Multi-Objective Optimisation of a Novel Bypass Graft with a Spiral Ridge
Abstract
1. Introduction
2. Optimisation Approach
2.1. Introduction
2.2. Optimisation Criteria
2.3. Design Parameters
2.4. Goal-Driven Optimisation
2.5. Computational Approach
3. Results and Discussion
3.1. Introduction
3.2. Goal-Driven Optimisation Study
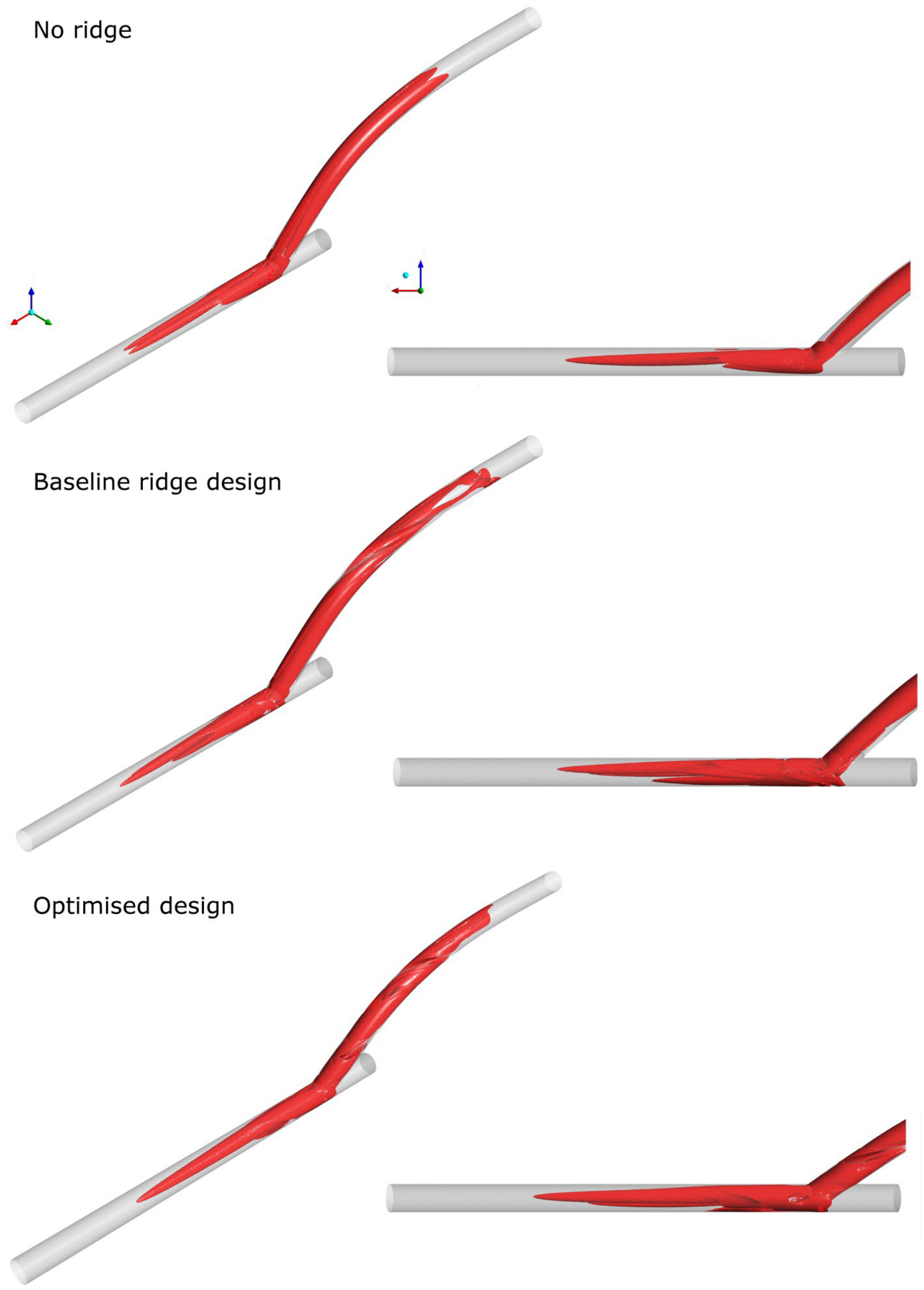
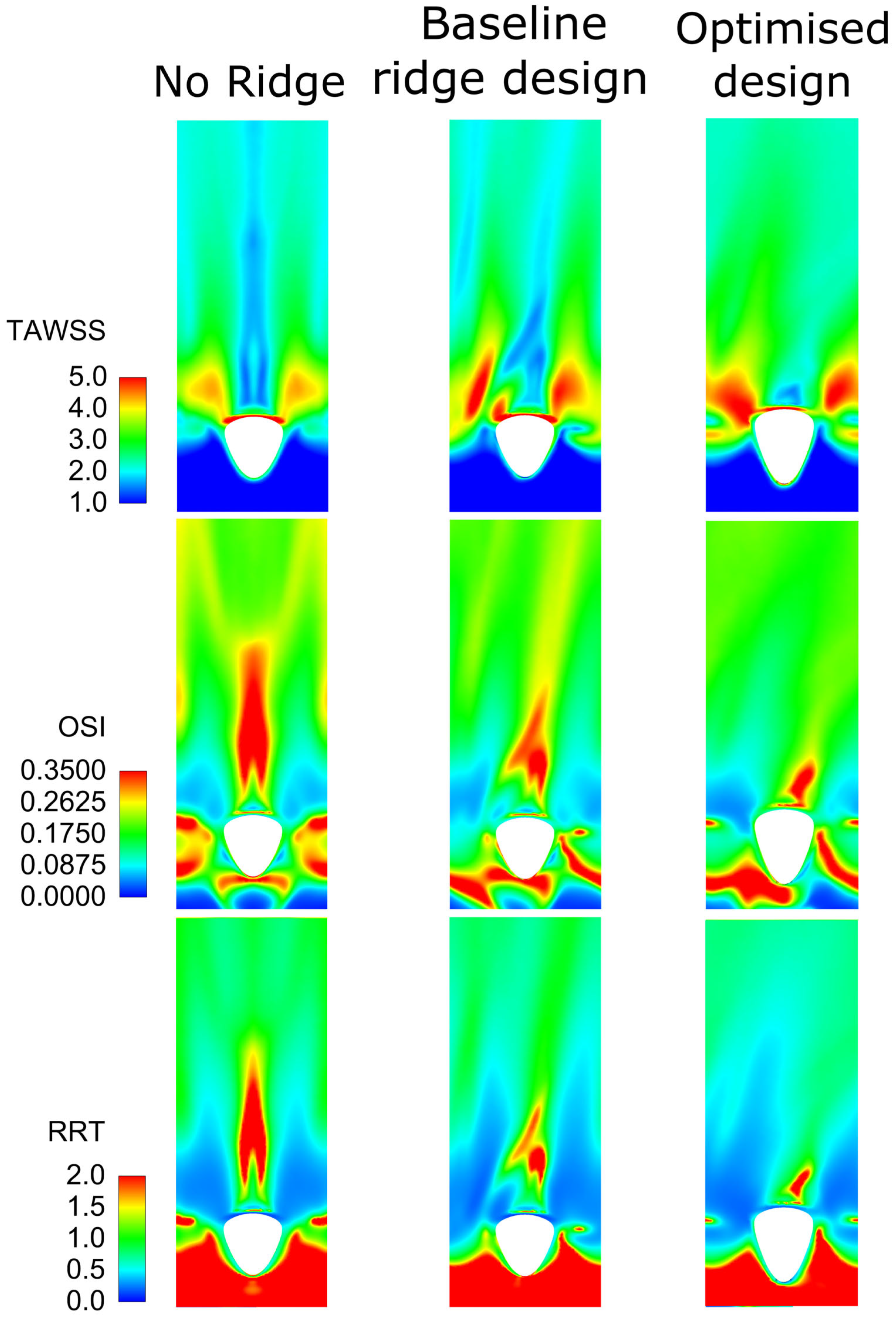
3.3. Assessment of the Optimised Design with Reference Geometries
3.3.1. Steady-State Simulations
3.3.2. Assessment of Optimised Geometry in Transient Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Jüni, P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [CrossRef] [PubMed]
- Haruguchi, H.; Teraoka, S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: A review. J. Artif. Organs 2003, 6, 227–235. [Google Scholar] [CrossRef]
- May, R.E.; Himmelfarb, J.; Yenicesu, M.; Knights, S.; Ikizler, T.A.; Schulman, G.; Hernanz-Schulman, M.; Shyr, Y.; Hakim, R.M. Predictive measures of vascular access thrombosis: A prospective study. Kidney Int. 1997, 52, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Hajati, O.; Zarrabi, K.; Karimi, R.; Hajati, A. CFD simulation of hemodynamics in sequential and individual coronary bypass grafts based on multislice CT scan datasets. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; Volume 2012, pp. 641–644. [Google Scholar]
- Roos, M.W.; Wadbro, E.; Berggren, M. Computational estimation of fluid mechanical benefits from a fluid deflector at the distal end of artificial vascular grafts. Comput. Biol. Med. 2013, 43, 164–168. [Google Scholar] [CrossRef]
- Kaufmann, T.A.S.; Neidlin, M.; Büsen, M.; Sonntag, S.J.; Steinseifer, U. Implementation of intrinsic lumped parameter modeling into computational fluid dynamics studies of cardiopulmonary bypass. J. Biomech. 2014, 47, 729–735. [Google Scholar] [CrossRef]
- Wen, J.; Liu, K.; Khoshmanesh, K.; Jiang, W.; Zheng, T. Numerical investigation of haemodynamics in a helical-type artery bypass graft using non-Newtonian multiphase model. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 760–768. [Google Scholar] [CrossRef]
- Ramachandra, A.B.; Kahn, A.M.; Marsden, A.L. Patient-Specific Simulations Reveal Significant Differences in Mechanical Stimuli in Venous and Arterial Coronary Grafts. J. Cardiovasc. Transl. Res. 2016, 9, 279–290. [Google Scholar] [CrossRef]
- Elliot, W.; Keshmiri, A.; Tan, W. Cardiovascular Mechanobiology. In Mechanobiology: Exploitation for Medical Benefit; Wiley: Hoboken, NJ, USA, 2017; pp. 373–400. ISBN 978-1-118-96614-3. [Google Scholar]
- Archie, J.P.; Hyun, S.; Kleinstreuer, C.; Longest, P.W.; Truskey, G.A.; Buchanan, J.R. Hemodynamic parameters and early intimal thickening in branching blood vessels. Crit. Rev. Biomed. Eng. 2001, 29, 1–64. [Google Scholar] [CrossRef]
- Ghista, D.N.; Kabinejadian, F. Coronary artery bypass grafting hemodynamics and anastomosis design: A biomedical engineering review. Biomed. Eng. Online 2013, 12, 129. [Google Scholar] [CrossRef]
- Stary, H.C.; Blankenhorn, D.H.; Chandler, A.B.; Glagov, S.; Insull, W.J.; Richardson, M.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1992, 85, 391–405. [Google Scholar] [CrossRef]
- Kabinejadian, F.; Ghista, D.N.; Su, B.; Nezhadian, M.K.; Chua, L.P.; Yeo, J.H.; Leo, H.L. In vitro measurements of velocity and wall shear stress in a novel sequential anastomotic graft design model under pulsatile flow conditions. Med. Eng. Phys. 2014, 36, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Boyle, F.J. Reducing In-Stent Restenosis Through Novel Stent Flow Field Augmentation. Cardiovasc. Eng. Technol. 2012, 3, 353–373. [Google Scholar] [CrossRef]
- Caro, C.G.; Cheshire, N.J.; Watkins, N. Preliminary comparative study of small amplitude helical and conventional ePTFE arteriovenous shunts in pigs. J. R. Soc. Interface 2005, 2, 261–266. [Google Scholar] [CrossRef]
- Stonebridge, P.A. Three-dimensional blood flow dynamics: Spiral/helical laminar flow. Methodist Debakey Cardiovasc. J. 2011, 7, 21–26. [Google Scholar] [CrossRef]
- Stonebridge, P.A.; Brophy, C.M. Spiral laminar flow in arteries? Lancet 1991, 338, 1360–1361. [Google Scholar] [CrossRef]
- Sherwin, S.J.; Shah, O.; Doorly, D.J.; Peiro, J.; Papaharilaou, Y.; Watkins, N.; Caro, C.G.; Dumoulin, C.L. The Influence of Out-of-Plane Geometry on the Flow Within a Distal End-to-Side Anastomosis. J. Biomech. Eng. 2000, 122, 86–95. [Google Scholar] [CrossRef]
- Papaharilaou, Y.; Doorly, D.; Sherwin, S. The influence of out-of-plane geometry on pulsatile flow within a distal end-to-side anastomosis. J. Biomech. 2002, 35, 1225–1239. [Google Scholar] [CrossRef]
- Cookson, A.N.; Doorly, D.J.; Sherwin, S.J. Mixing through stirring of steady flow in small amplitude helical tubes. Ann. Biomed. Eng. 2009, 37, 710–721. [Google Scholar] [CrossRef]
- Zheng, T.; Fan, Y.; Xiong, Y.; Jiang, W.; Deng, X. Hemodynamic performance study on small diameter helical grafts. ASAIO J. 2009, 55, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Fan, Y.; Deng, X. Numerical comparative study on the hemodynamic performance of a new helical graft with noncircular cross section and swirlGraft. Artif. Organs 2010, 34, 22–27. [Google Scholar] [CrossRef]
- Lee, K.E.; Lee, J.S.; Yoo, J.Y. A numerical study on steady flow in helically sinuous vascular prostheses. Med. Eng. Phys. 2011, 33, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, A.; Fan, Y.; Deng, X. Physiological Significance of Helical Flow in the Arterial System and its Potential Clinical Applications. Ann. Biomed. Eng. 2014, 43, 3–15. [Google Scholar] [CrossRef]
- Ruiz-Soler, A.; Kabinejadian, F.; Slevin, M.A.; Bartolo, P.J.; Keshmiri, A. Optimisation of a novel spiral-inducing bypass graft using computational fluid dynamics. Sci. Rep. 2017, 7, 1865. [Google Scholar] [CrossRef] [PubMed]
- Keshmiri, A.; Ruiz-Soler, A.; McElroy, M.; Kabinejadian, F. Numerical Investigation on the Geometrical Effects of Novel Graft Designs for Peripheral Artery Bypass Surgery. Procedia CIRP 2016, 49, 147–152. [Google Scholar] [CrossRef]
- Kabinejadian, F.; McElroy, M.; Ruiz-Soler, A.; Leo, H.L.; Slevin, M.A.; Badimon, L.; Keshmiri, A. Numerical assessment of novel helical/spiral grafts with improved hemodynamics for distal graft anastomoses. PLoS ONE 2016, 11, e0165892. [Google Scholar] [CrossRef] [PubMed]
- Deyranlou, A.; Naish, J.H.; Miller, C.A.; Revell, A.; Keshmiri, A. Numerical Study of Atrial Fibrillation Effects on Flow Distribution in Aortic Circulation. Ann. Biomed. Eng. 2020, 48, 1291–1308. [Google Scholar] [CrossRef]
- Deyranlou, A.; Miller, C.A.; Revell, A.; Keshmiri, A. Effects of Ageing on Aortic Circulation During Atrial Fibrillation; a Numerical Study on Different Aortic Morphologies. Ann. Biomed. Eng. 2021, 49, 2196–2213. [Google Scholar] [CrossRef]
- Swanson, L.; Owen, B.; Keshmiri, A.; Deyranlou, A.; Aldersley, T.; Lawrenson, J.; Human, P.; De Decker, R.; Fourie, B.; Comitis, G.; et al. A Patient-Specific CFD Pipeline Using Doppler Echocardiography for Application in Coarctation of the Aorta in a Limited Resource Clinical Context. Front. Bioeng. Biotechnol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Mcelroy, M.; Kim, Y.; Niccoli, G.; Vergallo, R.; Smith, A.L.; Crea, F.; Gijsen, F.; Johnson, T.; Keshmiri, A.; White, S. Identification of the haemodynamic environment permissive for plaque erosion. Sci. Rep. 2021, 11, 7253. [Google Scholar] [CrossRef]
- McElroy, M.; Xenakis, A.; Keshmiri, A. Impact of heart failure severity on ventricular assist device haemodynamics: A computational study. Res. Biomed. Eng. 2020, 36, 489–500. [Google Scholar] [CrossRef]
- McElroy, M.; Ruiz-Soler, A.; Keshmiri, A. Left Ventricular Assist Devices: Impact of Flow Ratios on the Localisation of Cardiovascular Diseases Using Computational Fluid Dynamics. Procedia CIRP 2016, 49, 163–169. [Google Scholar] [CrossRef]
- McElroy, M.; Keshmiri, A. Impact of using conventional inlet/outlet boundary conditions on haemodynamic metrics in a subject-specific rabbit aorta. Proc. Inst. Mech. Eng. H 2018, 232, 103–113. [Google Scholar] [CrossRef]
- Deyranlou, A.; Revell, A.; Keshmiri, A. Subject Specific Modelling of Aortic Flows. In Applied Complex Flow: Applications of Complex Flows and CFD; Azizi, A., Ed.; Springer Nature: Singapore, 2023; pp. 69–105. ISBN 978-981-19-7746-6. [Google Scholar]
- Shahbazi, F.; Jabbari, M.; Esfahani, M.N.; Keshmiri, A. Microfluidic-Integrated Biosensors. In Applied Complex Flow: Applications of Complex Flows and CFD; Azizi, A., Ed.; Springer Nature: Singapore, 2023; pp. 21–42. ISBN 978-981-19-7746-6. [Google Scholar]
- Shahbazi, F.; Jabbari, M.; Esfahani, M.N.; Keshmiri, A.; Nasr, M.; Keshmiri, A. Biosensors and Bioelectronics A computational simulation platform for designing real-time monitoring systems with application to COVID-19. Biosens. Bioelectron. 2021, 171, 112716. [Google Scholar] [CrossRef]
- Giannakoglou, K.C. Design of optimal aerodynamic shapes using stochastic optimization methods and computational intelligence. Prog. Aerosp. Sci. 2002, 38, 43–76. [Google Scholar] [CrossRef]
- Hilbert, R.; Janiga, G.; Baron, R.; Thévenin, D. Multi-objective shape optimization of a heat exchanger using parallel genetic algorithms. Int. J. Heat Mass Transf. 2006, 49, 2567–2577. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, Z.; Liu, W. Multi-objective genetic optimization of the heat transfer for tube inserted with porous media. Int. J. Heat Mass Transf. 2016, 101, 981–987. [Google Scholar] [CrossRef]
- De Maesschalck, C.; Lavagnoli, S.; Paniagua, G.; Verstraete, T.; Olive, R.; Picot, P. Heterogeneous optimization strategies for carved and squealer-like turbine blade tips. J. Turbomach. 2016, 138, 121011. [Google Scholar] [CrossRef]
- Safikhani, H.; Eiamsa-Ard, S. Multi-Objective Optimization of Turbulent Tube Flows Over Diamond-Shaped Turbulators. Heat Transf. Eng. 2016, 37, 1579–1584. [Google Scholar] [CrossRef]
- Sousa, L.C.; Castro, C.F.; António, C.C.; Chaves, R. Blood flow simulation and vascular reconstruction. J. Biomech. 2012, 45, 2549–2555. [Google Scholar] [CrossRef]
- Dur, O.; Tolga Coskun, S.; Oguz Coskun, K.; Frakes, D.; Burak Kara, L.; Pekkan, K. Computer-Aided Patient-Specific Coronary Artery Graft Design Improvements Using CFD Coupled Shape Optimizer. Cardiovasc. Eng. Technol. 2011, 2, 35–47. [Google Scholar] [CrossRef]
- Sankaran, S.; Esmaily Moghadam, M.; Kahn, A.M.; Tseng, E.E.; Guccione, J.M.; Marsden, A.L. Patient-Specific Multiscale Modeling of Blood Flow for Coronary Artery Bypass Graft Surgery. Ann. Biomed. Eng. 2012, 40, 2228–2242. [Google Scholar] [CrossRef] [PubMed]
- Perktold, K.; Resch, M. Numerical flow studies in human carotid artery bifurcations: Basic discussion of the geometric factor in atherogenesis. J. Biomed. Eng. 1990, 12, 111–123. [Google Scholar] [CrossRef]
- Goubergrits, L.; Affeld, K.; Fernandez-Britto, J.; Falcon, L. Atherosclerosis and flow in carotid arteries with authentic geometries. Biorheology 2002, 39, 519–524. [Google Scholar]
- Peiffer, V.; Sherwin, S.J.; Weinberg, P.D. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc. Res. 2013, 99, 242–250. [Google Scholar] [CrossRef]
- Steinman, D.A. Image-based computational fluid dynamics: A new paradigm for monitoring hemodynamics and atherosclerosis. Curr. Drug Targets-Cardiovasc. Haematol. Disord. 2004, 4, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Olgac, U.; Kurtcuoglu, V.; Saur, S.C.; Poulikakos, D. Identification of atherosclerotic lesion-prone sites through patient-specific simulation of low-density lipoprotein accumulation. In Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5242, pp. 774–781. [Google Scholar]
- Siogkas, P.; Sakellarios, A.; Exarchos, T.P.; Athanasiou, L.; Karvounis, E.; Stefanou, K.; Fotiou, E.; Fotiadis, D.I.; Naka, K.K.; Michalis, L.K.; et al. Multiscale-Patient-specific artery and atherogenesis models. IEEE Trans. Biomed. Eng. 2011, 58, 3464–3468. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Feldman, C.L.; Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Maynard, C.; Baker, A.B.; Papafaklis, M.I.; Edelman, E.R.; Stone, P.H. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: A serial, in vivo intravascular ultrasound study. Circulation 2010, 121, 2092–2101. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Jonas, M.; Coskun, A.U.; Beigel, R.; Stone, B.V.; Maynard, C.; Gerrity, R.G.; Daley, W.; Rogers, C.; Edelman, E.R.; et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress-an intravascular ultrasound and histopathology natural history study. Circulation 2008, 117, 993–1002. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Baker, A.B.; Sukhova, G.K.; Koskinas, K.C.; Papafaklis, M.I.; Beigel, R.; Jonas, M.; Coskun, A.U.; Stone, B.V.; Maynard, C.; et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation 2011, 123, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.L. Certain chemorheologic considerations regarding the blood vascular wall interface with particular reference to coronary artery disease. Circulation 1969, 40, 38–59. [Google Scholar] [CrossRef]
- DePaola, N.; Gimbrone, M.A.; Davies, P.F.; Dewey, C.F. Vascular endothelium responds to fluid shear stress gradients. Arter. Thromb. Vasc. Biol. 1992, 12, 1254–1257. [Google Scholar] [CrossRef]
- Loth, F.; Fischer, P.F.; Bassiouny, H.S. Blood Flow in End-to-Side Anastomoses. Annu. Rev. Fluid Mech. 2008, 40, 367–393. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic Shear Stress and Its Role in Atherosclerosis. J. Am. Med. Assoc. 1999, 282, 2035–2042. [Google Scholar] [CrossRef]
- Miettinen, K. Nonlinear Multiobjective Optimization; Kluwer Academic Publishers: Boston, MA, USA, 1999; ISBN 9780792382782. [Google Scholar]
- McKay, M.D.; Beckman, R.J.; Conover, W.J. Comparison of Three Methods for Selecting Values of Input Variables in the Analysis of Output from a Computer Code. Technometrics 1979, 21, 239–245. [Google Scholar] [CrossRef]
- ANSYS. Design Exploration User’s Guide; ANSYS, Inc.: Canonsburg, PA, USA, 2013. [Google Scholar]
- Morris, M.D. Factorial sampling plans for preliminary computational experiments. Technometrics 1991, 33, 161–174. [Google Scholar] [CrossRef]
- Durrleman, S.; Simon, R. Fkexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Bierens, H.J. Uniform Consistency of Kernel Estimators of a Regression Function Under Generalized Conditions. J. Am. Stat. Assoc. 1983, 78, 699–707. [Google Scholar] [CrossRef]
- Karush, W. Minima of Functions of Several Variables with Inequalities as Side Constraints. Master’s Thesis, Department of Mathematics, University of Chicago, Chicago, IL, USA, 1939. [Google Scholar]
- Kuhn, H.W.; Tucker, A.W. Nonlinear programming. In Proceedings of the 2nd Berkeley Symposium; University of California Press: Berkeley, CA, USA, 1951; pp. 481–492. [Google Scholar]
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T. A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Branke, J.; Miettinen, K.; Deb, K.; Slowinski, R. Multiobjective Optimization: Interactive and Evolutionary Approaches; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540889076. [Google Scholar]
- Coello Coello, C.A.; Lamont, G.B.; Van Veldhuisen, D.A. Evolutionary Algorithms for Solving Multi-Objective Problems; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9780387367972. [Google Scholar]
- Bird, R.B.; Armstrong, R.C.; Hassanger, O. Dynamics of Polymeric Liquids, Fluid Mechanics; Wiley-Interscience: New York, NY, USA, 1987; Volume 25, ISBN 978-0471802457. [Google Scholar]
- Gijsen, F.J.H.; van de Vosse, F.N.; Janssen, J.D. The influence of non-Newtonian properties of blood of the flow in large arteries: Steady flow in a carotid bifurcation model. J. Biomech. 1999, 32, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Kabinejadian, F.; Cui, F.; Su, B.; Danpinid, A.; Ho, P.; Leo, H.L. Effects of a carotid covered stent with a novel membrane design on the blood flow regime and hemodynamic parameters distribution at the carotid artery bifurcation. Med. Biol. Eng. Comput. 2015, 53, 165–177. [Google Scholar] [CrossRef]
- Kabinejadian, F.; Chua, L.P.; Ghista, D.N.; Tan, Y.S. CABG models flow simulation study on the effects of valve remnants in the venous graft. J. Mech. Med. Biol. 2010, 10, 593–609. [Google Scholar] [CrossRef]
- Moore, J.E., Jr.; Maier, S.E.; Ku, D.N.; Boesiger, P. Hemodynamics in the abdominal aorta: A comparison of in vitro and in vivo measurements. J. Appl. Physiol. 1994, 76, 1520–1527. [Google Scholar] [CrossRef]
- Perktold, K.; Rappitsch, G. Computer simulation of local blood flow and vessel mechanics in a compliant carotid artery bifurcation model. J. Biomech. 1995, 28, 845–856. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ku, D.N. Pulsatile flow in the human left coronary artery bifurcation: Average conditions. J. Biomech. Eng. 1996, 118, 74–82. [Google Scholar] [CrossRef]
- Lee, S.-W.; Antiga, L.; Steinman, D. A Correlations among indicators of disturbed flow at the normal carotid bifurcation. J. Biomech. Eng. 2009, 131, 061013. [Google Scholar] [CrossRef]
- Hellums, J.D. Whitaker lecture: Biorheology in thrombosis research. Ann. Biomed. Eng. 1993, 22, 445–455. [Google Scholar] [CrossRef]
- Ramstack, J.M.; Zuckerman, L.; Mockros, L.F. Shear-induced activation of platelets. J. Biomech. 1979, 12, 113–125. [Google Scholar] [CrossRef]
- Wurzinger, L.J.; Opitz, R.; Eckstein, H. Mechanical bloodtrauma. An overview. Angeiologie 1986, 38, 81–97. [Google Scholar]
- Wurzinger, L.J.; Optiz, R.; Wolf, M.; Schmid-Schonbein, H. “Shear induced platelet activation”—A cricitcal reappraisal. Biorheology 1985, 22, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Cito, S.; Mazzeo, M.D.; Badimon, L. A review of macroscopic thrombus modeling methods. Thromb. Res. 2013, 131, 116–124. [Google Scholar] [CrossRef] [PubMed]
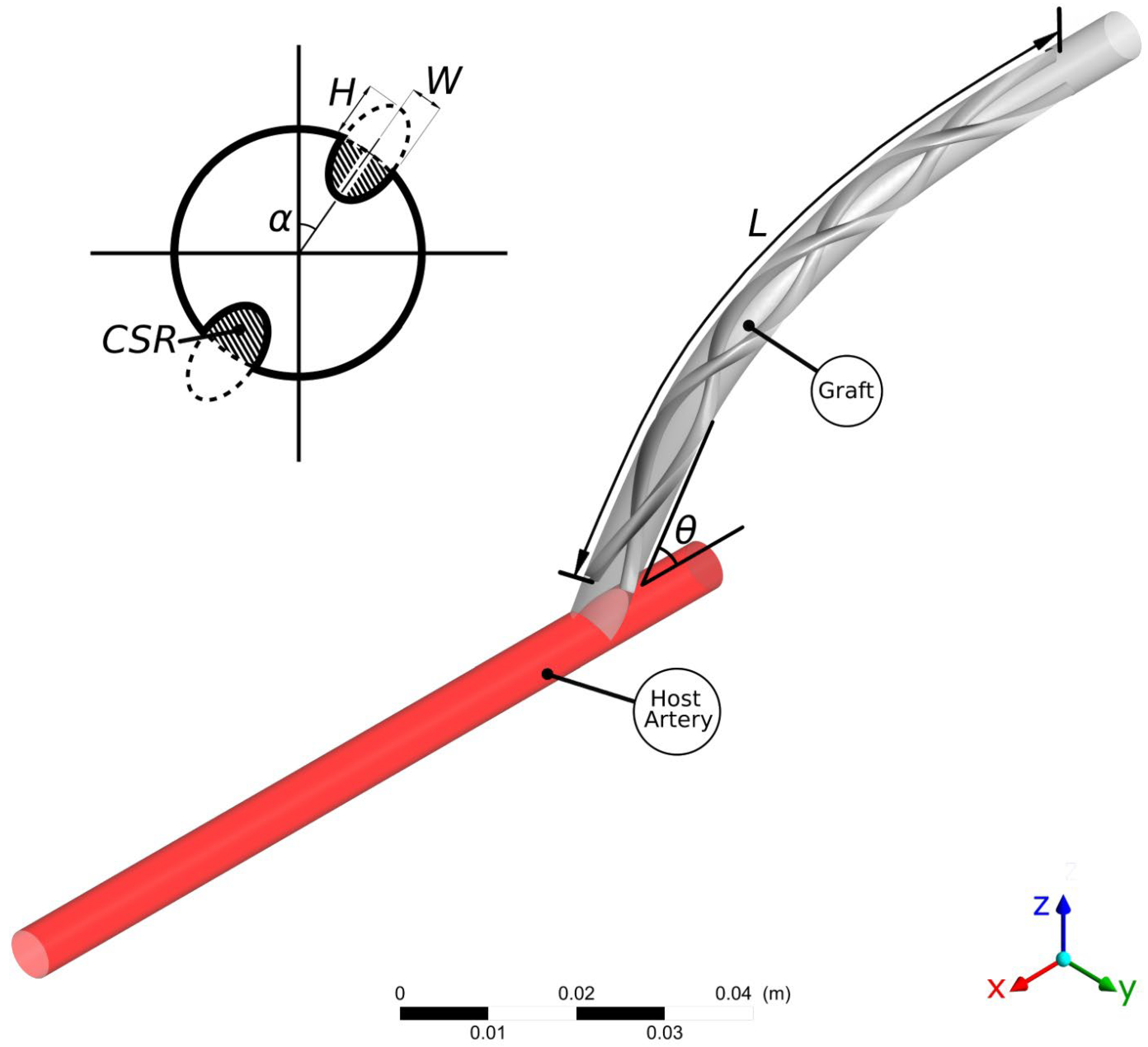



| Optimisation Criteria | Target |
|---|---|
| Helicity | Maximise |
| WSS on host artery | Maximise |
| WSSA < 1 Pa on host artery | Minimise |
| WSSG on host artery | Minimise |
| Reversing a portion of the flow | Minimise |
| Pressure drop along the graft region | Minimise |
| Design Parameter | Range |
|---|---|
| Ridge count | |
| Ridge Elliptical Height/Width ratio | |
| Cross-Sectional Ratio (CSR) | |
| Trailing Edge Orientation (TEO) | |
| Pitch (φ) [turns/L] | [turns/L] |
| Ridge length ratio (L/L0) | |
| Graft-artery anastomosis angle (θ) |
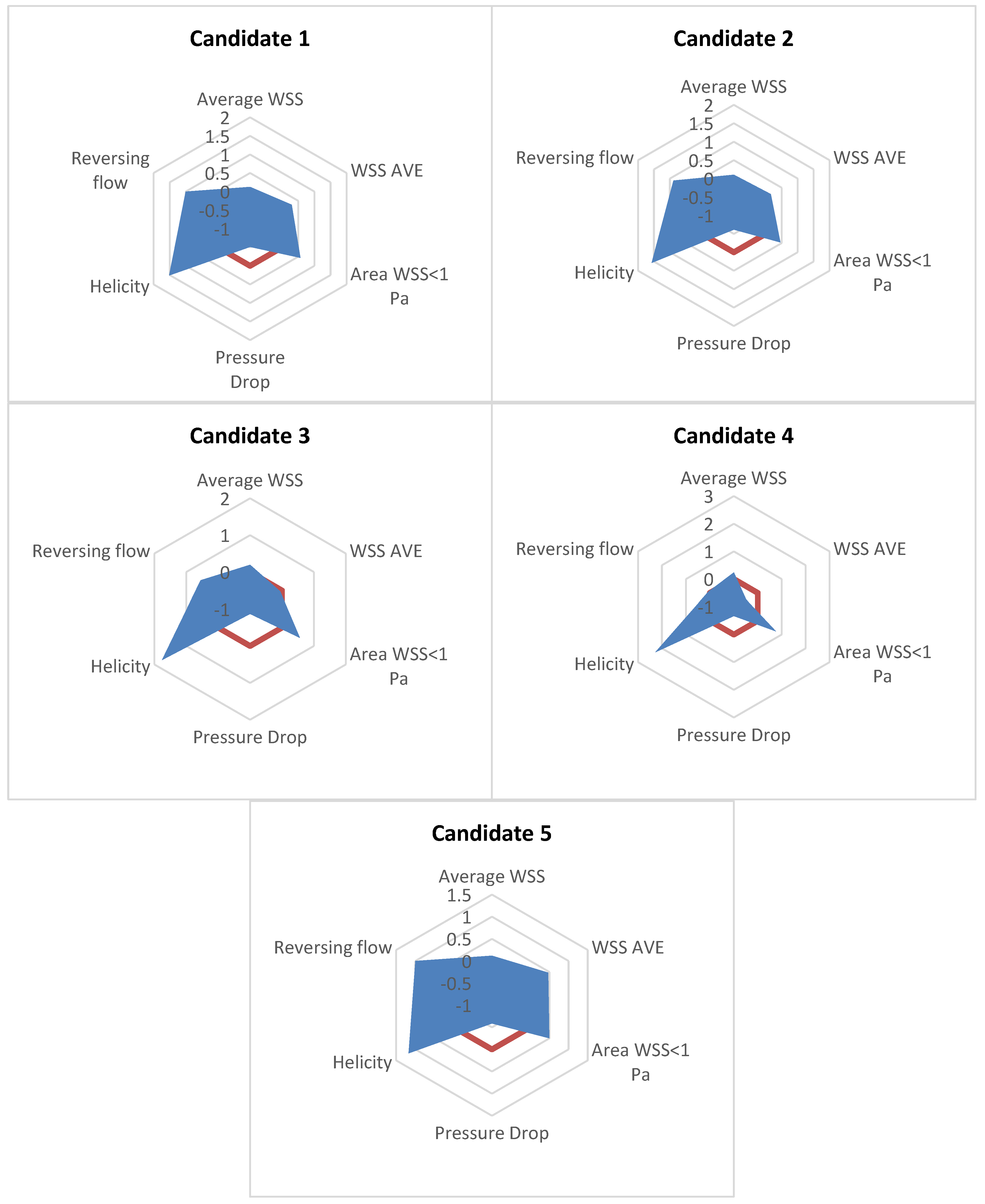
| Design Parameter | Candidate 1 | Candidate 2 | Candidate 3 | Candidate 4 | Candidate 5 | Reference |
|---|---|---|---|---|---|---|
| Ridge count | - | |||||
| Ridge Elliptical Height/Width ratio (HoW) | - | |||||
| Cross-Sectional Ratio (CSR) | - | |||||
| (TEO) | - | |||||
| ] | - | |||||
| - | ||||||
| ) | ||||||
| Optimisation Criteria | ||||||
| Helicity [J kg−1] | ||||||
| WSS on host artery [Pa] | ||||||
| Area of WSS < 1 Pa on host artery [mm2] | ||||||
| WSSG on host artery [kg m−2 s−2] | ||||||
| Reversing a portion of the flow | ||||||
| Pressure drop along the graft region [Pa] |
| Candidate Number | |
|---|---|
| 1 | 2.13 |
| 2 | 1.91 |
| 3 | 1.55 |
| 4 | 1.34 |
| 5 | 1.99 |
| Design Parameter | Reference | Single-Ridge Design [28] | Candidate 1 | |||
| Ridge count | - | |||||
| Ridge Elliptical Height/Width ratio (HoW) | - | |||||
| Occlusion area (%) | - | |||||
| (TEO) | - | |||||
| ] | - | |||||
| - | ||||||
| Design Parameter | Ref. | Ref. | Single Ridge [28] | |||
| WSS on host artery [Pa] | % | |||||
| Area of WSS < 1 Pa on host artery [mm2] | % | % | % | |||
| WSSG on host artery [kg m−2 s−2] | % | % | % | |||
| Reversing a portion of the flow | % | % | % | |||
| Helicity [J kg−1] | % | % | % | |||
| Pressure drop along the end anastomosis [Pa] | % | % | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xenakis, A.; Ruiz-Soler, A.; Keshmiri, A. Multi-Objective Optimisation of a Novel Bypass Graft with a Spiral Ridge. Bioengineering 2023, 10, 489. https://doi.org/10.3390/bioengineering10040489
Xenakis A, Ruiz-Soler A, Keshmiri A. Multi-Objective Optimisation of a Novel Bypass Graft with a Spiral Ridge. Bioengineering. 2023; 10(4):489. https://doi.org/10.3390/bioengineering10040489
Chicago/Turabian StyleXenakis, Antonios, Andres Ruiz-Soler, and Amir Keshmiri. 2023. "Multi-Objective Optimisation of a Novel Bypass Graft with a Spiral Ridge" Bioengineering 10, no. 4: 489. https://doi.org/10.3390/bioengineering10040489
APA StyleXenakis, A., Ruiz-Soler, A., & Keshmiri, A. (2023). Multi-Objective Optimisation of a Novel Bypass Graft with a Spiral Ridge. Bioengineering, 10(4), 489. https://doi.org/10.3390/bioengineering10040489






