Advances in Animal Models for Studying Bone Fracture Healing
Abstract
1. Introduction
2. Search Strategy
3. Mechanism of Fracture Healing
3.1. Three Key Elements in Fracture Healing
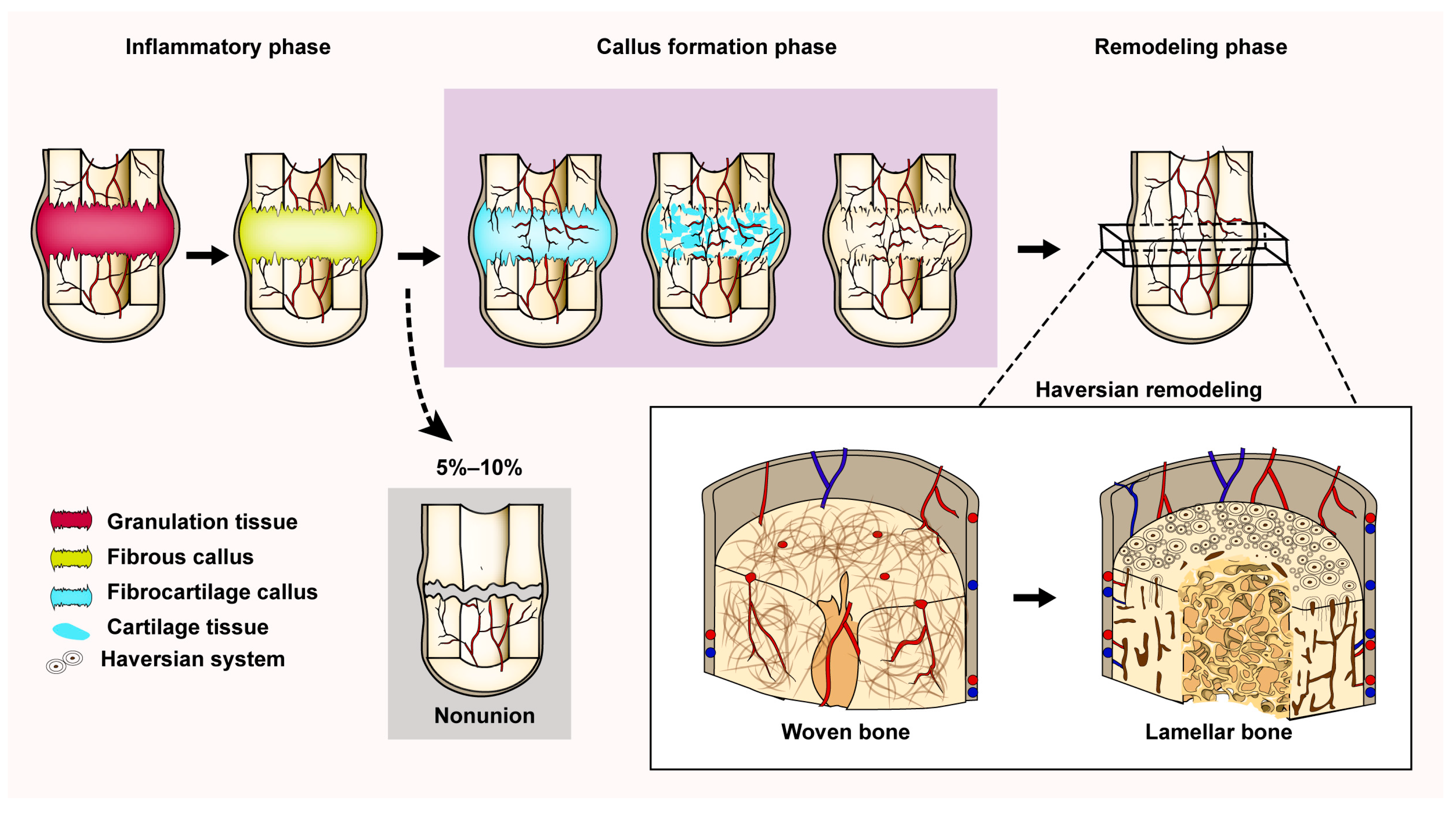
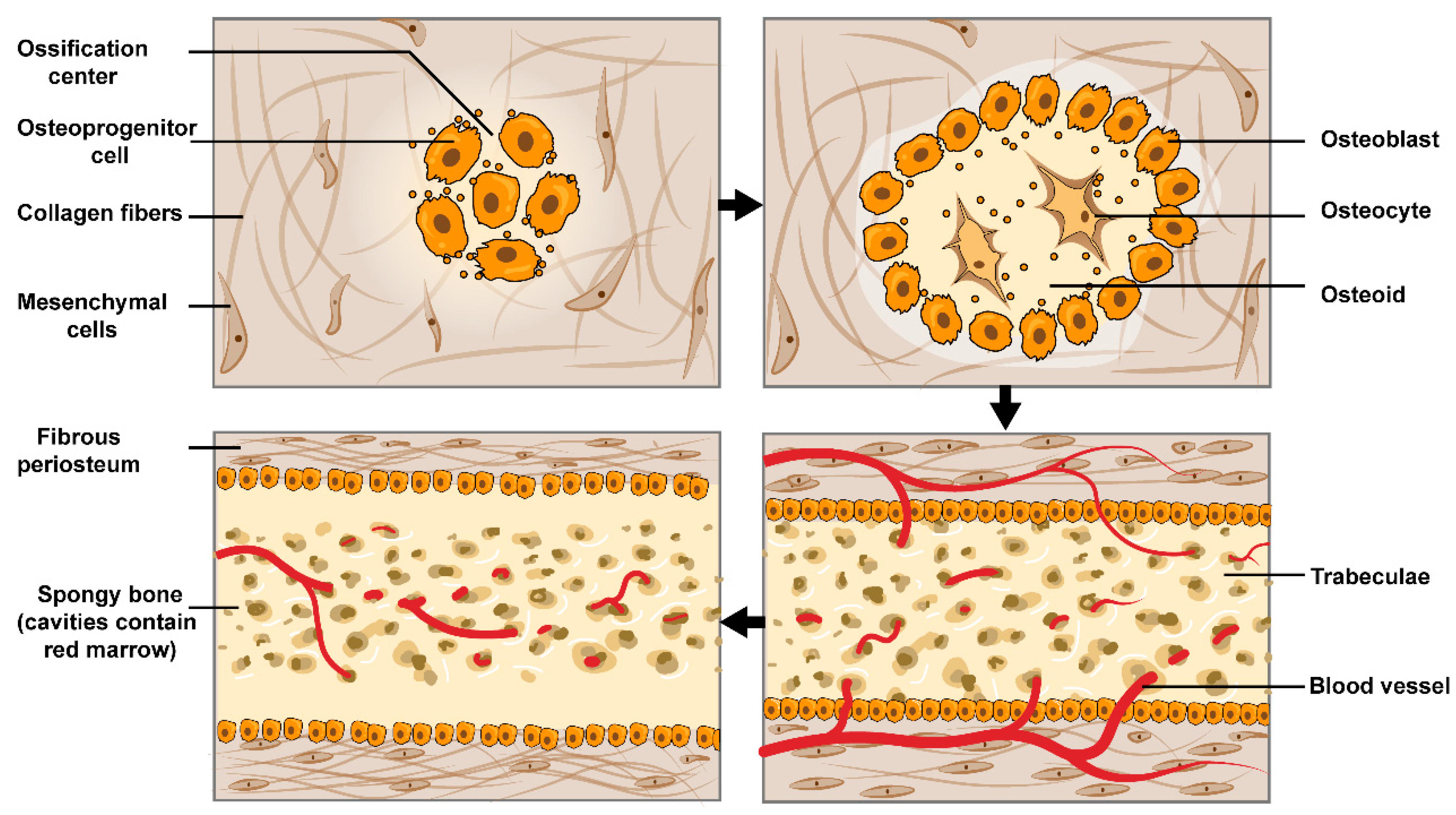
3.2. The Process of Indirect Fracture Healing
3.3. Reconstruction of the Haversian System
4. Animal Characteristics of Fracture Healing Models
5. Classification of Fracture Healing Model Applications
5.1. Traumatic Bone Fracture Animal Model
5.1.1. Closed Fracture Model
5.1.2. Open Fracture Model
5.2. Osteoporotic Fracture Model
5.2.1. Animal Model of Osteoporosis
- Postmenopausal osteoporosis model
- 2.
- Geriatric osteoporosis model
- 3.
- Secondary osteoporosis model
5.2.2. Osteoporotic Fracture Modeling
5.3. Bone Defect Model
5.4. Bone Nonunion
5.5. Fracture Fixation Types
- Intramedullary fixation
- 2.
- External fixation
| Animal Species | Sexual Maturity Time | Epiphyseal Plate | Haversian System * | Advantages | Disadvantages | Applicable Models |
|---|---|---|---|---|---|---|
| Mouse | 6 weeks | Existence For a Lifetime | None |
|
| |
| Rat | 8 weeks | Thinning with age | Rare |
|
| |
| Rabbit | 4–6 months | Closure after sexual maturity | Existence after sexual maturity |
| ||
| Dog | 8–10 months | Closure after sexual maturity | Existence |
|
| |
| Sheep and goat | 10–12 months | Closure after sexual maturity | Existence |
|
| |
| Pig | 4–6 months | Closure after sexual maturity | Existence |
|
|
|
| Nonhuman primates | 3–5 years | Closure after sexual maturity | Existence |
|
|
|
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Toosi, S.; Behravan, N.; Behravan, J. Nonunion fractures, mesenchymal stem cells and bone tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 2552–2562. [Google Scholar] [CrossRef]
- Nikitovic, M.; Wodchis, W.P.; Krahn, M.D.; Cadarette, S.M. Direct health-care costs attributed to hip fractures among seniors: A matched cohort study. Osteoporos. Int. 2013, 24, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Tewari, P.; Sweeney, B.F., Jr.; Lemos, J.L.; Shapiro, L.; Gardner, M.J.; Morris, A.M.; Baker, L.C.; Harris, A.S.; Kamal, R.N. Evaluation of Systemwide Improvement Programs to Optimize Time to Surgery for Patients With Hip Fractures: A Systematic Review. JAMA Netw. Open 2022, 5, e2231911. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Gray, A.M.; Prieto-Alhambra, D.; Arden, N.K.; Cooper, C.; Javaid, M.K.; Judge, A.; REFReSH Study Group. Impact of hip fracture on hospital care costs: A population-based study. Osteoporos. Int. 2016, 27, 549–558. [Google Scholar] [CrossRef]
- Robinson, Y.; Heyde, C.E.; Forsth, P.; Olerud, C. Kyphoplasty in osteoporotic vertebral compression fractures—Guidelines and technical considerations. J. Orthop. Surg. Res. 2011, 6, 43. [Google Scholar] [CrossRef]
- Ameis, A.; Randhawa, K.; Yu, H.; Cote, P.; Haldeman, S.; Chou, R.; Hurwitz, E.L.; Nordin, M.; Wong, J.J.; Shearer, H.M.; et al. The Global Spine Care Initiative: A review of reviews and recommendations for the non-invasive management of acute osteoporotic vertebral compression fracture pain in low- and middle-income communities. Eur. Spine J. 2018, 27, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Chien, L.-N.; Tsai, W.-L.; Chen, L.-Y.; Chiang, Y.-H.; Hsieh, Y.-C. Early vertebroplasty associated with a lower risk of mortality and respiratory failure in aged patients with painful vertebral compression fractures: A population-based cohort study in Taiwan. Spine J. 2017, 17, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Alpantaki, K.; Dohm, M.; Korovessis, P.; Hadjipavlou, A.G. Surgical options for osteoporotic vertebral compression fractures complicated with spinal deformity and neurologic deficit. Injury 2018, 49, 261–271. [Google Scholar] [CrossRef]
- Hoyt, D.; Urits, I.; Orhurhu, V.; Orhurhu, M.S.; Callan, J.; Powell, J.; Manchikanti, L.; Kaye, A.D.; Kaye, R.J.; Viswanath, O. Current Concepts in the Management of Vertebral Compression Fractures. Curr. Pain Headache Rep. 2020, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Beall, D.P.; Chambers, M.R.; Thomas, S.; Amburgy, J.; Webb, J.R., Jr.; Goodman, B.S.; Datta, D.K.; Easton, R.W.; Linville, D., II; Talati, S.; et al. Prospective and Multicenter Evaluation of Outcomes for Quality of Life and Activities of Daily Living for Balloon Kyphoplasty in the Treatment of Vertebral Compression Fractures: The EVOLVE Trial. Neurosurgery 2019, 84, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Wildemann, B.; Ignatius, A.; Leung, F.; Taitsman, L.A.; Smith, R.M.; Pesantez, R.; Stoddart, M.J.; Richards, R.G.; Jupiter, J.B. Non-union bone fractures. Nat. Rev. Dis. Prim. 2021, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Sayum Filho, J.; Lenza, M.; Tamaoki, M.J.; Matsunaga, F.T.; Belloti, J.C. Interventions for treating fractures of the patella in adults. Cochrane Database Syst. Rev. 2021, 2, Cd009651. [Google Scholar] [CrossRef] [PubMed]
- Naveiro, J.M.; Puértolas, S.; Rosell, J.; Hidalgo, A.; Ibarz, E.; Albareda, J.; Gracia, L. A new approach for initial callus growth during fracture healing in long bones. Comput. Methods Programs Biomed. 2021, 208, 106262. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, M.G.; Roe, A.K. The osteon: The micromechanical unit of compact bone. Front. Biosci. 2012, 17, 1551–1581. [Google Scholar] [CrossRef]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone Injury and Fracture Healing Biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried alive: How osteoblasts become osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Paiva, K.B.; Granjeiro, J.M. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 2014, 561, 74–87. [Google Scholar] [CrossRef]
- Camozzi, V.; Vescini, F.; Luisetto, G.; Moro, L. Bone organic matrix components: Their roles in skeletal physiology. J. Endocrinol. Investig. 2010, 33, 13–15. [Google Scholar]
- Boskey, A.L.; Coleman, R. Aging and bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef]
- Gupta, H.S.; Seto, J.; Wagermaier, W.; Zaslansky, P.; Boesecke, P.; Fratzl, P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc. Natl. Acad. Sci. USA 2006, 103, 17741–17746. [Google Scholar] [CrossRef] [PubMed]
- LaStayo, P.C.; Winters, K.M.; Hardy, M. Fracture healing: Bone healing, fracture management, and current concepts related to the hand. J. Hand. Ther. 2003, 16, 81–93. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Carter, D.R.; Beaupré, G.S.; Giori, N.J.; Helms, J.A. Mechanobiology of skeletal regeneration. Clin. Orthop. Relat. Res. 1998, 355, S41–S55. [Google Scholar] [CrossRef]
- Van den Bos, T.; Speijer, D.; Bank, R.A.; Brömme, D.; Everts, V. Differences in matrix composition between calvaria and long bone in mice suggest differences in biomechanical properties and resorption: Special emphasis on collagen. Bone 2008, 43, 459–468. [Google Scholar] [CrossRef]
- Lee, S.; Remark, L.H.; Josephson, A.M.; Leclerc, K.; Lopez, E.M.; Kirby, D.J.; Mehta, D.; Litwa, H.P.; Wong, M.Z.; Shin, S.Y.; et al. Notch-Wnt signal crosstalk regulates proliferation and differentiation of osteoprogenitor cells during intramembranous bone healing. NPJ. Regen. Med. 2021, 6, 29. [Google Scholar] [CrossRef]
- Weng, Y.; Wang, H.; Wu, D.; Xu, S.; Chen, X.; Huang, J.; Feng, Y.; Li, L.; Wang, Z. A novel lineage of osteoprogenitor cells with dual epithelial and mesenchymal properties govern maxillofacial bone homeostasis and regeneration after MSFL. Cell Res. 2022, 32, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; Della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z.; et al. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Liu, X. Osteon: Structure, Turnover, and Regeneration. Tissue Eng. Part B Rev. 2022, 28, 261–278. [Google Scholar] [CrossRef]
- Hauge, E.M.; Qvesel, D.; Eriksen, E.F.; Mosekilde, L.; Melsen, F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J. Bone Miner. Res. 2001, 16, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Fyhrie, D.P.; Palnitkar, S.; Rao, D.S. Histomorphometric assessment of Haversian canal and osteocyte lacunae in different-sized osteons in human rib. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 272, 520–525. [Google Scholar] [CrossRef]
- Casanova, M.; Schindeler, A.; Peacock, L.; Lee, L.; Schneider, P.; Little, D.G.; Müller, R. Characterization of the Developing Lacunocanalicular Network During Fracture Repair. JBMR Plus 2021, 5, e10525. [Google Scholar] [CrossRef]
- Van Tol, A.F.; Schemenz, V.; Wagermaier, W.; Roschger, A.; Razi, H.; Vitienes, I.; Fratzl, P.; Willie, B.M.; Weinkamer, R. The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture. Proc. Natl. Acad. Sci. USA 2020, 117, 32251–32259. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.D.; Sales, E.; Hiebert, B.D.; Panahifar, A.; Zhu, N.; Arnason, T.; Swekla, K.J.; Pivonka, P.; Chapman, L.D.; Cooper, D.M. Direct Assessment of Rabbit Cortical Bone Basic Multicellular Unit Longitudinal Erosion Rate: A 4D Synchrotron-Based Approach. J. Bone Miner. Res. 2022, 37, 2244–2258. [Google Scholar] [CrossRef]
- Dominguez, V.M.; Crowder, C.M. The utility of osteon shape and circularity for differentiating human and non-human Haversian bone. Am. J. Phys. Anthropol. 2012, 149, 84–91. [Google Scholar] [CrossRef]
- Gosain, A.K.; Song, L.; Riordan, P.; Amarante, M.T.; Nagy, P.G.; Wilson, C.R.; Toth, J.M.; Ricci, J.L. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: Part I. Plast. Reconstr. Surg. 2002, 109, 619–630. [Google Scholar] [CrossRef]
- Mori, R.; Kodaka, T.; Sano, T.; Yamagishi, N.; Asari, M.; Naito, Y. Comparative histology of the laminar bone between young calves and foals. Cells Tissues Organs 2003, 175, 43–50. [Google Scholar] [CrossRef]
- Fujihara, R.; Mashiba, T.; Yoshitake, S.; Komatsubara, S.; Iwata, K.; Takao-Kawabata, R.; Yamamoto, T. Weekly teriparatide treatment increases vertebral body strength by improving cortical shell architecture in ovariectomized cynomolgus monkeys. Bone 2019, 121, 80–88. [Google Scholar] [CrossRef]
- Nunamaker, D.M. Experimental models of fracture repair. Clin. Orthop. Relat. Res. 1998, 355, S56–S65. [Google Scholar] [CrossRef]
- Atkins, J.T.; George, G.C.; Hess, K.; Marcelo-Lewis, K.L.; Yuan, Y.; Borthakur, G.; Khozin, S.; LoRusso, P.; Hong, D.S. Pre-clinical animal models are poor predictors of human toxicities in phase 1 oncology clinical trials. Br. J. Cancer 2020, 123, 1496–1501. [Google Scholar] [CrossRef]
- Perel, P.; Roberts, I.; Sena, E.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMJ 2007, 334, 197. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A., Jr.; Meyer, M.H.; Tenholder, M.; Wondracek, S.; Wasserman, R.; Garges, P. Gene expression in older rats with delayed union of femoral fractures. J. Bone Jt. Surg. Am. 2003, 85, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Han da, C.; Zhang, P.X.; Han, N.; Kou, Y.H.; Yin, X.F.; Jiang, B.G. A special healing pattern in stable metaphyseal fractures. Acta Orthop. 2015, 86, 238–242. [Google Scholar] [CrossRef]
- Pozzi, A.; Risselada, M.; Winter, M.D. Assessment of fracture healing after minimally invasive plate osteosynthesis or open reduction and internal fixation of coexisting radius and ulna fractures in dogs via ultrasonography and radiography. J. Am. Vet. Med. Assoc. 2012, 241, 744–753. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Brits, D.; Steyn, M.; L’Abbé, E.N. A histomorphological analysis of human and non-human femora. Int. J. Legal Med. 2014, 128, 369–377. [Google Scholar] [CrossRef]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.Y.; Hou, J.X. Hepatocellular carcinoma: Insight from animal models. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Cauley, J.A.; Giangregorio, L. Physical activity and skeletal health in adults. Lancet Diabetes Endocrinol. 2020, 8, 150–162. [Google Scholar] [CrossRef]
- Rude, C. Management of closed fractures. Clin. Podiatry 1985, 2, 199–216. [Google Scholar]
- Sun, Y.; Xu, L.; Huang, S.; Hou, Y.; Liu, Y.; Chan, K.M.; Pan, X.H.; Li, G. mir-21 overexpressing mesenchymal stem cells accelerate fracture healing in a rat closed femur fracture model. BioMed Res. Int. 2015, 2015, 412327. [Google Scholar] [CrossRef]
- Histing, T.; Garcia, P.; Holstein, J.H.; Klein, M.; Matthys, R.; Nuetzi, R.; Steck, R.; Laschke, M.W.; Wehner, T.; Bindl, R.; et al. Small animal bone healing models: Standards, tips, and pitfalls results of a consensus meeting. Bone 2011, 49, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. The science of fracture healing. J. Orthop. Trauma 2005, 19, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, A.; Vuorio, E.; Aro, H.T. A standardized experimental fracture in the mouse tibia. J. Orthop. Res. 1993, 11, 305–312. [Google Scholar] [CrossRef]
- Marturano, J.E.; Cleveland, B.C.; Byrne, M.A.; O’Connell, S.L.; Wixted, J.J.; Billiar, K.L. An improved murine femur fracture device for bone healing studies. J. Biomech. 2008, 41, 1222–1228. [Google Scholar] [CrossRef]
- Bonnarens, F.; Einhorn, T.A. Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res. 1984, 2, 97–101. [Google Scholar] [CrossRef]
- Simon, A.M.; O’Connor, J.P. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J. Bone Jt. Surg. Am. 2007, 89, 500–511. [Google Scholar] [CrossRef]
- Freeman, K.T.; Koewler, N.J.; Jimenez-Andrade, J.M.; Buus, R.J.; Herrera, M.B.; Martin, C.D.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. A fracture pain model in the rat: Adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology 2008, 108, 473–483. [Google Scholar] [CrossRef]
- Zalavras, C.G.; Patzakis, M.J. Open fractures: Evaluation and management. J. Am. Acad. Orthop. Surg. 2003, 11, 212–219. [Google Scholar] [CrossRef]
- Bourque, W.T.; Gross, M.; Hall, B.K. A reproducible method for producing and quantifying the stages of fracture repair. Lab. Anim. Sci. 1992, 42, 369–374. [Google Scholar]
- Park, S.H.; O’Connor, K.; Sung, R.; McKellop, H.; Sarmiento, A. Comparison of healing process in open osteotomy model and closed fracture model. J. Orthop. Trauma 1999, 13, 114–120. [Google Scholar] [CrossRef]
- ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of Postmenopausal Osteoporosis: ACOG Clinical Practice Guideline No. 2. Obstet. Gynecol. 2022, 139, 698–717. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, ITC17–ITC32. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef]
- Jilka, R.L.; Hangoc, G.; Girasole, G.; Passeri, G.; Williams, D.C.; Abrams, J.S.; Boyce, B.; Broxmeyer, H.; Manolagas, S.C. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science 1992, 257, 88–91. [Google Scholar] [CrossRef]
- Li, M.; Shen, Y.; Wronski, T.J. Time course of femoral neck osteopenia in ovariectomized rats. Bone 1997, 20, 55–61. [Google Scholar] [CrossRef]
- Chavassieux, P.; Garnero, P.; Duboeuf, F.; Vergnaud, P.; Brunner-Ferber, F.; Delmas, P.D.; Meunier, P.J. Effects of a new selective estrogen receptor modulator (MDL 103,323) on cancellous and cortical bone in ovariectomized ewes: A biochemical, histomorphometric, and densitometric study. J. Bone Miner. Res. 2001, 16, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Shiga, T.; Hashimoto, J.; Yoshioka, M.; Honjo, H.; Urabe, M.; Kitajima, I.; Semba, I.; Hirasawa, Y. Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J. Steroid Biochem. Mol. Biol. 1999, 68, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.H.; Murray, I.R. Osteoporotic fracture models. Curr. Osteoporos. Rep. 2015, 13, 9–15. [Google Scholar] [CrossRef]
- Cao, J.; Venton, L.; Sakata, T.; Halloran, B.P. Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J. Bone Miner. Res. 2003, 18, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, V.L.; Ayers, R.A.; Bateman, T.A.; Simske, S.J. Bone development and age-related bone loss in male C57BL/6J mice. Bone 2003, 33, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Egermann, M.; Heil, P.; Tami, A.; Ito, K.; Janicki, P.; Von Rechenberg, B.; Hofstetter, W.; Richards, P.J. Influence of defective bone marrow osteogenesis on fracture repair in an experimental model of senile osteoporosis. J. Orthop. Res. 2010, 28, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Brodt, M.D.; Ettner, S.L. Long bones from the senescence accelerated mouse SAMP6 have increased size but reduced whole-bone strength and resistance to fracture. J. Bone Miner. Res. 2002, 17, 1597–1603. [Google Scholar] [CrossRef]
- Fitzpatrick, L.A. Secondary causes of osteoporosis. Mayo Clin. Proc. 2002, 77, 453–468. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Chapa, M.T.; Yallampalli, C.; Zhang, R.; Simmons, D.J. Prevention of corticosteroid-induced bone loss with nitric oxide donor nitroglycerin in male rats. Bone 1997, 21, 275–280. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Zheng, L.; Liu, Y.; Liu, G.; Li, N.; Wang, K.; Zou, L.; Wu, T.; Qin, L.; et al. Glucocorticoid-induced osteoporosis in growing rats. Calcif. Tissue Int. 2014, 95, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Grardel, B.; Sutter, B.; Flautre, B.; Viguier, E.; Lavaste, F.; Hardouin, P. Effects of glucocorticoids on skeletal growth in rabbits evaluated by dual-photon absorptiometry, microscopic connectivity and vertebral compressive strength. Osteoporos. Int. 1994, 4, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Cheng, L.; Bollen, P.; Schwarz, P.; Overgaard, S. Glucocorticoid induced osteopenia in cancellous bone of sheep: Validation of large animal model for spine fusion and biomaterial research. Spine 2010, 35, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Apseloff, G.; Girten, B.; Weisbrode, S.E.; Walker, M.; Stern, L.S.; Krecic, M.E.; Gerber, N. Effects of aminohydroxybutane bisphosphonate on bone growth when administered after hind-limb bone loss in tail-suspended rats. J. Pharmacol. Exp. Ther. 1993, 267, 515–521. [Google Scholar] [PubMed]
- Moriishi, T.; Fukuyama, R.; Ito, M.; Miyazaki, T.; Maeno, T.; Kawai, Y.; Komori, H.; Komori, T. Osteocyte network; a negative regulatory system for bone mass augmented by the induction of Rankl in osteoblasts and Sost in osteocytes at unloading. PLoS ONE 2012, 7, e40143. [Google Scholar] [CrossRef] [PubMed]
- Haffner-Luntzer, M.; Ignatius, A. Animal models for studying metaphyseal bone fracture healing. Eur. Cells Mater. 2020, 40, 172–188. [Google Scholar] [CrossRef]
- Alt, V.; Thormann, U.; Ray, S.; Zahner, D.; Durselen, L.; Lips, K.; El Khassawna, T.; Heiss, C.; Riedrich, A.; Schlewitz, G.; et al. A new metaphyseal bone defect model in osteoporotic rats to study biomaterials for the enhancement of bone healing in osteoporotic fractures. Acta Biomater. 2013, 9, 7035–7042. [Google Scholar] [CrossRef]
- Kampschulte, M.; Krombach, G.A.; Richards, D.C.; Sender, J.; Lips, K.S.; Thormann, U.; El Khassawna, T.; Ray, S.; Alt, V.; Langheinrich, A.C. Neovascularization of osteoporotic metaphyseal bone defects: A morphometric micro-CT study. Microvasc. Res. 2016, 105, 7–14. [Google Scholar] [CrossRef]
- Lips, K.S.; Kauschke, V.; Hartmann, S.; Thormann, U.; Ray, S.; Schumacher, M.; Gelinsky, M.; Heinemann, S.; Hanke, T.; Kautz, A.R.; et al. Cholinergic nerve fibers in bone defects of a rat osteoporosis model and their regulation by implantation of bone substitution materials. J. Musculoskelet. Neuronal Interact. 2014, 14, 173–188. [Google Scholar]
- Ray, S.; Thormann, U.; Eichelroth, M.; Budak, M.; Biehl, C.; Rupp, M.; Sommer, U.; El Khassawna, T.; Alagboso, F.I.; Kampschulte, M.; et al. Strontium and bisphosphonate coated iron foam scaffolds for osteoporotic fracture defect healing. Biomaterials 2018, 157, 1–16. [Google Scholar] [CrossRef]
- Nozaka, K.; Miyakoshi, N.; Kasukawa, Y.; Maekawa, S.; Noguchi, H.; Shimada, Y. Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone 2008, 42, 90–97. [Google Scholar] [CrossRef]
- Munhoz, M.; Pomini, K.T.; Plepis, A.M.G.; Martins, V.; Machado, E.G.; de Moraes, R.; Cunha, F.B.; Santos Junior, A.R.; Camargo Cardoso, G.B.; Duarte, M.A.H.; et al. Elastin-derived scaffolding associated or not with bone morphogenetic protein (BMP) or hydroxyapatite (HA) in the repair process of metaphyseal bone defects. PLoS ONE 2020, 15, e0231112. [Google Scholar] [CrossRef] [PubMed]
- Uhthoff, H.K.; Goto, S.; Cerckel, P.H. Influence of stable fixation on trabecular bone healing: A morphologic assessment in dogs. J. Orthop. Res. 1987, 5, 14–22. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [PubMed]
- Bigham-Sadegh, A.; Oryan, A. Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connect. Tissue Res. 2015, 56, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, W.; Zhang, C.; Thein-Han, W.; Hu, K.; Reynolds, M.A.; Bao, C.; Wang, P.; Zhao, L.; Xu, H.H.K. Co-Seeding Human Endothelial Cells with Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells on Calcium Phosphate Scaffold Enhances Osteogenesis and Vascularization in Rats. Tissue Eng. Part A 2017, 23, 546–555. [Google Scholar] [CrossRef]
- Claes, L.; Eckert-Hubner, K.; Augat, P. The fracture gap size influences the local vascularization and tissue differentiation in callus healing. Langenbecks Arch Surg 2003, 388, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, Y.; Kallai, I.; Gafni, Y.; Pelled, G.; Kossodo, S.; Yared, W.; Gazit, D. Fluorescence molecular tomography enables in vivo visualization and quantification of nonunion fracture repair induced by genetically engineered mesenchymal stem cells. J. Orthop Res. 2008, 26, 522–530. [Google Scholar] [CrossRef]
- Volpon, J.B. Nonunion using a canine model. Arch. Orthop. Trauma Surg. 1994, 113, 312–317. [Google Scholar] [CrossRef]
- Jackson, R.W.; Reed, C.A.; Israel, J.A.; Abou-Keer, F.K.; Garside, H. Production of a standard experimental fracture. Can. J. Surg. 1970, 13, 415–420. [Google Scholar] [PubMed]
- Lowe, J.; Bab, I.; Stein, H.; Sela, J. Primary calcification in remodeling haversian systems following tibial fracture in rats. Clin. Orthop. Relat. Res. 1983, 176, 291–297. [Google Scholar] [CrossRef]
- Gregory, P.; Sanders, R. The treatment of closed, unstable tibial shaft fractures with unreamed interlocking nails. Clin. Orthop. Relat. Res. 1995, 315, 48–55. [Google Scholar] [CrossRef]
- Kessler, S.B.; Hallfeldt, K.K.; Perren, S.M.; Schweiberer, L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. Relat. Res. 1986, 212, 18–25. [Google Scholar] [CrossRef]
- Sigurdsen, U.; Reikeras, O.; Utvag, S.E. Conversion of external fixation to definitive intramedullary nailing in experimental tibial fractures. J. Investig. Surg. 2010, 23, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.H.; Matthys, R.; Histing, T.; Becker, S.C.; Fiedler, M.; Garcia, P.; Meier, C.; Pohlemann, T.; Menger, M.D. Development of a stable closed femoral fracture model in mice. J. Surg. Res. 2009, 153, 71–75. [Google Scholar] [CrossRef]
- Wang, G.J.; Reger, S.I.; Mabie, K.N.; Richman, J.A.; Stamp, W.G. Semirigid rod fixation for long-bone fracture. Clin. Orthop. Relat. Res. 1985, 192, 291–298. [Google Scholar] [CrossRef]
- Krischak, G.D.; Augat, P.; Sorg, T.; Blakytny, R.; Kinzl, L.; Claes, L.; Beck, A. Effects of diclofenac on periosteal callus maturation in osteotomy healing in an animal model. Arch. Orthop. Trauma Surg. 2007, 127, 3–9. [Google Scholar] [CrossRef]
- Fujisawa, H.; Mori, Y.; Kogure, A.; Tanaka, H.; Kamimura, M.; Masahashi, N.; Hanada, S.; Itoi, E. Effects of intramedullary nails composed of a new beta-type Ti-Nb-Sn alloy with low Young’s modulus on fracture healing in mouse tibiae. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Jahn, K.; Saito, H.; Taipaleenmaki, H.; Gasser, A.; Hort, N.; Feyerabend, F.; Schluter, H.; Rueger, J.M.; Lehmann, W.; Willumeit-Romer, R.; et al. Intramedullary Mg2Ag nails augment callus formation during fracture healing in mice. Acta Biomater. 2016, 36, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, T.; Kurahashi, T.; Hara, T.; Hirata, H.; Kasuga, T. Novel intramedullary-fixation technique for long bone fragility fractures using bioresorbable materials. PLoS ONE 2014, 9, e104603. [Google Scholar] [CrossRef]
- Drosse, I.; Volkmer, E.; Seitz, S.; Seitz, H.; Penzkofer, R.; Zahn, K.; Matis, U.; Mutschler, W.; Augat, P.; Schieker, M. Validation of a femoral critical size defect model for orthotopic evaluation of bone healing: A biomechanical, veterinary and trauma surgical perspective. Tissue Eng. Part C Methods 2008, 14, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sangkaew, C. Distraction osteogenesis for the treatment of post traumatic complications using a conventional external fixator. A novel technique. Injury 2005, 36, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Knabe, C.; Radin, S.; Garino, J.; Ducheyne, P. Percutaneous external fixator pins with bactericidal micron-thin sol-gel films for the prevention of pin tract infection. Biomaterials 2015, 62, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; McKenzie, J.A.; Sykes, D.A.W.; Yoneda, S.; Hensley, A.; Buettmann, E.G.; Zheng, H.; Skouteris, D.; McAlinden, A.; Miller, A.N.; et al. Ablation of Proliferating Osteoblast Lineage Cells After Fracture Leads to Atrophic Nonunion in a Mouse Model. J. Bone Miner. Res. 2021, 36, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, A.I.; Zhu, S.; Loftin, A.H.; Weixian, X.; Niska, J.; Hegde, V.; Segura, T.; Bernthal, N.M. Controlled Release of Vancomycin and Tigecycline from an Orthopaedic Implant Coating Prevents Staphylococcus aureus Infection in an Open Fracture Animal Model. Biomed. Res. Int. 2019, 2019, 1638508. [Google Scholar] [CrossRef]
- Histing, T.; Klein, M.; Stieger, A.; Stenger, D.; Steck, R.; Matthys, R.; Holstein, J.H.; Garcia, P.; Pohlemann, T.; Menger, M.D. A new model to analyze metaphyseal bone healing in mice. J. Surg. Res. 2012, 178, 715–721. [Google Scholar] [CrossRef]
- Baker, R.M.; Tseng, L.F.; Iannolo, M.T.; Oest, M.E.; Henderson, J.H. Self-deploying shape memory polymer scaffolds for grafting and stabilizing complex bone defects: A mouse femoral segmental defect study. Biomaterials 2016, 76, 388–398. [Google Scholar] [CrossRef]
- Mills, R.; Cheng, T.L.; Mikulec, K.; Peacock, L.; Isaacs, D.; Genberg, C.; Savage, P.B.; Little, D.G.; Schindeler, A. CSA-90 Promotes Bone Formation and Mitigates Methicillin-resistant Staphylococcus aureus Infection in a Rat Open Fracture Model. Clin. Orthop. Relat. Res. 2018, 476, 1311–1323. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S. Reconstruction of radial bone defect in rat by calcium silicate biomaterials. Life Sci. 2018, 201, 45–53. [Google Scholar] [CrossRef]
- Mashiba, T.; Burr, D.B.; Turner, C.H.; Sato, M.; Cain, R.L.; Hock, J.M. Effects of human parathyroid hormone (1-34), LY333334, on bone mass, remodeling, and mechanical properties of cortical bone during the first remodeling cycle in rabbits. Bone 2001, 28, 538–547. [Google Scholar] [CrossRef]
- Tudisco, C.; Botti, F.; Bisicchia, S.; Ippolito, E. Ischemic necrosis of the femoral head: An experimental rabbit model. J. Orthop Res. 2015, 33, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Willett, T.L.; Wynnyckyj, C.; Wang, J.; Grynpas, M.D. The fatigue resistance of rabbit tibiae varies with age from youth to middle age. Osteoporos. Int. 2011, 22, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Altay, M.A.; Erturk, C.; Altay, N.; Ozturk, I.A.; Baykara, I.; Sert, C.; Isikan, U.E. Comparison of intracompartmental pressures in a rabbit model of open and closed tibial fractures: An experimental study. Bone Jt. J. 2013, 95-B, 111–114. [Google Scholar] [CrossRef]
- Bayrak, A.; Duramaz, A.; Kizilkaya, C.; Celik, M.; Kural, C.; Altinay, S.; Kural, A.; Basaran, S.H. Comparison of two types of fixation for proximal tibial epiphysiodesis: An experimental study in a rabbit model. Jt. Dis. Relat. Surg. 2021, 32, 468–477. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chan, Y.H.; Hsieh, S.C.; Lew, W.Z.; Feng, S.W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef]
- Arthur, E.G.; Arthur, G.L.; Keeler, M.R.; Bryan, J.N. Risk of Osteosarcoma in Dogs After Open Fracture Fixation. Vet. Surg. 2016, 45, 30–35. [Google Scholar] [CrossRef]
- Lazarus, M.A.; Lewis, D.D.; Johnson, M.D.; Porter, E.G. Use of a circular fixator construct to facilitate closed reduction and percutaneous stabilization of a distal femoral physeal fracture in a dog. Open Vet. J. 2021, 11, 89–95. [Google Scholar] [CrossRef]
- Hobbenaghi, R.; Mahboob, P.; Saifzadeh, S.; Javanbakht, J.; Manesh, J.Y.; Mortezaee, R.; Touni, S.R.; Hosseini, E.; Aghajanshakeri, S.; Moloudizargari, M.; et al. Histopathological features of bone regeneration in a canine segmental ulnar defect model. Diagn. Pathol. 2014, 9, 59. [Google Scholar] [CrossRef]
- Newman, E.; Turner, A.S.; Wark, J.D. The potential of sheep for the study of osteopenia—Current status and comparison with other animal-models. Bone 1995, 16, S277–S284. [Google Scholar] [CrossRef]
- Kay, W.; Hunt, C.; Nehring, L.; Barnum, B.; Ashton, N.; Williams, D. Biofilm Growth on Simulated Fracture Fixation Plates Using a Customized CDC Biofilm Reactor for a Sheep Model of Biofilm-Related Infection. Microorganisms 2022, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Alagboso, F.I.; Budak, M.; Sommer, U.; Ray, S.; Kaiser, A.; Kampschulte, M.; Henss, A.; Durselen, L.; Biehl, C.; Lips, K.S.; et al. Establishment of a clinically relevant large animal model to assess the healing of metaphyseal bone. Eur. Cell. Mater. 2019, 37, 444–466. [Google Scholar] [CrossRef]
- Large, T.M.; Douglas, G.; Erickson, G.; Grayson, J.K. Effect of negative pressure wound therapy on the elution of antibiotics from polymethylmethacrylate beads in a porcine simulated open femur fracture model. J. Orthop. Trauma 2012, 26, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Nakamura, K.; Tabata, Y.; Ikada, Y.; Aoyama, I.; Anzai, J.; Nakamura, T.; Hiyama, Y.; Tamura, M. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. J. Clin. Endocrinol. Metab. 2001, 86, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Hillier, M.L.; Bell, L.S. Differentiating human bone from animal bone: A review of histological methods. J. Forensic Sci. 2007, 52, 249–263. [Google Scholar] [CrossRef]
- O’Loughlin, P.F.; Morr, S.; Bogunovic, L.; Kim, A.D.; Park, B.; Lane, J.M. Selection and development of preclinical models in fracture-healing research. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. 1), 79–84. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Huang, J.; Wei, Q.; He, C. Advances in Animal Models for Studying Bone Fracture Healing. Bioengineering 2023, 10, 201. https://doi.org/10.3390/bioengineering10020201
Gao H, Huang J, Wei Q, He C. Advances in Animal Models for Studying Bone Fracture Healing. Bioengineering. 2023; 10(2):201. https://doi.org/10.3390/bioengineering10020201
Chicago/Turabian StyleGao, Hui, Jinming Huang, Quan Wei, and Chengqi He. 2023. "Advances in Animal Models for Studying Bone Fracture Healing" Bioengineering 10, no. 2: 201. https://doi.org/10.3390/bioengineering10020201
APA StyleGao, H., Huang, J., Wei, Q., & He, C. (2023). Advances in Animal Models for Studying Bone Fracture Healing. Bioengineering, 10(2), 201. https://doi.org/10.3390/bioengineering10020201






