Investigating the Extracellular-Electron-Transfer Mechanisms and Kinetics of Shewanella decolorationis NTOU1 Reducing Graphene Oxide via Lactate Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Cultural Conditions
2.2. Synthesis of Graphene Oxide
2.3. Microbial Reduction of Graphene Oxide via S. decolorationis NTOU1
2.4. Surface Characterizations and Materials Analyses
2.5. Organic-Acid Analyses
2.6. Colony Forming Units (CFU)
3. Results
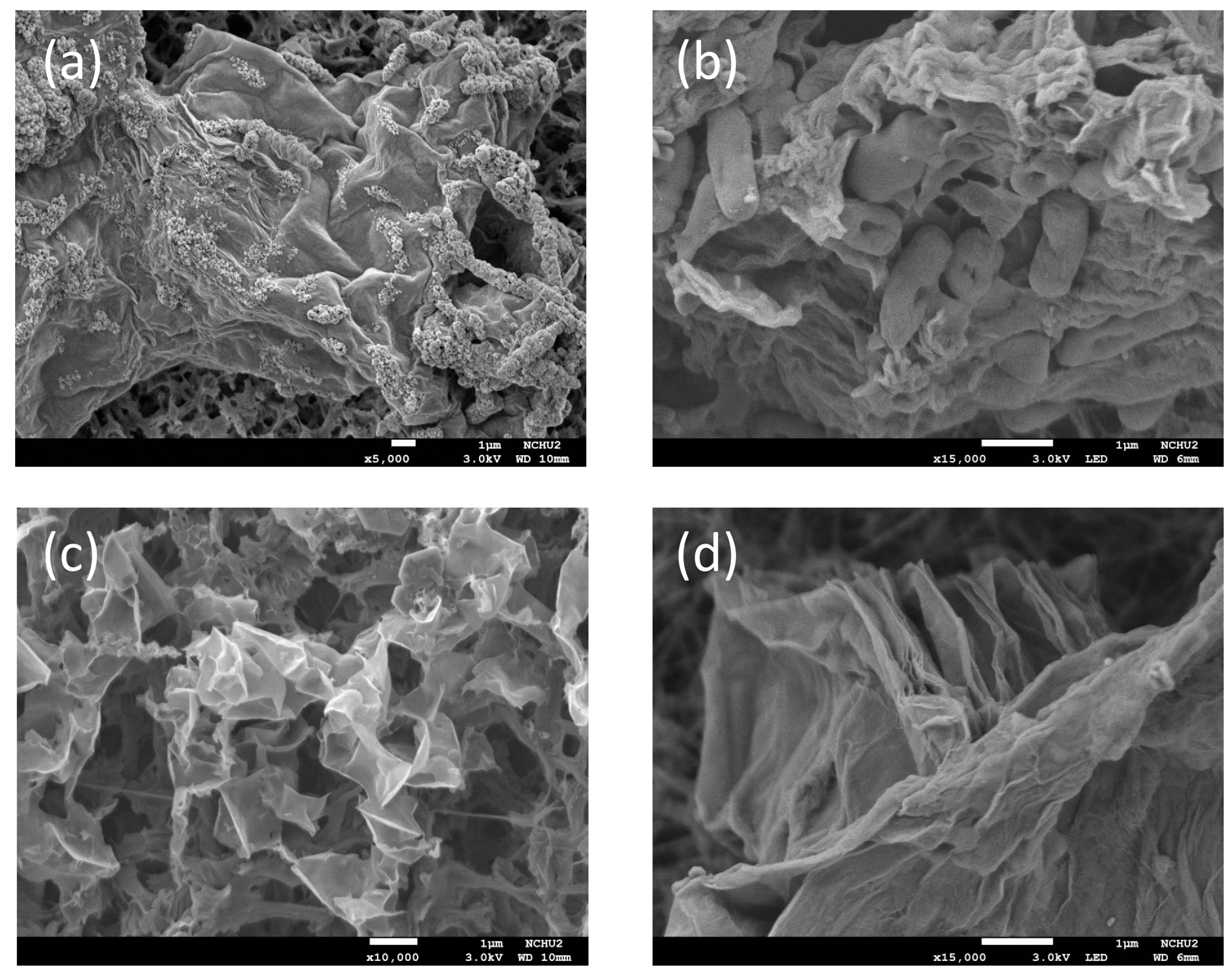
3.1. Characterizations of GO and rGO Interactions with S. decolorationis NTOU1
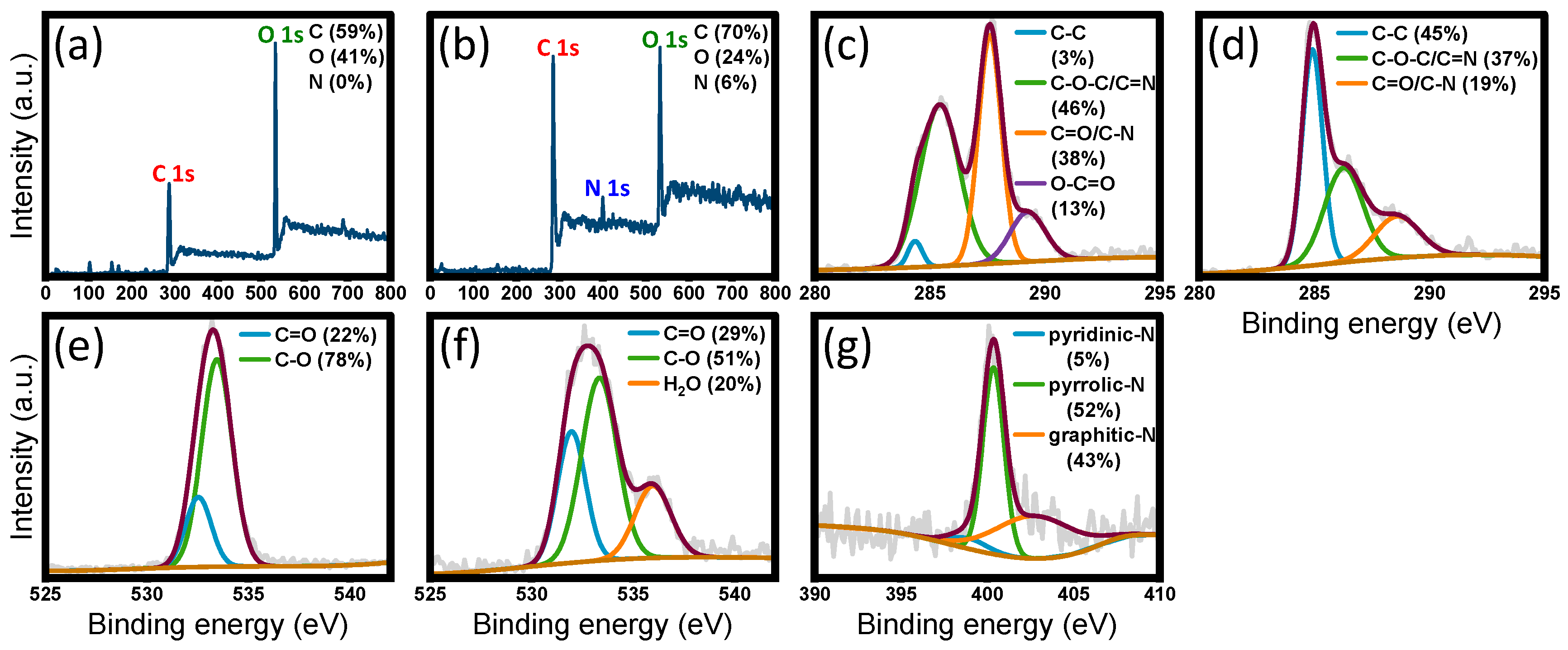
3.2. Elemental and Bond Composition Analysis of Graphene Materials
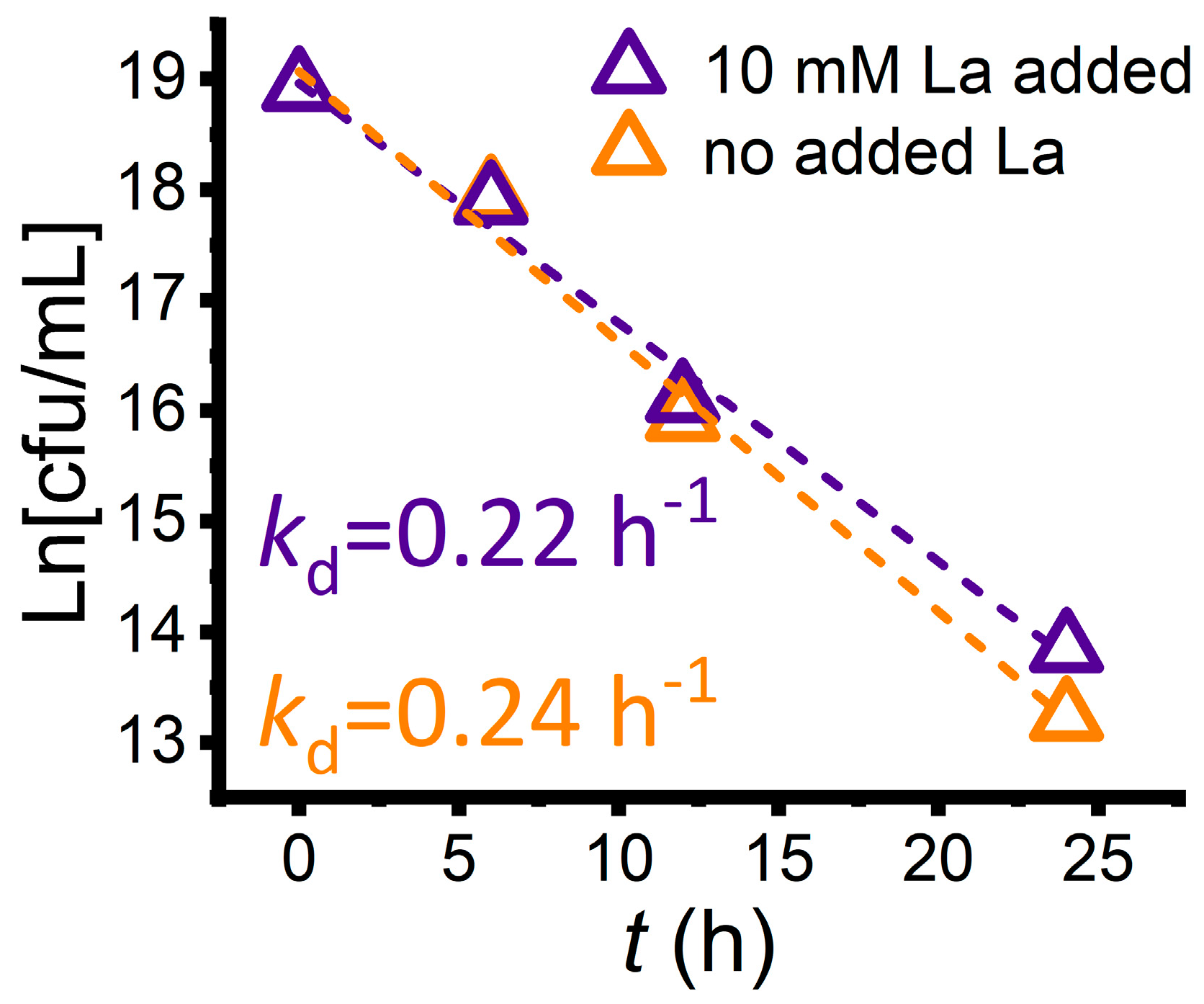
3.3. Bacterial Growth in the Reaction Process to Generate rGO
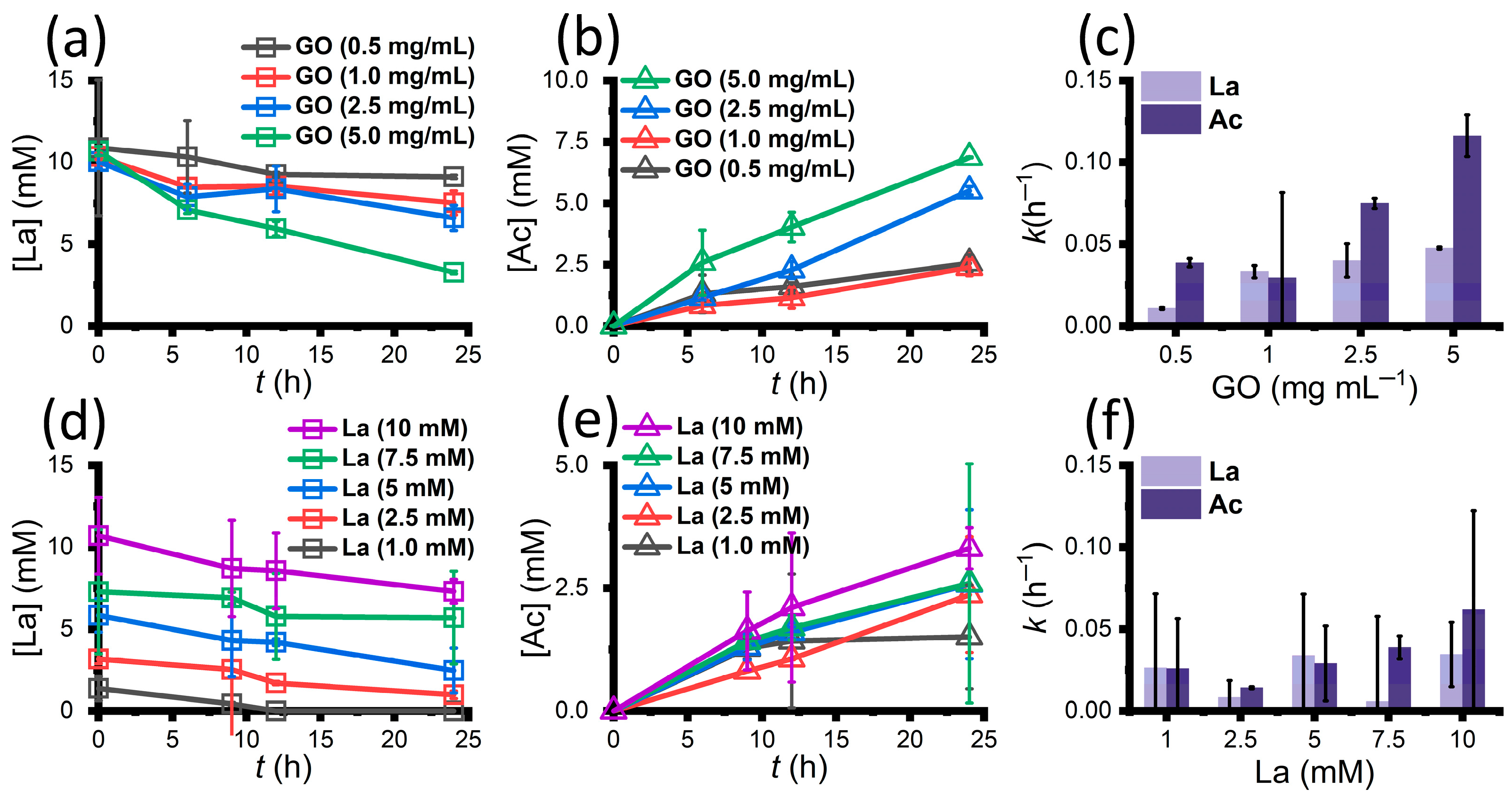
3.4. Organic Acid Analysis Results under Different Conditions
4. Discussion
5. Conclusions
- The number of bacterial cells gradually decreases with reaction time, showing the toxic effect during the GO reduction.
- While the initial bacteria concentrations were consistent in all the experiments, with the initial lactate concentration fixed, the rates of lactate metabolism and acetate generation increased when the added concentrations of GO increased; with the initial GO concentration fixed, the rates of lactate metabolism did not change significantly. These results indicate that GO concentration is the limiting factor governing the reaction rates of microbial GO reduction.
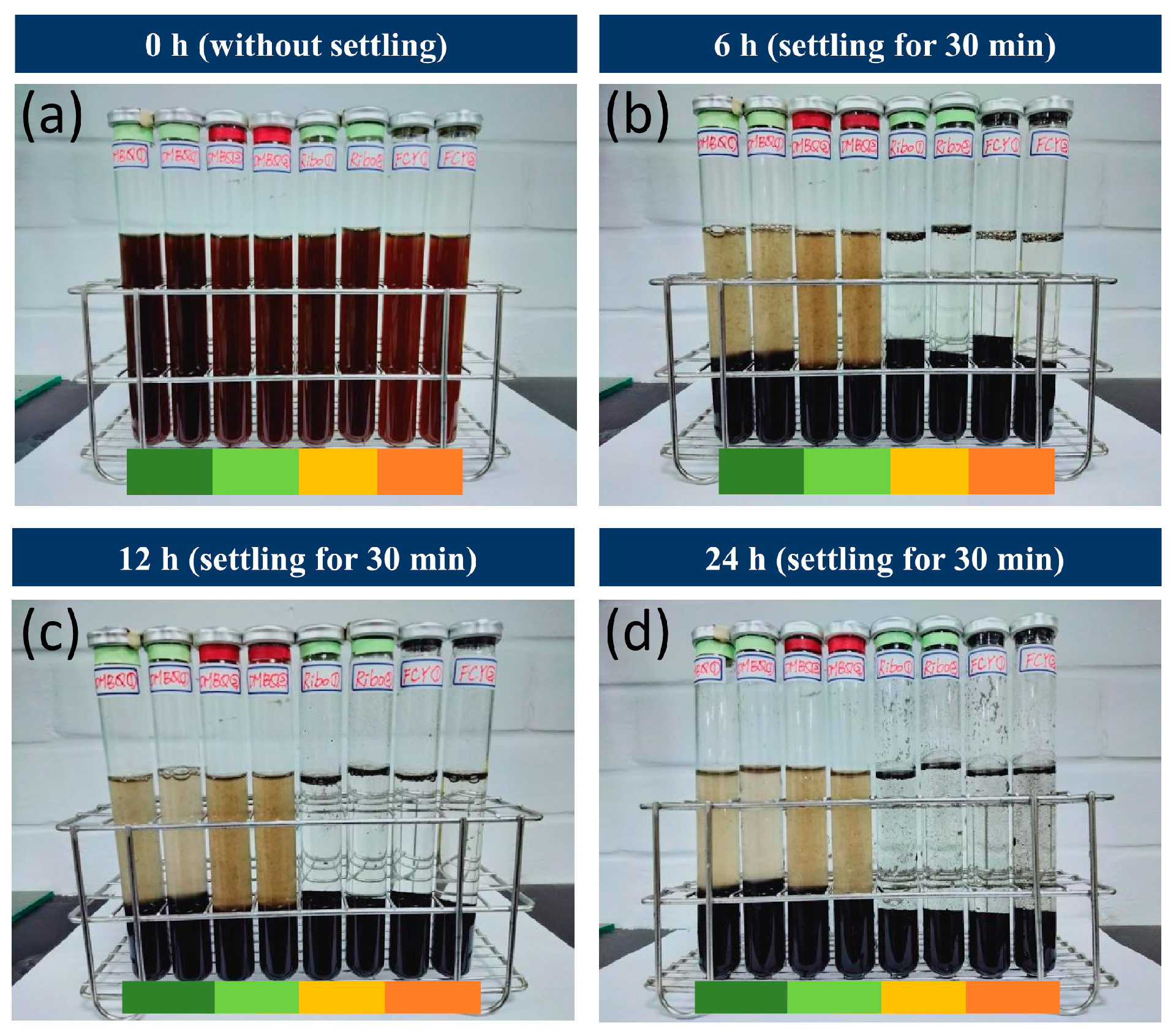
- Adding riboflavin and potassium ferricyanide as mediators boosts the generation of rGO, whereas adding DMoBQ and DMBQ eliminates the entire reaction, indicating that not all the redox compounds can help microbial GO reduction.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brownson, D.A.; Banks, C.E. The Handbook of Graphene Electrochemistry; Springer: London, UK, 2014; pp. 1–22. [Google Scholar]
- Wurstbauer, U.; Röling, C.; Wurstbauer, U.; Wegscheider, W.; Vaupel, M.; Thiesen, P.H.; Weiss, D. Imaging ellipsometry of graphene. Appl. Phys. Lett. 2010, 97, 231901. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef]
- Su, C.Y.; Lu, A.Y.; Xu, Y.; Chen, F.R.; Khlobystov, A.N.; Li, L.J. High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Low, C.T.J.; Walsh, F.C.; Chakrabarti, M.H.; Hashim, M.A.; Hussain, M.A. Electrochemical approaches to the production of graphene flakes and their potential applications. Carbon 2013, 54, 1–21. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Sofer, Z.; Šimek, P.; Pumera, M. Complex organic molecules are released during thermal reduction of graphite oxides. Phys. Chem. Chem. Phys. 2013, 15, 9257–9264. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- Chen, D.; Li, L.; Guo, L. An environment-friendly preparation of reduced graphene oxide nanosheets via amino acid. Nanotechnology 2011, 22, 325601. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Green reduction of graphene oxide by aqueous phytoextracts. Carbon 2012, 50, 5331–5339. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon 2012, 50, 1853–1860. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.; Wang, Y.; Chen, W.; Alvarez, P.J. Self-damaging aerobic reduction of graphene oxide by Escherichia coli: Role of GO-mediated extracellular superoxide formation. Environ. Sci. Technol. 2018, 52, 12783–12791. [Google Scholar] [CrossRef]
- Salas, E.C.; Sun, Z.; Lüttge, A.; Tour, J.M. Reduction of graphene oxide via bacterial respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef]
- Wang, G.; Qian, F.; Saltikov, C.W.; Jiao, Y.; Li, Y. Microbial reduction of graphene oxide by Shewanella. Nano Res. 2011, 4, 563–570. [Google Scholar] [CrossRef]
- Jiao, Y.; Qian, F.; Li, Y.; Wang, G.; Saltikov, C.W.; Gralnick, J.A. Deciphering the electron transport pathway for graphene oxide reduction by Shewanella oneidensis MR-1. J. Bacteriol. 2011, 193, 3662–3665. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe (III) and Mn (IV) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [PubMed]
- Kalathil, S.; Katuri, K.P.; Alazmi, A.S.; Pedireddy, S.; Kornienko, N.; Costa, P.M.; Saikaly, P.E. Bioinspired synthesis of reduced graphene oxide-wrapped Geobacter sulfurreducens as a hybrid electrocatalyst for efficient oxygen evolution reaction. Chem. Mater. 2019, 31, 3686–3693. [Google Scholar] [CrossRef]
- Lu, Y.; Zhong, L.; Tang, L.; Wang, H.; Yang, Z.; Xie, Q.; Feng, H.; Jia, M.; Fan, C. Extracellular electron transfer leading to the biological mediated production of reduced graphene oxide. Chemosphere 2020, 256, 127141. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Saito, K.; Inoue, K.; Nealson, K.H.; Hashimoto, K.; Nakamura, R. Uptake of self-secreted flavins as bound cofactors for extracellular electron transfer in Geobacter species. Energy Environ. Sci. 2014, 7, 1357–1361. [Google Scholar] [CrossRef]
- Smith, J.A.; Tremblay, P.L.; Shrestha, P.M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.P.; Lovley, D.R. Going wireless: Fe (III) oxide reduction without pili by Geobacter sulfurreducens strain JS-1. Appl. Environ. Microbiol. 2014, 80, 4331–4340. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lo, H.J.; Huang, M.C.; Lai, Y.H.; Chen, H.Y. Towards bi-functional all-solid-state supercapacitor based on nickel hydroxide-reduced graphene oxide composite electrodes. Mater. Chem. Phys. 2021, 262, 124306. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Trussell, R.R. Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Li, S.L.; Yen, J.H.; Kano, K.; Liu, S.M.; Liu, C.L.; Cheng, S.S.; Chen, H.Y. Using metabolic charge production in the tricarboxylic acid cycle (QTCA) to evaluate the extracellular-electron-transfer performances of Shewanella spp. Bioelectrochemistry 2018, 124, 119–126. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interface Anal. 1981, 3, 211225. [Google Scholar] [CrossRef]
- Karas, V.O.; Westerlaken, I.; Meyer, A.S. The DNA-binding protein from starved cells (Dps) utilizes dual functions to defend cells against multiple stresses. J. Bacteriol. 2015, 197, 3206–3215. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Xu, D.; Huang, Y.; Wu, Z.; Wang, L.M.; Zhang, X.B. Facile, mild and fast thermal-decomposition reduction of graphene oxide in air and its application in high-performance lithium batteries. Chem. Commun. 2012, 48, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Culley, D.E.; et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Srivastava, V.C. Simple synthesis of large graphene oxide sheets via electrochemical method coupled with oxidation process. ACS Omega 2018, 3, 10233–10242. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.X.; Chen, C.M.; Guo, S.Q.; Guo, F.; Li, X.M.; Wang, X.X.; Cui, H.T.; Zhou, L.F.; Li, W. Advanced visible-light-driven photocatalyst BiOBr–TiO2–graphene composite with graphene as a nano-filler. J. Mater. Chem. A 2014, 2, 4667–4675. [Google Scholar] [CrossRef]
- IR Spectrum Table & Chart, Sigma Aldrich. Available online: https://www.sigmaaldrich.com/TW/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 20 January 2023).
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Alkoxyamine with reduced homolysis temperature and its application in repeated autonomous self-healing of stiff polymers. Polym. Chem. 2013, 4, 4648–4654. [Google Scholar] [CrossRef]
- Lei, Z.; Lu, L.; Zhao, X.S. The electrocapacitive properties of graphene oxide reduced by urea. Energy Environ. Sci. 2012, 5, 6391–6399. [Google Scholar] [CrossRef]
- Shen, L.; Jin, Z.; Wang, D.; Wang, Y.; Lu, Y. Enhance wastewater biological treatment through the bacteria induced graphene oxide hydrogel. Chemosphere 2018, 190, 201–210. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, X.; Guo, D.; Qu, B.; Zhang, M.; Li, Q.; Wang, T. Synthesis of bacteria promoted reduced graphene oxide-nickel sulfide networks for advanced supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 7335–7340. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, J.; Tung, V.C.; Luo, J.; Kim, F.; Huang, J. Graphene oxide as surfactant sheets. Pure Appl. Chem. 2010, 83, 95–110. [Google Scholar] [CrossRef]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef] [PubMed]
- Ottow, J.C.G.; Glathe, H. Isolation and identification of iron-reducing bacteria from gley soils. Soil Biol. Biochem. 1971, 3, 43–55. [Google Scholar] [CrossRef]
- Burdige, D.J.; Nealson, K.H. Microbial manganese reduction by enrichment cultures from coastal marine sediments. Appl. Environ. Microbiol. 1985, 50, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef]
- von Canstein, H.; Ogawa, J.; Shimizu, S.; Lloyd, J.R. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 2008, 74, 615–623. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [CrossRef]
- Myers, C.R.; Nealson, K.H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 1988, 240, 1319–1321. [Google Scholar] [CrossRef]
- Strycharz-Glaven, S.M.; Snider, R.M.; Guiseppi-Elie, A.; Tender, L.M. On the electrical conductivity of microbial nanowires and biofilms. Energy Environ. Sci. 2011, 4, 4366–4379. [Google Scholar] [CrossRef]
- Harris, H.W.; El-Naggar, M.Y.; Nealson, K.H. Shewanella oneidensis MR-1 chemotaxis proteins and electron-transport chain components essential for congregation near insoluble electron acceptors. Biochem. Soc. Trans. 2012, 40, 1167–1177. [Google Scholar] [CrossRef]
- Xu, S.; Jangir, Y.; El-Naggar, M.Y. Disentangling the roles of free and cytochrome-bound flavins in extracellular electron transport from Shewanella oneidensis MR-1. Electrochim. Acta 2016, 198, 4955. [Google Scholar] [CrossRef]
- Li, S.L.; Bai, M.D.; Hsiao, C.J.; Cheng, S.S.; Nealson, K.H. A metabolic-activity-detecting approach to life detection: Restoring a chemostat from stop-feeding using a rapid bioactivity assay. Bioelectrochemistry 2017, 118, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.E.; Brantley, S.L.; Tien, M. Kinetic characterization of OmcA and MtrC, terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2009, 75, 5218–5226. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Xiao, F.; Liu, Q.; Liu, H.; Guo, Y.; Gong, J.R.; Wang, S.; Liu, Y. One-pot microbial method to synthesize dual-doped graphene and its use as high-performance electrocatalyst. Sci. Rep. 2013, 3, 3499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fu, P.; Wang, Y.; Sun, L.; Yuan, Y. Microbe-engaged synthesis of carbon dot-decorated reduced graphene oxide as high-performance oxygen reduction catalysts. J. Mater. Chem. A 2016, 4, 7222–7229. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Ren, W.; Cheng, H.; Tang, N.; Wu, W.; Zhong, W.; Du, Y. The doping of reduced graphene oxide with nitrogen and its effect on the quenching of the material’s photoluminescence. Carbon 2012, 50, 5286–5291. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Bao, S.-J.; Gou, Y.-T.; Cai, C.-J.; Ji, C.-C.; Xu, M.-W.; Song, J.; Wang, R. Nitrogen-doped reduced-graphene oxide as an efficient metal-free electrocatalyst for oxygen reduction in fuel cells. RSC Adv. 2013, 3, 3990–3995. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liou, Y.-X.; Li, S.-L.; Hsieh, K.-Y.; Li, S.-J.; Hu, L.-J. Investigating the Extracellular-Electron-Transfer Mechanisms and Kinetics of Shewanella decolorationis NTOU1 Reducing Graphene Oxide via Lactate Metabolism. Bioengineering 2023, 10, 311. https://doi.org/10.3390/bioengineering10030311
Liou Y-X, Li S-L, Hsieh K-Y, Li S-J, Hu L-J. Investigating the Extracellular-Electron-Transfer Mechanisms and Kinetics of Shewanella decolorationis NTOU1 Reducing Graphene Oxide via Lactate Metabolism. Bioengineering. 2023; 10(3):311. https://doi.org/10.3390/bioengineering10030311
Chicago/Turabian StyleLiou, Yu-Xuan, Shiue-Lin Li, Kun-Yi Hsieh, Sin-Jie Li, and Li-Jie Hu. 2023. "Investigating the Extracellular-Electron-Transfer Mechanisms and Kinetics of Shewanella decolorationis NTOU1 Reducing Graphene Oxide via Lactate Metabolism" Bioengineering 10, no. 3: 311. https://doi.org/10.3390/bioengineering10030311
APA StyleLiou, Y.-X., Li, S.-L., Hsieh, K.-Y., Li, S.-J., & Hu, L.-J. (2023). Investigating the Extracellular-Electron-Transfer Mechanisms and Kinetics of Shewanella decolorationis NTOU1 Reducing Graphene Oxide via Lactate Metabolism. Bioengineering, 10(3), 311. https://doi.org/10.3390/bioengineering10030311




_Li.png)


