Assessment of Dorsiflexion Ability across Tasks in Persons with Subacute SCI after Combined Locomotor Training and Transcutaneous Spinal Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
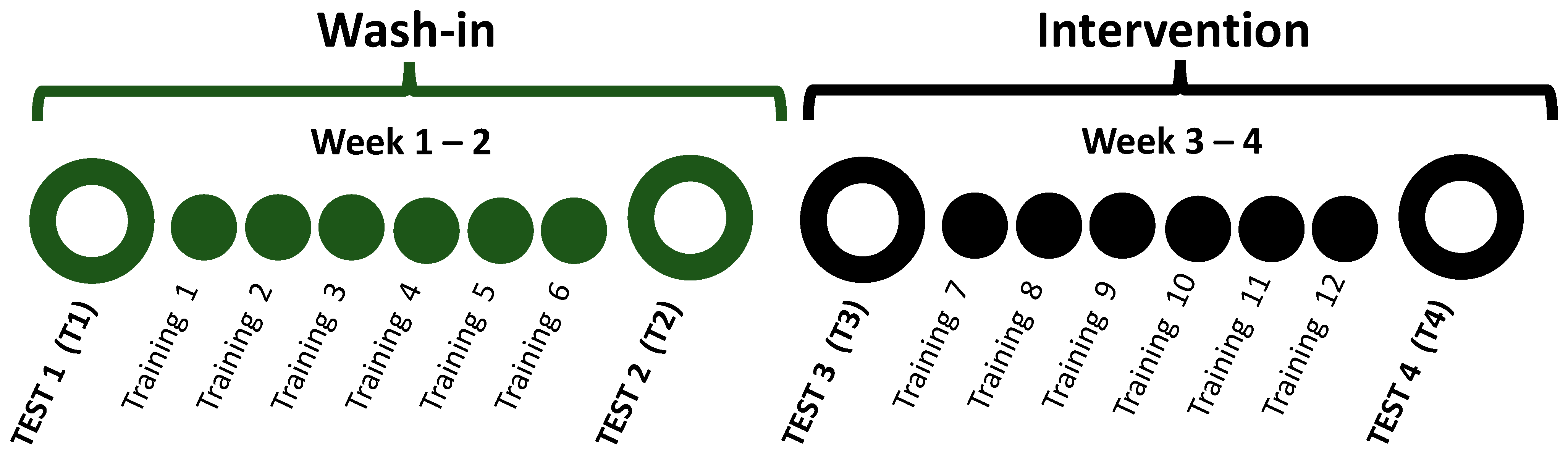
2.2. Study Design
2.3. Intervention
2.3.1. Locomotor Training (LT)
2.3.2. Transcutaneous Spinal Stimulation (TSS)
2.3.3. Sham-Control Stimulation
2.4. Outcome Measures
2.4.1. Dorsiflexion during Walking
2.4.2. Dorsiflexor Activation Task
2.4.3. Ankle Clonus Drop Test
2.5. Data Analysis
3. Results
3.1. Demographics
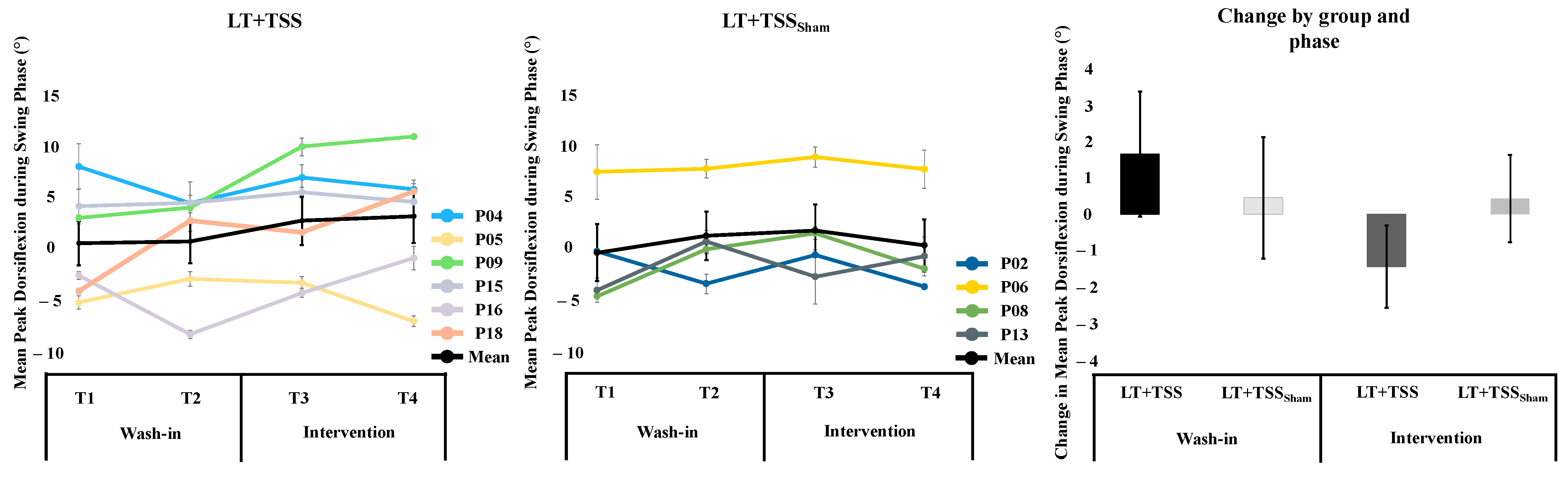
3.2. Dorsiflexion during Walking
3.3. Dorsiflexor Activation Task
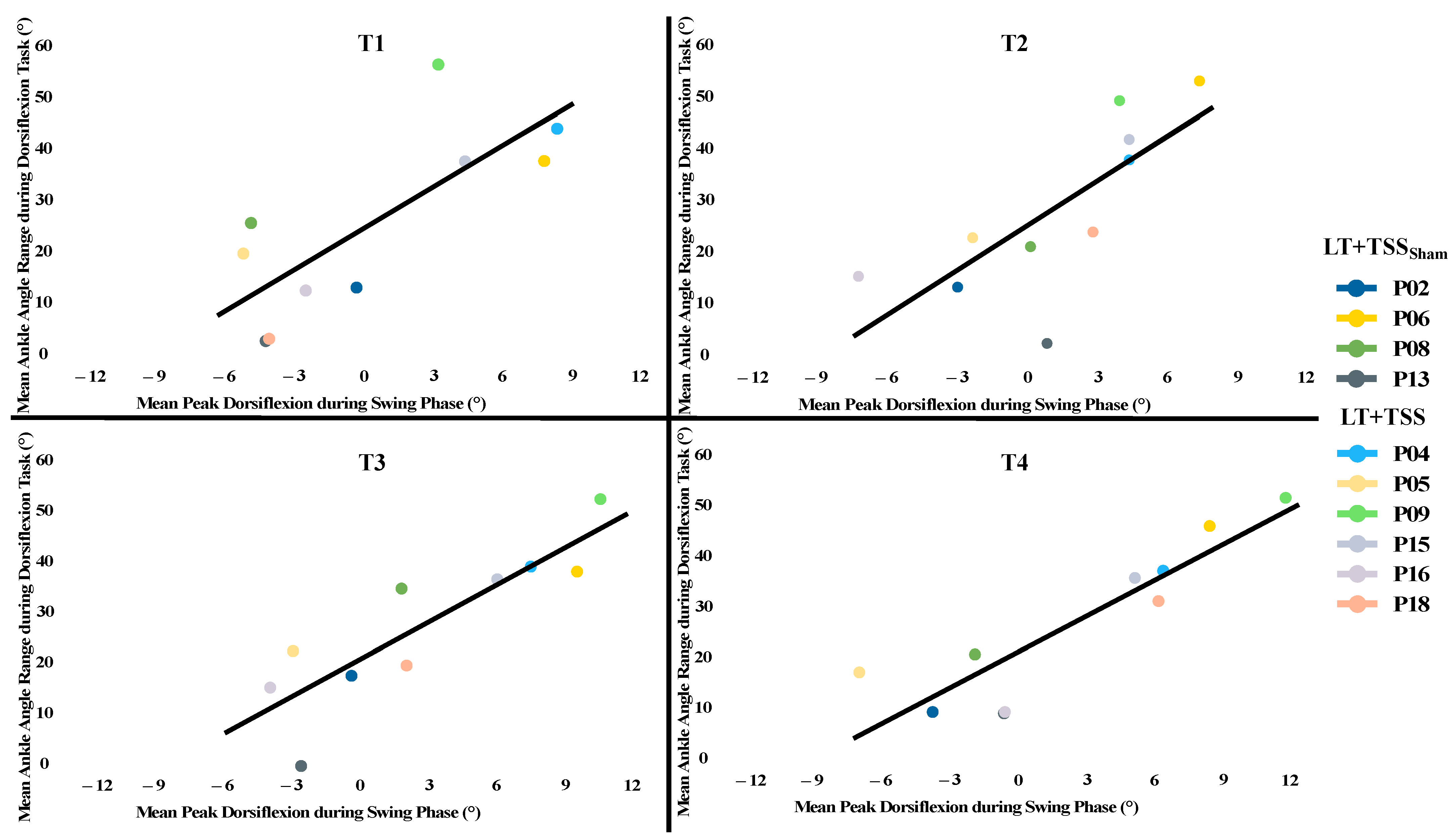
3.4. Overall Effects of Locomotor Training on Ankle-Related Outcomes and Relationships among Measures
4. Discussion
4.1. Dorsiflexion during Walking
4.2. Dorsiflexor Activation Task
4.3. Overall Effects of Locomotor Training on Ankle-Related Outcomes
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Clinical Trial Registration
References
- Ditunno, P.L.; Patrick, M.; Stineman, M.; Ditunno, J.F. Who wants to walk? Preferences for recovery after SCI: A longitudinal and cross-sectional study. Spinal Cord. 2008, 46, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Kemoun, G.; Thoumie, P.; Boisson, D.; Guieu, J.D. Ankle dorsiflexion delay can predict falls in the elderly. J. Rehabil. Med. 2002, 34, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Capaday, C.; Lavoie, B.A.; Barbeau, H.; Schneider, C.; Bonnard, M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J. Neurophysiol. 1999, 81, 129–139. [Google Scholar] [CrossRef]
- Schubert, M.; Curt, A.; Jensen, L.; Dietz, V. Corticospinal input in human gait: Modulation of magnetically evoked motor responses. Exp. Brain Res. 1997, 115, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Calancie, B.; Needham-Shropshire, B.; Jacobs, P.; Willer, K.; Zych, G.; Green, B.A. Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain 1994, 117 Pt 5, 1143–1159. [Google Scholar] [CrossRef] [PubMed]
- Minassian, K.; Hofstoetter, U.S.; Dzeladini, F.; Guertin, P.A.; Ijspeert, A. The Human Central Pattern Generator for Locomotion: Does It Exist and Contribute to Walking? Neuroscientist 2017, 23, 649–663. [Google Scholar] [CrossRef]
- Maegele, M.; Muller, S.; Wernig, A.; Edgerton, V.R.; Harkema, S.J. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J. Neurotrauma 2002, 19, 1217–1229. [Google Scholar] [CrossRef]
- Manella, K.J.; Roach, K.E.; Field-Fote, E.C. Operant conditioning to increase ankle control or decrease reflex excitability improves reflex modulation and walking function in chronic spinal cord injury. J. Neurophysiol. 2013, 109, 2666–2679. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Fung, J.; Edamura, M.; Blunt, R.; Stein, R.B.; Barbeau, H. H-reflex modulation during walking in spastic paretic subjects. Can. J. Neurol. Sci. 1991, 18, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Levine, J.M.; Wecht, J.R.; Maher, M.T.; LiMonta, J.M.; Saeed, S.; Santiago, T.M.; Bailey, E.; Kastuar, S.; Guber, K.S.; et al. Posteroanterior cervical transcutaneous spinal stimulation targets ventral and dorsal nerve roots. Clin. Neurophysiol. 2020, 131, 451–460. [Google Scholar] [CrossRef]
- de Freitas, R.M.; Capogrosso, M.; Nomura, T.; Milosevic, M. Preferential activation of proprioceptive and cutaneous sensory fibers compared to motor fibers during cervical transcutaneous spinal cord stimulation: A computational study. J. Neural Eng. 2022, 19, 036012. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Fernandes, S.R.; Miranda, P.C.; de Carvalho, M. Lumbar trans-spinal direct current stimulation: A modeling-experimental approach to dorsal root ganglia stimulation. Front. Neurosci. 2022, 16, 1041932. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Krenn, M.; Danner, S.M.; Hofer, C.; Kern, H.; McKay, W.B.; Mayr, W.; Minassian, K. Augmentation of Voluntary Locomotor Activity by Transcutaneous Spinal Cord Stimulation in Motor-Incomplete Spinal Cord-Injured Individuals. Artif. Organs 2015, 39, E176–E186. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Chemello, E.; Castellazzi, P.; Roncari, L.; Waldner, A.; Saltuari, L.; Smania, N. Combined effects of transcranial direct current stimulation (tDCS) and transcutaneous spinal direct current stimulation (tsDCS) on robot-assisted gait training in patients with chronic stroke: A pilot, double blind, randomized controlled trial. Restor. Neurol. Neurosci. 2015, 33, 357–368. [Google Scholar] [CrossRef]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous Spinal Cord Stimulation Restores Hand and Arm Function After Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef]
- Meyer, C.; Hofstoetter, U.S.; Hubli, M.; Hassani, R.H.; Rinaldo, C.; Curt, A.; Bolliger, M. Immediate Effects of Transcutaneous Spinal Cord Stimulation on Motor Function in Chronic, Sensorimotor Incomplete Spinal Cord Injury. J. Clin. Med. 2020, 9, 3541. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Lackner, P.; Binder, H. Transcutaneous Spinal Cord Stimulation Enhances Walking Performance and Reduces Spasticity in Individuals with Multiple Sclerosis. Brain Sci. 2021, 11, 472. [Google Scholar] [CrossRef]
- Kandel, E.R.; Hawkins, R.D. The biological basis of learning and individuality. Sci. Am. 1992, 267, 78–86. [Google Scholar] [CrossRef]
- Hubbard, I.J.; Parsons, M.W.; Neilson, C.; Carey, L.M. Task-specific training: Evidence for and translation to clinical practice. Occup. Ther. Int. 2009, 16, 175–189. [Google Scholar] [CrossRef]
- Nooijen, C.F.; Ter Hoeve, N.; Field-Fote, E.C. Gait quality is improved by locomotor training in individuals with SCI regardless of training approach. J. Neuroeng. Rehabil. 2009, 6, 36. [Google Scholar] [CrossRef]
- Sandler, E.B.; Roach, K.E.; Field-Fote, E.C. Dose-Response Outcomes Associated with Different Forms of Locomotor Training in Persons with Chronic Motor-Incomplete Spinal Cord Injury. J. Neurotrauma 2017, 34, 1903–1908. [Google Scholar] [CrossRef]
- Field-Fote, E.C. Exciting recovery: Augmenting practice with stimulation to optimize outcomes after spinal cord injury. Prog. Brain Res. 2015, 218, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.M.; Koter, R.Z.; Estes, S.P.; Field-Fote, E.C. Disrupted Ankle Control and Spasticity in Persons With Spinal Cord Injury: The Association Between Neurophysiologic Measures and Function. A Scoping Review. Front. Neurol. 2020, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, D.; Knudsen, H.; Willerslev-Olsen, M.; Lundell, H.; Nielsen, J.B.; Biering-Sorensen, F. Functional implications of corticospinal tract impairment on gait after spinal cord injury. Spinal Cord. 2013, 51, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.K.; Fiorenza, G.; Smyth, L.; Favale, B.; Brangaccio, J.; Sniffen, J. Operant conditioning of the motor-evoked potential and locomotion in people with and without chronic incomplete spinal cord injury. J. Neurophysiol. 2019, 121, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Adrian, C.; Franziska, M.; Irina, L.; Tim, K.; Bjorn, Z.; Armin, C.; Marc, B. Corticospinal Control of a Challenging Ankle Task in Incomplete Spinal Cord Injury. J. Neurotrauma 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Estes, S.; Zarkou, A.; Hope, J.M.; Suri, C.; Field-Fote, E.C. Combined Transcutaneous Spinal Stimulation and Locomotor Training to Improve Walking Function and Reduce Spasticity in Subacute Spinal Cord Injury: A Randomized Study of Clinical Feasibility and Efficacy. J. Clin. Med. 2021, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef]
- Kesar, T.M.; Binder-Macleod, S.A.; Hicks, G.E.; Reisman, D.S. Minimal detectable change for gait variables collected during treadmill walking in individuals post-stroke. Gait Posture 2011, 33, 314–317. [Google Scholar] [CrossRef]
- Koelman, J.H.; Bour, L.J.; Hilgevoord, A.A.; van Bruggen, G.J.; Ongerboer de Visser, B.W. Soleus H-reflex tests and clinical signs of the upper motor neuron syndrome. J. Neurol. Neurosurg. Psychiatry 1993, 56, 776–781. [Google Scholar] [CrossRef]
- Borenstein, M. Hypothesis testing and effect size estimation in clinical trials. Ann. Allergy Asthma Immunol. 1997, 78, 5–11. [Google Scholar] [CrossRef]
- Kinney, A.R.; Eakman, A.M.; Graham, J.E. Novel Effect Size Interpretation Guidelines and an Evaluation of Statistical Power in Rehabilitation Research. Arch. Phys. Med. Rehabil. 2020, 101, 2219–2226. [Google Scholar] [CrossRef]
- Barthelemy, D.; Willerslev-Olsen, M.; Lundell, H.; Conway, B.A.; Knudsen, H.; Biering-Sorensen, F.; Nielsen, J.B. Impaired transmission in the corticospinal tract and gait disability in spinal cord injured persons. J. Neurophysiol. 2010, 104, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Bonnyaud, C.; Geiger, M.; Bussel, B.; Bensmail, D. Relationship between hip flexion and ankle dorsiflexion during swing phase in chronic stroke patients. Clin. Biomech. 2015, 30, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H.; Firestine, A.; West, M.; Saremi, K.; Woods, R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage 2004, 23, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Gates, D.H.; Darter, B.J.; Dingwell, J.B.; Wilken, J.M. Comparison of walking overground and in a Computer Assisted Rehabilitation Environment (CAREN) in individuals with and without transtibial amputation. J. Neuroeng. Rehabil. 2012, 9, 81. [Google Scholar] [CrossRef]
- Mirbagheri, M.M.; Duffell, L.D.; Kotsapouikis, D.; Rogers, L.M. Reciprocal inhibition becomes facilitation after spinal cord injury: Clinical application of a system identification approach. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 4395–4398. [Google Scholar] [CrossRef]
- Crone, C.; Johnsen, L.L.; Biering-Sorensen, F.; Nielsen, J.B. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 2003, 126, 495–507. [Google Scholar] [CrossRef]

| Subject ID | Sex | Age (Years) | Time since Injury (Days) | AIS | Neurological Injury Level | LE (Tested) | LEMS (Tested) | LEMS (Total) | Clonus (Tested) | Group |
|---|---|---|---|---|---|---|---|---|---|---|
| P04 | F | 53 | 36 | D | C2 | L | 20 | 42 | 15.33 | LT + TSS |
| P05 | M | 56 | 84 | D | C4 | R | 20 | 44 | 2.67 | LT + TSS |
| P09 | M | 18 | 83 | D | C7 | R | 22 | 33 | 3.00 | LT + TSS |
| P15 | M | 54 | 141 | D | C5 | R | 18 | 32 | 3.33 | LT + TSS |
| P16 | M | 63 | 185 | D | C1 | R | 22 | 46 | 3.00 | LT + TSS |
| P18 | M | 18 | 47 | D | C5 | L | 8 | 33 | 22.33 | LT + TSS |
| P02 | M | 43 | 80 | C | C4 | R | 14 | 27 | 2.67 | LT + TSSSham |
| P06 | M | 37 | 103 | C | C3 | L | 22 | 39 | 32.00 | LT + TSSSham |
| P08 | M | 47 | 119 | D | C2 | L | 21 | 42 | 3.00 | LT + TSSSham |
| P13 | M | 20 | 68 | D | C4 | R | 11 | 36 | 24.33 | LT + TSSSham |
| Wash-in Phase | Intervention Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | Difference | Effect Size | T3 | T4 | Difference | Effect Size | |
| Peak Dorsiflexion during Swing Phase (°) | ||||||||
| LT + TSS | 0.76 (5.23) | 0.96 (5.21) | 0.20 (4.41) | 0.04 | 2.93 (5.67) | 3.36 (6.20) | 0.43 (2.92) | 0.07 |
| LT + TSSSham | −0.31 (5.63) | 1.34 (4.75) | 1.65 (3.83) | 0.32 | 1.85 (5.12) | 0.41 (5.14) | −1.44 (2.52) | −0.28 |
| Ankle Volitional Range during Dorsiflexion Task (°) | ||||||||
| LT + TSS | 25.32 (18.41) | 30.45 (12.97) | 5.13 (8.49) | 0.32 | 29.24 (12.85) | 30.30 (13.61) | 1.06 (5.68) | 0.08 |
| LT + TSSSham | 17.12 (13.71) | 21.17 (21.64) | 4.04 (9.60) | 0.22 | 21.68 (15.99) | 22.13 (15.62) | 0.45 (10.56) | 0.03 |
| EMG during Volitional Task (µv) | ||||||||
| LT + TSS | 54.49 (29.73) | 49.48 (13.57) | −5.01 (21.01) | −0.22 | 60.26 (32.59) | 52.48 (29.15) | −7.78 (19.75) | −0.25 |
| LT + TSSSham | 20.48 (20.53) | 31.15 (31.31) | 10.67 (14.40) | 0.4 | 43.65 (29.86) | 41.60 (24.42) | −2.05 (22.51) | −0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hope, J.M.; Field-Fote, E.C. Assessment of Dorsiflexion Ability across Tasks in Persons with Subacute SCI after Combined Locomotor Training and Transcutaneous Spinal Stimulation. Bioengineering 2023, 10, 528. https://doi.org/10.3390/bioengineering10050528
Hope JM, Field-Fote EC. Assessment of Dorsiflexion Ability across Tasks in Persons with Subacute SCI after Combined Locomotor Training and Transcutaneous Spinal Stimulation. Bioengineering. 2023; 10(5):528. https://doi.org/10.3390/bioengineering10050528
Chicago/Turabian StyleHope, Jasmine M., and Edelle C. Field-Fote. 2023. "Assessment of Dorsiflexion Ability across Tasks in Persons with Subacute SCI after Combined Locomotor Training and Transcutaneous Spinal Stimulation" Bioengineering 10, no. 5: 528. https://doi.org/10.3390/bioengineering10050528
APA StyleHope, J. M., & Field-Fote, E. C. (2023). Assessment of Dorsiflexion Ability across Tasks in Persons with Subacute SCI after Combined Locomotor Training and Transcutaneous Spinal Stimulation. Bioengineering, 10(5), 528. https://doi.org/10.3390/bioengineering10050528






