Ankle Joint Angle Influences Relative Torque Fluctuation during Isometric Plantar Flexion
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup
2.3. Experimental Protocol
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Absolute Variability for Torque
3.2. Absolute Variability for rsEMG
3.3. Relative Variability for Torque
3.4. Relative Variability for rsEMG
3.5. Contributions from Relative Variability of rsEMG to the Resultant Relative Torque Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enoka, R.M.; Farina, D. Force steadiness: From motor units to voluntary actions. Physiol. J. 2021, 36, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Newell, K.M. Aging and the time and frequency structure of force output variability. J. Appl. Physiol. 2003, 94, 903–912. [Google Scholar] [CrossRef]
- Lodha, N.; Misra, G.; Coombes, S.A.; Christou, E.A.; Cauraugh, J.H. Increased force variability in chronic stroke: Contributions of force modulation below 1 Hz. PLoS ONE 2013, 8, e83468. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A.; Callahan, D.M.; Fay, J.L.; Foulis, S.A.; Buonaccorsi, J.P. Muscle weakness, fatigue, and torque variability: Effects of age and mobility status. Muscle Nerve 2014, 49, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Christensen, J.C.; Marcus, R.L.; LaStayo, P.C. Muscle force and movement variability before and after total knee arthroplasty: A review. World J. Orthop. 2014, 5, 69. [Google Scholar] [CrossRef]
- Wang, W.-E.; Roy, A.; Misra, G.; Archer, D.B.; Ribeiro-Dasilva, M.C.; Fillingim, R.B.; Coombes, S.A. Motor-evoked pain increases force variability in chronic jaw pain. J. Pain Res. 2018, 19, 636–648. [Google Scholar] [CrossRef]
- Tracy, B.L.; Maluf, K.S.; Stephenson, J.L.; Hunter, S.K.; Enoka, R.M. Variability of motor unit discharge and force fluctuations across a range of muscle forces in older adults. Muscle Nerve 2005, 32, 533–540. [Google Scholar] [CrossRef]
- Taylor, A.M.; Christou, E.A.; Enoka, R.M. Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J. Neurophysiol. 2003, 90, 1350–1361. [Google Scholar] [CrossRef]
- Galganski, M.E.; Fuglevand, A.J.; Enoka, R.M. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J. Neurophysiol. 1993, 69, 2108–2115. [Google Scholar] [CrossRef]
- Jones, K.E.; Hamilton, A.F.d.C.; Wolpert, D.M. Sources of signal-dependent noise during isometric force production. J. Neurophysiol. 2002, 88, 1533–1544. [Google Scholar] [CrossRef]
- Dideriksen, J.L.; Negro, F.; Enoka, R.M.; Farina, D. Motor unit recruitment strategies and muscle properties determine the influence of synaptic noise on force steadiness. J. Neurophysiol. 2012, 107, 3357–3369. [Google Scholar] [CrossRef]
- Yoshitake, Y.; Shinohara, M. Oscillations in motor unit discharge are reflected in the low-frequency component of rectified surface EMG and the rate of change in force. Exp. Brain Res. 2013, 231, 267–276. [Google Scholar] [CrossRef]
- Yoshitake, Y.; Shinohara, M. Low-frequency component of rectified EMG is temporally correlated with force and instantaneous rate of force fluctuations during steady contractions. Muscle Nerve 2013, 47, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Casamento-Moran, A.; Yacoubi, B.; Christou, E.A. Voluntary reduction of force variability via modulation of low-frequency oscillations. Exp. Brain Res. 2017, 235, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Hali, K.; Zero, A.M.; Rice, C.L. Effect of ankle joint position on triceps surae contractile properties and motor unit discharge rates. Physiol. Rep. 2021, 8, e14680. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Awiszus, F. Physiological alterations of maximal voluntary quadriceps activation by changes of knee joint angle. Muscle Nerve 2001, 24, 667–672. [Google Scholar] [CrossRef]
- Shinohara, M.; Yoshitake, Y.; Kouzaki, M.; Fukunaga, T. The medial gastrocnemius muscle attenuates force fluctuations during plantar flexion. Exp. Brain Res. 2006, 169, 15–23. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Voudrie, S.J.; Ebersole, K.T. The effect of knee joint angle on torque control. J. Mot. Behav. 2009, 42, 5–10. [Google Scholar] [CrossRef]
- Pasquet, B.; Carpentier, A.; Duchateau, J. Change in muscle fascicle length influences the recruitment and discharge rate of motor units during isometric contractions. J. Neurophysiol. 2005, 94, 3126–3133. [Google Scholar] [CrossRef]
- Johansson, H.; Sjölander, P.; Sojka, P. A sensory role for the cruciate ligaments. Clin. Orthop. Relat. Res. 1991, 268, 161–178. [Google Scholar]
- George, D.; Mallery, P. IBM SPSS Statistics 26 Step by Step: A Simple Guide and Reference; Routledge: New York, NY, USA, 2019. [Google Scholar]
- Baweja, H.S.; Patel, B.K.; Martinkewiz, J.D.; Vu, J.; Christou, E.A. Removal of visual feedback alters muscle activity and reduces force variability during constant isometric contractions. Exp. Brain Res. 2009, 197, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Pakaslahti, J.; Komi, P. Medial gastrocnemius muscle behavior during human running and walking. Gait Posture 2007, 25, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Arnold, E.M.; Delp, S.L. Fibre operating lengths of human lower limb muscles during walking. Philos. Trans. R. Soc 2011, 366, 1530–1539. [Google Scholar] [CrossRef]
- Rubenson, J.; Pires, N.J.; Loi, H.O.; Pinniger, G.J.; Shannon, D.G. On the ascent: The soleus operating length is conserved to the ascending limb of the force–length curve across gait mechanics in humans. J. Exp. Biol. 2012, 215, 3539–3551. [Google Scholar] [CrossRef]
- Kanda, K.; Burke, R.; Walmsley, B. Differential control of fast and slow twitch motor units in the decerebrate cat. Exp. Brain Res. 1977, 29, 57–74. [Google Scholar] [CrossRef]
- Saltin, B. Skeletal muscle adaptability: Significant for metabolism and performance in Handbook of Physiology. Skeletal Muscle Bethesda. Am. Physiol. Soc. 1983, 10, 555–663. [Google Scholar]
- Chen, Y.-T.; Zhang, C.; Liu, Y.; Magat, E.; Verduzco-Gutierrez, M.; Francisco, G.E.; Zhou, P.; Zhang, Y.; Li, S. The effects of botulinum toxin injections on spasticity and motor performance in chronic stroke with spastic hemiplegia. Toxics 2020, 12, 492. [Google Scholar] [CrossRef] [PubMed]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part II: The role of proprioception in motor control and functional joint stability. J. Athl. Train. 2002, 37, 80. [Google Scholar] [PubMed]
- Le, P.; Best, T.M.; Khan, S.N.; Mendel, E.; Marras, W.S. A review of methods to assess coactivation in the spine. J. Electromyogr. Kinesiol. 2017, 32, 51–60. [Google Scholar] [CrossRef]
- Christou, E.A. Aging and variability of voluntary contractions. Exerc. Sport Sci. Rev. 2011, 39, 77. [Google Scholar] [CrossRef]
- McCrum, C.; Leow, P.; Epro, G.; König, M.; Meijer, K.; Karamanidis, K. Alterations in leg extensor muscle-tendon unit biomechanical properties with ageing and mechanical loading. Front. Physiol. 2018, 9, 150. [Google Scholar] [CrossRef] [PubMed]

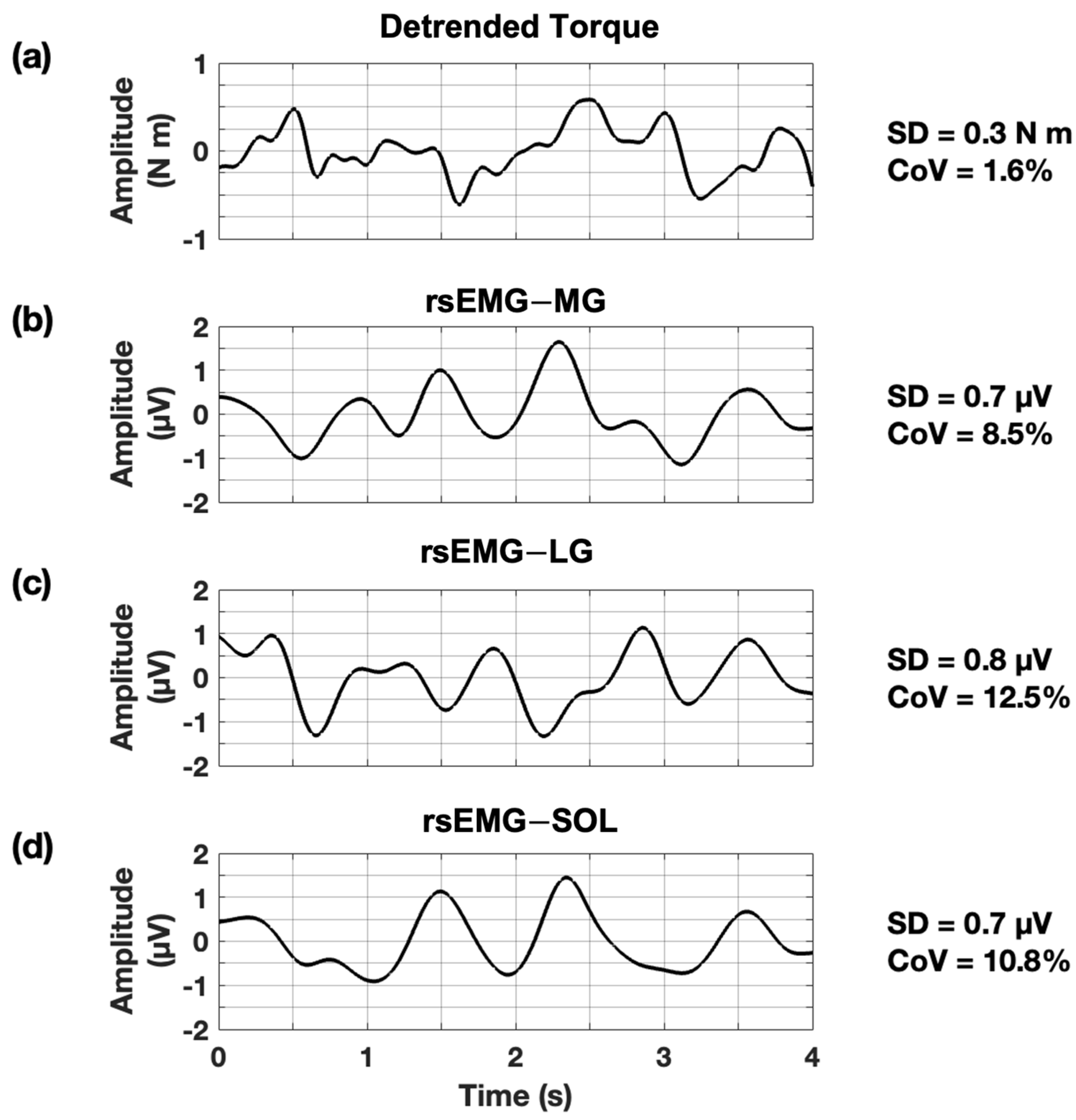
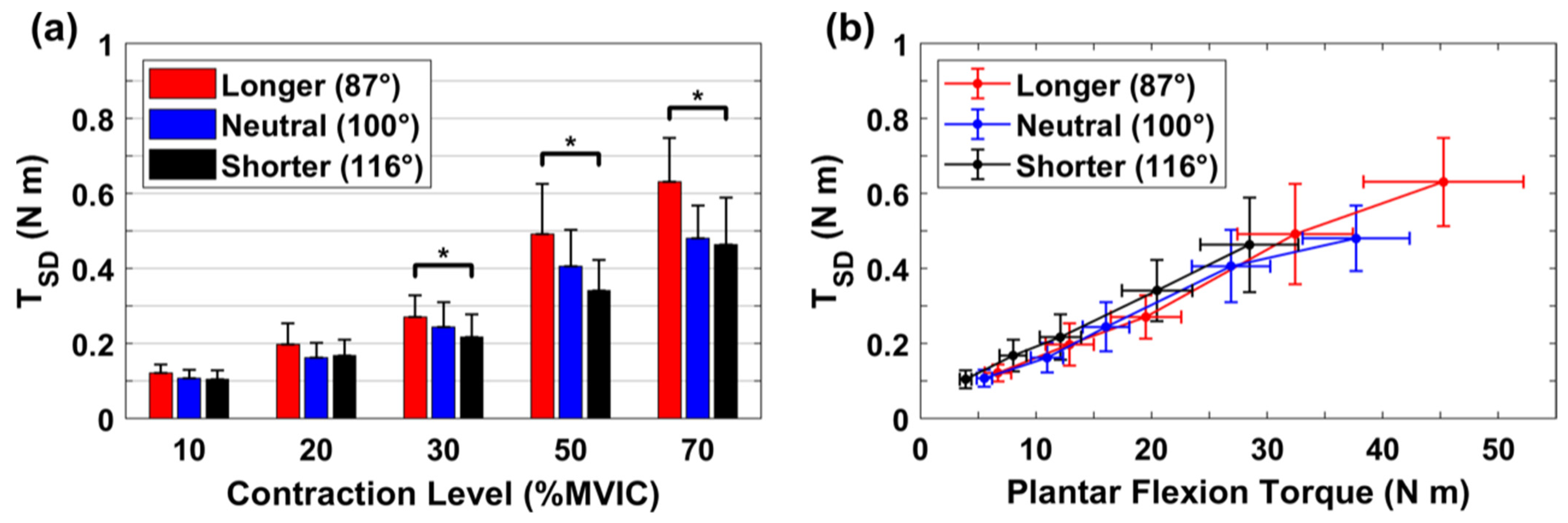
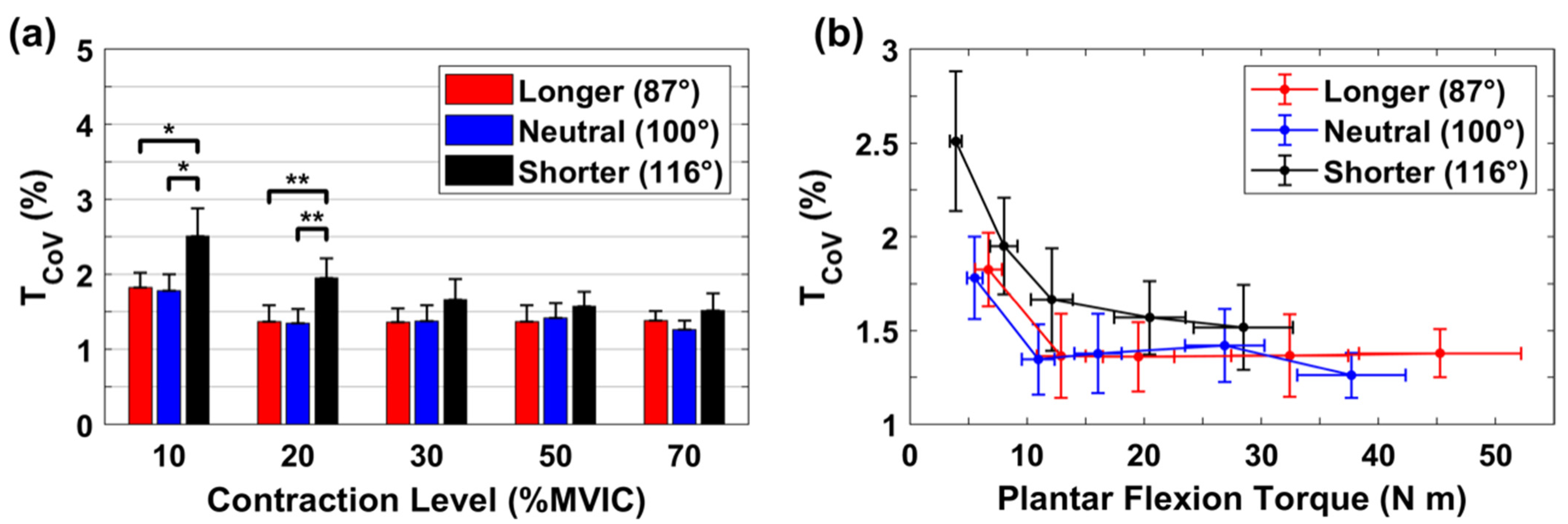
| Muscle | Position | 10% MVIC | 20% MVIC | 30% MVIC | 50% MVIC | 70% MVIC |
|---|---|---|---|---|---|---|
| MG | L | 0.62 ± 0.13 | 0.85 ± 0.17 | 1.18 ± 0.21 | 1.99 ± 0.40 | 2.56 ± 0.51 |
| N | 0.52 ± 0.09 | 0.87 ± 0.16 | 1.16 ± 0.24 | 2.04 ± 0.49 | 2.78 ± 0.64 | |
| S | 0.69 ± 0.12 | 0.98 ± 0.19 | 1.31 ± 0.27 | 2.10 ± 0.40 | 3.18 ± 0.70 | |
| LG | L | 0.42 ± 0.05 | 0.65 ± 0.07 | 0.94 ± 0.10 | 1.67 ± 0.27 | 2.37 ± 0.40 |
| N | 0.48 ± 0.09 | 0.59 ± 0.09 | 1.06 ± 0.24 | 1.52 ± 0.27 | 2.23 ± 0.39 | |
| S | 0.49 ± 0.08 | 0.69 ± 0.11 | 1.06 ± 0.20 | 1.75 ± 0.31 | 2.84 ± 0.46 | |
| SOL | L | 0.30 ± 0.03 | 0.30 ± 0.05 | 0.50 ± 0.06 | 0.78 ± 0.11 | 1.01 ± 0.14 |
| N | 0.35 ± 0.06 | 0.35 ± 0.08 | 0.57 ± 0.13 | 0.70 ± 0.14 | 0.84 ± 0.13 | |
| S | 0.31 ± 0.06 | 0.31 ± 0.05 | 0.48 ± 0.10 | 0.69 ± 0.14 | 1.02 ± 0.19 |
| Muscle | Position | 10% MVIC | 20% MVIC | 30% MVIC | 50% MVIC | 70% MVIC |
|---|---|---|---|---|---|---|
| MG | L | 9.98 ± 0.68 | 8.73 ± 0.50 | 8.41 ± 0.62 | 8.74 ± 0.62 | 8.25 ± 0.55 |
| N | 8.81 ± 0.70 | 7.77 ± 0.52 | 7.52 ± 0.43 | 8.07 ± 0.61 | 8.13 ± 0.62 | |
| S | 8.66 ± 9.68 | 8.03 ± 0.50 | 7.60 ± 0.67 | 8.28 ± 0.56 | 8.42 ± 0.55 | |
| LG | L | 8.30 ± 0.37 | 8.01 ± 0.72 | 8.04 ± 0.41 | 8.41 ± 0.76 | 8.77 ± 0.96 |
| N | 8.17 ± 0.14 | 7.77 ± 0.61 | 8.46 ± 0.80 | 7.47 ± 0.62 | 8.05 ± 0.68 | |
| S | 8.19 ± 0.48 | 7.46 ± 0.51 | 7.64 ± 0.51 | 8.16 ± 0.38 | 8.41 ± 0.53 | |
| SOL | L | 7.53 ± 0.40 | 6.76 ± 0.35 | 6.98 ± 0.33 | 7.31 ± 0.46 | 7.38 ± 0.31 |
| N | 7.78 ± 0.36 | 7.46 ± 0.47 | 7.08 ± 0.36 | 7.36 ± 0.67 | 8.83 ± 1.17 | |
| S | 9.02 ± 0.93 | 8.30 ± 0.37 | 8.63 ± 0.58 | 8.80 ± 0.82 | 9.29 ± 0.68 |
| MG | LG | SOL | |
|---|---|---|---|
| Longer | * 0.14 ± 0.08 | 0.04 ± 0.07 | * 0.18 ± 0.13 |
| Neutral | * 0.08 ± 0.07 | ** 0.13 ± 0.07 | 0.04 ± 0.06 |
| Shorter | * 0.19 ± 0.11 | * 0.18 ± 0.14 | −0.01 ± 0.09 |
| % MVIC | MG | LG | SOL |
|---|---|---|---|
| 10 | 0.03 ± 0.15 | 0.17 ± 0.28 | 0.04 ± 0.16 |
| 20 | * 0.22 ± 0.14 | 0.04 ± 0.13 | 0.08 ± 0.16 |
| 30 | ** 0.19 ± 0.08 | * 0.15 ± 0.09 | * 0.12 ± 0.10 |
| 50 | * 0.10 ± 0.09 | * 0.11 ± 0.09 | * 0.11 ± 0.08 |
| 70 | ** 0.19 ± 0.10 | 0.04 ± 0.09 | 0.03 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, F.; Rymer, W.Z.; Son, J. Ankle Joint Angle Influences Relative Torque Fluctuation during Isometric Plantar Flexion. Bioengineering 2023, 10, 373. https://doi.org/10.3390/bioengineering10030373
Shi F, Rymer WZ, Son J. Ankle Joint Angle Influences Relative Torque Fluctuation during Isometric Plantar Flexion. Bioengineering. 2023; 10(3):373. https://doi.org/10.3390/bioengineering10030373
Chicago/Turabian StyleShi, Fandi, William Zev Rymer, and Jongsang Son. 2023. "Ankle Joint Angle Influences Relative Torque Fluctuation during Isometric Plantar Flexion" Bioengineering 10, no. 3: 373. https://doi.org/10.3390/bioengineering10030373
APA StyleShi, F., Rymer, W. Z., & Son, J. (2023). Ankle Joint Angle Influences Relative Torque Fluctuation during Isometric Plantar Flexion. Bioengineering, 10(3), 373. https://doi.org/10.3390/bioengineering10030373








