Acellular Human Placenta Small-Diameter Vessels as a Favorable Source of Super-Microsurgical Vascular Replacements: A Proof of Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Vessel Isolation and Matrix Graft Fabrication
2.3. Animal Experimentation
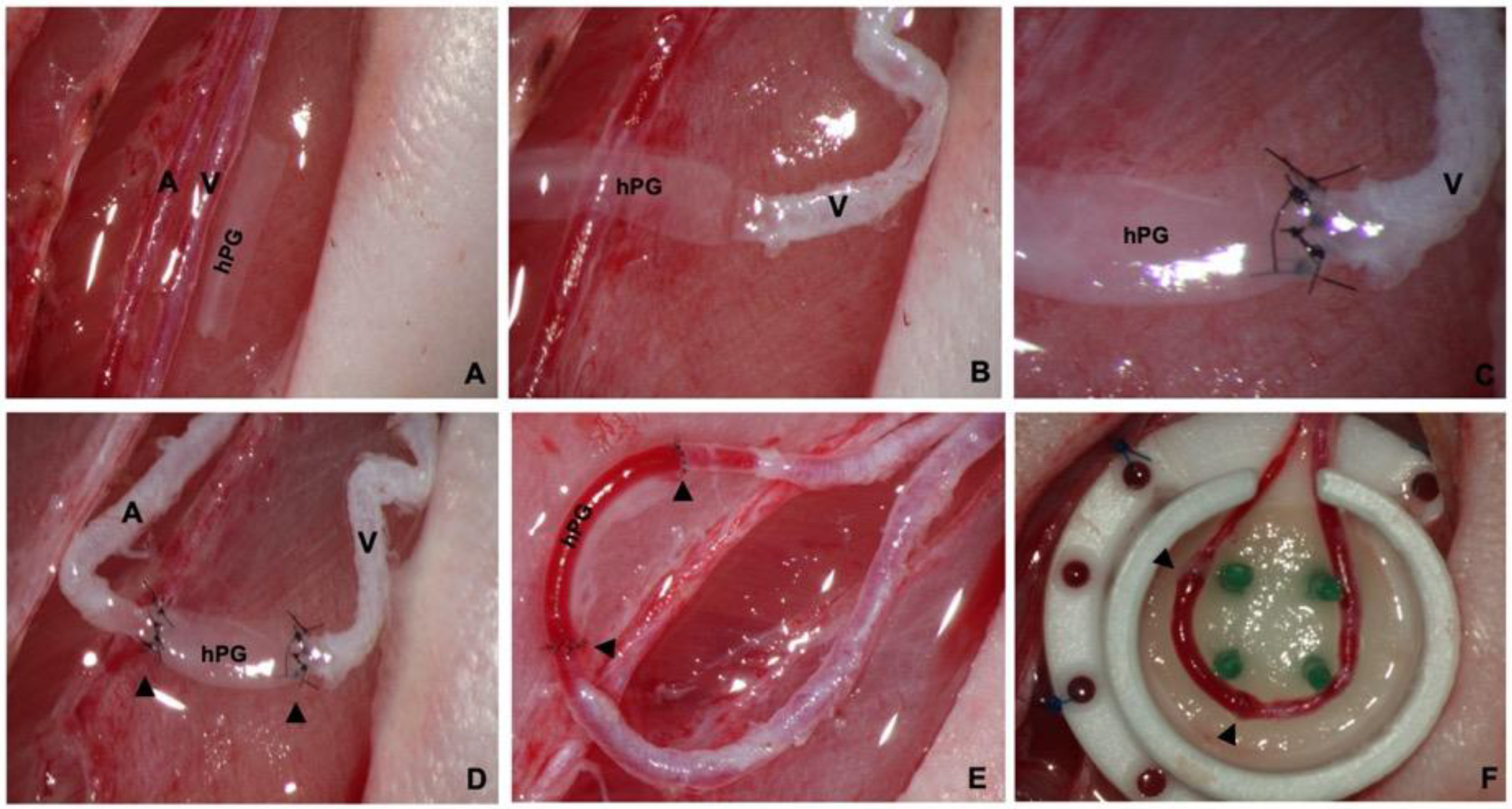
2.4. Arteriovenous Loop (AVL) Operations
2.5. Histology
2.6. Micro Computed Tomography
2.7. Statistical Analysis
3. Results
3.1. Production of Acellular Submillimeter hPGs
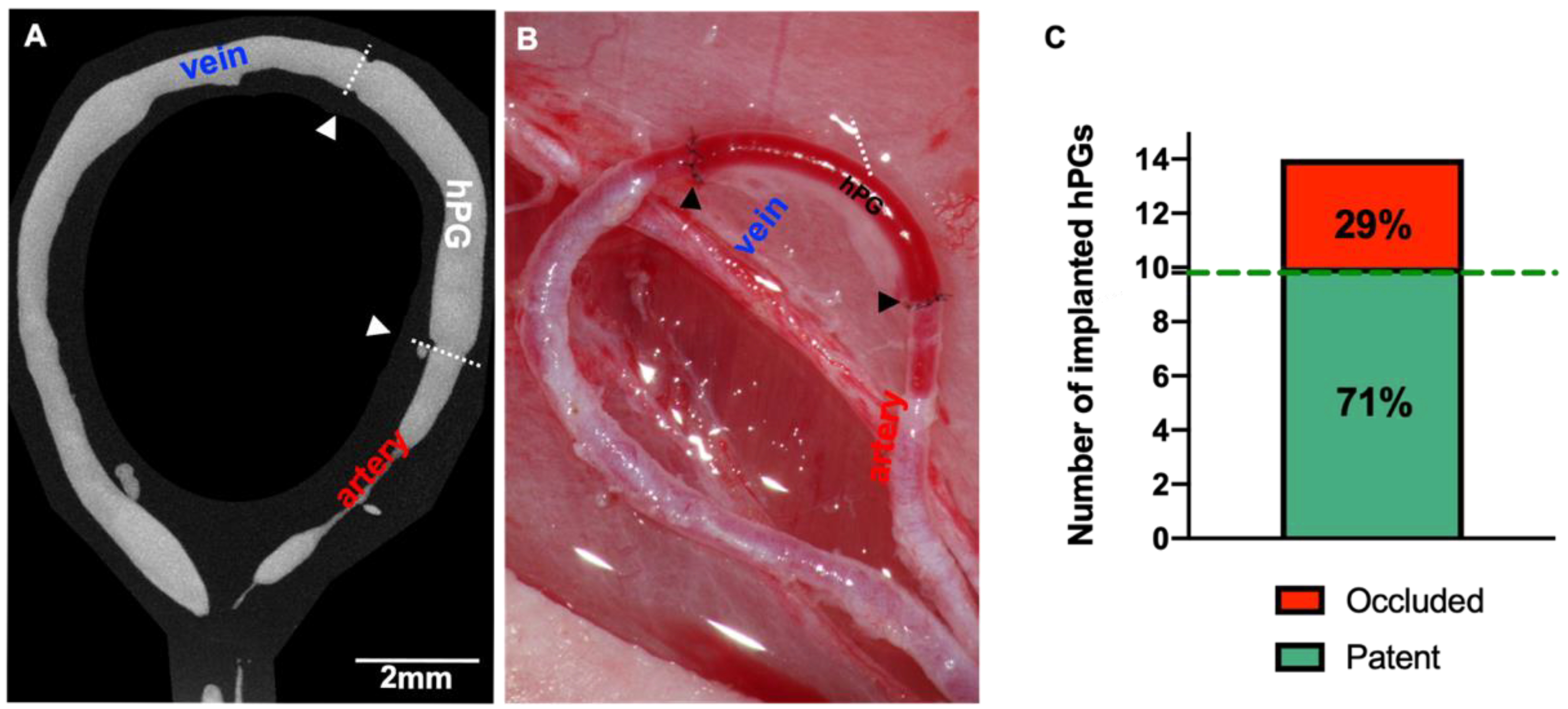
3.2. hPGs Have a Perfect Caliber Match and High Patency Rates
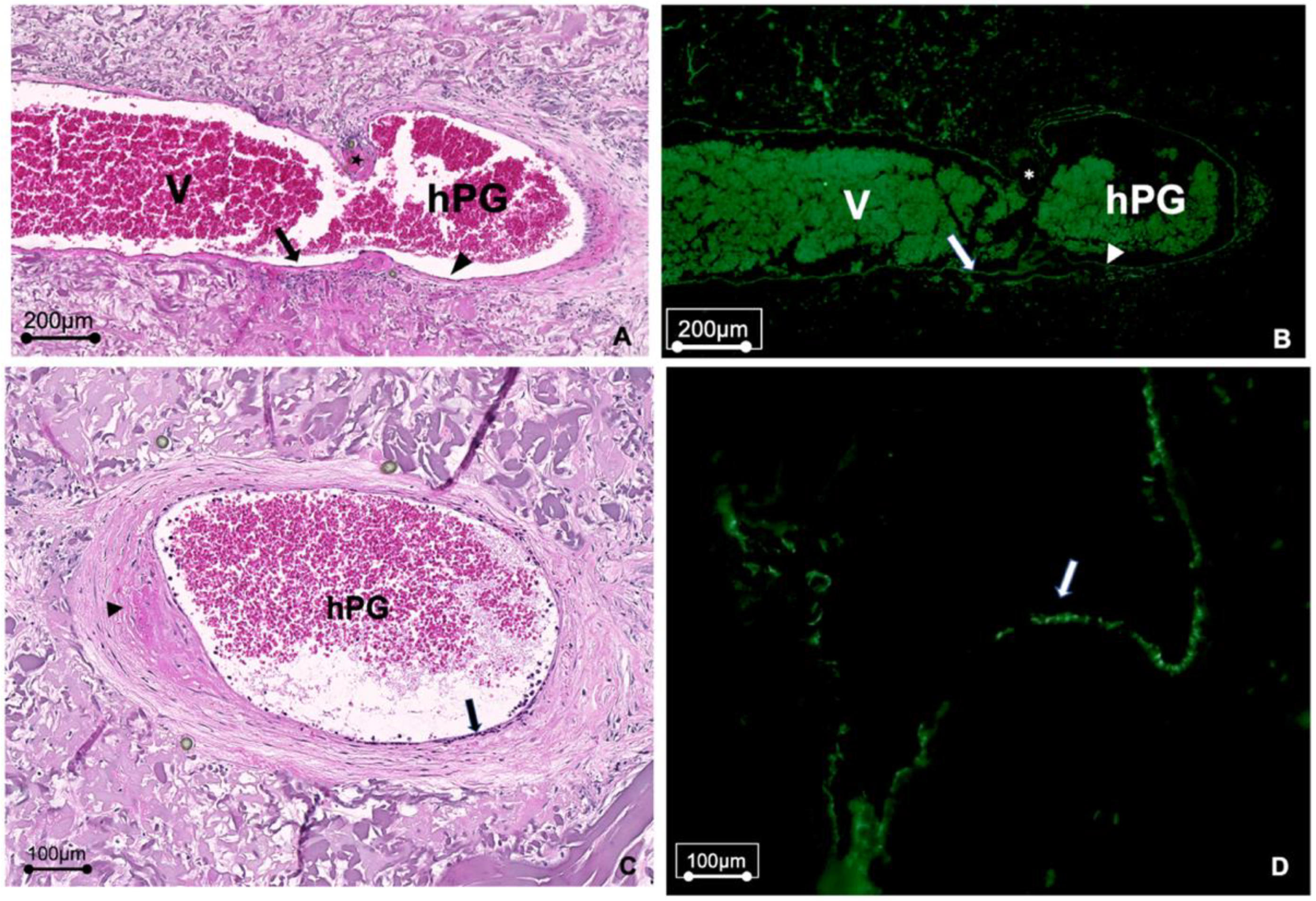
3.3. Vascular Remodeling Takes Place in hPGs
3.4. µCT Analysis of hPGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Germann, G.; Steinau, H.U. The clinical reliability of vein grafts in free-flap transfer. J. Reconstr. Microsurg. 1996, 12, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hyza, P.; Vesely, J.; Drazan, L.; Stupka, I.; Ranno, R.; Castagnetti, F. Primary vein grafting in treatment of ring avulsion injuries: A 5-year prospective study. Ann. Plast. Surg. 2007, 59, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Henn, D.; Wahmann, M.S.T.; Horsch, M.; Hetjens, S.; Kremer, T.; Gazyakan, E.; Hirche, C.; Schmidt, V.J.; Germann, G.; Kneser, U. One-Stage versus Two-Stage Arteriovenous Loop Reconstructions: An Experience on 103 Cases from a Single Center. Plast. Reconstr. Surg. 2019, 143, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.H.; Klifto, C.S.; Milone, M.T.; Cohen, J.M.; Daar, D.A.; Anzai, L.; Thanik, V.D.; Hacquebord, J.H. Survival after Digit Replantation and Revascularization Is Not Affected by the Use of Interpositional Grafts during Arterial Repair. Plast. Reconstr. Surg. 2019, 143, 551e–557e. [Google Scholar] [CrossRef]
- Chamiot-Clerc, P.; Copie, X.; Renaud, J.F.; Safar, M.; Girerd, X. Comparative reactivity and mechanical properties of human isolated internal mammary and radial arteries. Cardiovasc. Res. 1998, 37, 811–819. [Google Scholar] [CrossRef]
- Chlupac, J.; Filova, E.; Bacakova, L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009, 58 (Suppl. 2), S119–S140. [Google Scholar] [CrossRef]
- Ballotta, E.; Renon, L.; Toffano, M.; Da Giau, G. Prospective randomized study on bilateral above-knee femoropopliteal revascularization: Polytetrafluoroethylene graft versus reversed saphenous vein. J. Vasc. Surg. 2003, 38, 1051–1055. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; John, K.; Shoji, T.; Shinoka, T. The Evolution of Tissue Engineered Vascular Graft Technologies: From Preclinical Trials to Advancing Patient Care. Appl. Sci. 2019, 9, 1274. [Google Scholar] [CrossRef]
- Bergmeister, H.; Plasenzotti, R.; Walter, I.; Plass, C.; Bastian, F.; Rieder, E.; Sipos, W.; Kaider, A.; Losert, U.; Weigel, G. Decellularized, xenogeneic small-diameter arteries: Transition from a muscular to an elastic phenotype in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 95–104. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Baier, J.M. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef]
- Mancuso, L.; Gualerzi, A.; Boschetti, F.; Loy, F.; Cao, G. Decellularized ovine arteries as small-diameter vascular grafts. Biomed. Mater. 2014, 9, 045011. [Google Scholar] [CrossRef]
- Borschel, G.H.; Huang, Y.C.; Calve, S.; Arruda, E.M.; Lynch, J.B.; Dow, D.E.; Kuzon, W.M.; Dennis, R.G.; Brown, D.L. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005, 11, 778–786. [Google Scholar] [CrossRef]
- Galili, U.; Swanson, K. Gene sequences suggest inactivation of alpha-1,3-galactosyltransferase in catarrhines after the divergence of apes from monkeys. Proc. Natl. Acad. Sci. USA 1991, 88, 7401–7404. [Google Scholar] [CrossRef]
- Benirschke, K. Remarkable placenta. Clin. Anat. 1998, 11, 194–205. [Google Scholar] [CrossRef]
- Roy, A.; Mantay, M.; Brannan, C.; Griffiths, S. Placental Tissues as Biomaterials in Regenerative Medicine. Biomed. Res. Int. 2022, 2022, 6751456. [Google Scholar] [CrossRef]
- Fogarty, N.M.; Ferguson-Smith, A.C.; Burton, G.J. Syncytial knots (Tenney-Parker changes) in the human placenta: Evidence of loss of transcriptional activity and oxidative damage. Am. J. Pathol. 2013, 183, 144–152. [Google Scholar] [CrossRef]
- Schneider, K.H.; Enayati, M.; Grasl, C.; Walter, I.; Budinsky, L.; Zebic, G.; Kaun, C.; Wagner, A.; Kratochwill, K.; Redl, H.; et al. Acellular vascular matrix grafts from human placenta chorion: Impact of ECM preservation on graft characteristics, protein composition and in vivo performance. Biomaterials 2018, 177, 14–26. [Google Scholar] [CrossRef]
- Masia, J.; Olivares, L.; Koshima, I.; Teo, T.C.; Suominen, S.; Van Landuyt, K.; Demirtas, Y.; Becker, C.; Pons, G.; Garusi, C.; et al. Barcelona consensus on supermicrosurgery. J. Reconstr. Microsurg. 2014, 30, 53–58. [Google Scholar] [CrossRef]
- Hodde, J.; Hiles, M. Virus safety of a porcine-derived medical device: Evaluation of a viral inactivation method. Biotechnol. Bioeng. 2002, 79, 211–216. [Google Scholar] [CrossRef]
- Conklin, B.S.; Richter, E.R.; Kreutziger, K.L.; Zhong, D.S.; Chen, C. Development and evaluation of a novel decellularized vascular xenograft. Med. Eng. Phys. 2002, 24, 173–183. [Google Scholar] [CrossRef]
- Schmidt, V.J.; Wietbrock, J.O.; Leibig, N.; Hernekamp, J.F.; Henn, D.; Radu, C.A.; Kneser, U. Haemodynamically stimulated and in vivo generated axially vascularized soft-tissue free flaps for closure of complex defects: Evaluation in a small animal model. J. Tissue Eng. Regen. Med. 2018, 12, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.H.; Aigner, P.; Holnthoner, W.; Monforte, X.; Nurnberger, S.; Runzler, D.; Redl, H.; Teuschl, A.H. Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts. Acta Biomater. 2016, 29, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Muto, A.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng. Part A 2009, 15, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Mirabella, T. Decellularized matrices for cardiovascular tissue engineering. Am. J. Stem Cells 2014, 3, 1–20. [Google Scholar]
- Tuan-Mu, H.Y.; Yu, C.H.; Hu, J.J. On the decellularization of fresh or frozen human umbilical arteries: Implications for small-diameter tissue engineered vascular grafts. Ann. Biomed. Eng. 2014, 42, 1305–1318. [Google Scholar] [CrossRef]
- Leibig, N.; Wietbrock, J.O.; Bigdeli, A.K.; Horch, R.E.; Kremer, T.; Kneser, U.; Schmidt, V.J. Flow-Induced Axial Vascularization: The Arteriovenous Loop in Angiogenesis and Tissue Engineering. Plast. Reconstr. Surg. 2016, 138, 825–835. [Google Scholar] [CrossRef]
- Silini, A.R.; Cargnoni, A.; Magatti, M.; Pianta, S.; Parolini, O. The Long Path of Human Placenta, and Its Derivatives, in Regenerative Medicine. Front. Bioeng. Biotechnol. 2015, 3, 162. [Google Scholar] [CrossRef]
- Gordon, Z.; Elad, D.; Almog, R.; Hazan, Y.; Jaffa, A.J.; Eytan, O. Anthropometry of fetal vasculature in the chorionic plate. J. Anat. 2007, 211, 698–706. [Google Scholar] [CrossRef]
- Flynn, L.; Semple, J.L.; Woodhouse, K.A. Decellularized placental matrices for adipose tissue engineering. J. Biomed. Mater. Res. A 2006, 79, 359–369. [Google Scholar] [CrossRef]
- Mofikoya, B.O.; Ugburo, A.O.; Bankole, O.B. Does open guide suture technique improve the patency rate in submillimeter rat artery anastomosis? Handchir. Mikrochir. Plast. Chir. 2014, 46, 105–107. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Drakotos, D.; Arkudas, A.; Pryymachuk, G.; Rath, S.; Beier, J.P.; Klumpp, D.; Dragu, A.; Horch, R.E.; Kneser, U. Factors influencing successful outcome in the arteriovenous loop model: A retrospective study of 612 loop operations. J. Reconstr. Microsurg. 2011, 27, 11–18. [Google Scholar] [CrossRef]
- Dardik, H. A 30-year odyssey with the umbilical vein graft. J. Am. Coll. Surg. 2006, 203, 582–583. [Google Scholar] [CrossRef]
- Neufang, A.; Dorweiler, B.; Espinola-Klein, C.; Savvidis, S.; Doemland, M.; Schotten, S.; Vahl, C.F. Outcomes of complex femorodistal sequential autologous vein and biologic prosthesis composite bypass grafts. J. Vasc. Surg. 2014, 60, 1543–1553. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Tjiawi, J.; Euler, S.; Arkudas, A.; Hess, A.; Brune, K.; Greil, P.; Lametschwandtner, A.; Horch, R.E.; Kneser, U. The venous graft as an effector of early angiogenesis in a fibrin matrix. Microvasc. Res. 2008, 75, 25–33. [Google Scholar] [CrossRef]
- Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef]
- Wyburn, K.R.; Jose, M.D.; Wu, H.; Atkins, R.C.; Chadban, S.J. The role of macrophages in allograft rejection. Transplantation 2005, 80, 1641–1647. [Google Scholar] [CrossRef]
- Badylak, S.F.; Valentin, J.E.; Ravindra, A.K.; McCabe, G.P.; Stewart-Akers, A.M. Macrophage Phenotype as a Determinant of Biologic Scaffold Remodeling. Tissue Eng. Part A 2008, 14, 1835–1842. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef]
- Dardik, H.; Wengerter, K.; Qin, F.; Pangilinan, A.; Silvestri, F.; Wolodiger, F.; Kahn, M.; Sussman, B.; Ibrahim, I.M. Comparative decades of experience with glutaraldehyde-tanned human umbilical cord vein graft for lower limb revascularization: An analysis of 1275 cases. J. Vasc. Surg. 2002, 35, 64–71. [Google Scholar] [CrossRef]
- Smith, R.J., Jr.; Yi, T.; Nasiri, B.; Breuer, C.K.; Andreadis, S.T. Implantation of VEGF-functionalized cell-free vascular grafts: Regenerative and immunological response. FASEB J. 2019, 33, 5089–5100. [Google Scholar] [CrossRef] [PubMed]
- Rohringer, S.; Schneider, K.H.; Eder, G.; Hager, P.; Enayati, M.; Kapeller, B.; Kiss, H.; Windberger, U.; Podesser, B.K.; Bergmeister, H. Chorion-derived extracellular matrix hydrogel and fibronectin surface coatings show similar beneficial effects on endothelialization of expanded polytetrafluorethylene vascular grafts. Mater. Today Bio 2022, 14, 100262. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.J.; Wietbrock, J.O.; Leibig, N.; Gloe, T.; Henn, D.; Hernekamp, J.F.; Harhaus, L.; Kneser, U. Collagen-Elastin and Collagen-Glycosaminoglycan Scaffolds Promote Distinct Patterns of Matrix Maturation and Axial Vascularization in Arteriovenous Loop-Based Soft Tissue Flaps. Ann. Plast. Surg. 2017, 79, 92–100. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falkner, F.; Mayer, S.A.; Thomas, B.; Zimmermann, S.O.; Walter, S.; Heimel, P.; Thiele, W.; Sleeman, J.P.; Bigdeli, A.K.; Kiss, H.; et al. Acellular Human Placenta Small-Diameter Vessels as a Favorable Source of Super-Microsurgical Vascular Replacements: A Proof of Concept. Bioengineering 2023, 10, 337. https://doi.org/10.3390/bioengineering10030337
Falkner F, Mayer SA, Thomas B, Zimmermann SO, Walter S, Heimel P, Thiele W, Sleeman JP, Bigdeli AK, Kiss H, et al. Acellular Human Placenta Small-Diameter Vessels as a Favorable Source of Super-Microsurgical Vascular Replacements: A Proof of Concept. Bioengineering. 2023; 10(3):337. https://doi.org/10.3390/bioengineering10030337
Chicago/Turabian StyleFalkner, Florian, Simon Andreas Mayer, Benjamin Thomas, Sarah Onon Zimmermann, Sonja Walter, Patrick Heimel, Wilko Thiele, Jonathan Paul Sleeman, Amir Khosrow Bigdeli, Herbert Kiss, and et al. 2023. "Acellular Human Placenta Small-Diameter Vessels as a Favorable Source of Super-Microsurgical Vascular Replacements: A Proof of Concept" Bioengineering 10, no. 3: 337. https://doi.org/10.3390/bioengineering10030337
APA StyleFalkner, F., Mayer, S. A., Thomas, B., Zimmermann, S. O., Walter, S., Heimel, P., Thiele, W., Sleeman, J. P., Bigdeli, A. K., Kiss, H., Podesser, B. K., Kneser, U., Bergmeister, H., & Schneider, K. H. (2023). Acellular Human Placenta Small-Diameter Vessels as a Favorable Source of Super-Microsurgical Vascular Replacements: A Proof of Concept. Bioengineering, 10(3), 337. https://doi.org/10.3390/bioengineering10030337









