N.; Sanfilippo, J.S.; Valli-Pulaski, H. Female fertility preservation in the pediatric and adolescent cancer patient population. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Nandagopal, R.; Laverdière, C.; Mulrooney, D.; Hudson, M.M.; Meacham, L. Endocrine late effects of childhood cancer therapy: A report from the children’s oncology group. Horm. Res. Paediatr. 2008, 69, 65–74. [Google Scholar] [CrossRef]
- Yu, B.; Fritz, R.; Vega, M.; Merino, M. Dissociation of Pubertal Development Abnormality and Gonadal Dysfunction in Childhood Cancer Survivors. J. Adolesc. Young Adult Oncol. 2020, 9, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.R.; Horne, V.E.; Howell, J.; Lawson, S.A.; Rutter, M.M.; Trotman, G.E.; Corathers, S.D. Late endocrine effects of childhood cancer. Nat. Rev. Endocrinol. 2016, 12, 319–336. [Google Scholar] [CrossRef]
- Blumenfeld, Z. Fertility Preservation in Women with Malignancy: Future Endeavors. Clin. Med. Insights Reprod. Health 2019, 13, 117955811987249. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Andersen, C.Y. Cryopreservation of ovarian tissue: Opportunities beyond fertility preservation and a positive view into the future. Front. Endocrinol. 2018, 9, 347. [Google Scholar] [CrossRef]
- Sklar, C.A.; Antal, Z.; Chemaitilly, W.; Cohen, L.E.; Follin, C.; Meacham, L.R.; Murad, M.H. Hypothalamic-Pituitary and Growth Disorders in Survivors of Childhood Cancer: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 2761–2784. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Roemmich, J.N.; Richmond, E.J.; Rogol, A.D.; Lovejoy, J.C.; Sheffield-Moore, M.; Mauras, N.; Bowers, C.Y. Endocrine control of body composition in infancy, childhood, and puberty. Endocr. Rev. 2005, 26, 114–146. [Google Scholar] [CrossRef]
- Jensen, A.K.K.; Rechnitzer, C.; Macklon, K.T.T.; Ifversen, M.; Birkebæk, N.; Clausen, N.; Sørensen, K.; Fedder, J.; Ernst, E.; Andersen, C.Y. Cryopreservation of ovarian tissue for fertility preservation in a large cohort of young girls: Focus on pubertal development. Hum. Reprod. 2016, 32, 154–164. [Google Scholar] [CrossRef]
- von Wolff, M.; Stute, P.; Flück, C. Autologous transplantation of cryopreserved ovarian tissue to induce puberty—The endocrinologists’ view. Eur. J. Pediatr. 2016, 175, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.; Emery, B.R.; Huang, I.; Peterson, C.M.; Carrell, D.T. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J. Exp. Clin. Assist. Reprod. 2006, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhaimi, E.A.; Khan, F.A.; Homeida, A.M. Regulation of Male and Female Reproductive Functions. In Emerging Concepts in Endocrine Structure and Functions; Al-Suhaimi, E.A., Ed.; Springer Nature: Singapore, 2022; p. 287. [Google Scholar] [CrossRef]
- Beccuti, G.; Ghizzoni, L. Normal and Abnormal Puberty. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com: South Dartmouth, MA, USA, 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279024/?report=reader (accessed on 8 September 2020).
- Peper, J.S.; Dahl, R.E. The Teenage Brain: Surging Hormones-Brain-Behavior Interactions During Puberty. Curr. Dir. Psychol. Sci. 2013, 22, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.; Cariboni, A.M.; Marelli, M.M.; Moretti, R.M.; Andrè, V.; Marzagalli, M.; Limonta, P. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum. Reprod. Update 2016, 22, 358–381. [Google Scholar] [CrossRef]
- Kenigsberg, L.; Balachandar, S.; Prasad, K.; Shah, B. Exogenous Pubertal Induction by Oral versus Transdermal Estrogen Therapy. J. Pediatr. Adolesc. Gynecol. 2013, 26, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pinyerd, B.; Zipf, W.B. Puberty—Timing is everything! J. Pediatr. Nurs. 2005, 20, 75–82. [Google Scholar] [CrossRef]
- Dunkel, L.; Quinton, R. Transition in endocrinology: Induction of puberty. Eur. J. Endocrinol. 2014, 170, R229–R239. [Google Scholar] [CrossRef]
- Agarwal, S.; Alzahrani, F.A.; Ahmed, A. Hormone replacement therapy: Would it be possible to replicate a functional ovary? Int. J. Mol. Sci. 2018, 19, 3160. [Google Scholar] [CrossRef]
- Golub, M.S.; Hogrefe, C.E.; Germann, S.L.; Lasley, B.L.; Natarajan, K.; Tarantal, A.F. Effects of exogenous estrogenic agents on pubertal growth and reproductive system maturation in female rhesus monkeys. Toxicol. Sci. 2003, 74, 103–113. [Google Scholar] [CrossRef]
- Fish, J.D. Part 1: Hormone Replacement for Survivors of Childhood Cancer with Ovarian Failure—When Is It Worth the Risk? J. Pediatr. Adolesc. Gynecol. 2011, 24, 98–101. [Google Scholar] [CrossRef]
- Gargus, E.; Deans, R.; Anazodo, A.; Woodruff, T.K. Management of primary ovarian insufficiency symptoms in survivors of childhood and adolescent cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 1137–1149. [Google Scholar] [CrossRef]
- Poirot, C.; Abirached, F.; Prades, M.; Coussieu, C.; Bernaudin, F.; Piver, P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet 2012, 379, 588. [Google Scholar] [CrossRef]
- Dittrich, R.; Hackl, J.; Lotz, L.; Hoffmann, I.; Beckmann, M.W. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertil. Steril. 2015, 103, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Ra’anani, H.; Shapira, M.; Brenghausen, M.; Chaim, S.D.; Aviel-Ronen, S.; Amariglio, N.; Schiff, E.; Orvieto, R.; Dor, J. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil. Steril. 2016, 106, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.T.; Pors, S.E.; Poulsen, L.L.C.; Colmorn, L.B.; Macklon, K.T.; Ernst, E.; Humaidan, P.; Andersen, C.Y.; Kristensen, S.G. Ovarian stimulation and assisted reproductive technology outcomes in women transplanted with cryopreserved ovarian tissue: A systematic review. Fertil. Steril. 2019, 112, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Lotz, L.; Dittrich, R.; Hoffmann, I.; Beckmann, M.W. Ovarian Tissue Transplantation: Experience from Germany and Worldwide Efficacy. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119867357. [Google Scholar] [CrossRef]
- Silber, S.J.; Lenahan, K.M.; Levine, D.J.; Pineda, J.A.; Gorman, K.S.; Friez, M.J.; Crawford, E.C.; Gosden, R.G. Ovarian Transplantation between Monozygotic Twins Discordant for Premature Ovarian Failure. N. Engl. J. Med. 2005, 353, 58–63. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Soares, M. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Gonadal. Tissue Cryopreserv. Fertil. Preserv. 2016, 99, 161–173. [Google Scholar] [CrossRef]
- Soares, M.; Sahrari, K.; Amorim, C.A.; Saussoy, P.; Donnez, J.; Dolmans, M.M. Evaluation of a human ovarian follicle isolation technique to obtain disease-free follicle suspensions before safely grafting to cancer patients. Fertil. Steril. 2015, 104, 672–680.e2. [Google Scholar] [CrossRef]
- Corkum, K.S.; Rhee, D.S.; Wafford, Q.E.; Demeestere, I.; Dasgupta, R.; Baertschiger, R.; Malek, M.M.; Aldrink, J.H.; Heaton, T.E.; Weil, B.R.; et al. Fertility and hormone preservation and restoration for female children and adolescents receiving gonadotoxic cancer treatments: A systematic review. J. Pediatr. Surg. 2019, 54, 2200–2209. [Google Scholar] [CrossRef]
- Blumenfeld, Z. Ovarian tissue transplantation and leukemia. Fertil. Steril. 2018, 109, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; David, A.; Long, C.; Bushnell, G.G.; Woodruff, T.K.; Shea, L.D.; Shikanov, A. Immuno-Isolating Dual Poly(ethylene glycol) Capsule Prevents Cancer Cells from Spreading Following Mouse Ovarian Tissue Auto-Transplantation. Regen. Med. Front. 2019, 2019, e190006. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; David, A.; Cichon, A.L.; Kulkarni, T.; Cascalho, M.; Shikanov, A. Immunoisolating poly(ethylene glycol) based capsules support ovarian tissue survival to restore endocrine function. J. Biomed. Mater. Res. Part A 2018, 106, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; David, A.; Kim, J.; Farkash, E.A.; Cascalho, M.; Milašinović, N.; Shikanov, A. The impact of functional groups of poly (ethylene glycol) macromers on the physical properties of photo-polymerized hydrogels and the local inflammatory response in the host. Acta Biomater. 2018, 67, 42–52. [Google Scholar] [CrossRef]
- Day, J.R.; David, A.; Barbosa, M.G.d.M.; Brunette, M.A.; Cascalho, M.; Shikanov, A. Encapsulation of ovarian allograft precludes immune rejection and promotes restoration of endocrine function in immune-competent ovariectomized mice. Sci. Rep. 2019, 9, 16614. [Google Scholar] [CrossRef]
- David, A.; Day, J.R.; Cichon, A.L.; Lefferts, A.; Cascalho, M.; Shikanov, A. Restoring Ovarian Endocrine Function with Encapsulated Ovarian Allograft in Immune Competent Mice. Ann. Biomed. Eng. 2017, 45, 1685–1696. [Google Scholar] [CrossRef]
- Cascalho, M.; Platt, J.L. The Immunological Barrier Review to Xenotransplantation. Immunity 2001, 14, 437–446. [Google Scholar] [CrossRef]
- Platt, J.; Cascalho, M. Transplantation Immunology. In Greenfield’s Surgery: Scientific Principles and Practice, 5th ed.; Mulholland, M., Lillemoe, K., Doherty, G., Maier, R., Simeone, D., Upchurch, G.R.J., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 497–514. [Google Scholar]
- Weinbauer, G.F.; Niehoff, M.; Niehaus, M.; Srivastav, S.; Fuchs, A.; Van Esch, E.; Cline, J.M. Physiology and Endocrinology of the Ovarian Cycle in Macaques. Toxicol. Pathol. 2008, 36 (Suppl. S7), 7S–23S. [Google Scholar] [CrossRef]
- Stouffer, R.L.; Woodruff, T.K. Nonhuman primates: A vital model for basic and applied research on female reproduction, prenatal development, and women’s health. ILAR J. 2017, 58, 281–294. [Google Scholar] [CrossRef]
- Batchelder, C.A.; Duru, N.; Lee, C.I.; Baker, C.A.; Swainson, L.; McCune, J.M.; Tarantal, A.F. Myeloid-lymphoid ontogeny in the rhesus monkey (Macaca mulatta). Anat. Rec. 2014, 297, 1392–1406. [Google Scholar] [CrossRef]
- Elliott, R.B.; Escobar, L.; Calafiore, R.; Basta, G.; Garkavenko, O.; Vasconcellos, A.; Bambra, C. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant. Proc. 2005, 37, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.P.; Singh, J.; Baerwald, A.R. Large animal models for the study of ovarian follicular dynamics in women. Theriogenology 2012, 78, 1733–1748. [Google Scholar] [CrossRef] [PubMed]
- Tarantal, A.F.; Lee, C.C.I.; Itkin-Ansari, P. Real-time bioluminescence imaging of macroencapsulated fibroblasts reveals allograft protection in rhesus monkeys (Macaca mulatta). Transplantation 2009, 88, 38–41. [Google Scholar] [CrossRef]
- Shideler, S.E.; Gee, N.A.; Chen, J.; Laughlin, L.; Rapp, P.; Morrison, J.; Roberts, J.; Moran, F.; Lasley, B. Contribution of Ovarian Steroid Production to Urinary Estrone Conjugate Concentrations in Macaca mulatta. Am. J. Primatol. 2003, 61, 111–121. [Google Scholar] [CrossRef]
- Shideler, S.E.; Munro, C.J.; Tell, L.; Owiti, G.; Laughlin, L.; Chatterton, R.; Lasley, B.L. The relationship of serum estradiol and progesterone concentrations to the enzyme immunoassay measurements of urinary estrone conjugates and immunoreactive pregnanediol-3-glucuronide in Macaca mulatta. Am. J. Primatol. 1990, 22, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, C.L.; Vandevoort, C.A. Follicle growth, ovulation, and luteal formation in primates and rodents: A comparative perspective. Exp. Biol. Med. 2013, 238, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Zelinski, M.B.; Murphy, M.K.; Lawson, M.S.; Jurisicova, A.; Pau, K.Y.F.; Toscano, N.P.; Jacob, D.S.; Fanton, J.K.; Casper, R.F.; Dertinger, S.D.; et al. In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates. Fertil. Steril. 2011, 95, 1440–1445.e7. [Google Scholar] [CrossRef]
- Monfort, S.L.; Hess, D.L.; Shideler, S.E.; Samuels, S.J.; Hendrickx, A.G.; Lasley, B.L. Comparison of Serum Estradiol to Urinary Estrone Conjugates in the Rhesus Macaque (Macaca mulatta). Biol. Reprod. 1987, 37, 832–837. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Day, J.R.; Flanagan, C.L.; David, A.; Hartigan-O’Connor, D.J.; Garcia de Mattos Barbosa, M.; Martinez, M.L.; Lee, C.; Barnes, J.; Farkash, E.; Zelinski, M.; et al. Encapsulated Allografts Preclude Host Sensitization and Promote Ovarian Endocrine Function in Ovariectomized Young Rhesus Monkeys and Sensitized Mice. Bioengineering 2023, 10, 550. https://doi.org/10.3390/bioengineering10050550
Day JR, Flanagan CL, David A, Hartigan-O’Connor DJ, Garcia de Mattos Barbosa M, Martinez ML, Lee C, Barnes J, Farkash E, Zelinski M, et al. Encapsulated Allografts Preclude Host Sensitization and Promote Ovarian Endocrine Function in Ovariectomized Young Rhesus Monkeys and Sensitized Mice. Bioengineering. 2023; 10(5):550. https://doi.org/10.3390/bioengineering10050550
Chicago/Turabian StyleDay, James R., Colleen L. Flanagan, Anu David, Dennis J. Hartigan-O’Connor, Mayara Garcia de Mattos Barbosa, Michele L. Martinez, Charles Lee, Jenna Barnes, Evan Farkash, Mary Zelinski, and et al. 2023. "Encapsulated Allografts Preclude Host Sensitization and Promote Ovarian Endocrine Function in Ovariectomized Young Rhesus Monkeys and Sensitized Mice" Bioengineering 10, no. 5: 550. https://doi.org/10.3390/bioengineering10050550
APA StyleDay, J. R., Flanagan, C. L., David, A., Hartigan-O’Connor, D. J., Garcia de Mattos Barbosa, M., Martinez, M. L., Lee, C., Barnes, J., Farkash, E., Zelinski, M., Tarantal, A., Cascalho, M., & Shikanov, A. (2023). Encapsulated Allografts Preclude Host Sensitization and Promote Ovarian Endocrine Function in Ovariectomized Young Rhesus Monkeys and Sensitized Mice. Bioengineering, 10(5), 550. https://doi.org/10.3390/bioengineering10050550





