Metaverse and Healthcare: Machine Learning-Enabled Digital Twins of Cancer
Abstract
1. Introduction
2. Cancer Digital Twins
2.1. Digital Twins
2.2. Healthcare Digital Twin
2.3. Machine Learning Digital Twins
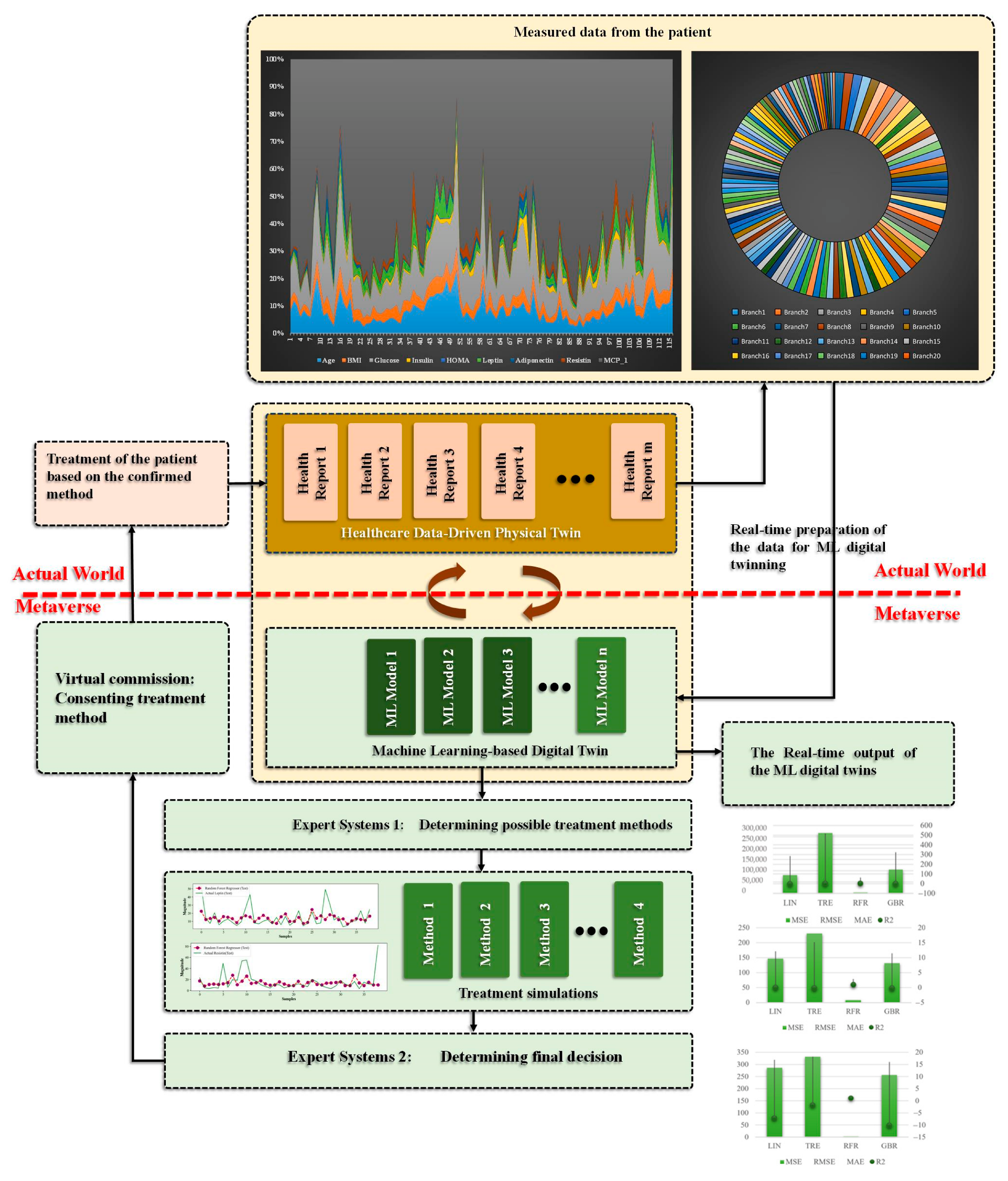
3. The Proposed Cancer Digital Twin
3.1. Case Study—Breast Cancer
3.2. Participants
3.3. Sample Analysis
4. Results
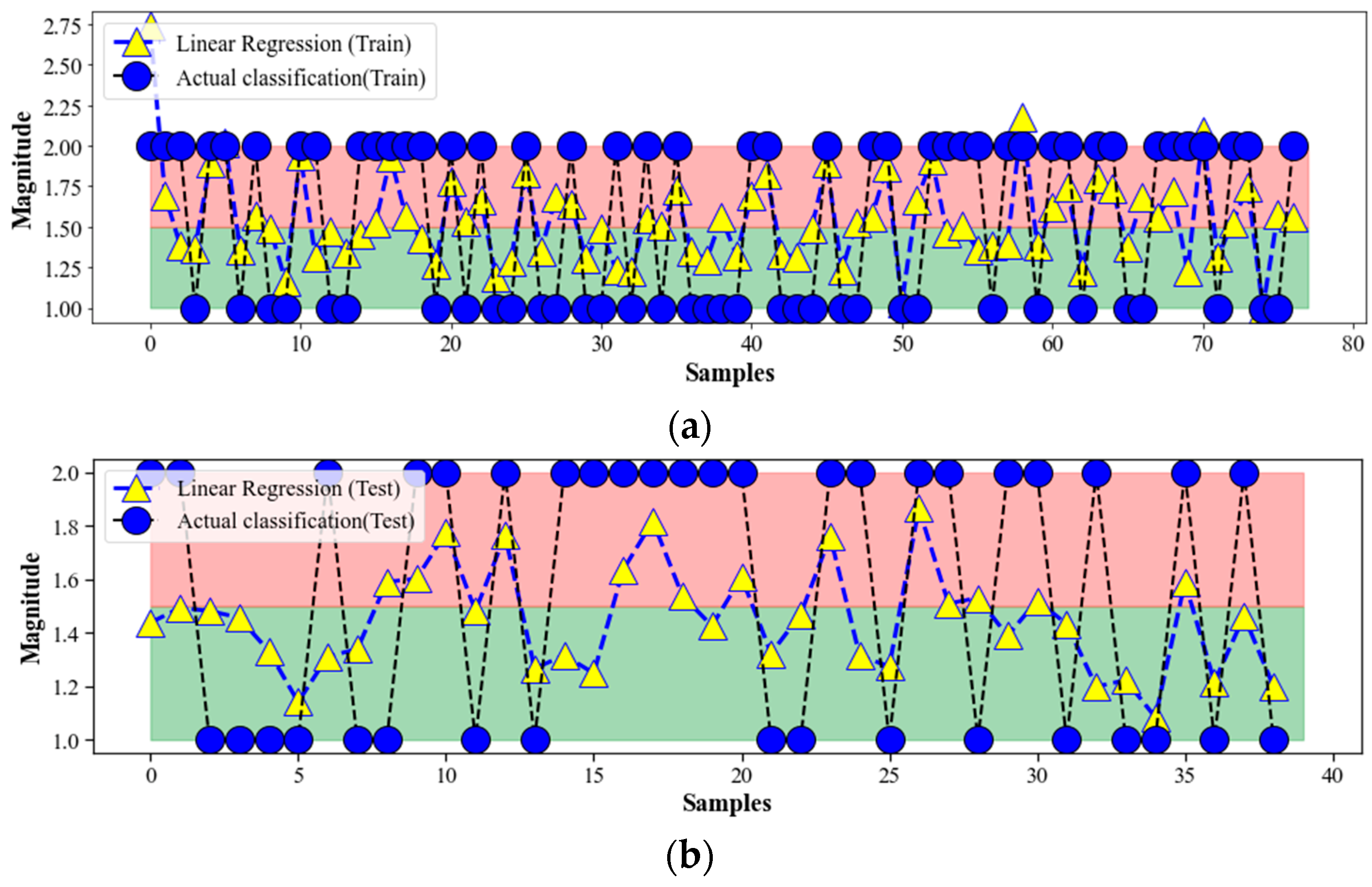
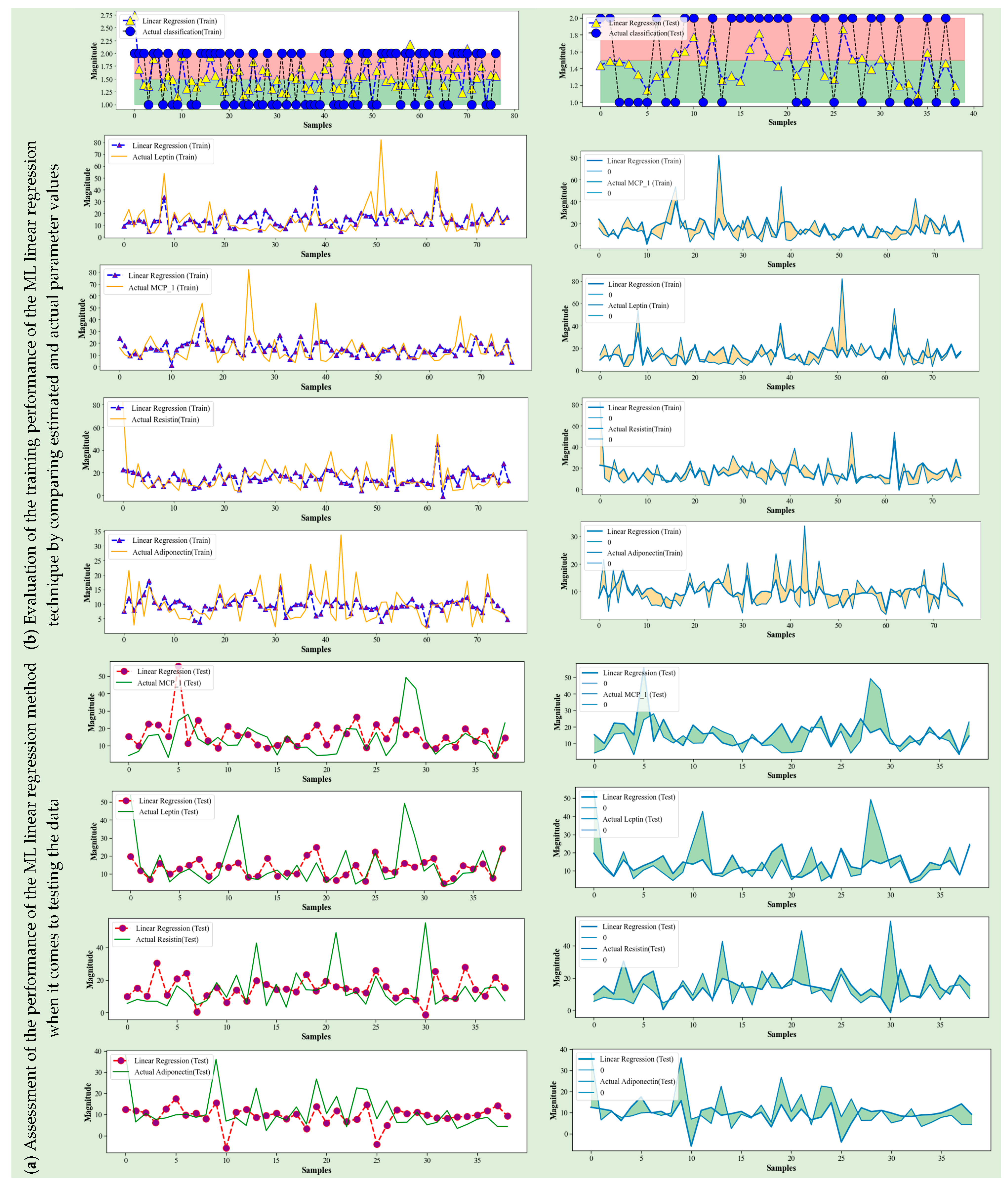
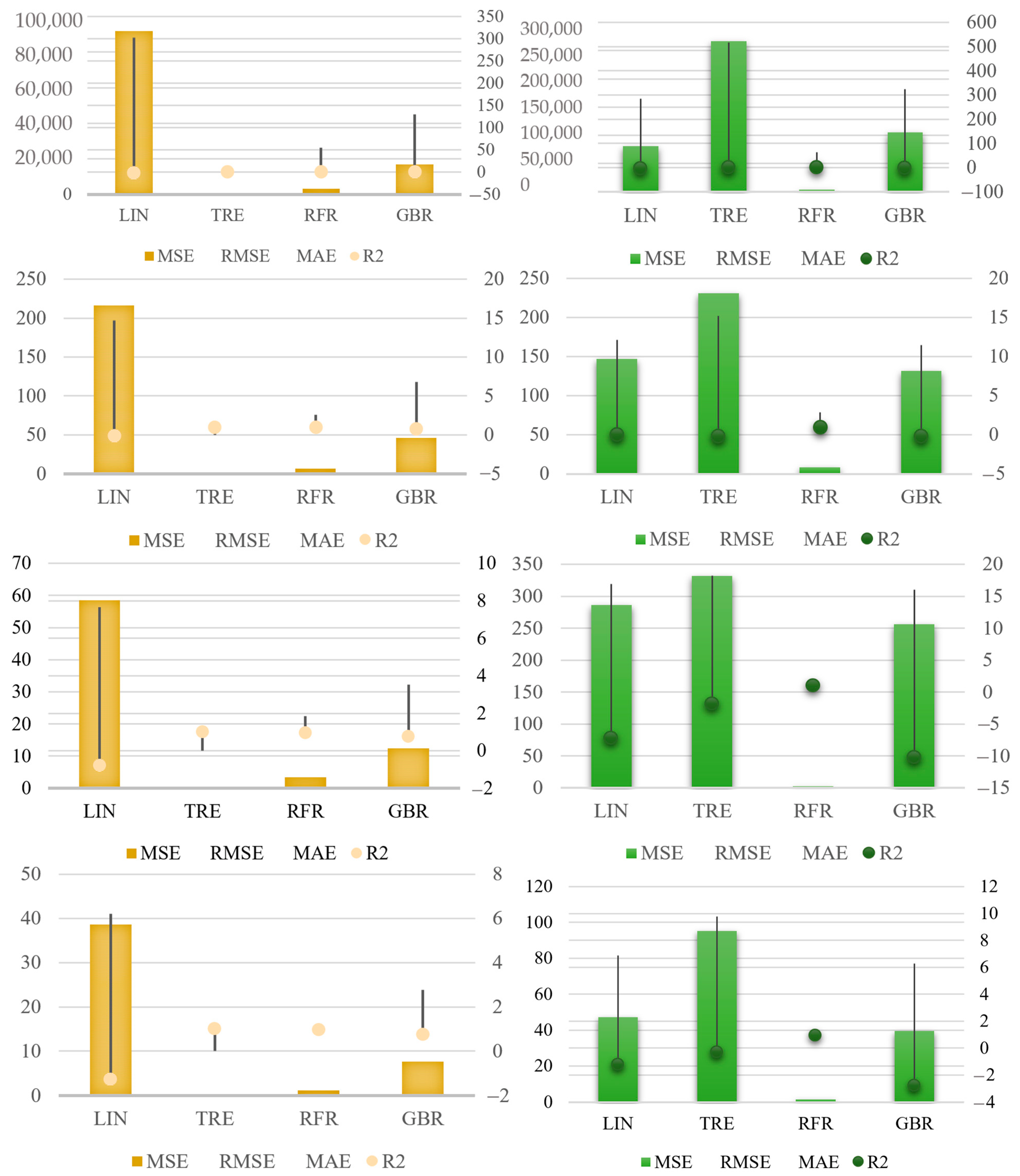
4.1. ML Linear Regression
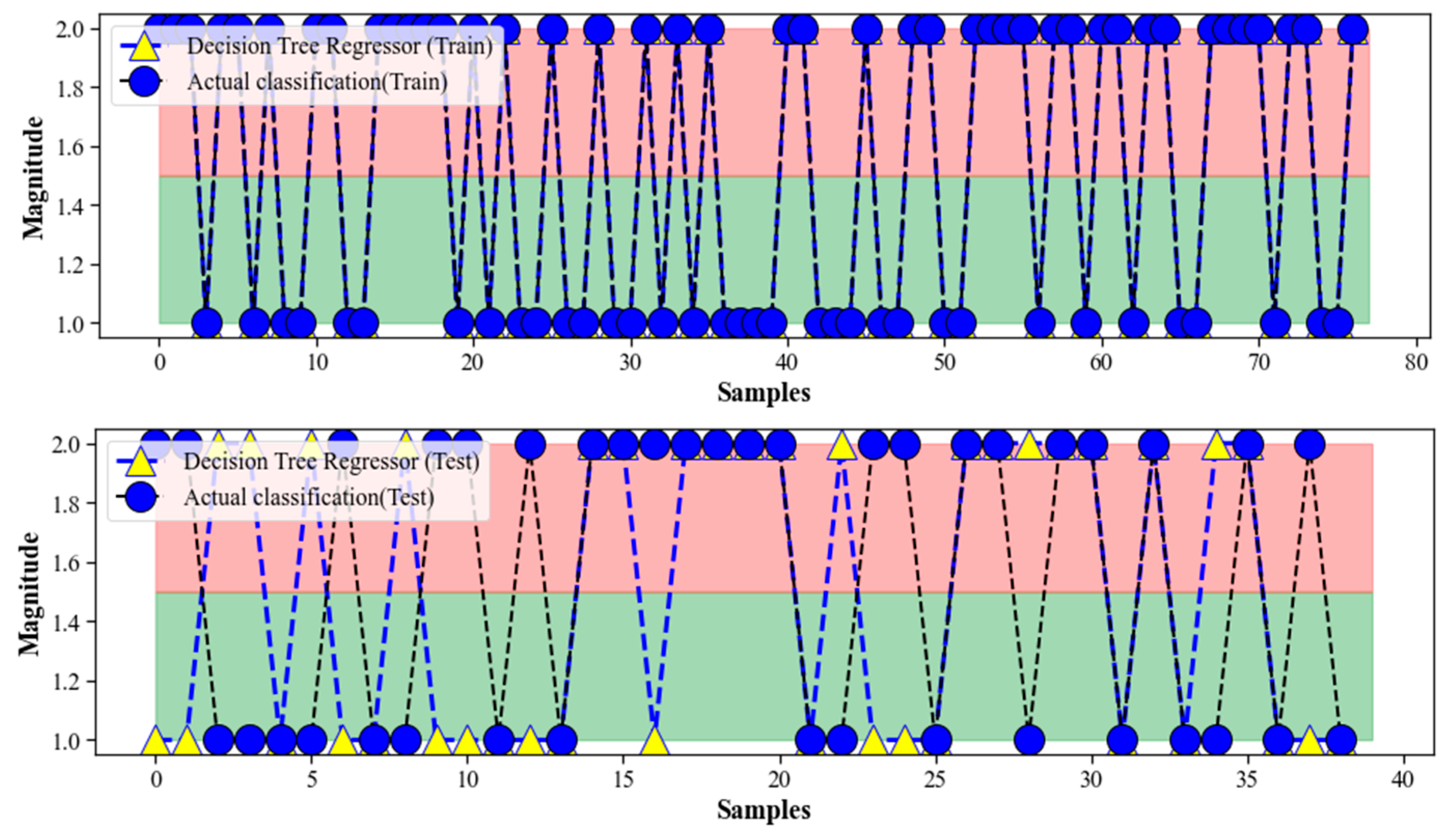
4.2. Decision Tree Regression
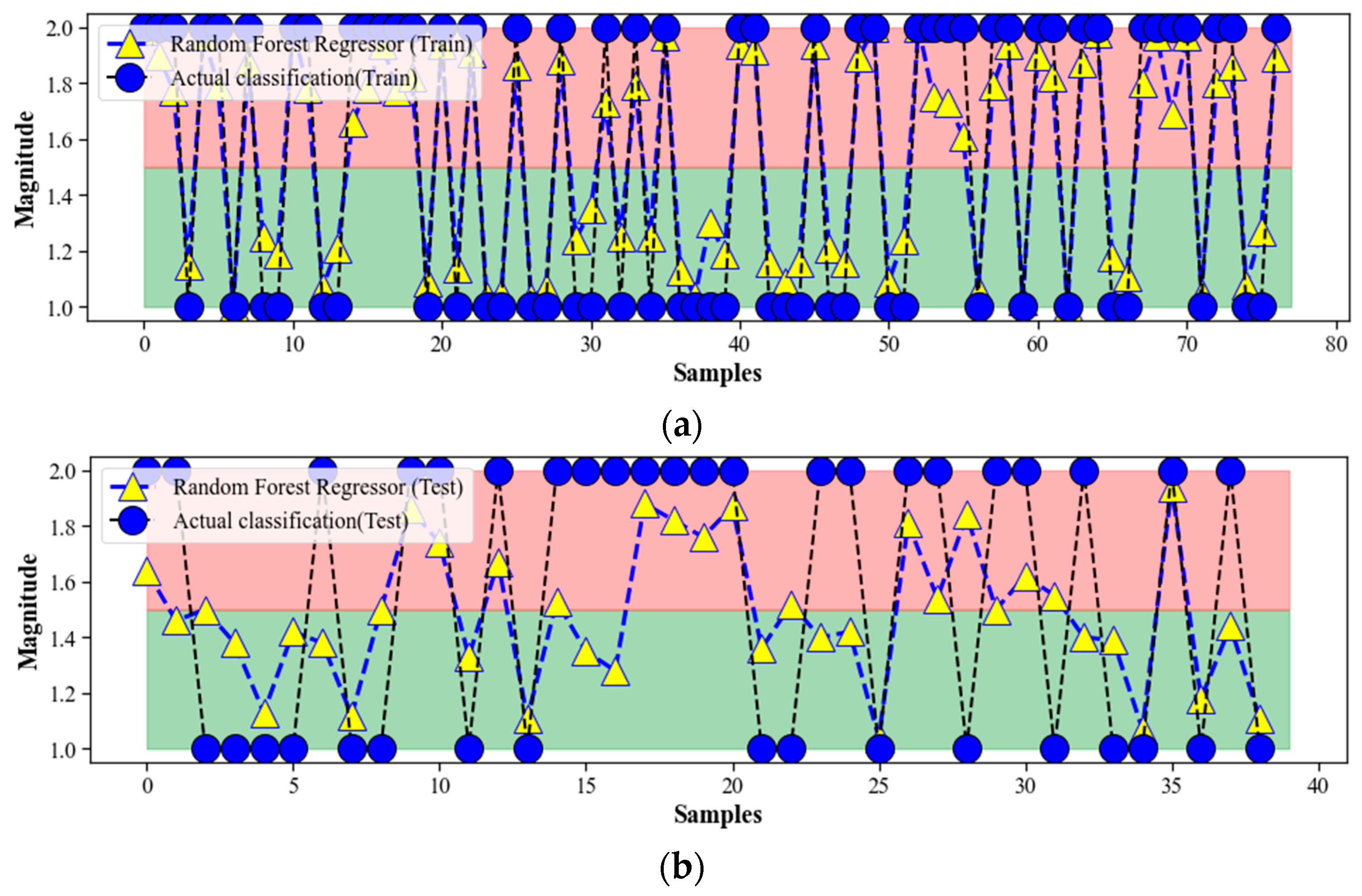
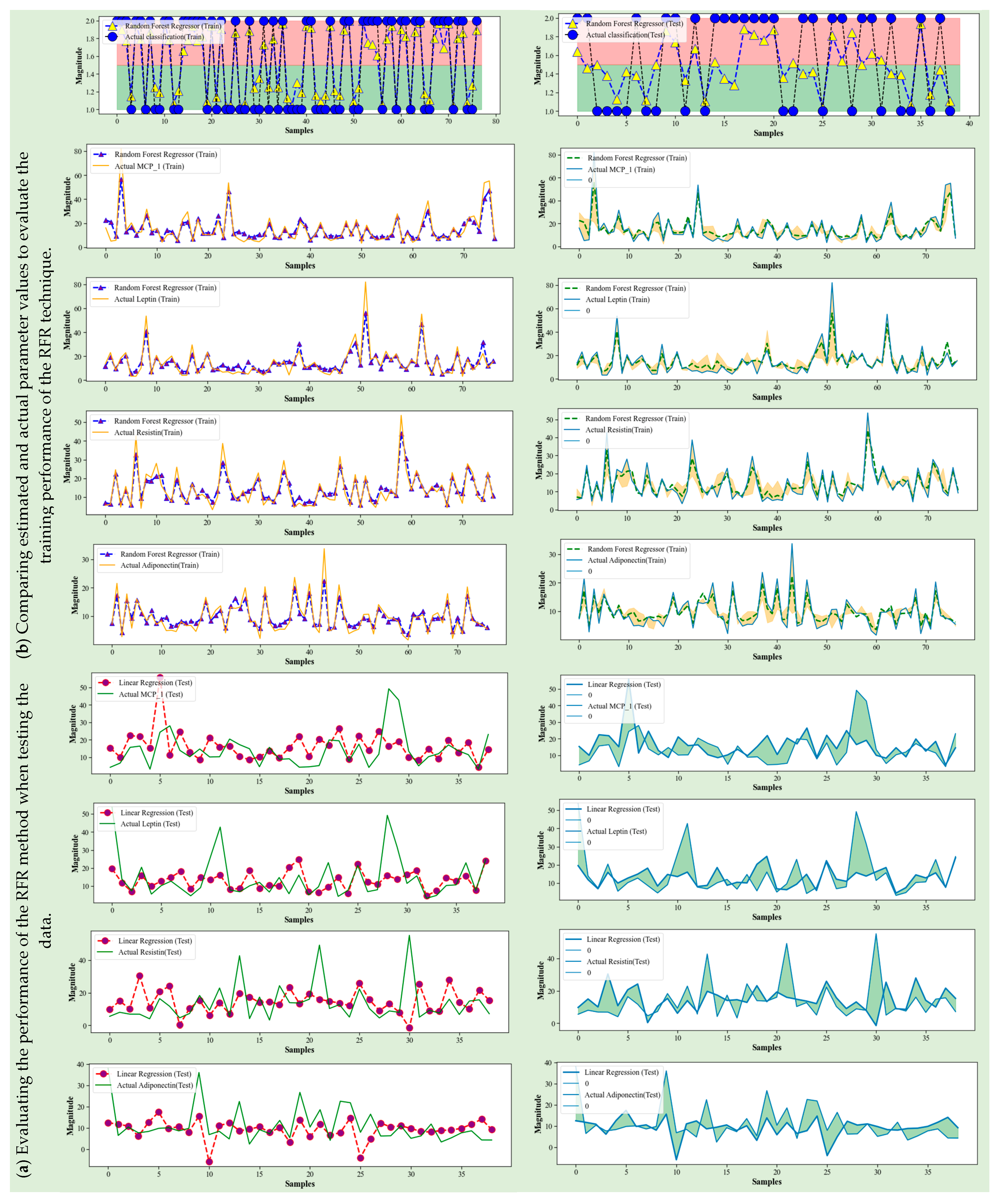
4.3. Random Forest Regression
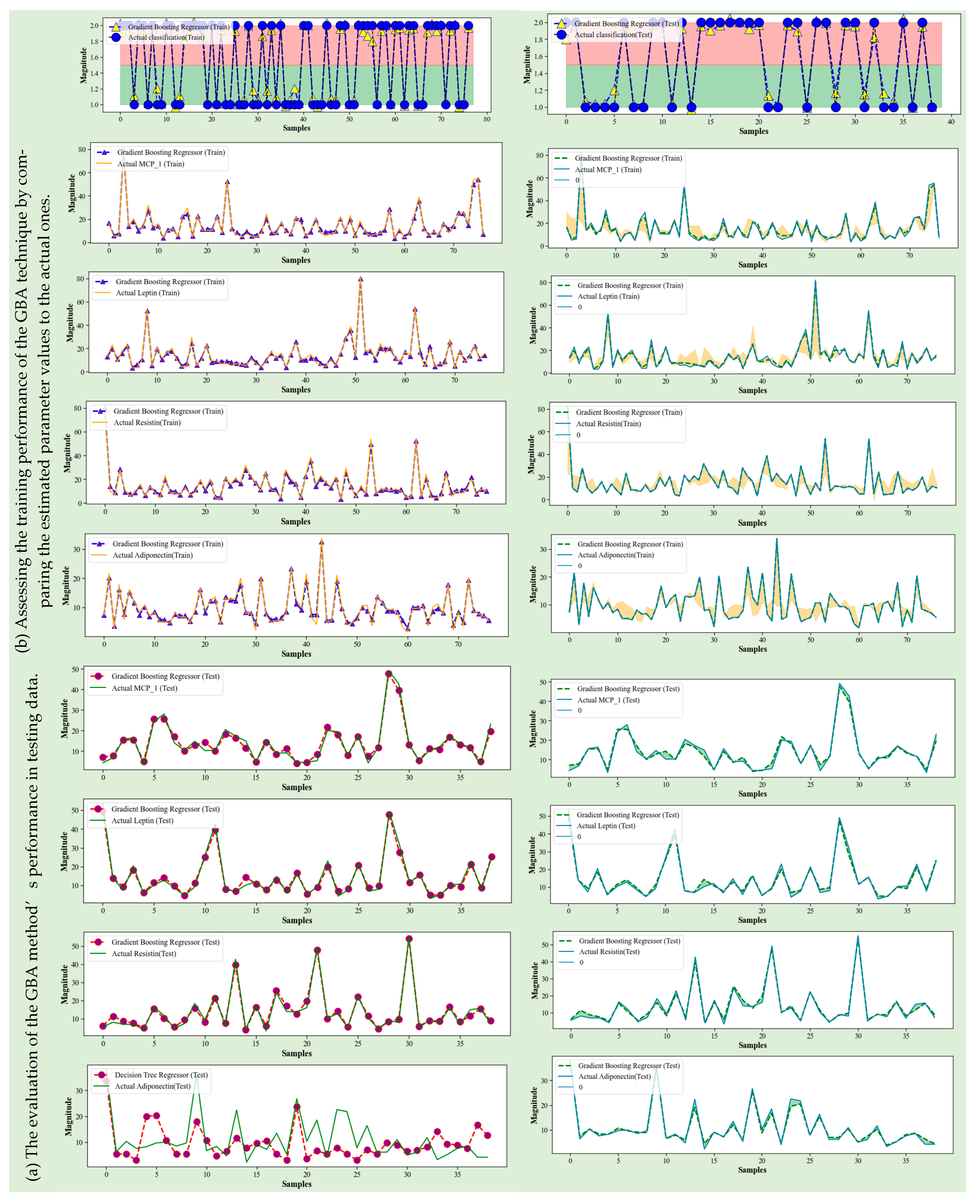
4.4. Gradient Boosting Algorithm
4.5. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jamshidi, M.; Lalbakhsh, A.; Talla, J.; Peroutka, Z.; Hadjilooei, F.; Lalbakhsh, P.; Jamshidi, M.; La Spada, L.; Mirmozafari, M.; Dehghani, M. Artificial intelligence and COVID-19: Deep learning approaches for diagnosis and treatment. IEEE Access 2020, 8, 109581–109595. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.B.; Talla, J.; Lalbakhsh, A.; Sharifi-Atashgah, M.S.; Sabet, A.; Peroutka, Z. A Conceptual Deep Learning Framework for COVID-19 Drug Discovery. In Proceedings of the 2021 IEEE 12th Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON), New York, NY, USA, 1–4 December 2021; pp. 30–34. [Google Scholar]
- Kaul, R.; Ossai, C.; Forkan, A.R.M.; Jayaraman, P.P.; Zelcer, J.; Vaughan, S.; Wickramasinghe, N. The role of AI for developing digital twins in healthcare: The case of cancer care. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2022, 13, e1480. [Google Scholar] [CrossRef]
- Meraghni, S.; Benaggoune, K.; Al Masry, Z.; Terrissa, L.S.; Devalland, C.; Zerhouni, N. Towards digital twins driven breast cancer detection. In Proceedings of the Intelligent Computing: Proceedings of the 2021 Computing Conference, Virtually. 15–16 July 2021; Springer: Berlin/Heidelberg, Germany, 2021; Volume 3, pp. 87–99. [Google Scholar]
- Wickramasinghe, N.; Jayaraman, P.P.; Zelcer, J.; Forkan, A.R.M.; Ulapane, N.; Kaul, R.; Vaughan, S. A vision for leveraging the concept of digital twins to support the provision of personalised cancer care. IEEE Internet Comput. 2021, 26, 17–24. [Google Scholar] [CrossRef]
- Jamshidi, M.B.; Daneshfar, F. A Hybrid Echo State Network for Hypercomplex Pattern Recognition, Classification, and Big Data Analysis. In Proceedings of the 2022 12th International Conference on Computer and Knowledge Engineering (ICCKE), Mashhad, Iran, 17–18 November 2022; pp. 7–12. [Google Scholar]
- Wang, G.; Badal, A.; Jia, X.; Maltz, J.S.; Mueller, K.; Myers, K.J.; Niu, C.; Vannier, M.; Yan, P.; Yu, Z. Development of metaverse for intelligent healthcare. Nat. Mach. Intell. 2022, 4, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Daneshfar, F.; Jamshidi, M.B. An Octonion-Based Nonlinear Echo State Network for Speech Emotion Recognition in Metaverse. Neural Netw. 2023. [Google Scholar] [CrossRef]
- Keshmiri Neghab, H.; Jamshidi, M.; Keshmiri Neghab, H. Digital twin of a magnetic medical microrobot with stochastic model predictive controller boosted by machine learning in cyber-physical healthcare systems. Information 2022, 13, 321. [Google Scholar] [CrossRef]
- Carey, B. Metaverse Technologies, Behavioral Predictive Analytics, and Customer Location Tracking Tools in Blockchain-based Virtual Worlds. Rev. Contemp. Philos. 2022, 21, 188–204. [Google Scholar]
- Musamih, A.; Yaqoob, I.; Salah, K.; Jayaraman, R.; Al-Hammadi, Y.; Omar, M.; Ellahham, S. Metaverse in Healthcare: Applications, Challenges, and Future Directions. IEEE Consum. Electron. Mag. 2022, 1–13. [Google Scholar] [CrossRef]
- Dwivedi, Y.K.; Hughes, L.; Wang, Y.; Alalwan, A.A.; Ahn, S.J.; Balakrishnan, J.; Barta, S.; Belk, R.; Buhalis, D.; Dutot, V. Metaverse marketing: How the metaverse will shape the future of consumer research and practice. Psychol. Mark. 2022, 40, 750–776. [Google Scholar] [CrossRef]
- Yang, Y.; Siau, K.; Xie, W.; Sun, Y. Smart Health: Intelligent Healthcare Systems in the Metaverse, Artificial Intelligence, and Data Science Era. J. Organ. End User Comput. (JOEUC) 2022, 34, 1–14. [Google Scholar] [CrossRef]
- Alazab, M.; Khan, L.U.; Koppu, S.; Ramu, S.P.; Iyapparaja, M.; Boobalan, P.; Baker, T.; Maddikunta, P.K.R.; Gadekallu, T.R.; Aljuhani, A. Digital twins for healthcare 4.0-recent advances, architecture, and open challenges. IEEE Consum. Electron. Mag. 2022, 1–8. [Google Scholar] [CrossRef]
- De Benedictis, A.; Mazzocca, N.; Somma, A.; Strigaro, C. Digital twins in healthcare: An architectural proposal and its application in a social distancing case study. IEEE J. Biomed. Health Inform. 2022, 1–12. [Google Scholar] [CrossRef]
- Sahal, R.; Alsamhi, S.H.; Brown, K.N. Personal digital twin: A close look into the present and a step towards the future of personalised healthcare industry. Sensors 2022, 22, 5918. [Google Scholar] [CrossRef]
- Björnsson, B.; Borrebaeck, C.; Elander, N.; Gasslander, T.; Gawel, D.R.; Gustafsson, M.; Jörnsten, R.; Lee, E.J.; Li, X.; Lilja, S. Digital twins to personalize medicine. Genome Med. 2020, 12, 4. [Google Scholar] [CrossRef]
- Jamshidi, M.B.; Ebadpour, M.; Moghani, M.M. Cancer Digital Twins in Metaverse. In Proceedings of the 2022 20th International Conference on Mechatronics-Mechatronika (ME), Pilsen, Czech Republic, 7–9 December 2022; pp. 1–6. [Google Scholar]
- Zhang, J.; Li, L.; Lin, G.; Fang, D.; Tai, Y.; Huang, J. Cyber resilience in healthcare digital twin on lung cancer. IEEE Access 2020, 8, 201900–201913. [Google Scholar] [CrossRef]
- Filippo, M.D.; Damiani, C.; Vanoni, M.; Maspero, D.; Mauri, G.; Alberghina, L.; Pescini, D. Single-cell digital twins for cancer preclinical investigation. In Metabolic Flux Analysis in Eukaryotic Cells; Springer: Berlin/Heidelberg, Germany, 2020; pp. 331–343. [Google Scholar]
- Thiong’o, G.M.; Rutka, J.T. Digital Twin Technology: The Future of Predicting Neurological Complications of Pediatric Cancers and Their Treatment. Front. Oncol. 2021, 11, 781499. [Google Scholar] [CrossRef]
- Ebadpour, M.; Talla, J.; Jamshidi, M.B.; Peroutka, Z. EKF Digital Twinning of Induction Motor Drives for the Metaverse. In Proceedings of the 2022 20th International Conference on Mechatronics-Mechatronika (ME), Pilsen, Czech Republic, 7–9 December 2022; pp. 1–6. [Google Scholar]
- Zhou, W.; Jia, Y.; Peng, A.; Zhang, Y.; Liu, P. The effect of iot new features on security and privacy: New threats, existing solutions, and challenges yet to be solved. IEEE Internet Things J. 2018, 6, 1606–1616. [Google Scholar] [CrossRef]
- Banerjee, I.; Sofela, M.; Yang, J.; Chen, J.H.; Shah, N.H.; Ball, R.; Mushlin, A.I.; Desai, M.; Bledsoe, J.; Amrhein, T. Development and performance of the pulmonary embolism result forecast model (PERFORM) for computed tomography clinical decision support. JAMA Netw. Open 2019, 2, e198719. [Google Scholar] [CrossRef]
- Miguel, P.; José, P.; Joana, C.; Paulo, M.; Manuel, G. Using Resistin, glucose, age and BMI to predict the presence of breast cancer. BMC Cancer 2018, 18, 123–130. [Google Scholar]
- Maulud, D.; Abdulazeez, A.M. A review on linear regression comprehensive in machine learning. J. Appl. Sci. Technol. Trends 2020, 1, 140–147. [Google Scholar] [CrossRef]
- Rong, S.; Bao-Wen, Z. The research of regression model in machine learning field. MATEC Web Conf. 2018, 176, 01033. [Google Scholar] [CrossRef]
- Tso, G.K.; Yau, K.K. Predicting electricity energy consumption: A comparison of regression analysis, decision tree and neural networks. Energy 2007, 32, 1761–1768. [Google Scholar] [CrossRef]
- Xu, M.; Watanachaturaporn, P.; Varshney, P.K.; Arora, M.K. Decision tree regression for soft classification of remote sensing data. Remote Sens. Environ. 2005, 97, 322–336. [Google Scholar] [CrossRef]
- Kwak, S.; Kim, J.; Ding, H.; Xu, X.; Chen, R.; Guo, J.; Fu, H. Machine learning prediction of the mechanical properties of γ-TiAl alloys produced using random forest regression model. J. Mater. Res. Technol. 2022, 18, 520–530. [Google Scholar] [CrossRef]
- Dai, L.; Ge, J.; Wang, L.; Zhang, Q.; Liang, T.; Bolan, N.; Lischeid, G.; Rinklebe, J. Influence of soil properties, topography, and land cover on soil organic carbon and total nitrogen concentration: A case study in Qinghai-Tibet plateau based on random forest regression and structural equation modeling. Sci. Total Environ. 2022, 821, 153440. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, G.; Huang, Y.; Aslani, F.; Nener, B. Modelling uniaxial compressive strength of lightweight self-compacting concrete using random forest regression. Constr. Build. Mater. 2019, 210, 713–719. [Google Scholar] [CrossRef]
- Dyer, A.S.; Zaengle, D.; Nelson, J.R.; Duran, R.; Wenzlick, M.; Wingo, P.C.; Bauer, J.R.; Rose, K.; Romeo, L. Applied machine learning model comparison: Predicting offshore platform integrity with gradient boosting algorithms and neural networks. Mar. Struct. 2022, 83, 103152. [Google Scholar] [CrossRef]
- Jafari, S.; Shahbazi, Z.; Byun, Y.-C.; Lee, S.-J. Lithium-ion battery estimation in online framework using extreme gradient boosting machine learning approach. Mathematics 2022, 10, 888. [Google Scholar] [CrossRef]
- Das, K.; Jiang, J.; Rao, J. Mean squared error of empirical predictor. Ann. Statist. 2004, 32, 818–840. [Google Scholar] [CrossRef]
- Chai, T.; Draxler, R.R. Root mean square error (RMSE) or mean absolute error (MAE)?—Arguments against avoiding RMSE in the literature. Geosci. Model Dev. 2014, 7, 1247–1250. [Google Scholar] [CrossRef]
- Willmott, C.J.; Matsuura, K. Advantages of the mean absolute error (MAE) over the root mean square error (RMSE) in assessing average model performance. Clim. Res. 2005, 30, 79–82. [Google Scholar] [CrossRef]
- Angulo, C.; Gonzalez-Abril, L.; Raya, C.; Ortega, J.A. A proposal to evolving towards digital twins in healthcare. In Proceedings of the International Work-Conference on Bioinformatics and Biomedical Engineering, Granada, Spain, 6–8 May 2020; pp. 418–426. [Google Scholar]
- Apidianakis, Y.; Eliopoulos, A.G. A holo’ome approach in colon cancer: We change as we age. EMBO Rep. 2015, 16, 1239–1240. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moztarzadeh, O.; Jamshidi, M.; Sargolzaei, S.; Jamshidi, A.; Baghalipour, N.; Malekzadeh Moghani, M.; Hauer, L. Metaverse and Healthcare: Machine Learning-Enabled Digital Twins of Cancer. Bioengineering 2023, 10, 455. https://doi.org/10.3390/bioengineering10040455
Moztarzadeh O, Jamshidi M, Sargolzaei S, Jamshidi A, Baghalipour N, Malekzadeh Moghani M, Hauer L. Metaverse and Healthcare: Machine Learning-Enabled Digital Twins of Cancer. Bioengineering. 2023; 10(4):455. https://doi.org/10.3390/bioengineering10040455
Chicago/Turabian StyleMoztarzadeh, Omid, Mohammad (Behdad) Jamshidi, Saleh Sargolzaei, Alireza Jamshidi, Nasimeh Baghalipour, Mona Malekzadeh Moghani, and Lukas Hauer. 2023. "Metaverse and Healthcare: Machine Learning-Enabled Digital Twins of Cancer" Bioengineering 10, no. 4: 455. https://doi.org/10.3390/bioengineering10040455
APA StyleMoztarzadeh, O., Jamshidi, M., Sargolzaei, S., Jamshidi, A., Baghalipour, N., Malekzadeh Moghani, M., & Hauer, L. (2023). Metaverse and Healthcare: Machine Learning-Enabled Digital Twins of Cancer. Bioengineering, 10(4), 455. https://doi.org/10.3390/bioengineering10040455






