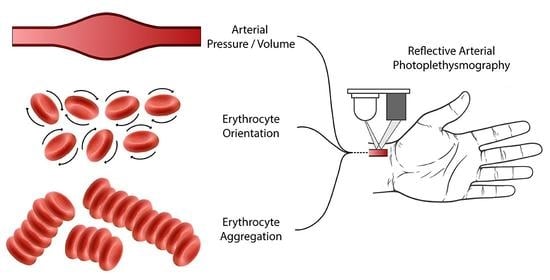
Quantification of the Phenomena Affecting Reflective Arterial Photoplethysmography
Abstract
1. Introduction
2. Materials and Methods
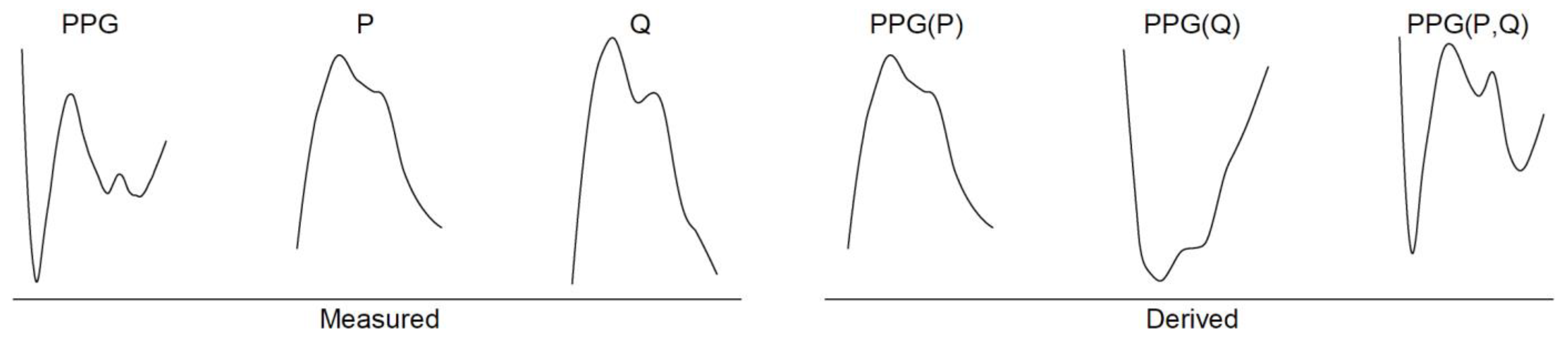
2.1. Theoretical Approach
2.2. Blood Preparation
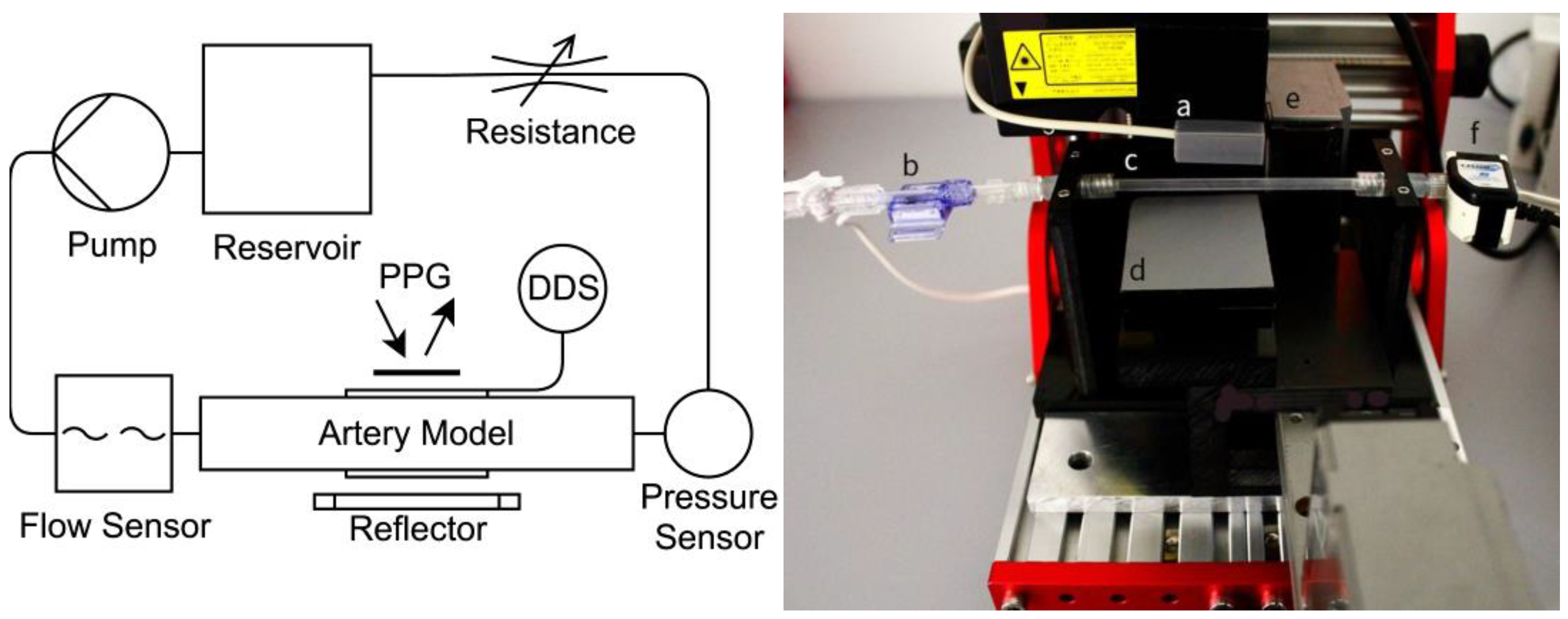
2.3. Experimental Setup
2.4. Artery Models
2.5. Data Acquisition and Processing
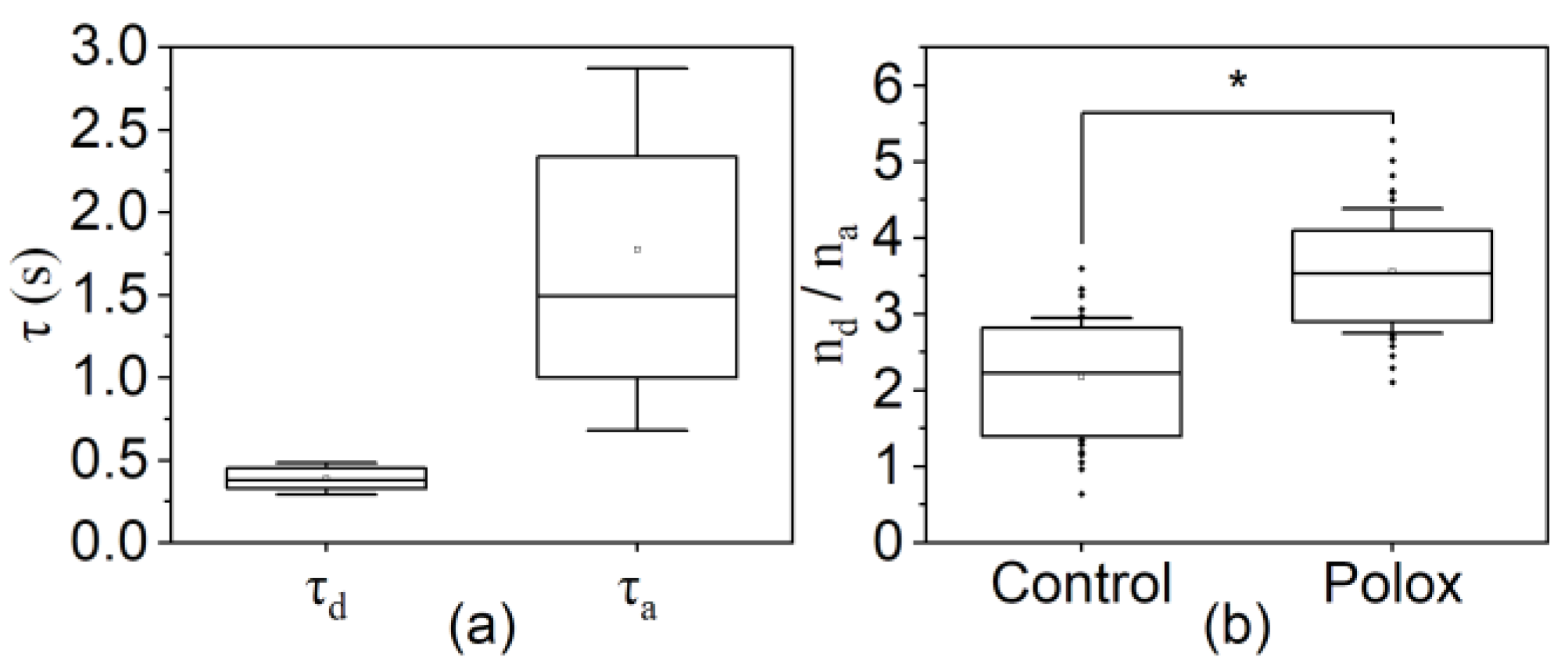
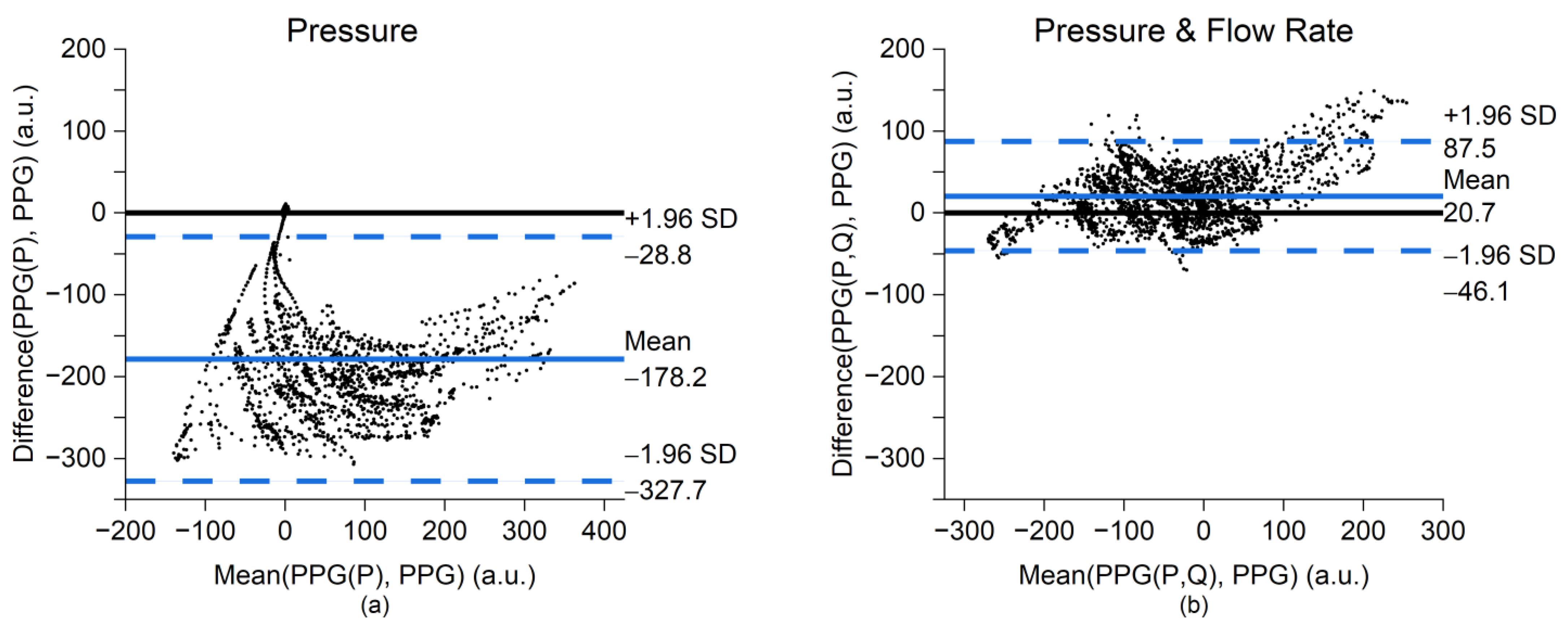
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chan, G.; Cooper, R.; Hosanee, M.; Welykholowa, K.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Lovell, N.H.; Fletcher, R.; et al. Multi-Site Photoplethysmography Technology for Blood Pressure Assessment: Challenges and Recommendations. J. Clin. Med. 2019, 8, 1827. [Google Scholar] [CrossRef]
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.H.; Abbott, D.; Lim, K.; Ward, R. The Use of Photoplethysmography for Assessing Hypertension. NPJ Digit. Med. 2019, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.L.; Rocha, M.X.; Vasconcelos, G.G.; Vasconcelos Filho, J.E.; De Albuquerque, V.H.C.; Alexandria, A.R. Advances in Photopletysmography Signal Analysis for Biomedical Applications. Sensors 2018, 18, 1894. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M. On the Analysis of Fingertip Photoplethysmogram Signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef]
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-ananta, T.; Ajmal; Ramella-Roman, J.C.; McShane, M.J.; Coté, G.L. Sources of Inaccuracy in Photoplethysmography for Continuous Cardiovascular Monitoring. Biosensors 2021, 11, 126. [Google Scholar] [CrossRef]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating Sources of Inaccuracy in Wearable Optical Heart Rate Sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef]
- Shcherbina, A.; Mattsson, C.M.; Waggott, D.; Salisbury, H.; Christle, J.W.; Hastie, T.; Wheeler, M.T.; Ashley, E.A. Accuracy in Wrist-Worn, Sensor-Based Measurements of Heart Rate and Energy Expenditure in a Diverse Cohort. J. Pers. Med. 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, P.; Garde, A.; Molavi, B.; Ansermino, J.M.; Dumont, G.A. Extracting Instantaneous Respiratory Rate From Multiple Photoplethysmogram Respiratory-Induced Variations. Front. Physiol. 2018, 9, 948. [Google Scholar] [CrossRef]
- Alian, A.A.; Shelley, K.H. Photoplethysmography. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Longmore, S.K.; Lui, G.Y.; Naik, G.; Breen, P.P.; Jalaludin, B.; Gargiulo, G.D. A Comparison of Reflective Photoplethysmography for Detection of Heart Rate, Blood Oxygen Saturation, and Respiration Rate at Various Anatomical Locations. Sensors 2019, 19, 1874. [Google Scholar] [CrossRef]
- Moço, A.V.; Stuijk, S.; de Haan, G. Ballistocardiographic Artifacts in PPG Imaging. IEEE Trans. Biomed. Eng. 2016, 63, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Seok, H.S.; Kim, S.-S.; Shin, H. Photoplethysmogram Analysis and Applications: An Integrative Review. Front. Physiol. 2022, 12, 2511. [Google Scholar] [CrossRef] [PubMed]
- Pi, I.; Pi, I.; Wu, W. External Factors That Affect the Photoplethysmography Waveforms. SN Appl. Sci. 2021, 4, 21. [Google Scholar] [CrossRef]
- Nijboer, J.A.; Dorlas, J.C.; Mahieu, H.F. Photoelectric Plethysmography-Some Fundamental Aspects of the Reflection and Transmission Methods. Clin. Phys. Physiol. Meas. 1981, 2, 205–215. [Google Scholar] [CrossRef]
- Kamshilin, A.A.; Nippolainen, E.; Sidorov, I.S.; Vasilev, P.V.; Erofeev, N.P.; Podolian, N.P.; Romashko, R.V. A New Look at the Essence of the Imaging Photoplethysmography. Sci. Rep. 2015, 5, 10494. [Google Scholar] [CrossRef]
- Moço, A.V.; Stuijk, S.; de Haan, G. New Insights into the Origin of Remote PPG Signals in Visible Light and Infrared. Sci. Rep. 2018, 8, 8501. [Google Scholar] [CrossRef]
- Lindberg, L.-G.; Oberg, P.A. Optical Properties of Blood in Motion. Opt. Eng. 1993, 32, 253–257. [Google Scholar] [CrossRef]
- Miwa, Z.; Ikawa, M.; Iijima, H.; Saito, M.; Takagi, Y. Pulpal Blood Flow in Vital and Nonvital Young Permanent Teeth Measured by Transmitted-Light Photoplethysmography: A Pilot Study. Pediatr. Dent. 2002, 5, 24. [Google Scholar]
- Näslund, J.; Pettersson, J.; Lundeberg, T.; Linnarsson, D.; Lindberg, L.-G. Non-Invasive Continuous Estimation of Blood Flow Changes in Human Patellar Bone. Med. Bio. Eng. Comput. 2006, 44, 501–509. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef]
- Verkruysse, W.; Lucassen, G.W.; de Boer, J.F.; Smithies, D.J.; Nelson, J.S.; van Gemert, M.J.C. Modelling Light Distributions of Homogeneous versus Discrete Absorbers in Light Irradiated Turbid Media. Phys. Med. Biol. 1997, 42, 51. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Kanai, H. Electrical Characteristics of Flowing Blood. IEEE Trans. Biomed. Eng. 1979, BME-26, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Shvartsman, L.D.; Fine, I. Optical Transmission of Blood: Effect of Erythrocyte Aggregation. IEEE Trans. Biomed. Eng. 2003, 50, 1026–1033. [Google Scholar] [CrossRef]
- Weinman, J.; Hayat, A.; Raviv, G. Reflection Photoplethysmography of Arterial-Blood-Volume Pulses. Med. Biol. Eng. Comput. 1977, 15, 22–31. [Google Scholar] [CrossRef]
- Allen, J.; Murray, A. Modelling the Relationship between Peripheral Blood Pressure and Blood Volume Pulses Using Linear and Neural Network System Identification Techniques. Physiol. Meas. 1999, 20, 287–301. [Google Scholar] [CrossRef]
- Martinez, G.; Howard, N.; Abbott, D.; Lim, K.; Ward, R.; Elgendi, M. Can Photoplethysmography Replace Arterial Blood Pressure in the Assessment of Blood Pressure? J. Clin. Med. 2018, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Boonya-ananta, T.; Rodriguez, A.J.; Ajmal, A.; Du Le, V.N.; Hansen, A.K.; Hutcheson, J.D.; Ramella-Roman, J.C. Synthetic Photoplethysmography (PPG) of the Radial Artery through Parallelized Monte Carlo and Its Correlation to Body Mass Index (BMI). Sci. Rep. 2021, 11, 2570. [Google Scholar] [CrossRef]
- Janjua, G.M.W.; Finlay, D.; Guldenring, D.; Haq, A.U.; Mclaughlin, J. A Low-Cost Tonometer Alternative: A Comparison Between Photoplethysmogram and Finger Ballistocardiogram and Validation Against Tonometric Waveform. IEEE Access 2019, 7, 142787–142795. [Google Scholar] [CrossRef]
- Remizovich, V.S.; Rogozkin, D.B.; Ryazanov, M.I. Propagation of a Narrow Modulated Light Beam in a Scattering Medium with Fluctuations of the Photon Pathlengths in Multiple Scattering. Radiophys. Quantum. Electron. 1982, 25, 639–645. [Google Scholar] [CrossRef]
- Sun, Y.; Thakor, N. Photoplethysmography Revisited: From Contact to Noncontact, From Point to Imaging. IEEE Trans. Biomed. Eng. 2016, 63, 463–477. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Giannattasio, C.; Brunani, A.; Failla, M.; Colombo, M.; Bolla, G.; Cavagnini, F.; Grassi, G.; Mancia, G. Radial Artery Compliance in Young, Obese, Normotensive Subjects. Hypertension 1995, 26, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Giannattasio, C.; Failla, M.; Pesenti, A.; Peretti, G.; Marinoni, E.; Fraschini, N.; Vailati, S.; Mancia, G. Sympathetic Modulation of Radial Artery Compliance in Congestive Heart Failure. Hypertension 1995, 26, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Bitbol, M.; Quemada, D. Measurement of Erythrocyte Orientation in Flow by Spin Labeling II--Phenomenological Models for Erythrocyte Orientation Rate. Biorheology 1985, 22, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Tsinopoulos, S.V.; Sellountos, E.J.; Polyzos, D. Light Scattering by Aggregated Red Blood Cells. Appl. Opt. AO 2002, 41, 1408–1417. [Google Scholar] [CrossRef]
- Minetti, C.; Audemar, V.; Podgorski, T.; Coupier, G. Dynamics of a Large Population of Red Blood Cells under Shear Flow. J. Fluid Mech. 2019, 864, 408–448. [Google Scholar] [CrossRef]
- Bitbol, M. Red Blood Cell Orientation in Orbit C = 0. Biophys. J. 1986, 49, 1055–1068. [Google Scholar] [CrossRef]
- Wen, J.; Wan, N.; Bao, H.; Li, J. Quantitative Measurement and Evaluation of Red Blood Cell Aggregation in Normal Blood Based on a Modified Hanai Equation. Sensors 2019, 19, 1095. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Uyuklu, M.; Hardeman, M.R.; Meiselman, H.J. Photometric Measurements of Red Blood Cell Aggregation: Light Transmission versus Light Reflectance. J. Biomed. Opt. 2009, 14, 054044. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Yalcin, O.; Ozdem, S.; Armstrong, J.K.; Meiselman, H.J. Modulation of Endothelial Nitric Oxide Synthase Expression by Red Blood Cell Aggregation. Am. J. Physiol. -Heart Circ. Physiol. 2004, 286, H222–H229. [Google Scholar] [CrossRef]
- Fatkin, D.; Loupas, T.; Low, J.; Feneley, M. Inhibition of Red Cell Aggregation Prevents Spontaneous Echocardiographic Contrast Formation in Human Blood. Circulation 1997, 96, 889–896. [Google Scholar] [CrossRef]
- Carter, C.; Fisher, T.C.; Hamai, H.; Johnson, C.S.; Meiselman, H.J.; Nash, G.B.; Stuart, J. Haemorheological Effects of a Nonionic Copolymer Surfactant (Poloxamer 188). Clin. Hemorheol. Microcirc. 1992, 12, 109–120. [Google Scholar] [CrossRef]
- Sandor, B.; Marin, M.; Lapoumeroulie, C.; Rabaï, M.; Lefevre, S.D.; Lemonne, N.; El Nemer, W.; Mozar, A.; Français, O.; Le Pioufle, B.; et al. Effects of Poloxamer 188 on Red Blood Cell Membrane Properties in Sickle Cell Anaemia. Br. J. Haematol. 2016, 173, 145–149. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kyriacou, P.A. Monte Carlo Analysis of Optical Interactions in Reflectance and Transmittance Finger Photoplethysmography. Sensors 2019, 19, 789. [Google Scholar] [CrossRef]
- van der Meer, F.J.; Faber, D.J.; Cilesiz, I.F.; van Gemert, M.J.C.; van Leeuwen, T.G. Temperature-Dependent Optical Properties of Individual Vascular Wall Components Measured by Optical Coherence Tomography. J. Biomed. Opt. 2006, 11, 041120. [Google Scholar] [CrossRef]
- Martin Bland, J.; Altman; Douglas, G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Maraun, D.; Kurths, J.; Holschneider, M. Nonstationary Gaussian Processes in Wavelet Domain: Synthesis, Estimation, and Significance Testing. Phys. Rev. E Stat. Nonlin. Soft. Matter Phys. 2007, 75, 016707. [Google Scholar] [CrossRef]
- Bitbol, M.; Leterrier, F.; Dufaux, J.; Quemada, D. Measurement of Erythrocyte Orientation in Flow by Spin Labeling III--Erythrocyte Orientation and Rheological Conditions. Biorheology 1985, 22, 43–53. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues. In Biomechanics: Mechanical Properties of Living Tissues; Springer: New York, NY, USA, 1981; pp. 139–150. ISBN 978-0-387-90472-6. [Google Scholar]
- Weng, X.; Cloutier, G.; Pibarot, P.; Durand, L.-G. Comparison and Simulation of Different Levels of Erythrocyte Aggregation with Pig, Horse, Sheep, Calf, and Normal Human Blood. Biorheology 1996, 33, 365–377. [Google Scholar] [CrossRef]
- Prado, G.; Farutin, A.; Misbah, C.; Bureau, L. Viscoelastic Transient of Confined Red Blood Cells. Biophys. J. 2015, 108, 2126–2136. [Google Scholar] [CrossRef]
- Charlton, P.H.; Mariscal Harana, J.; Vennin, S.; Li, Y.; Chowienczyk, P.; Alastruey, J. Modeling Arterial Pulse Waves in Healthy Aging: A Database for in Silico Evaluation of Hemodynamics and Pulse Wave Indexes. Am. J. Physiol.-Heart Circ. Physiol. 2019, 317, H1062–H1085. [Google Scholar] [CrossRef]
- Bikia, V.; Segers, P.; Rovas, G.; Pagoulatou, S.; Stergiopulos, N. On the Assessment of Arterial Compliance from Carotid Pressure Waveform. Am. J. Physiol.-Heart Circ. Physiol. 2021, 321, H424–H434. [Google Scholar] [CrossRef]
- Bikia, V.; Lazaroska, M.; Scherrer Ma, D.; Zhao, M.; Rovas, G.; Pagoulatou, S.; Stergiopulos, N. Estimation of Left Ventricular End-Systolic Elastance From Brachial Pressure Waveform via Deep Learning. Front. Bioeng. Biotechnol. 2021, 9, 754003. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.S.; Maryam, S.; Amissah, M.; Lu, H.; Lynch, N.; Killeen, S.; O’Riordain, M.; Andersson-Engels, S. Evaluation of Wavelength Ranges and Tissue Depth Probed by Diffuse Reflectance Spectroscopy for Colorectal Cancer Detection. Sci. Rep. 2021, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Shimizu, K. Development of a Technique to Measure Local Scattering in Turbid Media Using Backscattered Light at the Surface for Noninvasive Turbidity Evaluation of Blood in Subcutaneous Blood Vessels. Jpn. J. Appl. Phys. 2021, 60, 022002. [Google Scholar] [CrossRef]
- Bosschaart, N.; Edelman, G.J.; Aalders, M.C.G.; van Leeuwen, T.G.; Faber, D.J. A Literature Review and Novel Theoretical Approach on the Optical Properties of Whole Blood. Lasers Med. Sci. 2014, 29, 453–479. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovas, G.; Bikia, V.; Stergiopulos, N. Quantification of the Phenomena Affecting Reflective Arterial Photoplethysmography. Bioengineering 2023, 10, 460. https://doi.org/10.3390/bioengineering10040460
Rovas G, Bikia V, Stergiopulos N. Quantification of the Phenomena Affecting Reflective Arterial Photoplethysmography. Bioengineering. 2023; 10(4):460. https://doi.org/10.3390/bioengineering10040460
Chicago/Turabian StyleRovas, Georgios, Vasiliki Bikia, and Nikolaos Stergiopulos. 2023. "Quantification of the Phenomena Affecting Reflective Arterial Photoplethysmography" Bioengineering 10, no. 4: 460. https://doi.org/10.3390/bioengineering10040460
APA StyleRovas, G., Bikia, V., & Stergiopulos, N. (2023). Quantification of the Phenomena Affecting Reflective Arterial Photoplethysmography. Bioengineering, 10(4), 460. https://doi.org/10.3390/bioengineering10040460