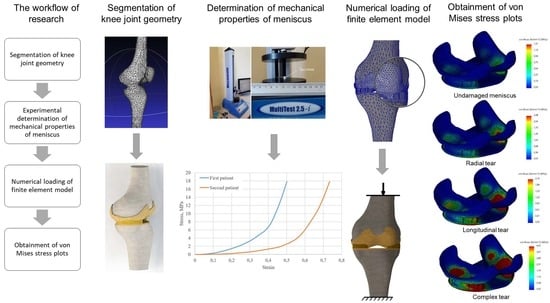
Modeling the Impact of Meniscal Tears on von Mises Stress of Knee Cartilage Tissue
Abstract
1. Introduction
2. Materials and Methods
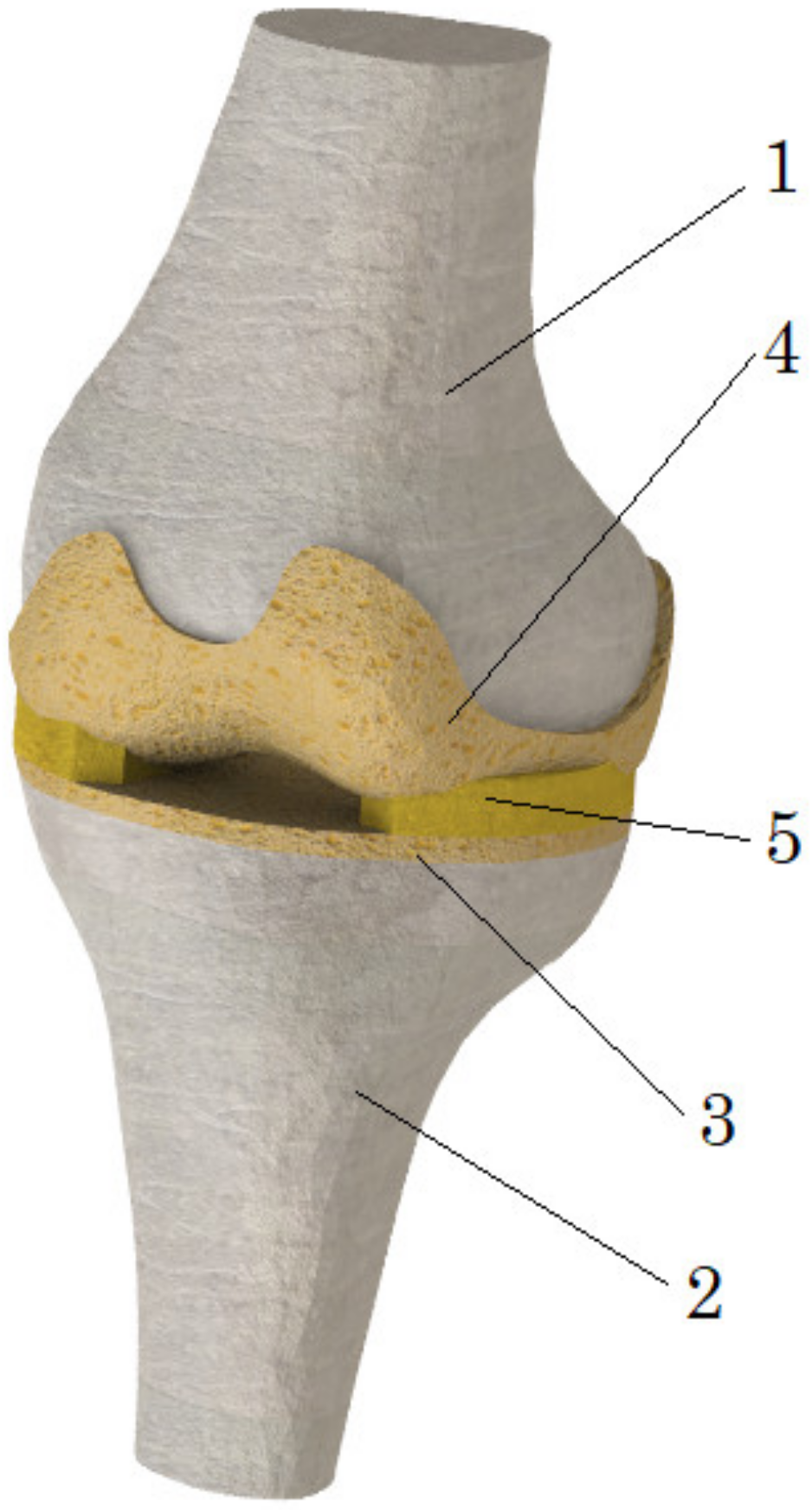
2.1. Geometry of the Model
2.2. Problem Formulation
2.3. Mechanical Properties of Model Components
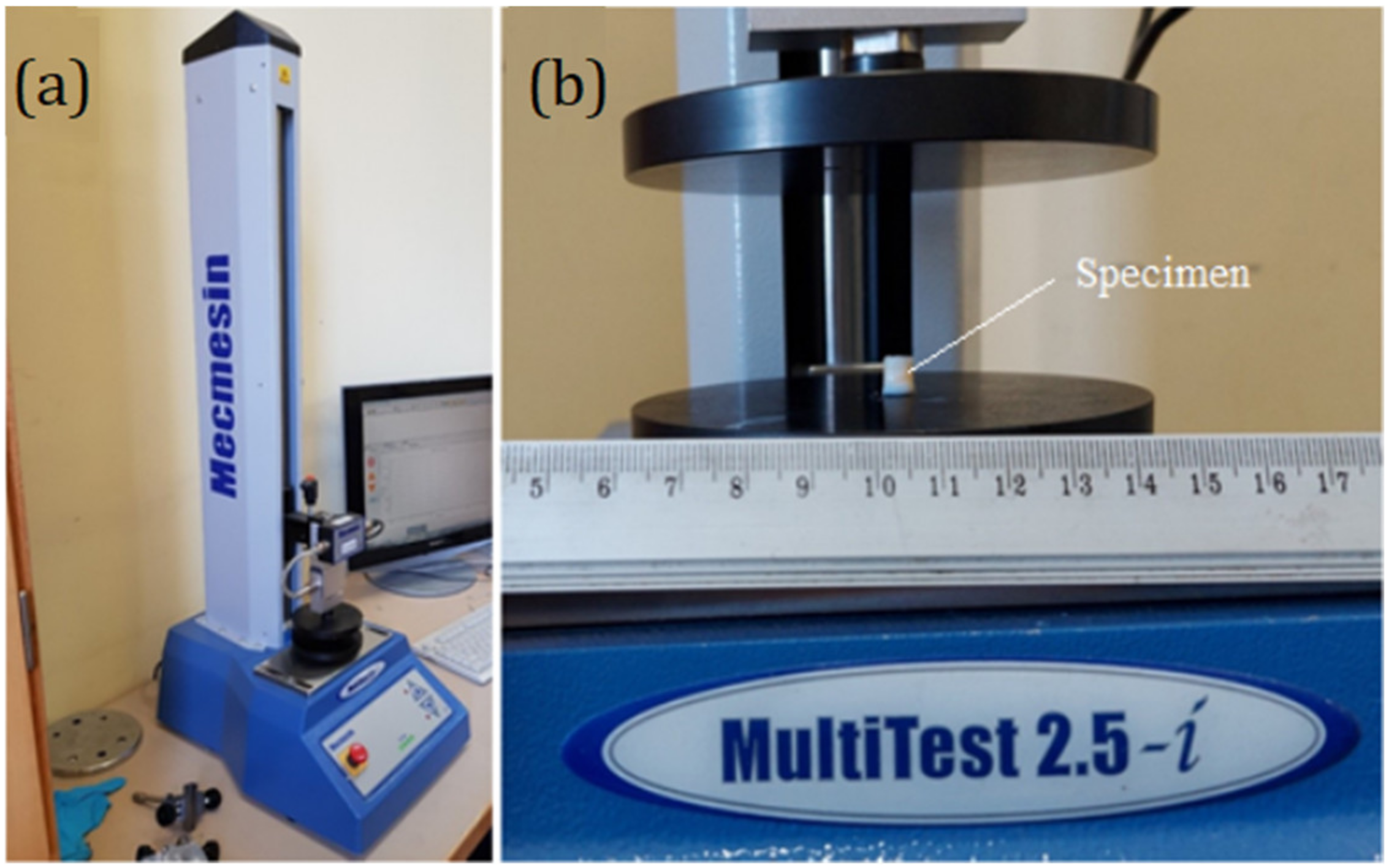
2.4. Experimental Determination of Meniscus Mechanical Properties
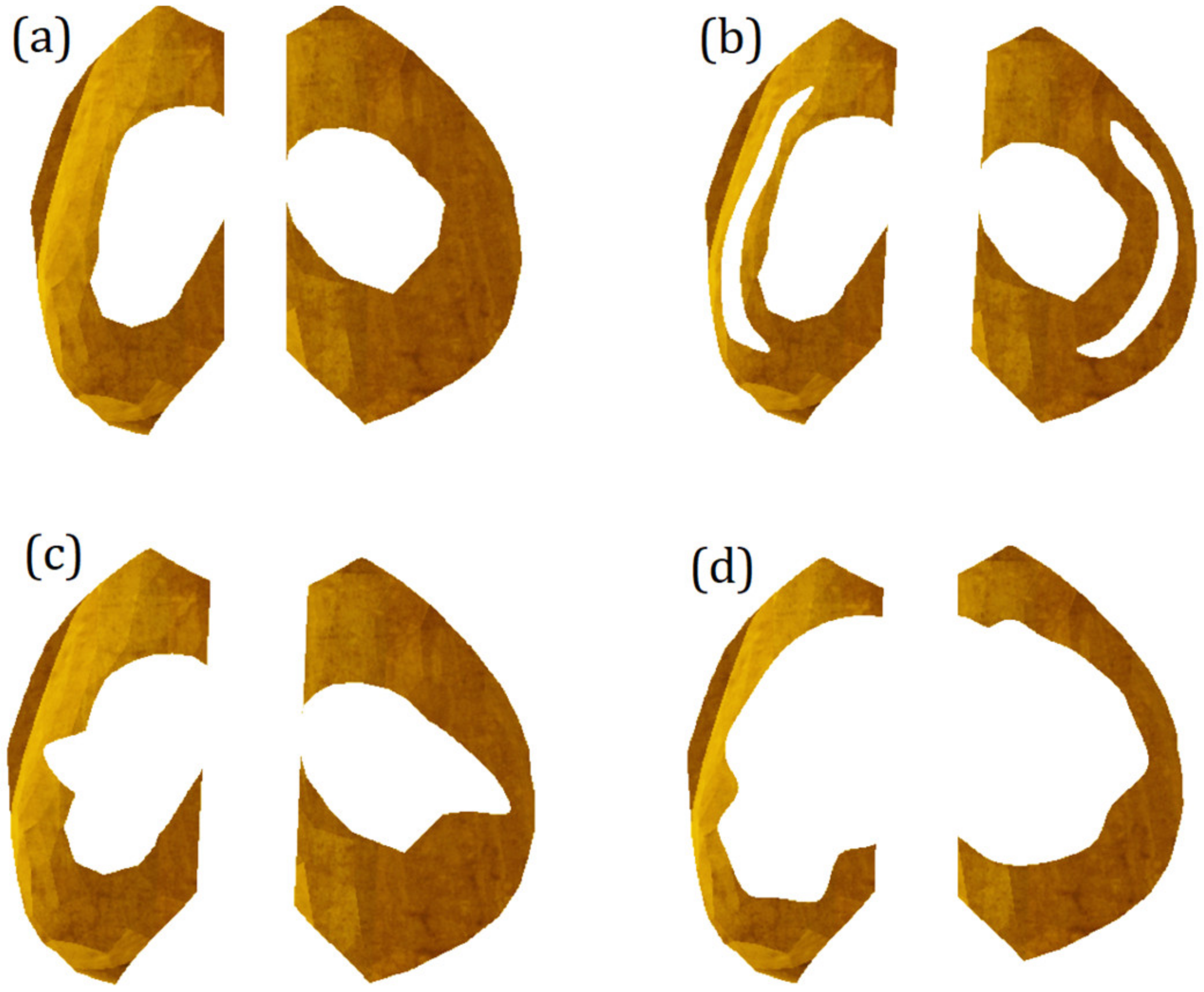
2.5. Modeling of Meniscus Damage
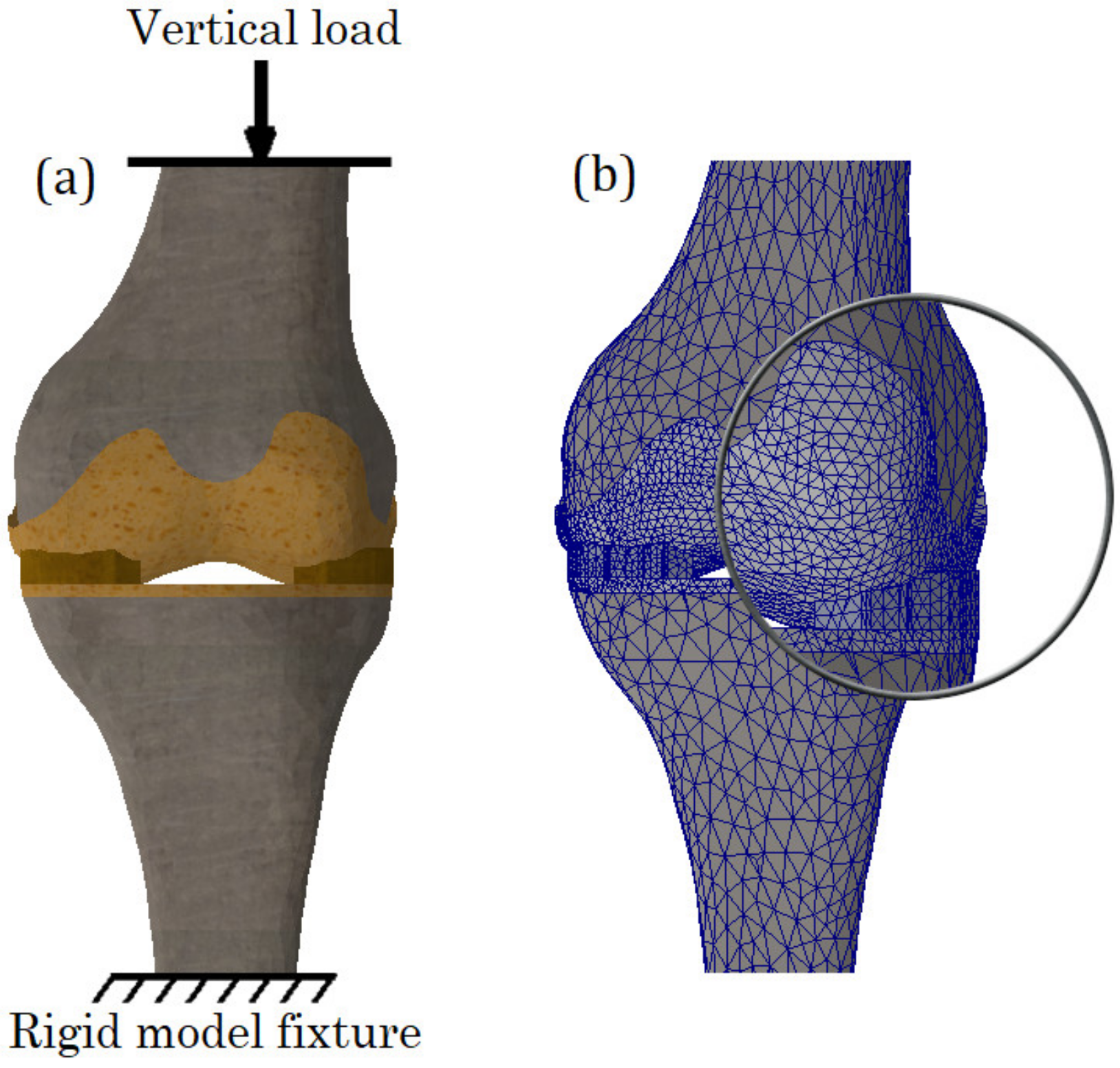
2.6. Calculation Cases, Boundary Conditions and Mesh
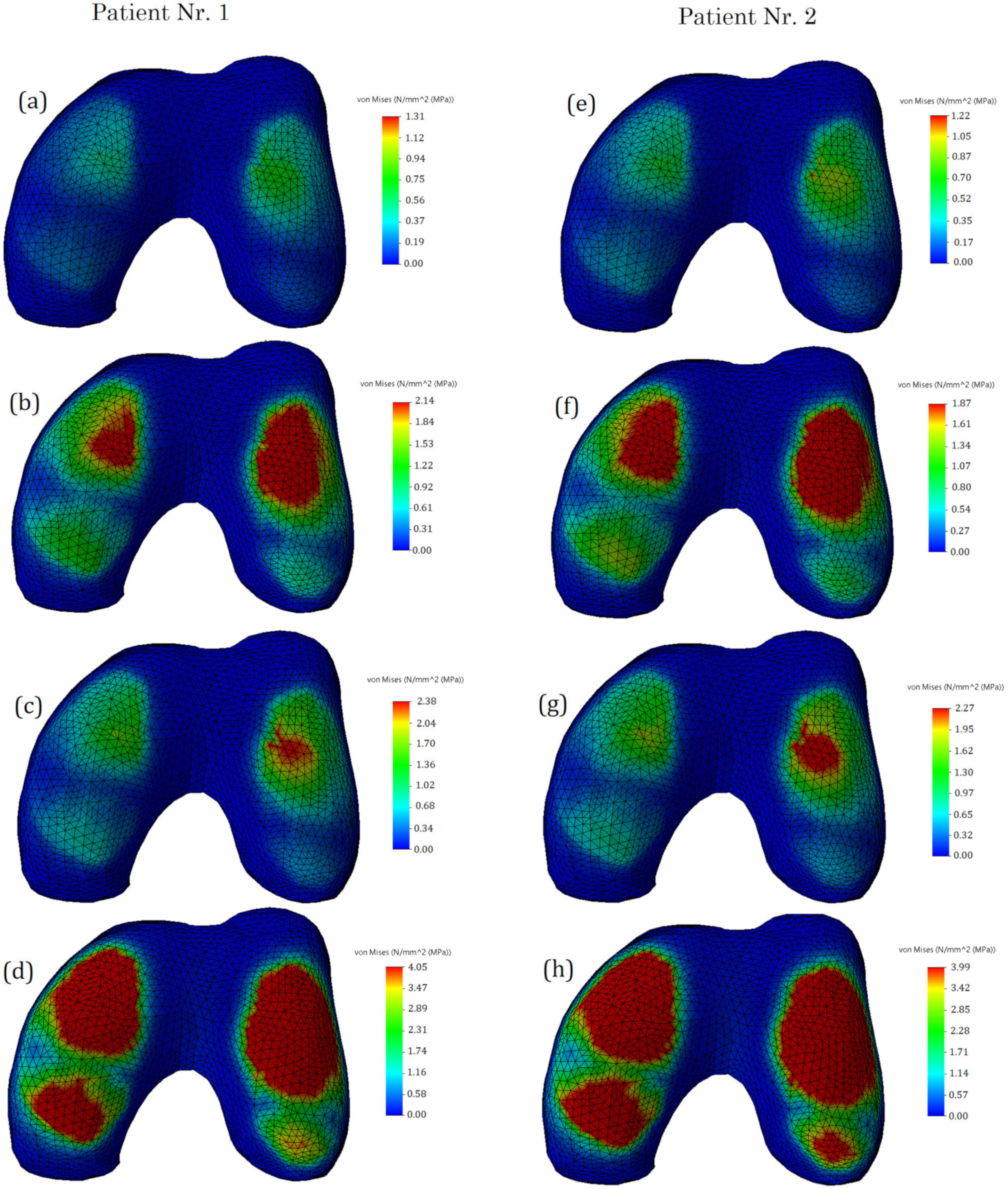
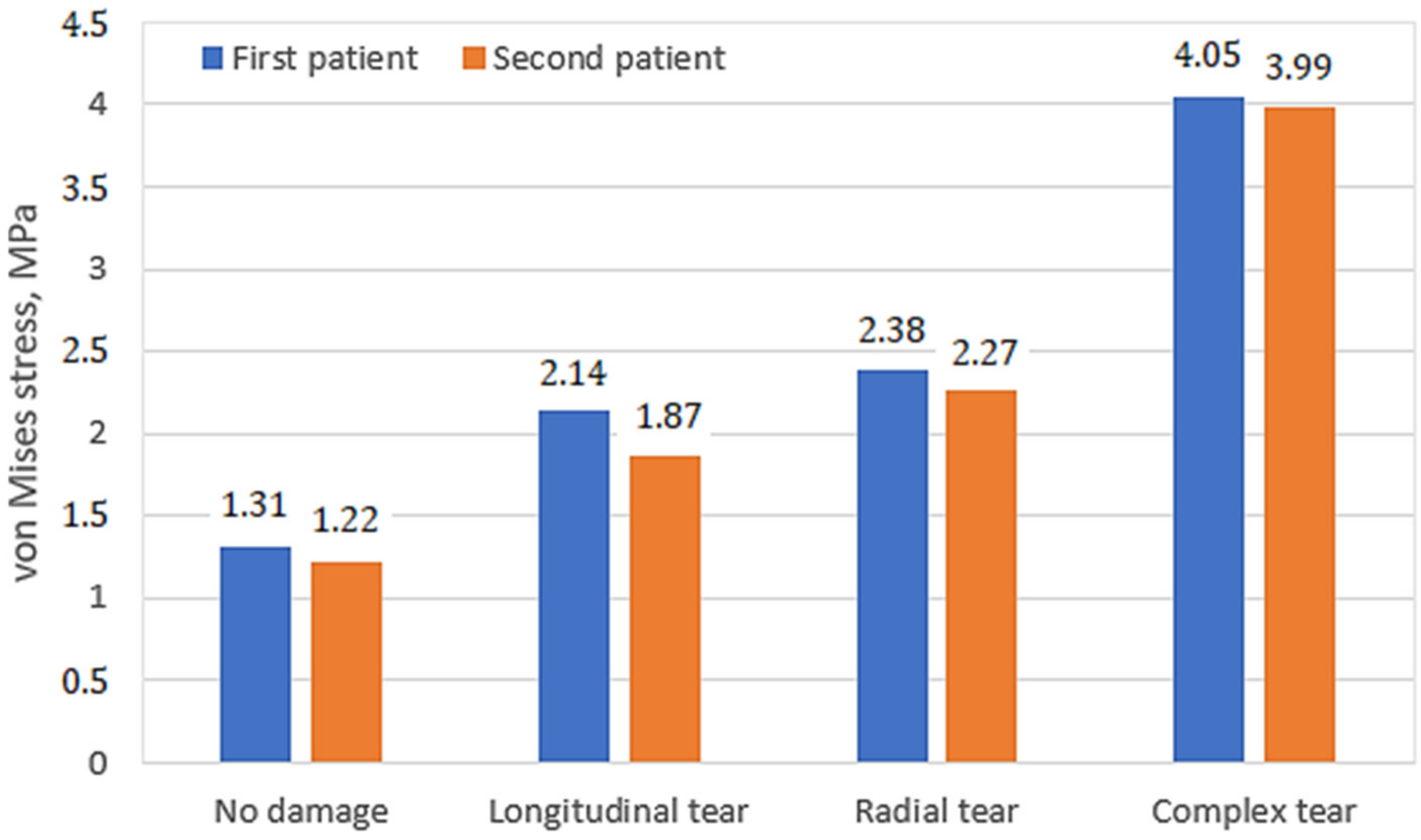
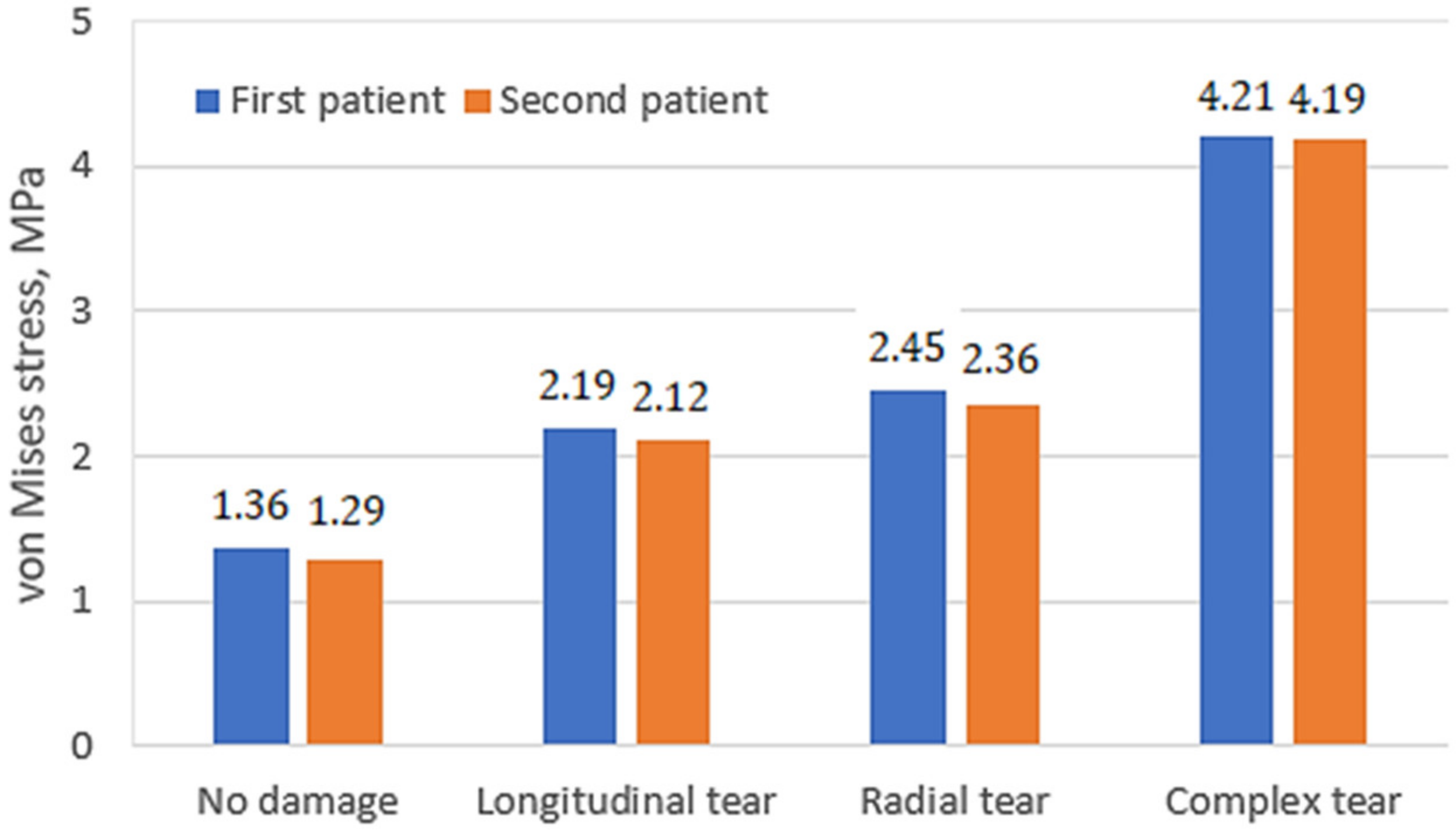
3. Results and Discussion
4. Conclusions
- The distribution of stresses on the cartilage surfaces varied slightly depending on the mechanical properties of the undamaged meniscus. The differences in stress distribution were more noticeable in cases of different damage types. Thus, the geometric shape and mechanical condition of the knee joint have the greatest influences on stress distribution;
- The major factors, such as stress values, their distribution on the contact surfaces, as well as the clinical condition of the meniscus and cartilage tissues, should be evaluated together, since they may be individually uninformative;
- The results of the study and the developed methodology can be useful for further studies of the mechanical aspects occurring in the knee joint, as well as for modeling artificial meniscus and predicting cartilage responses.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hede, A.; Jensen, D.B.; Blyme, P.; Sonne-Holm, S. Epidemiology of meniscal lesions in the knee: 1,215 open operations in copenhagen 1982-84. Acta Orthop. Scand. 1990, 61, 435–437. [Google Scholar] [CrossRef]
- Chambers, H.G.; Chambers, R.C. The Natural History of Meniscus Tears. J. Pediatr. Orthop. 2019, 39, S53–S55. [Google Scholar] [CrossRef] [PubMed]
- Klimkiewicz, J.J.; Shaffer, B. Meniscal surgery 2002 update: Indications and techniques for resection, repair, regeneration, and replacement. Arthroscopy 2002, 18, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Longo, U.G.; Campi, S.; Denaro, V. Meniscal tears. Open. Access J. Sports Med. 2010, 1, 45–54. [Google Scholar] [CrossRef]
- Magee, T.; Shapiro, M.; Williams, D. Comparison of high-field-strength versus low-field-strength MRI of the shoulder. AJR Am. J. Roentgenol. 2003, 181, 1211–1215. [Google Scholar] [CrossRef]
- Ozeki, N.; Koga, H.; Sekiya, I. Degenerative Meniscus in Knee Osteoarthritis: From Pathology to Treatment. Life 2022, 12, 603. [Google Scholar] [CrossRef]
- Lange, A.K.; Fiatarone Singh, M.A.; Smith, R.M.; Foroughi, N.; Baker, M.K.; Shnier, R.; Vanwanseele, B. Degenerative meniscus tears and mobility impairment in women with knee osteoarthritis. Osteoarthr. Cartil. 2007, 15, 701–708. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Lohmander, S.L. The role of the meniscus in knee osteoarthritis: A cause or consequence? Radiol. Clin. North. Am. 2009, 47, 703–712. [Google Scholar] [CrossRef]
- López-Franco, M.; Gómez-Barrena, E. Cellular and molecular meniscal changes in the degenerative knee: A review. J. Exp. Orthop. 2018, 5, 11. [Google Scholar] [CrossRef]
- López-Franco, M.; López-Franco, O.; Murciano-Antón, M.A.; Cañamero-Vaquero, M.; Fernández-Aceñero, M.J.; Herrero-Beaumont, G.; Gómez-Barrena, E. Meniscal degeneration in human knee osteoarthritis: In situ hybridization and immunohistochemistry study. Arch. Orthop. Trauma. Surg. 2016, 136, 175–183. [Google Scholar] [CrossRef]
- Katsuragawa, Y.; Saitoh, K.; Tanaka, N.; Wake, M.; Ikeda, Y.; Furukawa, H.; Tohma, S.; Sawabe, M.; Ishiyama, M.; Yagishita, S.; et al. Changes of human menisci in osteoarthritic knee joints. Osteoarthr. Cartil. 2010, 18, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Pullig, O.; Weseloh, G.; Swoboda, B. Expression of type VI collagen in normal and osteoarthritic human cartilage. Osteoarthr. Cartil. 1999, 7, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.A.; Liu, X.; Barnabe, C.; Yee, K.; Faris, P.D.; Barber, C.; Mosher, D.; Noseworthy, T.; Werle, J.; Lix, L. Existing comorbidities in people with osteoarthritis: A retrospective analysis of a population-based cohort in Alberta, Canada. BMJ Open 2019, 9, e033334. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed Pharm. 2019, 109, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Potočnik, B.; Zazula, D.; Cigale, B.; Heric, D.; Cibula, E.; Donlagic, D. A Patient-specific Knee Joint Computer Model Using MRI Data and ‘in vivo’ Compressive Load from the Optical Force Measuring System. J. Comput. Inf. Technol. 2008, 16, 209–222. [Google Scholar] [CrossRef]
- Ethier, C.R.; Simmons, C. Introductory Biomechanics: From Cells to Organisms; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Öhman, C.; Espino, D.M.; Heinmann, T.; Baleani, M.; Delingette, H.; Viceconti, M. Subject-specific knee joint model: Design of an experiment to validate a multi-body finite element model. Vis. Comput. 2011, 27, 153–159. [Google Scholar] [CrossRef]
- Erdemir, A. Open Knee: Open Source Modeling and Simulation in Knee Biomechanics. J. Knee Surg. 2016, 29, 107–116. [Google Scholar] [CrossRef]
- Brown, C.P.; Nguyen, T.C.; Moody, H.R.; Crawford, R.W.; Oloyede, A. Assessment of common hyperelastic constitutive equations for describing normal and osteoarthritic articular cartilage. Proc. Inst. Mech. Eng. H 2009, 223, 643–652. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, G.; Dong, Y.; Hu, Y.; Xu, Q. The effect of meniscal tears and resultant partial meniscectomies on the knee contact stresses: A finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 1452–1463. [Google Scholar] [CrossRef]
- Venäläinen, M.S.; Mononen, M.E.; Jurvelin, J.S.; Töyräs, J.; Virén, T.; Korhonen, R.K. Importance of material properties and porosity of bone on mechanical response of articular cartilage in human knee joint—A two-dimensional finite element study. J. Biomech. Eng. 2014, 136, 121005. [Google Scholar] [CrossRef]
- Peña, E.; Calvo, B.; Martínez, M.A.; Palanca, D.; Doblaré, M. Finite element analysis of the effect of meniscal tears and meniscectomies on human knee biomechanics. Clin. Biomech. 2005, 20, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Park, K.S.; Seon, J.K.; Kwak, D.S.; Jeon, I.; Song, E.K. Biomechanical analysis of the effects of medial meniscectomy on degenerative osteoarthritis. Med. Biol. Eng. Comput. 2012, 50, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, L.; Yu, F.; Shi, J.; Zhu, L.; Yang, X.; Teng, H.; Wang, X.; Jiang, Q. 3D printing individualized heel cup for improving the self-reported pain of plantar fasciitis. J. Transl. Med. 2018, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- 3D Slicer Image Computer Platform. Available online: https://www.slicer.org/ (accessed on 11 November 2022).
- MESHLAB. Available online: https://meshlab.net/ (accessed on 11 November 2022).
- SOLIDWORKS Systemes. Available online: https://help.solidworks.com/ (accessed on 14 November 2022).
- Jaramillo, H.E. Evaluation of the Use of the Yeoh and Mooney-Rivlin Functions as Strain Energy Density Functions for the Ground Substance Material of the Annulus Fibrosus. Math. Probl. Eng. 2018, 2018, 1570142. [Google Scholar] [CrossRef]
- Butz, K.D.; Chan, D.D.; Nauman, E.A.; Neu, C.P. Stress distributions and material properties determined in articular cartilage from MRI-based finite strains. J. Biomech. 2011, 44, 2667–2672. [Google Scholar] [CrossRef]
- Lecouvet, F.; Van Haver, T.; Acid, S.; Perlepe, V.; Kirchgesner, T.; Vande Berg, B.; Triqueneaux, P.; Denis, M.L.; Thienpont, E.; Malghem, J. Magnetic resonance imaging (MRI) of the knee: Identification of difficult-to-diagnose meniscal lesions. Diagn. Interv. Imaging 2018, 99, 55–64. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Zhang, K.; Zhu, L.; Wang, X.; Jiang, Q. Three-dimensional finite-element analysis of aggravating medial meniscus tears on knee osteoarthritis. J. Orthop. Transl. 2020, 20, 47–55. [Google Scholar] [CrossRef]
- Badlani, J.T.; Borrero, C.; Golla, S.; Harner, C.D.; Irrgang, J.J. The Effects of Meniscus Injury on the Development of Knee Osteoarthritis:Data From the Osteoarthritis Initiative. Am. J. Sport. Med. 2013, 41, 1238–1244. [Google Scholar] [CrossRef]



| Model Component | C1, MPa | C2, MPa |
|---|---|---|
| Cartilage (tibia) | 1.13 | 0.32 |
| Cartilage (femur) | 1.08 | 0.63 |
| Patient No. | Sex | Age | Body Mass Index | Diagnosis |
|---|---|---|---|---|
| 1 | Female | 61 | 41.0 | Knee osteoarthritis stage IV |
| 2 | Female | 60 | 34.4 | Knee osteoarthritis stage III |
| Calculations Cases | Number of Finite Elements | Number of Nodes |
|---|---|---|
| No meniscus damage | 53,443 | 89,871 |
| Longitudinal meniscal tear | 51,252 | 87,799 |
| Radial meniscal tear | 52,884 | 88,345 |
| Complex meniscal tear | 50,986 | 87,658 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardatov, O.; Aleksiuk, V.; Maknickas, A.; Stonkus, R.; Uzieliene, I.; Vaiciuleviciute, R.; Pachaleva, J.; Kvederas, G.; Bernotiene, E. Modeling the Impact of Meniscal Tears on von Mises Stress of Knee Cartilage Tissue. Bioengineering 2023, 10, 314. https://doi.org/10.3390/bioengineering10030314
Ardatov O, Aleksiuk V, Maknickas A, Stonkus R, Uzieliene I, Vaiciuleviciute R, Pachaleva J, Kvederas G, Bernotiene E. Modeling the Impact of Meniscal Tears on von Mises Stress of Knee Cartilage Tissue. Bioengineering. 2023; 10(3):314. https://doi.org/10.3390/bioengineering10030314
Chicago/Turabian StyleArdatov, Oleg, Viktorija Aleksiuk, Algirdas Maknickas, Rimantas Stonkus, Ilona Uzieliene, Raminta Vaiciuleviciute, Jolita Pachaleva, Giedrius Kvederas, and Eiva Bernotiene. 2023. "Modeling the Impact of Meniscal Tears on von Mises Stress of Knee Cartilage Tissue" Bioengineering 10, no. 3: 314. https://doi.org/10.3390/bioengineering10030314
APA StyleArdatov, O., Aleksiuk, V., Maknickas, A., Stonkus, R., Uzieliene, I., Vaiciuleviciute, R., Pachaleva, J., Kvederas, G., & Bernotiene, E. (2023). Modeling the Impact of Meniscal Tears on von Mises Stress of Knee Cartilage Tissue. Bioengineering, 10(3), 314. https://doi.org/10.3390/bioengineering10030314