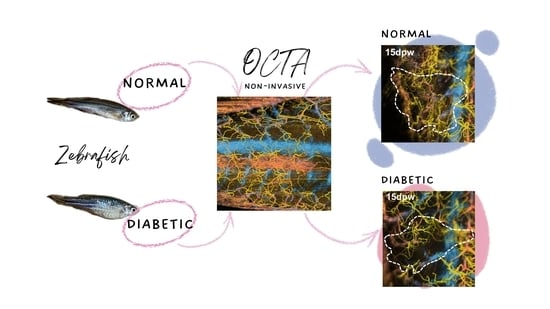
Non-Invasive Monitoring of Cutaneous Wound Healing in Non-Diabetic and Diabetic Model of Adult Zebrafish Using OCT Angiography
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish and Wounding
2.2. MTZ Treatment
2.3. Measurement of Blood Glucose Level
2.4. Fluorescence Imaging
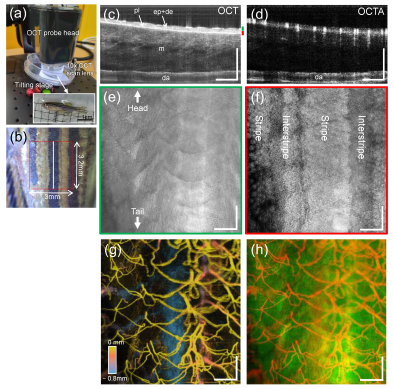
2.5. OCTA Imaging
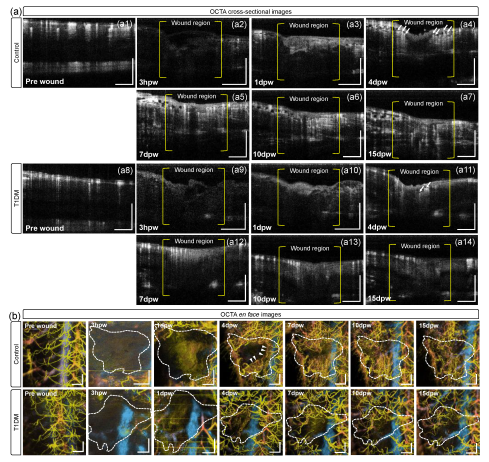
2.6. Quantification of Vascular Response in the Wound Site
2.7. Statistical Analysis
3. Results
3.1. Identification of Diabetic Zebrafish Model
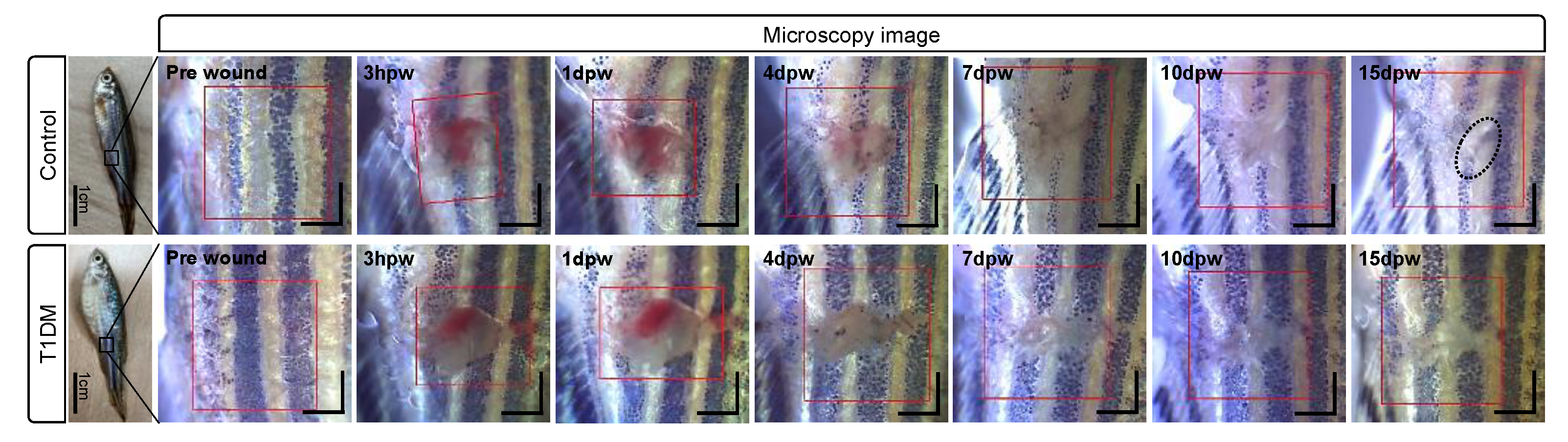
3.2. Structural Changes during Cutaneous Wound Healing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas 2021: IDF Diabetes Atlas 10th Edition. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 29 November 2022).
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Weledji, E.P.; Fokam, P. Treatment of the diabetic foot–to amputate or not? BMC Surg. 2014, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Desmoulière, A.; Chaponnier, C.; Gabbiani, G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef]
- Hinz, B. Formation and Function of the Myofibroblast during Tissue Repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Monika, P.; Waiker, P.V.; Chandraprabha, M.N.; Rangarajan, A.; Murthy, K.N.C. Myofibroblast progeny in wound biology and wound healing studies. Wound Repair Regen. 2021, 29, 531–547. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Murthy, K.N.C. Challenges in Healing Wound: Role of Complementary and Alternative Medicine. Front. Nutr. 2021, 8, 791899. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e2091. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult Zebrafish as a Model System for Cutaneous Wound-Healing Research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Metzger, M.; Knyphausen, P.; Ramezani, T.; Slanchev, K.; Kraus, C.; Schmelzer, E.; Hammerschmidt, M. Re-epithelialization of cutaneous wounds in adult zebrafish combines mechanisms of wound closure in embryonic and adult mammals. Development 2016, 15, 2077–2088. [Google Scholar] [CrossRef]
- Yen, J.; White, R.M.; Wedge, D.C.; van Loo, P.; de Ridder, J.; Capper, A.; Richardson, J.; Jones, D.; Raine, K.; Watson, I.R.; et al. The genetic heterogeneity and mutational burden of engineered melanomas in zebrafish models. Genome Biol. 2013, 14, R113. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Sallinen, V.; Sundvik, M.; Kolehmainen, J.; Torkko, V.; Tiittula, A.; Moshnyakov, M.; Podlasz, P. Modulatory Neurotransmitter Systems and Behavior: Towards Zebrafish Models of Neurodegenerative Diseases. Zebrafish 2006, 3, 235–247. [Google Scholar] [CrossRef]
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Abbate, F.; Laurà, R.; Navarra, M.; Vega, J.A.; Ciriaco, E.; Germana, A. Morphological differences in adipose tissue and changes in BDNF/Trkb expression in brain and gut of a diet induced obese zebrafish model. Ann. Anat. 2016, 204, 36–44. [Google Scholar] [CrossRef]
- Tsuji, N.; Ninov, N.; Delawary, M.; Osman, S.; Roh, A.S.; Gut, P.; Stainier, D.Y.R. Whole Organism High Content Screening Identifies Stimulators of Pancreatic Beta-Cell Proliferation. PLoS ONE 2014, 9, e104112. [Google Scholar] [CrossRef]
- Richardson, R.J. Parallels between vertebrate cardiac and cutaneous wound healing and regeneration. NPJ Regen. Med. 2018, 3, 21. [Google Scholar] [CrossRef]
- Noishiki, C.; Yuge, S.; Ando, K.; Wakayama, Y.; Mochizuki, N.; Ogawa, R.; Fukuhara, S. Live imaging of angiogenesis during cutaneous wound healing in adult zebrafish. Angiogenesis 2019, 22, 341–354. [Google Scholar] [CrossRef]
- Schmitt, J.M. Optical coherence tomography (OCT): A review. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1205–1215. [Google Scholar] [CrossRef]
- Mason, C.; Markusen, J.F.; Town, M.A.; Dunnill, P.; Wang, R.K. The potential of optical coherence tomography in the engineering of living tissue. Phys. Med. Biol. 2004, 49, 1097–1115. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Alexandrov, S.; Rani, S.; Zhou, Y.; Ritter, T.; Leahy, M. Nanosensitive optical coherence tomography to assess wound healing within the cornea. Biomed. Opt. Express 2020, 11, 3407–3422. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Mandal, M.; Mitra, P.; Chatterjee, J. Attenuationcorrected-opticalcoherence tomography for quantitative assessment of skin wound healing and scar morphology. J. Biophotonics 2020, 14, e202000357. [Google Scholar] [CrossRef] [PubMed]
- Vakoc, B.J.; Lanning, R.M.; Tyrrell, J.A.; Padera, T.P.; Bartlett, L.A.; Stylianopoulos, T.; Munn, L.L.; Tearney, G.J.; Fukumura, D.; Jain, R.K.; et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009, 15, 1219–1223. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Deegan, A.J.; Wang, W.; Men, S.; Li, Y.; Song, S.; Xu, J.; Wang, R.K. Optical coherence tomography angiography monitors human cutaneous wound healing over time. Quant. Imaging Med. Surg. 2018, 8, 135–150. [Google Scholar] [CrossRef]
- Gong, P.; Es’Haghian, S.; Wood, F.M.; Sampson, D.D.; McLaughlin, R.A. Optical coherence tomography angiography for longitudinal monitoring of vascular changes in human cutaneous burns. Exp. Dermatol. 2016, 25, 722–724. [Google Scholar] [CrossRef]
- Liew, Y.M.; McLaughlin, R.A.; Gong, P.; Wood, F.M.; Sampson, D.D. In vivo assessment of human burn scars through automated quantification of vascularity using optical coherence tomography. J. Biomed. Opt. 2013, 18, 061213. [Google Scholar] [CrossRef]
- Argarini, R.; McLaughlin, R.A.; Joseph, S.Z.; Naylor, L.H.; Carter, H.H.; Yeap, B.B.; Jansen, S.J.; Green, D.J. Optical coherence tomography: A novel imaging approach to visualize and quantify cutaneous microvascular structure and function in patients with diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001479. [Google Scholar] [CrossRef]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Untracht, G.R.; Dikaios, N.; Durrani, A.K.; Bapir, M.; Sarunic, M.V.; Sampson, D.D.; Heiss, C.; Sampson, D.M. Pilot study of optical coherence tomography angiography-derived microvascular metrics in hands and feet of healthy and diabetic people. Sci. Rep. 2023, 13, 1122. [Google Scholar] [CrossRef] [PubMed]
- Rieger, S. Ablation of β-Cells in Tg(ins:NTR mCherry) Transgenic Zebrafish Using Metronidazole. Available online: https://www.diacomp.org/shared/document.aspx?id=221&docType=Protocol (accessed on 15 December 2022).
- Curado, S.; Anderson, R.M.; Jungblut, B.; Mumm, J.; Schroeter, E.; Stainier, D.Y. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev. Dyn. 2007, 236, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Kahanovitz, L.M.; Sluss, P.M.; Russell, S.J. Type 1 Diabetes—A Clinical Perspective. Point Care 2017, 16, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Wang, R.K. Highly efficient eigen decomposition based statistical optical microangiography. Quant. Imaging Med. Surg. 2016, 6, 557–563. [Google Scholar] [CrossRef]
- Baran, U.; Zhu, W.; Choi, W.J.; Omori, M.; Zhang, W.; Alkayed, N.J.; Wang, R.K. Automated segmentation and enhancement of optical coherence tomography-acquired images of rodent brain. J. Neurosci. Methods 2016, 270, 132–137. [Google Scholar] [CrossRef]
- Pourghadamyari, H.; Rezaei, M.; Basiri, M.; Tahamtani, Y.; Asgari, B.; Hassani, S.-N.; Meshkani, R.; Golmohammadi, T.; Baharvand, H. Generation of a Transgenic Zebrafish Model for Pancreatic Beta Cell Regeneration. Galen Med. J. 2019, 8, e1056. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- Sveen, L.; Karlsen, C.; Ytteborg, E. Mechanical induced wounds in fish—A review on models and healing mechanisms. Rev. Aquac. 2020, 12, 2446–2465. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; Chen, L.; Ma, D.; Haywood, V.A.; Barakat, M.; Urao, N.; DiPietro, L.A. Compromised angiogenesis and vascular Integrity in impaired diabetic wound healing. PLoS ONE 2020, 15, e0231962. [Google Scholar] [CrossRef] [PubMed]
- Pfister, M.; Schützenberger, K.; Schäfer, B.J.; Puchner, S.; Stegmann, H.; Hohenadl, C.; Mildner, M.; Garhöfer, G.; Schmetterer, L.; Werkmeister, R.M. Optical Coherence Tomography Angiography Monitors Cutaneous Wound Healing under Angiogenesis-Promoting Treatment in Diabetic and Non-Diabetic Mice. Appl. Sci. 2021, 11, 2447. [Google Scholar] [CrossRef]
- Moss, J.B.; Koustubhan, P.; Greenman, M.; Parsons, M.J.; Walter, I.; Moss, L.G. Regeneration of the Pancreas in Adult Zebrafish. Diabetes 2009, 58, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Wang, R.K. Optical coherence tomography based angiography [Invited]. Biomed. Opt. Express 2017, 8, 1056–1082. [Google Scholar] [CrossRef]
- Bozic, I.; Li, X.; Tao, Y. Quantitative biometry of zebrafish retinal vasculature using optical coherence tomographic angiography. Biomed. Opt. Express 2018, 9, 1244–1255. [Google Scholar] [CrossRef]
- Yang, D.; Yuan, Z.; Yang, Z.; Hu, M.; Liang, Y. High-resolution polarization-sensitive optical coherence tomography and optical coherence tomography angiography for zebrafish skin imaging. J. Innov. Opt. Health Sci. 2021, 14, 2150022. [Google Scholar] [CrossRef]
- Lichtenegger, A.; Mukherjee, P.; Tamaoki, J.; Bian, L.; Zhu, L.; El-Sadek, I.A.; Makita, S.; Leskovar, K.; Kobayashi, M.; Baumann, B.; et al. Multicontrast investigation of in vivo wildtype zebrafish in three development stages using polarization-sensitive optical coherence tomography. J. Biomed. Opt. 2022, 27, 016001. [Google Scholar] [CrossRef]
- Sharma, P.; Kumawat, J.; Kumar, S.; Sahu, K.; Verma, Y.; Gupta, P.K.; Rao, K.D. Feasibility of speckle variance OCT for imaging cutaneous microvasculature regeneration during healing of wounds in diabetic mice. Laser Phys. 2018, 28, 025601. [Google Scholar] [CrossRef]
- Rico-Jimenez, J.; Lee, J.H.; Alex, A.; Musaad, S.; Chaney, E.; Barkalifa, R.; Spillman, D.R., Jr.; Olson, E.; Adams, D.; Marjanovic, M.; et al. Non-invasive monitoring of pharmacodynamics during the skin wound healing process using multimodal optical microscopy. BMJ Open Diabetes Res. Care 2020, 8, e000974. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Tang, P.; Xie, Z.; Li, Y.; Wang, R.K. Dynamic imaging and quantification of subcellular motion with eigen-decomposition optical coherence tomography-based variance analysis. J. Biophotonics 2019, 12, e201900076. [Google Scholar] [CrossRef] [PubMed]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical Vascular Endothelial Growth Factor Accelerates Diabetic Wound Healing through Increased Angiogenesis and by Mobilizing and Recruiting Bone Marrow-Derived Cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Hall, A.P. Review of the Pericyte during Angiogenesis and its Role in Cancer and Diabetic Retinopathy. Toxicol. Pathol. 2006, 34, 763–775. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Fadini, G.P.; Miorin, M.; Facco, M.; Bonamico, S.; Baesso, I.; Grego, F.; Menegolo, M.; de Kreutzenberg, S.V.; Tiengo, A.; Agostini, C.; et al. Circulating Endothelial Progenitor Cells Are Reduced in Peripheral Vascular Complications of Type 2 Diabetes Mellitus. J. Am. Coll. Cardiol. 2005, 45, 1449–1457. [Google Scholar] [CrossRef]
- Pisharath, H.; Rhee, J.M.; Swanson, M.A.; Leach, S.D.; Parsons, M.J. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech. Dev. 2007, 124, 218–229. [Google Scholar] [CrossRef]
- Sarras, M.P., Jr. Genetic and chemically-induced Zebrafish models for the study of diabetes mellitus. MOJ Anat. Physiol. 2018, 5, 319–321. [Google Scholar] [CrossRef]
- Matsuda, H. Zebrafish as a model for studying functional pancreatic β cells development and regeneration. Dev. Growth Differ. 2018, 60, 393–399. [Google Scholar] [CrossRef]
- Li, Y.; Tang, P.; Song, S.; Rakymzhan, A.; Wang, R.K. Electrically tunable lens integrated with optical coherence tomography angiography for cerebral blood flow imaging in deep cortical layers in mice. Opt. Lett. 2019, 44, 5037–5040. [Google Scholar] [CrossRef] [PubMed]
- Untracht, G.R.; Matos, R.S.; Dikaios, N.; Bapir, M.; Durrani, A.K.; Butsabong, T.; Campagnolo, P.; Sampson, D.D.; Heiss, C.; Sampson, D.M. OCTAVA: An open-source toolbox for quantitative analysis of optical coherence tomography angiography images. PLoS ONE 2021, 16, e0261052. [Google Scholar] [CrossRef] [PubMed]
- Armi, L.; Fekri-Ershad, S. Texture image analysis and texture classification methods—A review. arXiv 2019, arXiv:1904.06554. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, S.; Choi, W.J. Non-Invasive Monitoring of Cutaneous Wound Healing in Non-Diabetic and Diabetic Model of Adult Zebrafish Using OCT Angiography. Bioengineering 2023, 10, 538. https://doi.org/10.3390/bioengineering10050538
Kim J, Kim S, Choi WJ. Non-Invasive Monitoring of Cutaneous Wound Healing in Non-Diabetic and Diabetic Model of Adult Zebrafish Using OCT Angiography. Bioengineering. 2023; 10(5):538. https://doi.org/10.3390/bioengineering10050538
Chicago/Turabian StyleKim, Jaeyoung, Suhyun Kim, and Woo June Choi. 2023. "Non-Invasive Monitoring of Cutaneous Wound Healing in Non-Diabetic and Diabetic Model of Adult Zebrafish Using OCT Angiography" Bioengineering 10, no. 5: 538. https://doi.org/10.3390/bioengineering10050538
APA StyleKim, J., Kim, S., & Choi, W. J. (2023). Non-Invasive Monitoring of Cutaneous Wound Healing in Non-Diabetic and Diabetic Model of Adult Zebrafish Using OCT Angiography. Bioengineering, 10(5), 538. https://doi.org/10.3390/bioengineering10050538