Spatial Dependence of Log-Transformed Electromyography–Force Relation: Model-Based Sensitivity Analysis and Experimental Study of Biceps Brachii
Abstract
1. Introduction
2. Materials and Methods
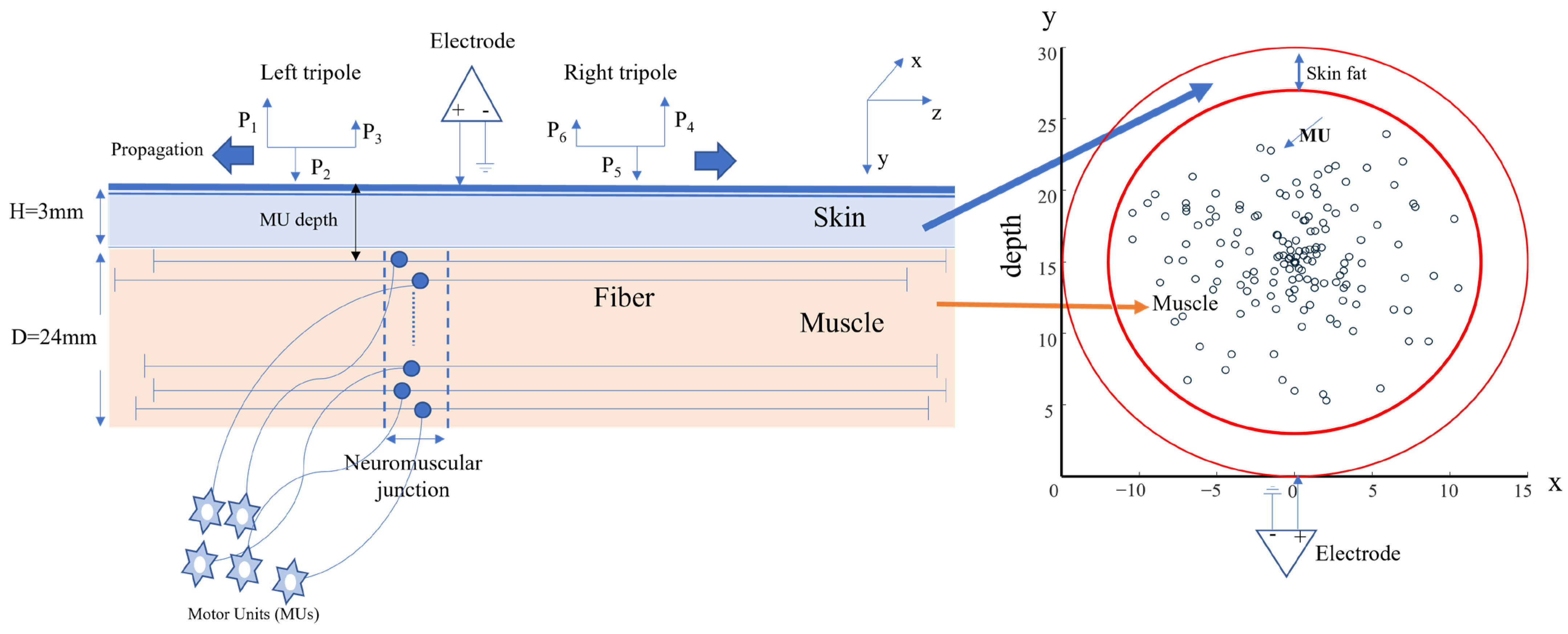
2.1. Simulation Study
2.2. Experimental Study
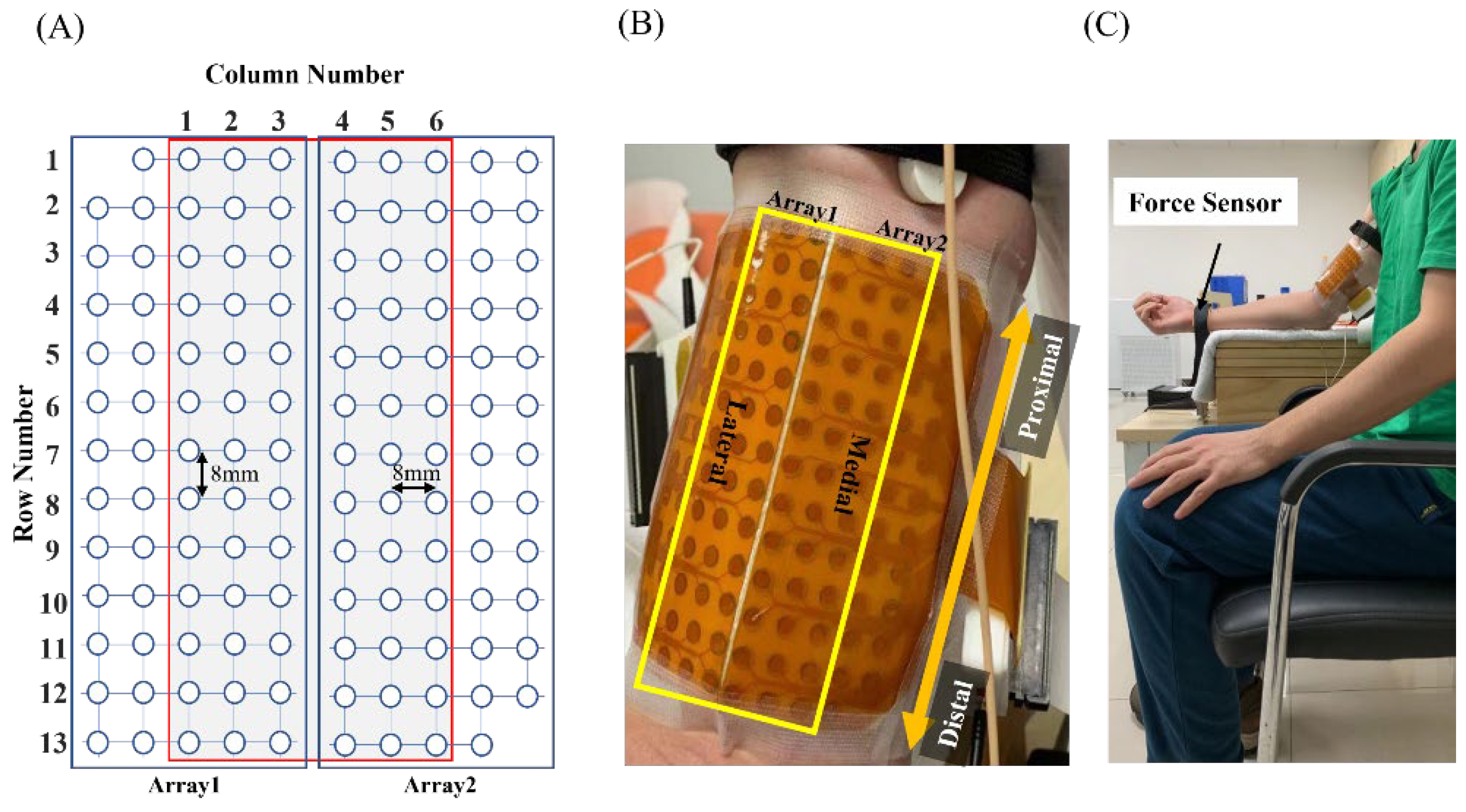
2.2.1. Participants and Consent
2.2.2. Experiment Protocol
2.3. Log-Transformed EMG–Force Relation
2.4. Data Analysis
3. Results
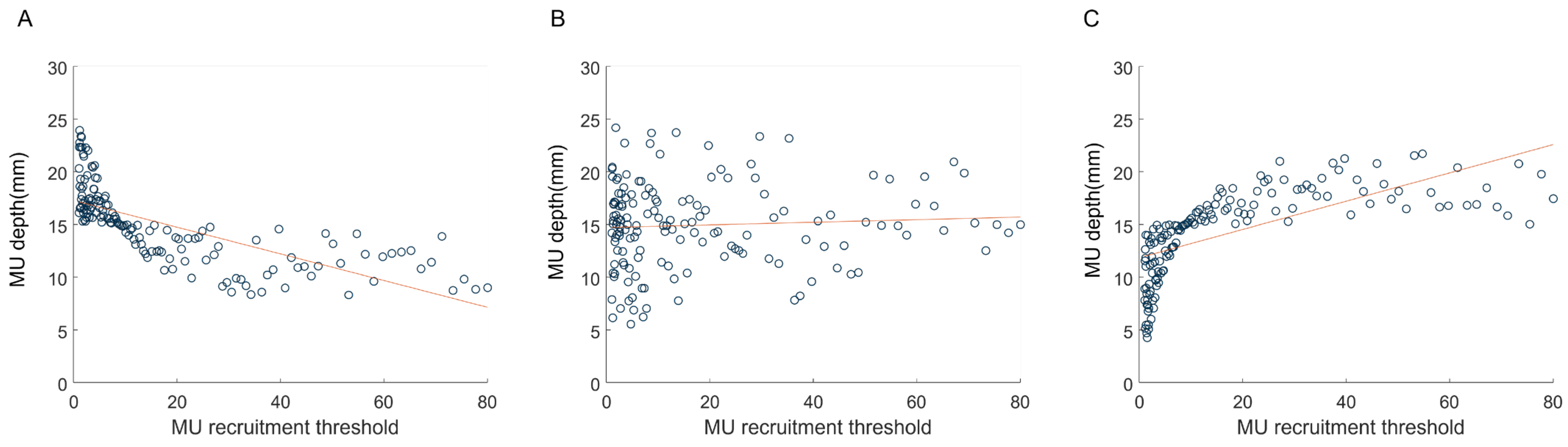
3.1. Simulation of the EMG–Force Relation
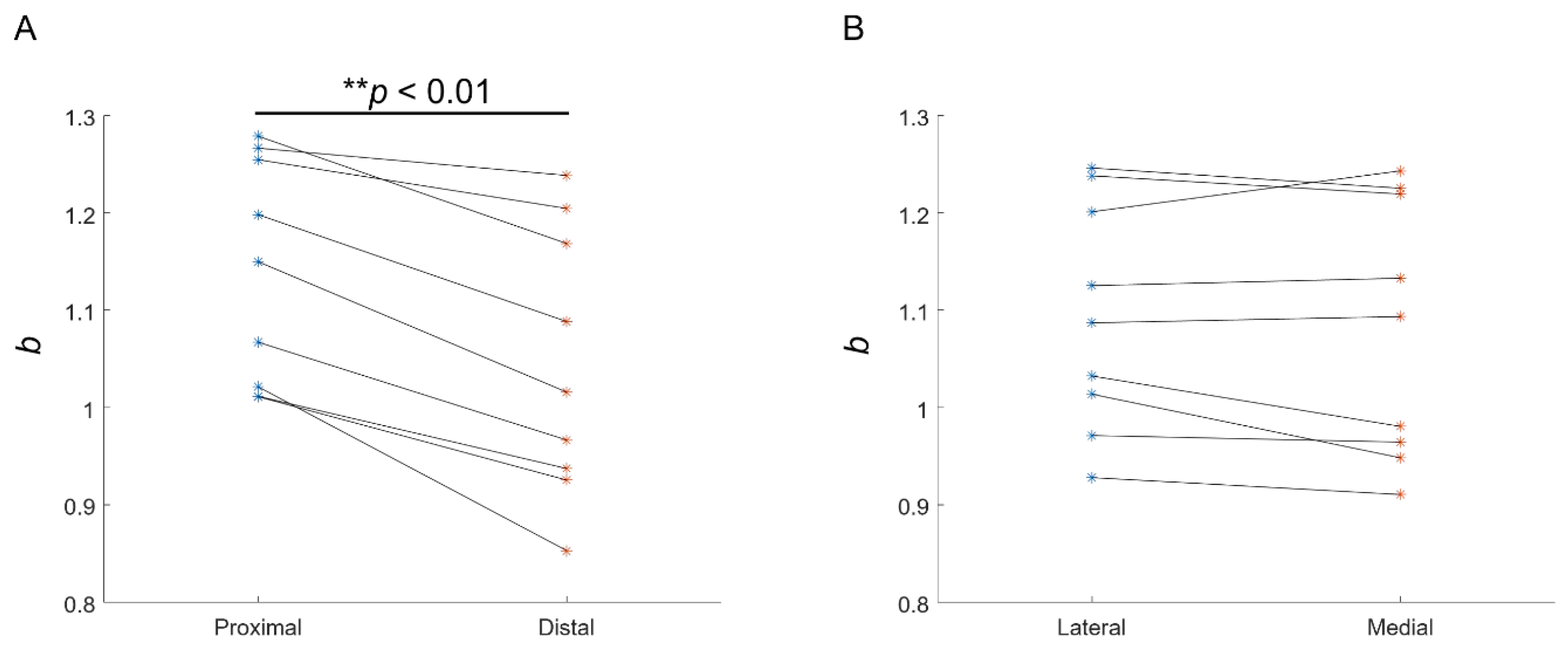
3.2. Experimental Recording of the EMG–Force Relation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| au | arbitrary unit |
| EMG | electromyography |
| ISI | inter-spike intervals |
| MUAP | motor unit action potential |
| PFR | peak firing rate |
| RMS | root mean square amplitude |
| RTE | recruitment threshold |
References
- Manta, P.; Kalfakis, N.; Kararizou, E.; Vassilopoulos, D.; Papageorgiou, K. Distribution of muscle fibre types in human skeletal muscle fascicles: An autopsy study of three human muscles. Funct. Neurol. 1995, 10, 137–141. [Google Scholar] [PubMed]
- Buchthal, F.; Schmalbruch, H. Motor unit of mammalian muscle. Physiol. Rev. 1980, 60, 90–142. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Smith, J.L.; Simpson, D.R. Muscle fibre type populations of human leg muscles. Histochem. J. 1975, 7, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Susheela, A.; Walton, J. Note on the distribution of histochemical fibre types in some normal human muscles: A study on autopsy material. J. Neurol. Sci. 1969, 8, 201–207. [Google Scholar] [CrossRef]
- Elder, G.C.; Bradbury, K.; Roberts, R. Variability of fiber type distributions within human muscles. J. Appl. Physiol. 1982, 53, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Polgar, J.; Weightman, D.; Appleton, D. Data on the distribution of fbre types in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 1973, 18, 111–129. [Google Scholar] [CrossRef]
- Lexell, J.; Henriksson-Larsén, K.; Sjöström, M. Distribution of different fibre types in human skeletal muscles. 2. A study of cross-sections of whole m. vastus lateralis. Acta Physiol. Scand. 1983, 117, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Dahmane, R.; Djordjevič, S.; Šimunič, B.; Valenčič, V. Spatial fiber type distribution in normal human muscle: Histochemical and tensiomyographical evaluation. J. Biomech. 2005, 38, 2451–2459. [Google Scholar] [CrossRef]
- Beck, T.W.; Housh, T.J.; Cramer, J.T.; Mielke, M.; Hendrix, R. The influence of electrode shift over the innervation zone and normalization on the electromyographic amplitude and mean power frequency versus isometric torque relationships for the vastus medialis muscle. J. Neurosci. Methods 2008, 169, 100–108. [Google Scholar] [CrossRef]
- Herda, T.J.; Zuniga, J.M.; Ryan, E.D.; Camic, C.L.; Bergstrom, H.C.; Smith, D.B.; Weir, J.P.; Cramer, J.T.; Housh, T.J. Quantifying the effects of electrode distance from the innervation zone on the electromyographic amplitude versus torque relationships. Physiol. Meas. 2013, 34, 315–324. [Google Scholar] [CrossRef]
- Bhadane, M.; Liu, J.; Rymer, W.Z.; Zhou, P.; Li, S. Re-evaluation of EMG-torque relation in chronic stroke using linear electrode array EMG recordings. Sci. Rep. 2016, 6, 28957. [Google Scholar] [CrossRef] [PubMed]
- Fuglevand, A.J.; Winter, D.A.; Patla, A.E. Models of recruitment and rate coding organization in motor-unit pools. J. Neurophysiol. 1993, 70, 2470–2488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Rymer, W.Z. Factors Governing the Form of the Relation Between Muscle Force and the EMG: A Simulation Study. J. Neurophysiol. 2004, 92, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Suresh, N.L.; Rymer, W.Z. Model Based Sensitivity Analysis of EMG–Force Relation with Respect to Motor Unit Properties: Applications to Muscle Paresis in Stroke. Ann. Biomed. Eng. 2007, 35, 1521–1531. [Google Scholar] [CrossRef]
- Huang, C.; Chen, M.; Zhang, Y.; Li, S.; Zhou, P. Model-Based Analysis of Muscle Strength and EMG-Force Relation with respect to Different Patterns of Motor Unit Loss. Neural Plast. 2021, 2021, 5513224. [Google Scholar] [CrossRef]
- Robertson, J.W.; Johnston, J.A. Modifying motor unit territory placement in the Fuglevand model. Med. Biol. Eng. Comput. 2017, 55, 2015–2025. [Google Scholar] [CrossRef]
- Herda, T.J.; Weir, J.P.; Ryan, E.D.; Walter, A.A.; Costa, P.B.; Hoge, K.M.; Beck, T.W.; Stout, J.R.; Cramer, J.T. Reliability of absolute versus log-transformed regression models for examining the torque-related patterns of response for mechanomyographic amplitude. J. Neurosci. Methods 2009, 179, 240–246. [Google Scholar] [CrossRef]
- Herda, T.J.; Walter, A.A.; Costa, P.B.; Ryan, E.D.; Stout, J.R.; Cramer, J.T. Differences in the log-transformed electromyographic–force relationships of the plantar flexors between high- and moderate-activated subjects. J. Electromyogr. Kinesiol. 2011, 21, 841–846. [Google Scholar] [CrossRef]
- Eason, T.; Gavel, C.R.; Hawley, K.A.; Galen, S.S.; Malek, M.H. Reliability of the log-transformed EMG amplitude-power output relationship for incremental knee-extensor ergometry. Muscle Nerve 2015, 52, 428–434. [Google Scholar] [CrossRef]
- De Luca, C.J.; Hostage, E.C. Relationship Between Firing Rate and Recruitment Threshold of Motoneurons in Voluntary Isometric Contractions. J. Neurophysiol. 2010, 104, 1034–1046. [Google Scholar] [CrossRef]
- De Luca, C.J.; Contessa, P. Biomechanical benefits of the onion-skin motor unit control scheme. J. Biomech. 2015, 48, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Piotrkiewicz, M.; Türker, K.S. Onion Skin or Common Drive? Front. Cell. Neurosci. 2017, 11, 2. [Google Scholar] [CrossRef]
- Inglis, J.G.; Gabriel, D.A. Is the ‘reverse onion skin’ phenomenon more prevalent than we thought during intramuscular myoelectric recordings from low to maximal force outputs? Neurosci. Lett. 2021, 743, 135583. [Google Scholar] [CrossRef] [PubMed]
- Erim, Z.; De Luca, C.J.; Mineo, K.; Aoki, T. Rank-ordered regulation of motor units. Muscle Nerve 1996, 19, 563–573. [Google Scholar] [CrossRef]
- Oya, T.; Riek, S.; Cresswell, A.G. Recruitment and rate coding organisation for soleus motor units across entire range of voluntary isometric plantar flexions. J. Physiol. 2009, 587, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Jesunathadas, M.; Klass, M.; Duchateau, J.; Enoka, R.M. Discharge properties of motor units during steady isometric contractions performed with the dorsiflexor muscles. J. Appl. Physiol. 2012, 112, 1897–1905. [Google Scholar] [CrossRef]
- Merletti, R.; Conte, L.L.; Avignone, E.; Guglielminotti, P. Modeling of surface myoelectric signals. I. Model implementation. IEEE Trans. Biomed. Eng. 1999, 46, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.H.; De Luca, C.J. Myoelectric signal versus force relationship in different human muscles. J. Appl. Physiol. 1983, 54, 1653–1659. [Google Scholar] [CrossRef]
- Knight, C.A.; Kamen, G. Superficial motor units are larger than deeper motor units in human vastus lateralis muscle. Muscle Nerve 2005, 31, 475–480. [Google Scholar] [CrossRef]
- Stalberg, E. Macro EMG, a new recording technique. J. Neurol. Neurosurg. Psychiatry 1980, 43, 475–482. [Google Scholar] [CrossRef]
- Farina, D.; Blanchietti, A.; Pozzo, M.; Merletti, R. M-wave properties during progressive motor unit activation by transcutaneous stimulation. J. Appl. Physiol. 2004, 97, 545–555. [Google Scholar] [CrossRef]
- Mesin, L.; Merlo, E.; Merletti, R.; Orizio, C. Investigation of motor unit recruitment during stimulated contractions of tibialis anterior muscle. J. Electromyogr. Kinesiol. 2010, 20, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.-T.; Zhang, C.; Zhou, P.; Li, S.; Zhang, Y. Motor unit distribution and recruitment in spastic and non-spastic bilateral biceps brachii muscles of chronic stroke survivors. J. Neural Eng. 2022, 19, 046047. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Rymer, W.Z. Abnormal force—EMG relations in paretic limbs of hemiparetic human subjects. J. Neurol. Neurosurg. Psychiatry 1981, 44, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Gemperline, J.J.; Allen, S.; Walk, D.; Rymer, W.Z. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve 1995, 18, 1101–1114. [Google Scholar] [CrossRef]
- Zhou, P.; Li, X.; Rymer, W.Z. EMG-Force Relations During Isometric Contractions of the First Dorsal Interosseous Muscle After Stroke. Top. Stroke Rehabil. 2013, 20, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Suresh, N.L.; Concepcion, N.S.; Madoff, J.; Rymer, W.Z. Anomalous EMG–force relations during low-force isometric tasks in hemiparetic stroke survivors. Exp. Brain Res. 2015, 233, 15–25. [Google Scholar] [CrossRef]
- Jahanmiri-Nezhad, F.; Hu, X.; Suresh, N.L.; Rymer, W.Z.; Zhou, P. EMG-force relation in the first dorsal interosseous muscle of patients with amyotrophic lateral sclerosis. Neurorehabilitation 2014, 35, 307–314. [Google Scholar] [CrossRef]
- Li, L.; Hu, H.; Yao, B.; Huang, C.; Lu, Z.; Klein, C.S.; Zhou, P. Electromyography–Force Relation and Muscle Fiber Conduction Velocity Affected by Spinal Cord Injury. Bioengineering 2023, 10, 217. [Google Scholar] [CrossRef]
- Lukács, M.; Vécsei, L.; Beniczky, S. Large motor units are selectively affected following a stroke. Clin. Neurophysiol. 2008, 119, 2555–2558. [Google Scholar] [CrossRef]
- Lowery, M.; Vaughan, C.; Nolan, P.; O’Malley, M. Spectral compression of the electromyographic signal due to decreasing muscle fiber conduction velocity. IEEE Trans. Rehabil. Eng. 2000, 8, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, B.; Carpentier, A.; Duchateau, J. Change in Muscle Fascicle Length Influences the Recruitment and Discharge Rate of Motor Units During Isometric Contractions. J. Neurophysiol. 2005, 94, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Del Santo, F.; Gelli, F.; Ginanneschi, F.; Popa, T.; Rossi, A. Relation between isometric muscle force and surface EMG in intrinsic hand muscles as function of the arm geometry. Brain Res. 2007, 1163, 79–85. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Chen, M.; Lu, Z.; Klein, C.S.; Zhou, P. Spatial Dependence of Log-Transformed Electromyography–Force Relation: Model-Based Sensitivity Analysis and Experimental Study of Biceps Brachii. Bioengineering 2023, 10, 469. https://doi.org/10.3390/bioengineering10040469
Huang C, Chen M, Lu Z, Klein CS, Zhou P. Spatial Dependence of Log-Transformed Electromyography–Force Relation: Model-Based Sensitivity Analysis and Experimental Study of Biceps Brachii. Bioengineering. 2023; 10(4):469. https://doi.org/10.3390/bioengineering10040469
Chicago/Turabian StyleHuang, Chengjun, Maoqi Chen, Zhiyuan Lu, Cliff S. Klein, and Ping Zhou. 2023. "Spatial Dependence of Log-Transformed Electromyography–Force Relation: Model-Based Sensitivity Analysis and Experimental Study of Biceps Brachii" Bioengineering 10, no. 4: 469. https://doi.org/10.3390/bioengineering10040469
APA StyleHuang, C., Chen, M., Lu, Z., Klein, C. S., & Zhou, P. (2023). Spatial Dependence of Log-Transformed Electromyography–Force Relation: Model-Based Sensitivity Analysis and Experimental Study of Biceps Brachii. Bioengineering, 10(4), 469. https://doi.org/10.3390/bioengineering10040469








