Novel, Blended Polymeric Microspheres for the Controlled Release of Methotrexate: Characterization and In Vivo Antifibrotic Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of MTX-Loaded Microspheres
2.3. Characterization of the Fabricated Microspheres
2.4. Quantification of MTX Using Spectrophotometer
2.5. Evaluation of the Encapsulation Efficiency
2.6. MTX Release Profile
2.7. Evaluation of the Stability of Encapsulated MTX and Released from Microspheres In Vitro
2.8. Fibroblast Cell Culture
2.9. In Vivo Animal Studies
2.10. Histological Analysis of Fibrosis
2.11. In Vivo Biological Activity; Quantification of Collagen
2.12. Total Tissue Cellularity
2.13. In Vivo Biological Activity; MMP-13, Type-1 Collagen, and α-SMA Gene Expression
2.14. In Vivo Biological Activity; α-SMA Protein Expression
2.15. Statistical Analysis
3. Results
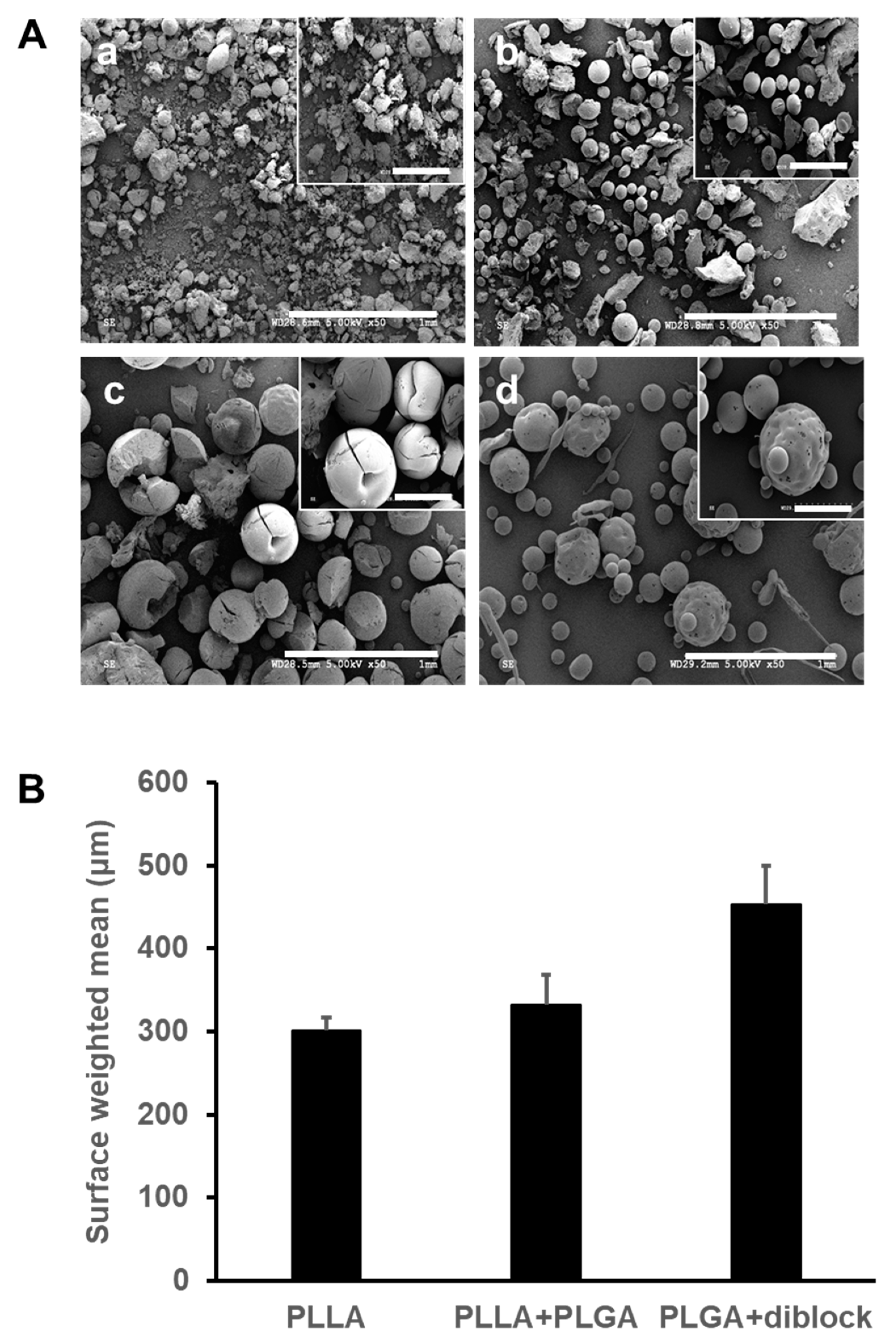
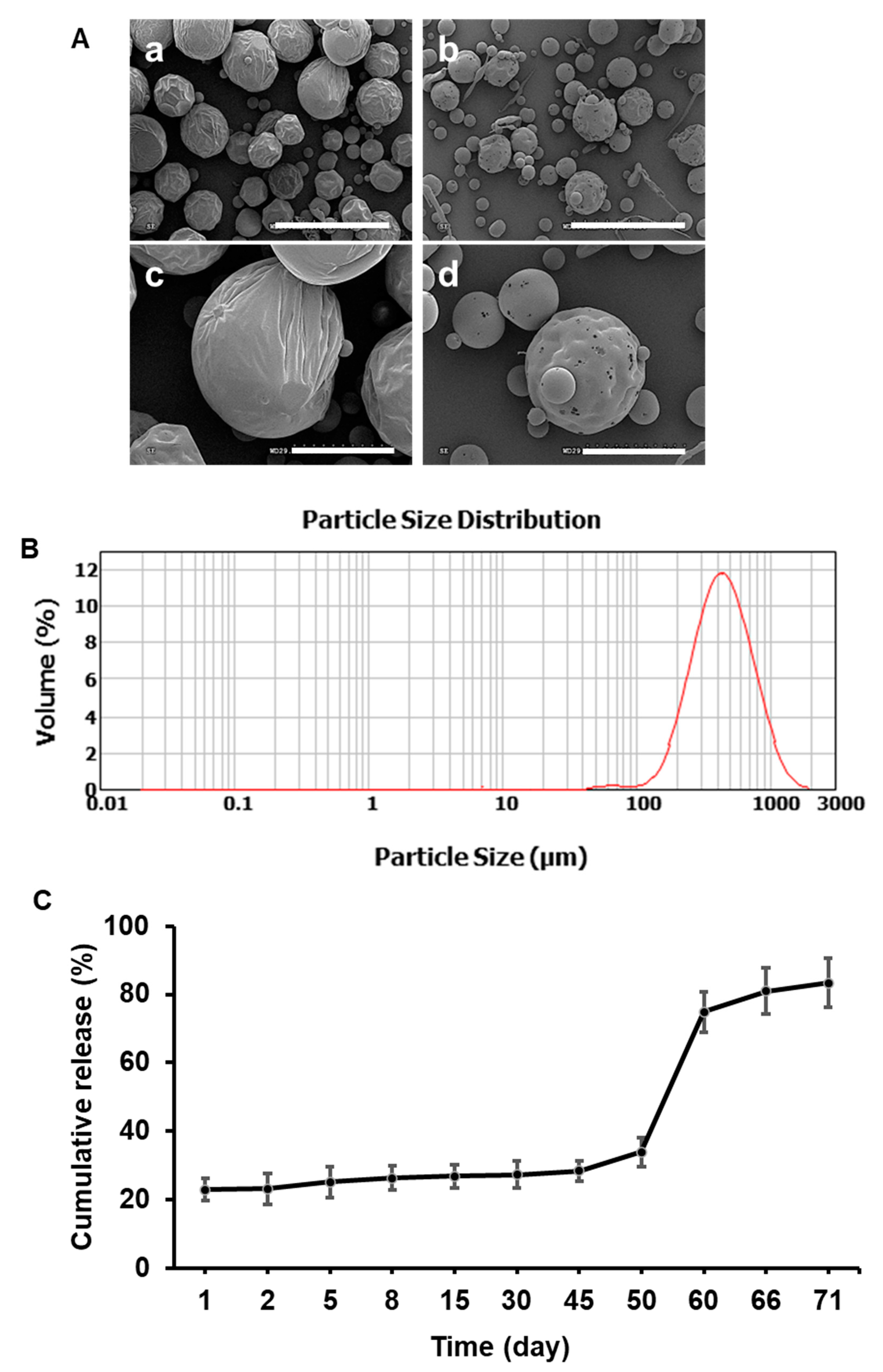
3.1. Morphology and Size Distribution of the Fabricated Microspheres
3.2. Encapsulation Efficiency and In Vitro Release Profile
3.3. Morphology of the Polymer-Only and Extended-Release Kinetics of MTX-Loaded Microspheres In Vitro
3.4. MTX Released from Microspheres Decreases Collagen and Increases MMP-1 Expression at Protein Level in Human Dermal Fibroblasts In Vitro
3.5. MTX Microspheres Decrease Total Cellularity inside Implanted PVA Sponges
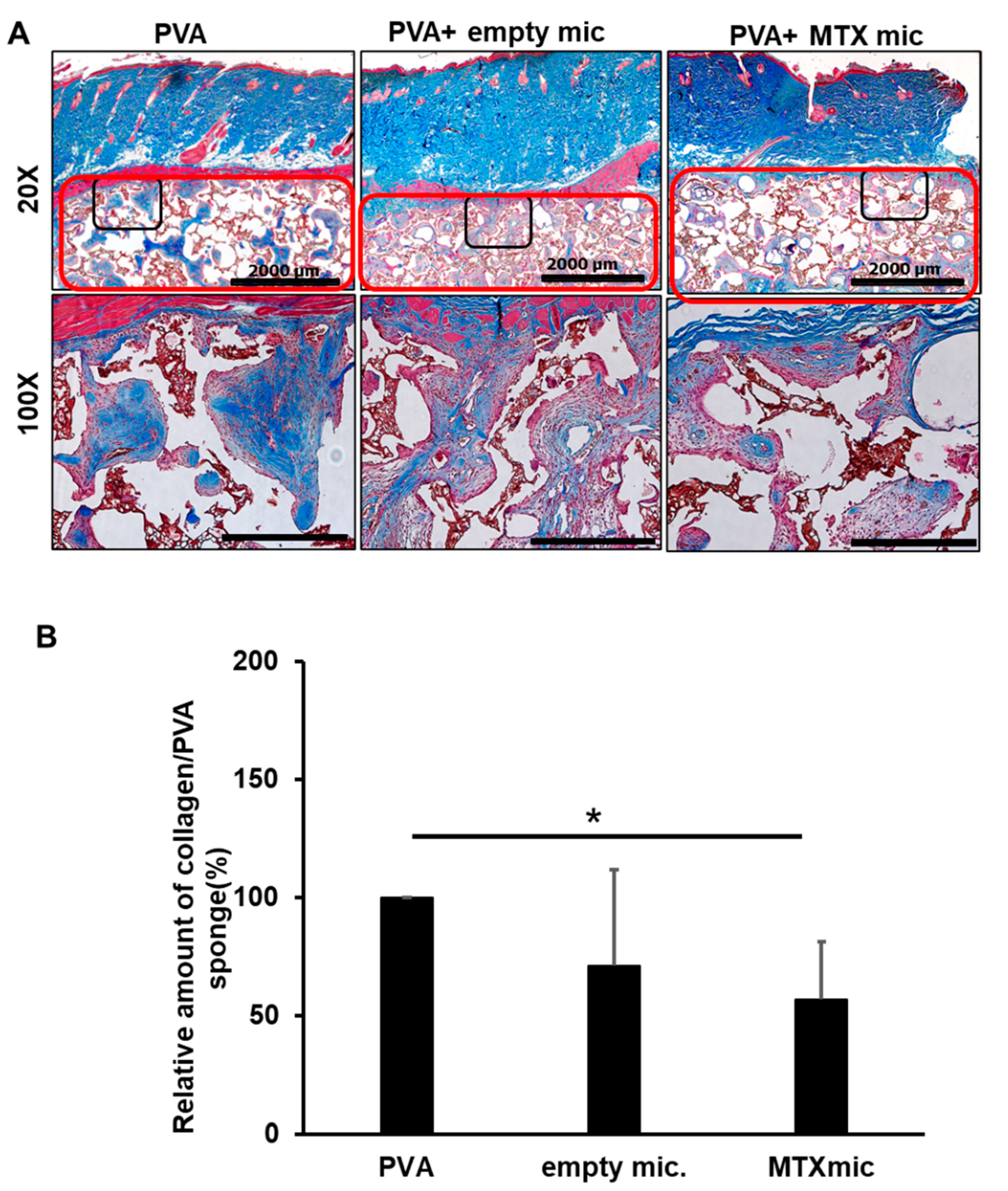
3.6. MTX Microspheres Decrease Collagen Deposition In Vivo
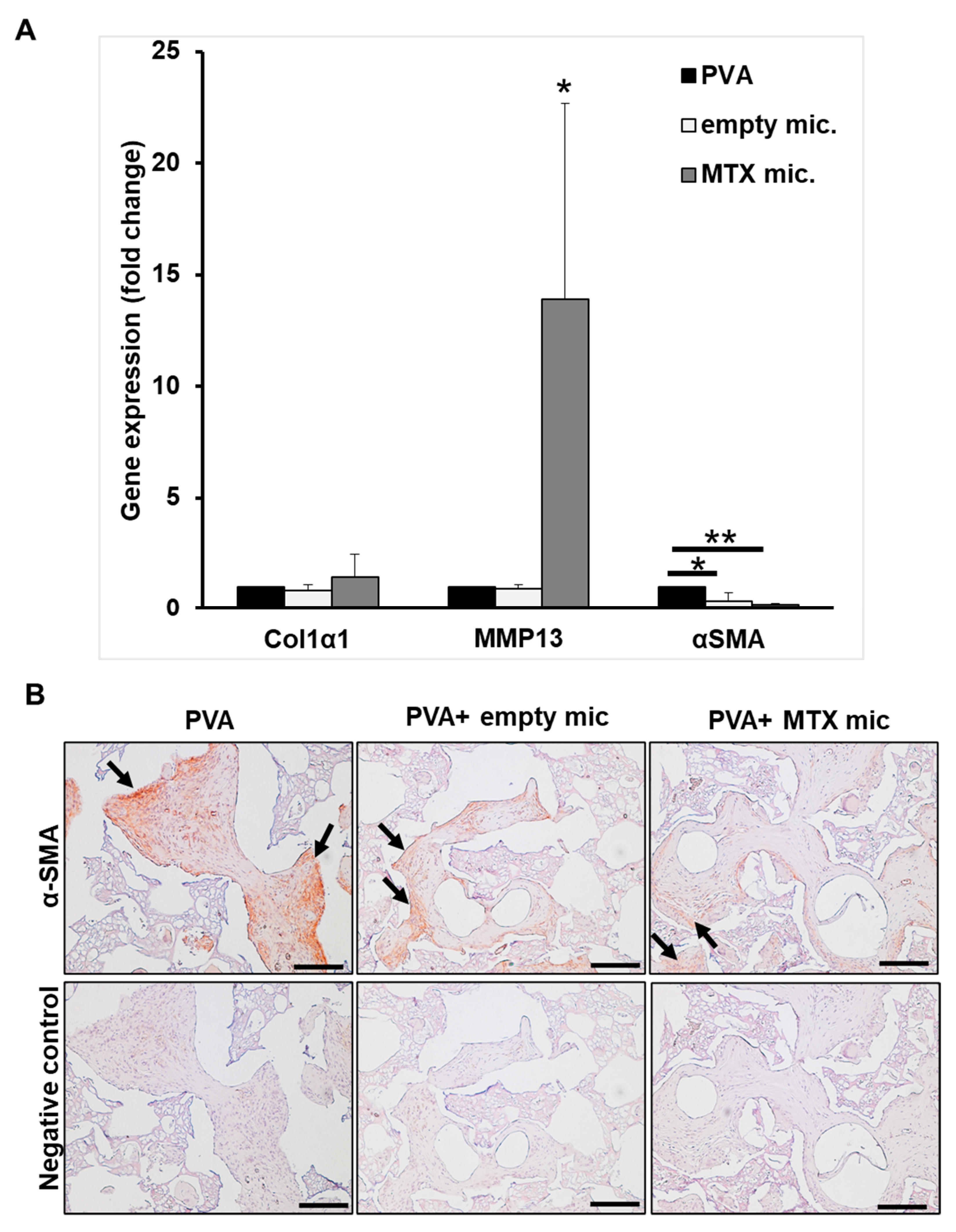
3.7. Col1α1, MMP-13, and α-SMA Expression In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malaviya, A.N. Landmark papers on the discovery of methotrexate for the treatment of rheumatoid arthritis and other systemic inflammatory rheumatic diseases: A fascinating story. Int. J. Rheum. Dis. 2016, 19, 844–851. [Google Scholar] [CrossRef]
- Roenigk, H.H.J.; Maibach, H.I.; Weinstein, G.D. Use of methotrexate in psoriasis. Arch. Dermatol. 1972, 105, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.L.; Cronstein, B.N. Methotrexate—How does it really work? Nat. Rev. Rheumatol. 2010, 6, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Siva, S.; Cook, G.K.; Jones, D.R.; Fadda, H.M. Methotrexate Polyglutamate Monitoring in Patients with Crohn’s Disease. Clin. Pharmacol. Drug Dev. 2017, 6, 240–245. [Google Scholar] [CrossRef]
- Yates, M. Commonly used medication for Lupus. Lupus 2018, 27 (Suppl. S1), 8–10. [Google Scholar] [CrossRef]
- Ruperto, N.; Pistorio, A.; Oliveira, S.; Zulian, F.; Cuttica, R.; Ravelli, A.; Fischbach, M.; Magnusson, B.; Sterba, G.; Avcin, T.; et al. Prednisone versus prednisone plus ciclosporin versus prednisone plus methotrexate in new-onset juvenile dermatomyositis: A randomised trial. Lancet 2016, 387, 671–678. [Google Scholar] [CrossRef]
- Onwukwe, M.F. Treating keloids by surgery and methotrexate. Arch. Dermatol. 1980, 116, 158. [Google Scholar] [CrossRef]
- Muzaffar, A.R.; Rafols, F.; Masson, J.; Ezaki, M.; Carter, P.R. Keloid formation after syndactyly reconstruction: Associated conditions, prevalence, and preliminary report of a treatment method. J. Hand Surg. 2004, 29, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Tolerton, S.K.; Tonkin, M.A. Keloid formation after syndactyly release in patients with associated macrodactyly: Management with methotrexate therapy. J. Hand Surg. 2011, 36, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.D.; McCullough, J.L.; Olsen, E. Topical methotrexate therapy for psoriasis. Arch. Dermatol. 1989, 125, 227–230. [Google Scholar] [CrossRef]
- Sutton, L.; Swinehart, J.M.; Cato, A.; Kaplan, A.S. A clinical study to determine the efficacy and safety of 1% methotrexate/Azone (MAZ) gel applied topically once daily in patients with psoriasis vulgaris. Int. J. Dermatol. 2001, 40, 464–467. [Google Scholar] [CrossRef]
- Wigginton, S.M.; Chu, B.C.F.; Weisman, M.H.; Howell, S.B. Methotrexate pharmacokinetics after intraarticular injection in patients with rheumatoid arthritis. Arthritis Rheum. 1980, 23, 119–122. [Google Scholar] [CrossRef]
- Sharma, A.; Arora, S. Formulation and in vitro evaluation of ufasomes for dermal administration of methotrexate. ISRN Pharm. 2012, 2012, 873653. [Google Scholar] [CrossRef]
- Liang, L.S.; Jackson, J.; Min, W.; Risovic, V.; Wasan, K.M.; Burt, H.M. Methotrexate loaded poly(L-lactic acid) microspheres for intra-articular delivery of methotrexate to the joint. J. Pharm. Sci. 2004, 93, 943–956. [Google Scholar] [CrossRef]
- Ferreira, M.; Chaves, L.L.; Lima, S.A.C.; Reis, S. Optimization of nanostructured lipid carriers loaded with methotrexate: A tool for inflammatory and cancer therapy. Int. J. Pharm. 2015, 492, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Tripathi, R.; Mishra, B. Methotrexate: A detailed review on drug delivery and clinical aspects. Expert Opin. Drug Deliv. 2012, 9, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Desmoulière, A.; Chaponnier, C.; Gabbiani, G. Tissue Repair, Contraction, and the Myofibroblast; Springer: Berlin/Heidelberg, Germany, 2005; Volume 13, pp. 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Hinz, B. The myofibroblast: Paradigm for a mechanically active cell. J. Biomech. 2010, 43, 146–155. [Google Scholar] [CrossRef]
- Nabai, L.; Kilani, R.T.; Aminuddin, F.; Li, Y.; Ghahary, A. Methotrexate modulates the expression of MMP-1 and type 1 collagen in dermal fibroblast. Mol. Cell Biochem. 2015, 409, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.K.; Hung, T.; Letchford, K.; Burt, H.M. The characterization of paclitaxel-loaded microspheres manufactured from blends of poly(lactic-co-glycolic acid) (PLGA) and low molecular weight diblock copolymers. Int. J. Pharm. 2007, 342, 6–17. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.J.; Davies, M.C.; Melia, C.D. Microencapsulation using emulsification/solvent evaporation: An overview of techniques and applications. Crit. Rev. Ther. Drug Carr. Syst. 1990, 7, 235–259. [Google Scholar]
- Carr, M.; Chavez-Munoz, C.; Lai, A.; Ghahary, A. Dermal fibroblasts influence the expression profile of 14-3-3 proteins in human keratinocytes. Mol. Cell Biochem. 2011, 353, 205–214. [Google Scholar] [CrossRef]
- Jorgensen, L.N.; Olsen, L.; Kallehave, F.; Karlsmark, T.; Diegelmann, R.F.; Cohen, I.K.; Gottruo, F. The wound healing process in surgical patients evaluated by the expanded polytetrafluoroethylene and the polyvinyl alcohol sponge: A comparison with special reference to intrapatient variability. Wound Repair Regen. 1995, 3, 527–532. [Google Scholar] [CrossRef]
- Alaish, S.M.; Bettinger, D.A.; Olutoye, O.O.; Gould, L.J.; Yager, D.R.; Davis, A.; Crossland, M.C.; Diegelmann, R.F.; Cohen, I.K. Comparison of the polyvinyl alcohol sponge and expanded polytetrafluoroethylene subcutaneous implants as models to evaluate wound healing potential in human beings. Wound Repair Regen. 1995, 3, 292–298. [Google Scholar] [CrossRef]
- Carson, F.L.; Cappellano, C.H. Histotechnology a Self Instructional Text, 4th ed.; ASCP Press: Chicago, IL, USA, 2015. [Google Scholar]
- Nabai, L.; Ghahary, A.; Jackson, J. Localized Controlled Release of Kynurenic Acid Encapsulated in Synthetic Polymer Reduces Implant-Induced Dermal Fibrosis. Pharmaceutics 2022, 14, 1546. [Google Scholar] [CrossRef]
- Sasano, Y.; Zhu, J.-X.; Tsubota, M.; Takahashi, I.; Onodera, K.; Mizoguchi, I.; Kagayama, M. Gene expression of MMP8 and MMP13 during embryonic development of bone and cartilage in the rat mandible and hind limb. J. Histochem. Cytochem. 2002, 50, 325–332. [Google Scholar] [CrossRef]
- Peterson, J.T.; Li, H.; Dillon, L.; Bryant, J.W. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc. Res. 2000, 46, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.C.; McKenna, S.P.; Siddhi, K.; McGrouther, D.A.; Bayat, A. The hidden cost of skin scars: Quality of life after skin scarring. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 1049–1058. [Google Scholar] [CrossRef]
- Bock, O.; Schmid-Ott, G.; Malewski, P.; Mrowietz, U. Quality of life of patients with keloid and hypertrophic scarring. Arch. Dermatol. Res. 2006, 297, 433–438. [Google Scholar] [CrossRef]
- van Goor, H. Consequences and complications of peritoneal adhesions. Colorectal. Dis. 2007, 9 (Suppl. S2), 25–34. [Google Scholar] [CrossRef]
- DiEgidio, P.; Friedman, H.I.; Gourdie, R.G.; Riley, A.E.; Yost, M.J.; Goodwin, R.L. Biomedical implant capsule formation: Lessons learned and the road ahead. Ann. Plast. Surg. 2014, 73, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Spano, A.; Palmieri, B.; Taidelli, T.P.; Nava, M.B. Reduction of capsular thickness around silicone breast implants by zafirlukast in rats. Eur. Surg. Res. 2008, 41, 8–14. [Google Scholar] [CrossRef]
- Zimman, O.A.; Toblli, J.; Stella, I.; Ferder, M.; Ferder, L.; Inserra, F. The effects of angiotensin-converting-enzyme inhibitors on the fibrous envelope around mammary implants. Plast. Reconstr. Surg. 2007, 120, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Gancedo, M.; Ruiz-Corro, L.; Salazar-Montes, A.; Rincon, A.R.; Armendariz-Borunda, J. Pirfenidone prevents capsular contracture after mammary implantation. Aesthetic Plast. Surg. 2008, 32, 32–40. [Google Scholar] [CrossRef]
- Cronstein, B. How does methotrexate suppress inflammation? Clin. Exp. Rheumatol. 2010, 28 (Suppl. S61), S21–S23. [Google Scholar]
- Liang, L.S.; Wong, W.; Burt, H.M. Pharmacokinetic study of methotrexate following intra-articular injection of methotrexate loaded poly (L-lactic acid) microspheres in rabbits. J. Pharm. Sci. 2005, 94, 1204–1215. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch. Pharm. Res. 2004, 27, 1–12. [Google Scholar] [CrossRef]
- Mao, S.; Shi, Y.; Li, L.; Xu, J.; Schaper, A.; Kissel, T. Effects of process and formulation parameters on characteristics and internal morphology of poly(d,l-lactide-co-glycolide) microspheres formed by the solvent evaporation method. Eur. J. Pharm. Biopharm. 2008, 68, 214–223. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, V.; Kesch, C.; Jackson, J.K.; Bidnur, S.; Beraldi, E.; Yago, V.; Bowden, M.; Gleave, M.E. Design and Characterization of Injectable Poly (Lactic-Co-Glycolic Acid) Pastes for Sustained and Local Drug Release. Pharm. Res. 2020, 37, 36. [Google Scholar] [CrossRef] [PubMed]
- Owen, G.R.; Jackson, J.K.; Chehroudi, B.; Brunette, D.M.; Burt, H.M. An in vitro study of plasticized poly (lactic-co-glycolic acid) films as possible guided tissue regeneration membranes: Material properties and drug release kinetics. J. Biomed. Mater. Res. 2010, 95, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-P.; Huang, H.-L.; Lai, W.-W.; Chung, J.-G.; Yang, J.-H. Antiproliferative effects of lactic acid via the induction of apoptosis and cell cycle arrest in a human keratinocyte cell line (HaCaT). J. Dermatol. Sci. 2009, 54, 175–184. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabai, L.; Ghahary, A.; Jackson, J. Novel, Blended Polymeric Microspheres for the Controlled Release of Methotrexate: Characterization and In Vivo Antifibrotic Studies. Bioengineering 2023, 10, 298. https://doi.org/10.3390/bioengineering10030298
Nabai L, Ghahary A, Jackson J. Novel, Blended Polymeric Microspheres for the Controlled Release of Methotrexate: Characterization and In Vivo Antifibrotic Studies. Bioengineering. 2023; 10(3):298. https://doi.org/10.3390/bioengineering10030298
Chicago/Turabian StyleNabai, Layla, Aziz Ghahary, and John Jackson. 2023. "Novel, Blended Polymeric Microspheres for the Controlled Release of Methotrexate: Characterization and In Vivo Antifibrotic Studies" Bioengineering 10, no. 3: 298. https://doi.org/10.3390/bioengineering10030298
APA StyleNabai, L., Ghahary, A., & Jackson, J. (2023). Novel, Blended Polymeric Microspheres for the Controlled Release of Methotrexate: Characterization and In Vivo Antifibrotic Studies. Bioengineering, 10(3), 298. https://doi.org/10.3390/bioengineering10030298




