Silver Nanoparticles in Dental Applications: A Descriptive Review
Abstract
1. Introduction
2. Silver Nanoparticle Chemistry and Synthesis
3. Dental Implications of Nanotechnology
3.1. Ethics and Implications
3.2. Society and Nanotechnology
3.3. Implications for Health
3.4. Biocompatibility
4. Clinical Applications of Nanoparticles in Dentistry
4.1. Preventive Dentistry
4.2. Endodontics
4.3. Dental Implants
4.4. Orthodontics
4.5. Pediatric Dentistry
4.6. Anticancer Treatment
5. Dental Biomaterials: Effect of Silver Nanoparticles (AgNPs)
5.1. Denture Acrylic Resin
5.2. Composite Resins
5.3. Endodontic Materials
5.4. Periodontology
5.5. Orthodontic Adhesives and Cement
5.6. Anticancer Treatment
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitra, D.; Kang, E.T.; Neoh, K.G. Polymer-based coatings with integrated antifouling and bactericidal properties for targeted biomedical applications. ACS Appl. Polym. Mater. 2021, 3, 2233–2263. [Google Scholar] [CrossRef]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of silver nanoparticles in dentistry: Advances and technological innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Mukherjee, S.; Bag, J.; Nayak, N.; Mishra, M. Application of nanoparticles in dentistry: Current trends. In Nanoparticles in Medicine; Springer: Singapore, 2020; pp. 55–98. [Google Scholar]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef]
- Senocak, E.; Sarıkaya, I.; Hatırlı, H.; Coşgun, A. Dental Students’ Emotions, Knowledge, Awareness and Learning Intention on the Applications of Nanotechnology in Dentistry. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Sen, D.; Patil, V.; Smriti, K.; Varchas, P.; Ratnakar, R.; Naik, N.; Kumar, S.; Saxena, J.; Kapoor, S. Nanotechnology and Nanomaterials in Dentistry: Present and Future Perspectives in Clinical Applications. Eng. Sci. 2022, 20, 14–24. [Google Scholar] [CrossRef]
- Gronwald, B.; Kozłowska, L.; Kijak, K.; Lietz-Kijak, D.; Skomro, P.; Gronwald, K.; Gronwald, H. Nanoparticles in Dentistry—Current Literature Review. Coatings 2023, 13, 102. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Ray, A.; Nath, D. Dose dependent intra-testicular accumulation of silver nanoparticles triggers morphometric changes in seminiferous tubules and Leydig cells and changes the structural integrity of spermatozoa chromatin. Theriogenology 2022, 192, 122–131. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Hanif, A.; Ghani, F. Mechanical properties of an experimental resin based composite containing silver nanoparticles and bioactive glass. Pak. J. Med. Sci. 2020, 36, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, N.; Wang, X.; Zhai, J.; Hu, B.; Chen, M.; Wang, J. Dual functional AgNPs-M13 phage composite serves as antibacterial film and sensing probe for monitoring the corrosion of chromium-containing dental alloys. Chin. Chem. Lett. 2020, 31, 145–149. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and biological assessment of silver nanoparticles immobilized Halloysite nanotubes-based resin composite for dental applications. Heliyon 2020, 6, e03601. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The influence of graphene in improvement of physico-mechanical properties in PMMA Denture Base Resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef]
- Tekade, M.; Maheshwari, N.; Choudhury, H.; Gorain, B.; Deb, P.K.; Tekade, R.K.; Sharma, M.C. Up-to-Date Implications of Nanomaterials in Dental Science. In Biomaterials and Bionanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 301–336. [Google Scholar]
- Ibrahim, N.A.; Zaini, M.A.A. Nanomaterials in detergents and cosmetics products: The mechanisms and implications. In Handbook of Nanomaterials for Manufacturing Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 23–49. [Google Scholar]
- Vaishali, S.; Nashra, K. Nanotechnology in Periodontics: A Review. J. Res. Med. Dent. Sci. 2021, 9, 339–344. [Google Scholar]
- Hamdy, T.M.; Abdelnabi, A.; Abdelraouf, R.M. Reinforced dental plaster with low setting expansion and enhanced microhardness. Bull. Natl. Res. Cent. 2020, 44, 78. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Dobrzański, L.B.; Dobrzańska-Danikiewicz, A.D.; Dobrzańska, J.; Kraszewska, M. The synergistic ethics interaction with nanoengineering, dentistry, and dental engineering. In Ethics in Nanotechnology; Walter de Gruyter GmbH: Berlin/Heidelberg, Germany; Munich, Germany; Boston, MA, USA, 2020; pp. 119–189. [Google Scholar]
- Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Alagawany, M.; Hashem, N.M.; Farag, M.R.; Alghamdi, E.S.; Hassan, F.U.; Bilal, R.M.; Elnesr, S.S.; Dawood, M.A.; Nagadi, S.A.; et al. Nanominerals: Fabrication methods, benefits and hazards, and their applications in ruminants with special reference to selenium and zinc nanoparticles. Animals 2021, 11, 1916. [Google Scholar] [CrossRef]
- Hamidia, Z.; Shahanipour, K.; Talebian, N.; Monajemi, R. Preparation of chelidonine highly loaded poly (lactide-co-glycolide)-based nanoparticles using a single emulsion method: Cytotoxic effect on MDA-MB-231 cell line. J. Herbmed Pharmacol. 2021, 11, 114–120. [Google Scholar] [CrossRef]
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle technology and its implications in endodontics: A review. Biomater. Res. 2020, 24, 21. [Google Scholar] [CrossRef]
- Esmaili, Z.; Soukhaklari, R.; Farokhi, M.R.; Absalan, S.; Moosavi, M. The impairing effect of oral aluminum oxide nanoparticle on novel object recognition memory coincides with Akt/GSK-3β signaling deregulation in mice hippocampus. BioNanoScience 2021, 11, 1119–1126. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in nanotechnology for restorative dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef]
- Nelson, K.; Hesse, B.; Addison, O.; Morrell, A.P.; Gross, C.; Lagrange, A.; Suárez, V.I.; Kohal, R.; Fretwurst, T. Distribution and chemical speciation of exogenous micro-and nanoparticles in inflamed soft tissue adjacent to titanium and ceramic dental implants. Anal. Chem. 2020, 92, 14432–14443. [Google Scholar] [CrossRef]
- Muraleetharan, V.; Mantaj, J.; Swedrowska, M.; Vllasaliu, D. Nanoparticle modification in biological media: Implications for oral nanomedicines. RSC Adv. 2019, 9, 40487–40497. [Google Scholar] [CrossRef]
- Mansoor, A.; Khan, M.T.; Mehmood, M.; Khurshid, Z.; Ali, M.I.; Jamal, A. Synthesis and Characterization of Titanium Oxide Nanoparticles with a Novel Biogenic Process for Dental Application. Nanomaterials 2022, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Dutra-Correa, M.; Leite, A.A.; de Cara, S.P.; Diniz, I.M.; Marques, M.M.; Suffredini, I.B.; Fernandes, M.S.; Toma, S.H.; Araki, K.; Medeiros, I.S. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J. Dent. 2018, 77, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, Y.; Liu, J.; Li, Z.; Fan, Q.; Jiang, Z.; Yan, F.; Wang, Z.; Huang, P.; Feng, N. Chitosan-functionalized lipid-polymer hybrid nanoparticles for oral delivery of silymarin and enhanced lipid-lowering effect in NAFLD. J. Nanobiotechnol. 2018, 16, 64. [Google Scholar] [CrossRef]
- Dudhipala, N.; Gorre, T. Neuroprotective effect of ropinirole lipid nanoparticles enriched hydrogel for parkinson’s disease: In vitro, ex vivo, pharmacokinetic and pharmacodynamic evaluation. Pharmaceutics 2020, 12, 448. [Google Scholar] [CrossRef]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural strength, biocompatibility, and antimi-crobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nano-particles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in glass ionomer cements: Nano-sized fillers and bioactive nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef]
- Yassaei, S.; Nasr, A.; Zandi, H.; Motallaei, M.N. Comparison of antibacterial effects of orthodontic composites containing different nanoparticles on Streptococcus mutans at different times. Dent. Press J. Orthod. 2020, 25, 52–60. [Google Scholar] [CrossRef]
- Amin, F.; Rahman, S.; Khurshid, Z.; Zafar, M.S.; Sefat, F.; Kumar, N. Effect of Nanostructures on the Properties of Glass Ionomer Dental Restoratives/Cements: A Comprehensive Narrative Review. Materials 2021, 14, 6260. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Chávez-Andrade, G.M.; Tanomaru-Filho, M.; Bernardi, M.I.B.; de Toledo Leonardo, R.; Faria, G.; Guerreiro-Tanomaru, J.M. Antimicrobial and biofilm anti-adhesion activities of silver nanoparticles and farnesol against endodontic microorganisms for possible application in root canal treatment. Arch. Oral Biol. 2019, 107, 104481. [Google Scholar] [CrossRef]
- Aydın, H.; Er, K.; Kuştarcı, A.; Akarsu, M.; Gençer, G.M.; Er, H.; Felek, R. Antibacterial activity of silver nanoparticles activated by photodynamic therapy in infected root canals. Dent. Med. Probl. 2020, 57, 393–400. [Google Scholar] [CrossRef]
- Obaid, H.M.; Shareef, H.A. Evaluation of the inhibitory impact of biosynthesized silver nanoparticles using Bacillus cereus and Chromobacterium violaceum bacteria on some intestinal protozoa. Ann. Parasitol. 2022, 68, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, A.; Khurshid, Z.; Mansoor, E.; Khan, M.T.; Ratnayake, J.; Jamal, A. Effect of Currently Available Nanoparticle Synthesis Routes on Their Biocompatibility with Fibroblast Cell Lines. Molecules 2022, 27, 6972. [Google Scholar] [CrossRef] [PubMed]
- Vence, M.G.; del Pilar Chantada-Vazquez, M.; Vázquez-Estévez, S.; Cameselle-Teijeiro, J.M.; Bravo, S.B.; Nunez, C. Potential clinical applications of the personalized, disease-specific protein corona on nanoparticles. Clin. Chim. Acta 2020, 501, 102–111. [Google Scholar] [CrossRef]
- Fatemeh, K.; Mohammad, J.; Samaneh, K. The effect of silver nanoparticles on composite shear bond strength to dentin with different adhesion protocols. J. Appl. Oral Sci. 2017, 25, 367–373. [Google Scholar] [CrossRef]
- Porenczukl, A.; Grzeczkowicz, A.; Maciejewska, I.; Gołaś, M.; Piskorska, K.; Kolenda, A.; Gozdowski, D.; Kopeć-Swoboda, E.; Granicka, L.; Olczak-Kowalczyk, D. An initial evaluation of cytotoxicity, genotoxicity and antibacterial effectiveness of a disinfection liquid containing silver nanoparticles alone and combined with a glass-ionomer cement and dentin bonding systems. Adv. Clin. Exp. Med. 2019, 28, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Salas-López, E.K.; Pierdant-Pérez, M.; Hernández-Sierra, J.F.; Ruíz, F.; Mandeville, P.; Pozos-Guillén, A.J. Effect of silver nanoparticle-added pit and fissure sealant in the prevention of dental caries in children. J. Clin. Pediatr. Dent. 2017, 41, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.A.M.; Ahmed, A.B.; Al-Ahmed, H.I. Green synthesis and characterization of silver nanoparticles for reducing the damage to sperm parameters in diabetic compared to metformin. Sci. Rep. 2023, 13, 2256. [Google Scholar] [CrossRef]
- Jonaidi-Jafari, N.; Izadi, M.; Javidi, P. The effects of silver nanoparticles on antimicrobial activity of ProRoot mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM). J. Clin. Exp. Dent. 2016, 8, e22. [Google Scholar] [CrossRef]
- Thangavelu, L.; Adil, A.H.; Arshad, S.; Devaraj, E.; Mallineni, S.K.; Sajja, R.; Chakradhar, A.; Karobari, M.I. Antimicrobial Properties of Silver Nitrate Nanoparticle and Its Application in Endodontics and Dentistry: A Review of Literature. J. Nanomater. 2021, 2021, 9132714. [Google Scholar] [CrossRef]
- Afkhami, F.; Akbari, S.; Chiniforush, N. Entrococcus faecalis elimination in root canals using silver nanoparticles, photodynamic therapy, diode laser, or laser-activated nanoparticles: An in vitro study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A.; Shi, Z.; Shrestha, A.; Neoh, K.G. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J. Endod. 2008, 34, 1515–1520. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotocixology 2017, 11, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhang, X.; Savino, K.; Gabrys, P.; Gao, Y.; Chaimayo, W.; Miller, B.L.; Yates, M.Z. Antimicrobial silver-hydroxyapatite composite coatings through two-stage electrochemical synthesis. Surf. Coat. Technol. 2016, 30, 13–19. [Google Scholar] [CrossRef]
- Niska, K.; Knap, N.; Kędzia, A.; Jaskiewicz, M.; Kamysz, W.; Inkielewicz-Stepniak, I. Capping agent-dependent toxicity and antimicrobial activity of silver nanoparticles: An in vitro study. Concerns about potential application in dental practice. Int. J. Med. Sci. 2016, 13, e772. [Google Scholar] [CrossRef]
- Sasabe, E.; Tomomura, A.; Kitamura, N.; Yamamoto, T. Metal nanoparticles-induced activation of NLRP3 inflammasome in human oral keratinocytes is a possible mechanism of oral lichenoid lesions. Toxicol. In Vitro 2020, 62, e104663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Melo, M.A.S.; Antonucci, J.M.; Lin, N.J.; Lin-Gibson, S.; Bai, Y.; Xu, H.H.K. Novel dental cement to combat biofilms and reduce acids for orthodontic applications to avoid enamel demineralization. Materials 2016, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.; Bussaneli, D.G.; Jeremias, F.; Cordeiro, R.C.; Magalhães, A.C.; Palomari Spolidorio, D.M.; Santos-Pinto, L. Control of white spot lesion adjacent to orthodontic bracket with use of fluoride varnish or chlorhexidine gel. Sci. World J. 2015, 2015, 218452. [Google Scholar] [CrossRef]
- Pellegrini, P.; Sauerwein, R.; Finlayson, T.; McLeod, J.; Covell, D.A., Jr.; Maier, T.; Machida, C.A. Plaque retention by self-ligating vs elastomeric orthodontic brackets: Quantitative comparison of oral bacteria and detection with adenosine triphosphate-driven bioluminescence. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 426.e1–426.e9. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, R.; Pourakbari, B.; Bahador, A. Effects of baseplates of orthodontic appliances with in situ generated silver nanoparticles on cariogenic bacteria: A randomized, double-blind cross-over clinical. J. Contemp. Dent. Pract. 2015, 16, 291–298. [Google Scholar] [CrossRef]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic aspects of restorative dentistry biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Alla, R.K.; Guduri, V.; Tiruveedula, N.B.P.; Narasimha Rao, G.; Swamy, K.N.R.; Vyas, R. Effect of silver nanoparticles incorporation on microhardness of Heat-cure denture base resins. Int. J. Dent. Mater. 2020, 2, 103–110. [Google Scholar] [CrossRef]
- Porter, G.C.; Tompkins, G.R.; Schwass, D.R.; Li, K.C.; Waddell, J.N.; Meledandri, C.J. Anti-biofilm activity of silver nanoparticle-containing glass ionomer cements. Dent. Mater. 2020, 36, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug delivery (nano) platforms for oral and dental applications: Tissue regeneration, infection control, and cancer management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Ahmed, S.F. Therapeutic effect of Aloe vera and silver nanoparticles on acid-induced oral ulcer in gamma-irradiated mice. Braz. Oral. Res. 2018, 32, e004. [Google Scholar] [CrossRef]
- Şuhani, M.F.; Băciuţ, G.; Băciuţ, M.; Şuhani, R.; Bran, S. Current perspectives regarding the application and incorporation of silver nanoparticles into dental biomaterials. Clujul Med. 2018, 91, 274–279. [Google Scholar] [CrossRef]
- Ginjupalli, K.; Shaw, T.; Tellapragada, C.; Alla, R.; Gupta, L.; Perampalli, N.U. Does the size matter? Evaluation of effect of incorporation of silver nanoparticles of varying particle size on the antimicrobial activity and properties of irreversible hydrocolloid impression material. Dent. Mater. 2018, 34, e158–e165. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Rolim, W.R.; Viana, M.M.; Souza, T.R.; Gonçalves, F.; Tanaka, C.J.; Bueno-Silva, B.; Seabra, A.B. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J. Dent. 2020, 96, 103327. [Google Scholar] [CrossRef] [PubMed]
- Bhandi, S.; Alkahtani, A.; Reda, R.; Mashyakhy, M.; Boreak, N.; Maganur, P.C.; Vishwanathaiah, S.; Mehta, D.; Vyas, N.; Patil, V.; et al. Parathyroid Hormone Secretion and Receptor Expression Determine the Age-Related Degree of Osteogenic Differentiation in Dental Pulp Stem Cells. J. Pers. Med. 2021, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Pajares-Chamorro, N.; Lensmire, J.M.; Hammer, N.D.; Hardy, J.W.; Chatzistavrou, X. Unraveling the mechanisms of inhibition of silver-doped bioactive glass-ceramic particles. J. Biomed. Mater. Res. A. 2022. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Bakopoulou, A.; Tsouknidas, A.; Prymak, O.; Papi, R.; Choli-Papadopoulou, T.; Epple, M.; Michailidis, N.; Koidis, P.; et al. Assessment of cytotoxicity and antibacterial effects of silver nanoparticle-doped titanium alloy surfaces. Dent. Mater. 2019, 35, e220–e233. [Google Scholar] [CrossRef]
- Sihivahanan, D.; Maniyan Vijayakumari, M.; Yadalam, P.K.; Boreak, N.; Binalrimal, S.; Alqahtani, S.M.; Wadei, M.H.D.A.; Vinothkumar, T.S.; Chohan, H.; Dewan, H.; et al. Evaluation of Micro-Tensile Bond Strength of Fibre Post with Titanium Dioxide Nanoparticles as Fillers in Experimental Dental Composite Resin. Materials 2022, 15, 3312. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Q.; Peng, J.; Yang, X.; Yu, D.; Zhao, W. Antibacterial and mechanical properties of reduced graphene-silver nanoparticle nanocomposite modified glass ionomer cements. J. Dent. 2020, 96, 103332. [Google Scholar] [CrossRef]
- de Souza Neto, F.N.; Sala, R.L.; Fernandes, R.A.; Xavier, T.P.O.; Cruz, S.A.; Paranhos, C.M.; Monteiro, D.R.; Barbosa, D.B.; Delbem, A.C.B.; de Camargo, E.R. Effect of synthetic colloidal nanoparticles in acrylic resin of dental use. Eur. Polym. J. 2019, 112, 531–538. [Google Scholar] [CrossRef]
- Ferreira, I.; Vidal, C.L.; Botelho, A.L.; Ferreira, P.S.; da Costa Valente, M.L.; Schiavon, M.A.; Alves, O.L.; Dos Reis, A.C. Effect of nanomaterial incorporation on the mechanical and microbiological properties of dental porcelain. J. Prosthet. Dent. 2020, 123, 529-e1. [Google Scholar] [CrossRef]
- Ren, L.; Pan, Y.; Liang, Q.; He, S.; Liu, Y.; Fan, Y.; Meng, X.; Chen, M. In situ synthesis of dental resin matrix containing silver nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 5774–5782. [Google Scholar] [CrossRef]
- Bahador, A.; Ayatollahi, B.; Akhavan, A.; Pourhajibagher, M.; Kharazifard, M.J.; Sodagar, A. Antimicrobial efficacy of silver nanoparticles incorporated in an orthodontic adhesive: An animal study. Front. Dent. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Aras, M.A.; Chitre, V.; Nagarsekar, A.; Ferreira, A.N. Evaluation and comparison of flexural strength, surface roughness and porosity percentage of denture base resins incorporated with Thymoquinone and silver nano-antimicrobial agents-an in vitro study. J. Oral Biol. Craniofacial Res. 2022, 12, 716–720. [Google Scholar] [CrossRef]
- Salas-Orozco, M.; Niño-Martínez, N.; Martínez-Castañón, G.A.; Méndez, F.T.; Jasso, M.E.C.; Ruiz, F. Mechanisms of resistance to silver nanoparticles in endodontic bacteria: A literature review. J. Nanomater. 2019, 2019, 7630316. [Google Scholar] [CrossRef]
- Salunkhe, J.D.; Mohite, B.V.; Patil, S.V. Naringenin biosynthesis and fabrication of naringenin mediated nano silver conjugate for antimicrobial potential. Nat. Prod. Res. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.; Hussen, Y.F. Influence of Silver Nanoparticles on Selected Properties of Dental Porcelain: An in vitro study. Open Access Maced. J. Med. Sci. 2022, 10, 359–364. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and acid etched titanium dental implant surfaces systematic review and confocal microscopy evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef]
- Ahmed, A.; Muhammad, N.; Ali, A.; Mutahir, Z.; Khan, A.S.; Sharif, F.; Shah, A.T.; Haq, Z.U.; Liaqat, S.; Khan, M.A. Effect of augmentin-coated silver nanoparticles on biological and mechanical properties of orthodontic bracket cement. Mater. Technol. 2022, 37, 2983–2994. [Google Scholar] [CrossRef]
- Alshehri, T.D.; Kotha, S.B.; Abed, F.M.; Barry, M.J.; AlAsmari, A.; Mallineni, S.K. Effect of the Addition of Varying Concentrations of Silver Nanoparticles on the Fluoride Uptake and Recharge of Glass Ionomer Cement. Nanomaterials 2022, 12, 1971. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Raee, M.J.; Manafi, Z.; Sotoodeh Jahromi, A.; Ghasemi, Y. Ancient and novel forms of silver in medicine and biomedicine. J. Adv. Med. Sci. Appl. Technol. 2016, 2, 122–128. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Shah, M.Z.; Guan, Z.H.; Din, A.U.; Ali, A.; Rehman, A.U.; Jan, K.; Faisal, S.; Saud, S.; Adnan, M.; Wahid, F.; et al. Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci. Rep. 2021, 11, 20754. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; López-Ruiz, N. Antiadherence and antimicrobial properties of silver nanoparticles against Streptococcus mutans on brackets and wires used for orthodontic treatments. J. Nanomater. 2018, 2018, 9248527. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Antibacterial Effect of Silver Nanoparticles Is Stronger If the Production Host and the Targeted Pathogen Are Closely Related. Biomedicines 2022, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Peretz, B. Nanomaterials: The leading edge of the rapidly developing nanotechnology in dentistry. Refu’at Ha-peh Veha-shinayim (1993) 2005, 22, 88. [Google Scholar]
- Bhandi, S.; Alkahtani, A.; Mashyakhy, M.; Abumelha, A.S.; Albar, N.H.M.; Renugalakshmi, A.; Alkahtany, M.F.; Robaian, A.; Almeslet, A.S.; Patil, V.R.; et al. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs). J. Pers. Med. 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, C.; Danielsen, A.K.; Enevold, C.; Reinholdt, J.; Holmstrup, P.; Nielsen, C.H.; Massarenti, L. Circulating antibodies against leukotoxin A as marker of periodontitis Grades B and C and oral infection with Aggregatibacter actinomycetemcomitans. J. Periodontol. 2021, 92, 1795–1804. [Google Scholar] [CrossRef]
- Lubojanski, A.; Dobrzynski, M.; Nowak, N.; Rewak-Soroczynska, J.; Sztyler, K.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Wiglusz, K.; et al. Application of Selected Nanomaterials and Ozone in Modern Clinical Dentistry. Nanomaterials 2021, 11, 259. [Google Scholar] [CrossRef]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- Juan Carlos, F.-A.; Rene, G.-C.; Germán, V.-S.; Laura Susana, A.-T. Antimicrobial Poly (methyl methacrylate) with Silver Nanoparticles for Dentistry: A Systematic Review. Appl. Sci. 2020, 10, 4007. [Google Scholar] [CrossRef]
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Lara-Carrillo, E.; Toral-Rizo, V.H.; López-Castañares, R.; Morales-Luckie, R.A. Synthesis and Characterization of Silver Nanoparticles on Orthodontic Brackets: A New Alternative in the Prevention of White Spots. Coatings 2019, 9, 480. [Google Scholar] [CrossRef]
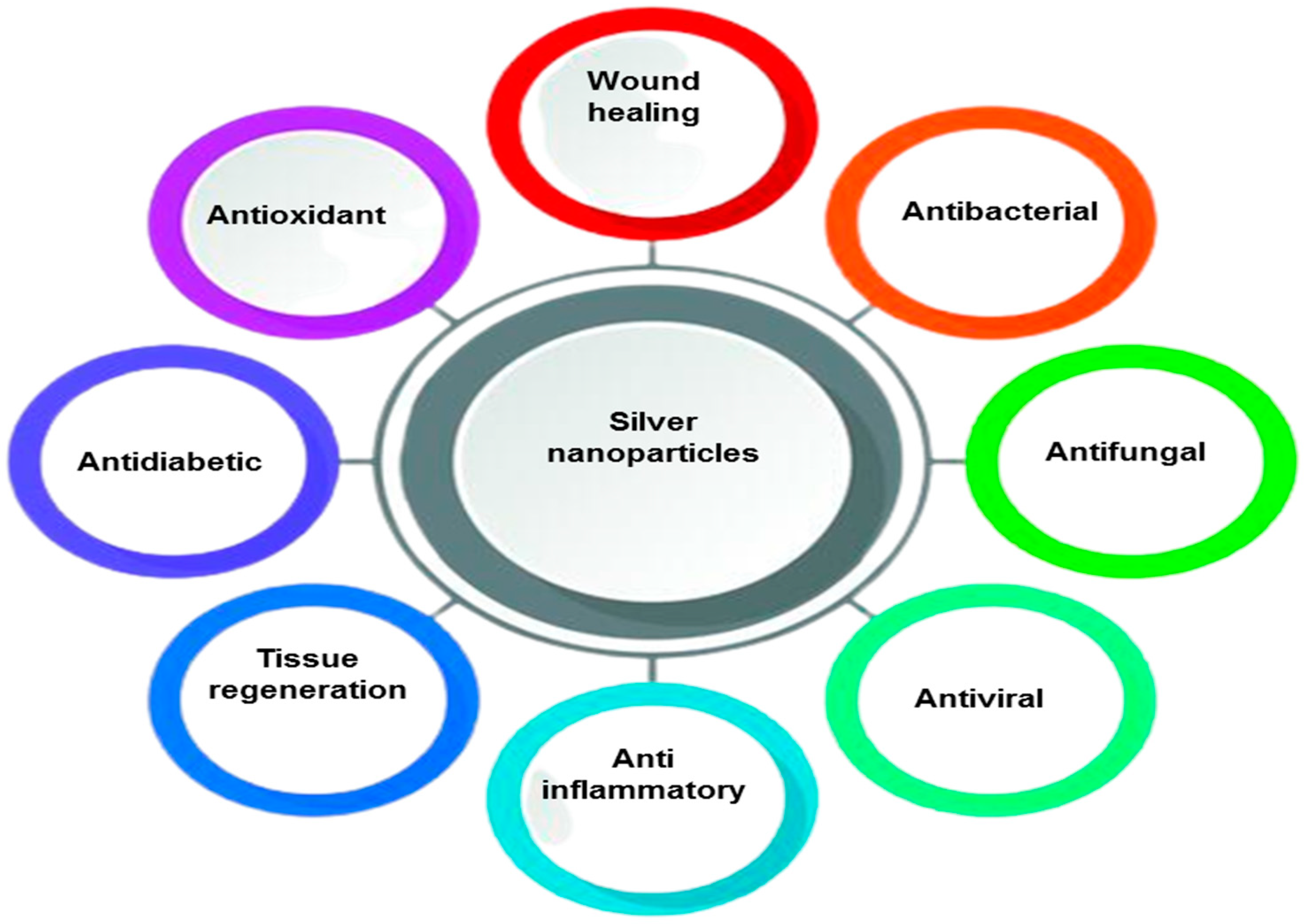
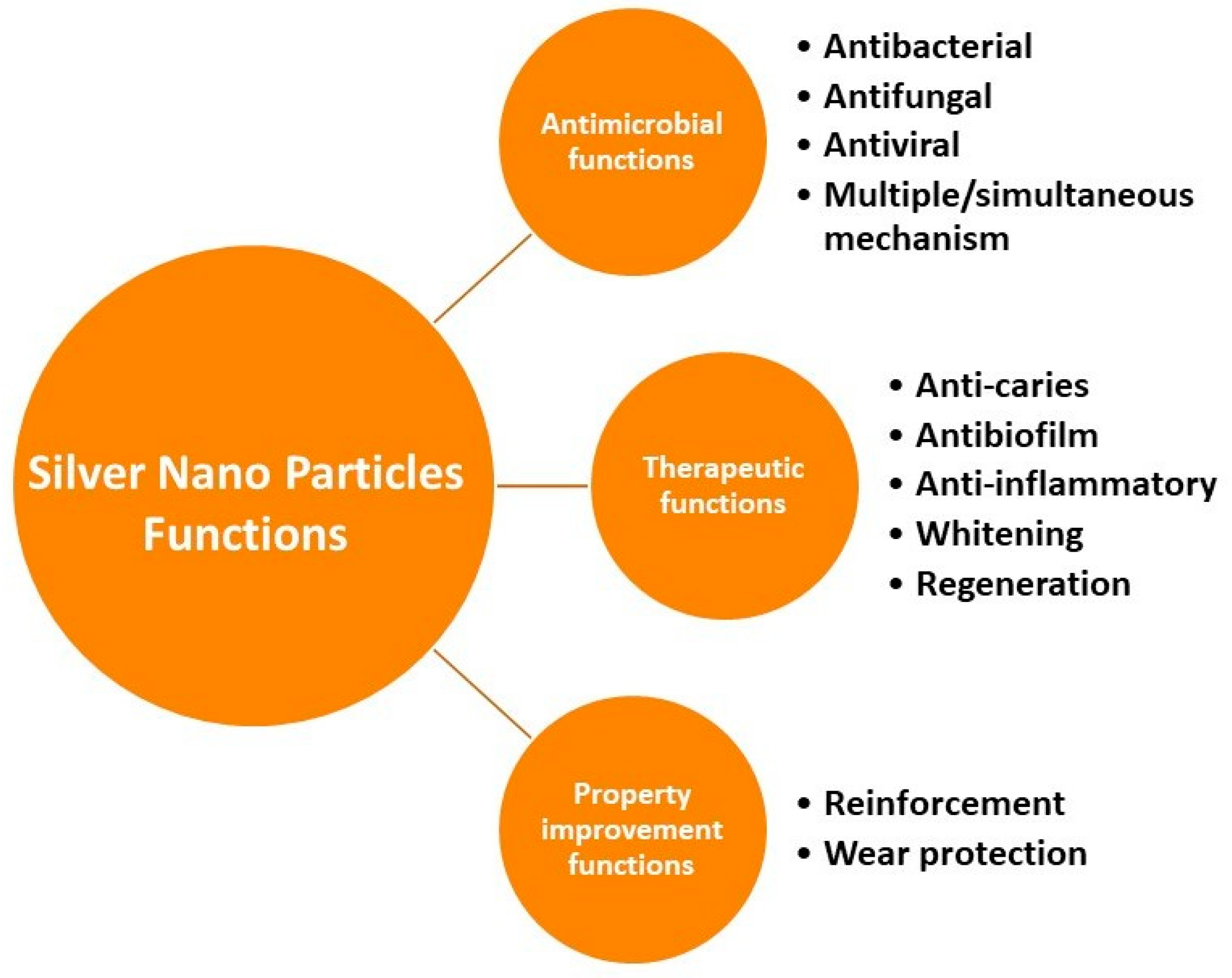
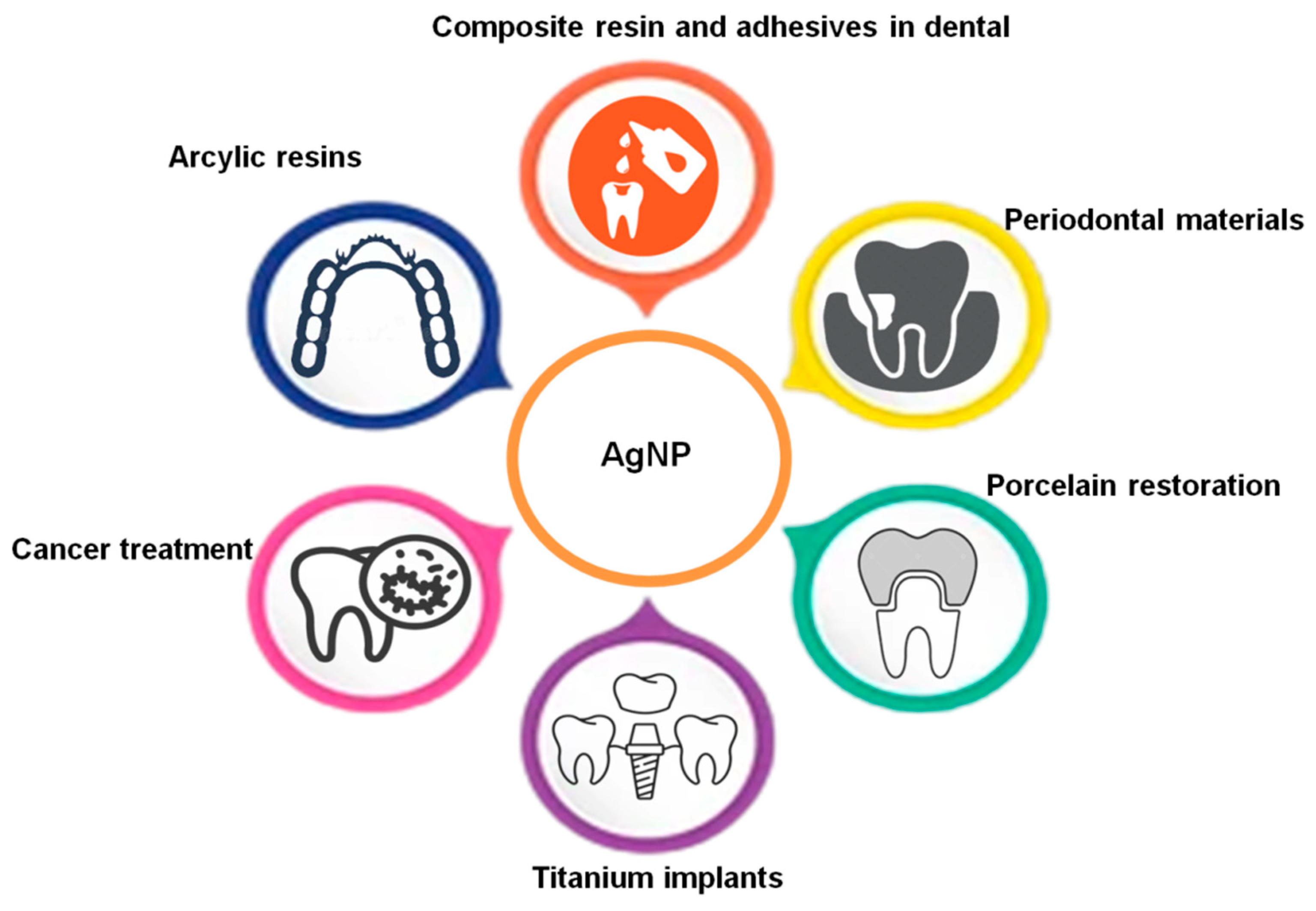

| S. No | Author | Synthesis Method | Technique Used | Merits |
|---|---|---|---|---|
| 1 | Hanif et al. [13] | Physical Synthesis | Evaporation and condensation approach and laser ablation technique | Both approaches may produce large amounts of pure AgNPs without using chemicals that damage humans and the environment. |
| 2 | Yang et al. [14] | Biosynthesis | Species such as Allium cepa, Azadirachta indica, Solanum lycopersicum | Low-cost, quick, one-step synthesis with manageable size/shape, good stability, efficacy, and security. |
| 3 | Barot et al. [15] | Chemical Synthesis | Wet chemical method | Low cost and high yield |
| 4 | Bacali et al. [16] | Physicochemical Synthesis | Variety of irradiation methods | Enormous biodiversity, environmental compatibility, scalability, |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallineni, S.K.; Sakhamuri, S.; Kotha, S.L.; AlAsmari, A.R.G.M.; AlJefri, G.H.; Almotawah, F.N.; Mallineni, S.; Sajja, R. Silver Nanoparticles in Dental Applications: A Descriptive Review. Bioengineering 2023, 10, 327. https://doi.org/10.3390/bioengineering10030327
Mallineni SK, Sakhamuri S, Kotha SL, AlAsmari ARGM, AlJefri GH, Almotawah FN, Mallineni S, Sajja R. Silver Nanoparticles in Dental Applications: A Descriptive Review. Bioengineering. 2023; 10(3):327. https://doi.org/10.3390/bioengineering10030327
Chicago/Turabian StyleMallineni, Sreekanth Kumar, Srinivasulu Sakhamuri, Sree Lalita Kotha, Abdul Rahman Gharamah M. AlAsmari, Galiah Husam AlJefri, Fatmah Nasser Almotawah, Sahana Mallineni, and Rishitha Sajja. 2023. "Silver Nanoparticles in Dental Applications: A Descriptive Review" Bioengineering 10, no. 3: 327. https://doi.org/10.3390/bioengineering10030327
APA StyleMallineni, S. K., Sakhamuri, S., Kotha, S. L., AlAsmari, A. R. G. M., AlJefri, G. H., Almotawah, F. N., Mallineni, S., & Sajja, R. (2023). Silver Nanoparticles in Dental Applications: A Descriptive Review. Bioengineering, 10(3), 327. https://doi.org/10.3390/bioengineering10030327







