Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives
Abstract
1. Introduction
2. Characteristics of MSCs for the OA Therapy
3. Underlying Treating Mechanism of MSCs for OA
4. Methods for Supplying MSCs
5. Preclinical Trials
5.1. MSCs for the Treatment of Rat OA
5.2. MSCs for the Treatment of Rabbit OA
5.3. MSCs for the Treatment of Goat OA
5.4. MSCs for the Treatment of Horse OA
5.5. MSCs for the Treatment of Dog OA
5.6. MSCs for the Treatment of Pig OA
6. Clinical Trials
6.1. OA Treatment with BM-MSCs
6.1.1. Autologous MSCs Generated from Bone Marrow
6.1.2. Allogeneic MSCs Generated from Bone Marrow
6.2. OA Treatment with AD-MSCs
6.3. OA Treatment with HUC-MSCs
6.4. OA Treatment with HUCB-MSCs
7. Meta-Analysis
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, J.; Kim, J.; Cheon, S. A Deep Neural Network-Based Method for Early Detection of Osteoarthritis Using Statistical Data. Int. J. Environ. Res. Public Health 2019, 16, 1281. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.; Agricola, R.; Price, A.; Vincent, T.; Weinans, H.; Carr, A. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Mandl, L. Osteoarthritis year in review 2018: Clinical. Osteoarthr. Cartil. 2019, 27, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Puig-Junoy, J.; Zamora, A.R. Socio-economic costs of osteoarthritis: A systematic review of cost-of-illness studies. Semin. Arthritis Rheum. 2015, 44, 531–541. [Google Scholar] [CrossRef]
- Iban, M.A.R.; Benavides, J.; Forero, J.P.; Bittelman, S.; Martinez, R.; Mite, M.A.; Heredia, J.D.; Ulloa, S.; Ferrand, M.M.L. Use of strong opioids for chronic pain in osteoarthritis: An insight into the Latin American reality. Expert Rev. Clin. Pharmacol. 2017, 11, 47–59. [Google Scholar] [CrossRef]
- Mahendira, L.; Jones, C.; Papachristos, A.; Waddell, J.; Rubin, L. Comparative clinical and cost analysis between surgical and non-surgical intervention for knee osteoarthritis. Int. Orthop. 2019, 44, 77–83. [Google Scholar] [CrossRef]
- White, R.H.; Romano, P.; Zhou, H.; Rodrigo, J.; Bargar, W. Incidence and Time Course of Thromboembolic Outcomes Following Total Hip or Knee Arthroplasty. Arch. Intern. Med. 1998, 158, 1525–1531. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic Burden of Periprosthetic Joint Infection in the United States. J. Arthroplast. 2012, 27, 61–65.e1. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Javaid, M.K.; Lynch, J.A.; Niu, J.; Zhang, Y.; Felson, D.T.; Lewis, C.E.; Torner, J.; Nevitt, M.C. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: The MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann. Rheum. Dis. 2008, 68, 1461–1465. [Google Scholar] [CrossRef]
- Hayes, A.J.; MacPherson, S.; Morrison, H.; Dowthwaite, G.; Archer, C.W. The development of articular cartilage: Evidence for an appositional growth mechanism. Anat. Embryol. 2001, 203, 469–479. [Google Scholar] [CrossRef]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.-P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.R.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.; Kapfinger, E.; Geiss, J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr. Cartil. 2007, 15, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.; Wu, C.-L.; Louer, C.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Intra-articular Delivery of Purified Mesenchymal Stem Cells from C57BL/6 or MRL/MpJ Superhealer Mice Prevents Posttraumatic Arthritis. Cell Transplant. 2013, 22, 1395–1408. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Kisiday, J.D.; Kawcak, C.E.; Werpy, N.M.; McIlwraith, C.W. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J. Orthop. Res. 2009, 27, 1675–1680. [Google Scholar] [CrossRef]
- Huh, J.-E.; Baek, Y.-H.; Ryu, S.-R.; Lee, J.-D.; Choi, D.-Y.; Park, D.-S. Efficacy and mechanism of action of KHBJ-9B, a new herbal medicine, and its major compound triterpenoids in human cartilage culture and in a rabbit model of collagenase-induced osteoarthritis. Int. Immunopharmacol. 2009, 9, 230–240. [Google Scholar] [CrossRef]
- Cosenza, S.; Ruiz, M.; Maumus, M.; Jorgensen, C.; Noël, D. Pathogenic or Therapeutic Extracellular Vesicles in Rheumatic Diseases: Role of Mesenchymal Stem Cell-Derived Vesicles. Int. J. Mol. Sci. 2017, 18, 889. [Google Scholar] [CrossRef]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.-A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef]
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018, 28, 918–933. [Google Scholar] [CrossRef]
- Dalby, M.J.; García, A.J.; Salmeron-Sanchez, M. Receptor control in mesenchymal stem cell engineering. Nat. Rev. Mater. 2018, 3, 17091. [Google Scholar] [CrossRef]
- Battula, V.L.; Cabreira, M.D.G.; Wang, Z.; Ma, W.; Benito, J.; Ruvolo, P.P.; Davis, R.E.; Konopleva, M.; Andreeff, M. Connective Tissue Growth Factor (CTGF) Is Essential for Self Renewal and Proliferation of Mesenchymal Stromal Cells (MSCs) and Affects Leukemia-Stromal Interactions. Blood 2010, 116, 3845. [Google Scholar] [CrossRef]
- Friedenstein, A.; Gorskaja, J.; Kulagina, N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Thromb. Haemost. 2003, 5, 32–45. [Google Scholar] [CrossRef]
- Hwang, J.J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front. Immunol. 2021, 12, 631291. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.-J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Umbilical Cord Blood as Sources of Cell Therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Fa, X.; Lixia, W.; Hou, J.; Zhang, R.; Wang, H.; Yang, C. Biological characteristics of human bone marrow mesenchymal stem cell cultured in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005, 25, 307–309. [Google Scholar]
- Kim, Y.J.; Kim, H.K.; Cho, H.H.; Bae, Y.C.; Suh, K.T.; Jung, J.S. Direct Comparison of Human Mesenchymal Stem Cells Derived from Adipose Tissues and Bone Marrow in Mediating Neovascularization in Response to Vascular Ischemia. Cell. Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef]
- Panepucci, R.; Siufi, J.; Silva, W., Jr.; Proto-Siquiera, R.; Neder, L.; Orellana, M.; Rocha, V.; Covas, D.; Zago, M. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells 2004, 22, 1263–1278. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, X.-Y.; Tong, J.-B.; Yang, X.-X.; Zhao, J.-L.; Zheng, Q.-F.; Zhao, G.-B.; Ma, Z.-J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Breit, S.; Parsch, D.; Benz, K.; Steck, E.; Hauner, H.; Weber, R.M.; Ewerbeck, V.; Richter, W. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003, 48, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Koch, C.; Gupta, M.K.; Lin, Q.; Lenz, M.; Laufs, S.; Denecke, B.; Schmidt, M.; Linke, M.; Hennies, H.C.; et al. Induced Pluripotent Mesenchymal Stromal Cell Clones Retain Donor-derived Differences in DNA Methylation Profiles. Mol. Ther. 2013, 21, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Aust, L.; Devlin, B.; Foster, S.; Halvorsen, Y.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.; Gimble, J. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef]
- Collins, K.; Lenz, K.; Pollitt, E.; Ferguson, D.; Hutson, I.; Springer, L.; Oestreich, A.; Tang, R.; Choi, Y.; Meyer, G.; et al. Adipose tissue is a critical regulator of osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021096118. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Deng, M.-X.; He, J.; Zeng, Q.-X.; Wen, W.; Wong, D.S.H.; Tse, H.-F.; Xu, G.; Lian, Q.; Shi, J.; et al. Human Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Allergic Airway Inflammation in Mice. Stem Cells 2012, 30, 2692–2699. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Diederichs, S.; Tuan, R.S. Functional Comparison of Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Cells and Bone Marrow-Derived Mesenchymal Stromal Cells from the Same Donor. Stem Cells Dev. 2014, 23, 1594–1610. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Connick, P.; Kolappan, M.; Crawley, C.; Webber, D.; Patani, R.; Michell, A.; Du, M.; Luan, S.; Altmann, D.; Thompson, A.; et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012, 11, 150–156. [Google Scholar] [CrossRef]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2012, 91, 19–26. [Google Scholar] [CrossRef]
- Hoogduijn, M.J. Are mesenchymal stromal cells immune cells? Arthritis Res. Ther. 2015, 17, 88. [Google Scholar] [CrossRef]
- Van den Bosch, M.; van Lent, P.; van der Kraan, P. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthr. Cartil. 2020, 28, 532–543. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef]
- Benvenuto, F.; Ferrari, S.; Gerdoni, E.; Gualandi, F.; Frassoni, F.; Pistoia, V.; Mancardi, G.; Uccelli, A. Human Mesenchymal Stem Cells Promote Survival of T Cells in a Quiescent State. Stem Cells 2007, 25, 1753–1760. [Google Scholar] [CrossRef]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Erratum: Corrigendum: Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 462. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jankovic, M.G.; Fellabaum, C.; Volarevic, A.; Djonov, V.; Arsenijevic, A.; Volarevic, V. Molecular Mechanisms Responsible for Anti-inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv. Exp. Med. Biol. 2018, 1084, 187–206. [Google Scholar] [CrossRef]
- Mokbel, A.N.; El Tookhy, O.S.; Shamaa, A.A.; Rashed, L.A.; Sabry, D.; El Sayed, A.M. Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet. Disord. 2011, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Uchida, K.; Nakajima, H.; Miyazaki, T.; Guerrero, A.R.; Watanabe, S.; Roberts, S.; Baba, H. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Thromb. Haemost. 2012, 14, R31. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.; Jablonski, C.L.; Leonard, C.A.; Dunn, J.F.; Raharjo, E.; Matyas, J.R.; Biernaskie, J.; Krawetz, R.J. Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci. Rep. 2016, 6, 23076. [Google Scholar] [CrossRef] [PubMed]
- Bárdos, T.; Kamath, R.V.; Mikecz, K.; Glant, T.T. Anti-Inflammatory and Chondroprotective Effect of TSG-6 (Tumor Necrosis Factor-α-Stimulated Gene-6) in Murine Models of Experimental Arthritis. Am. J. Pathol. 2001, 159, 1711–1721. [Google Scholar] [CrossRef]
- Von Bahr, L.; Batsis, I.; Moll, G.; Hägg, M.; Szakos, A.; Sundberg, B.; Uzunel, M.; Ringden, O.; Le Blanc, K. Analysis of Tissues Following Mesenchymal Stromal Cell Therapy in Humans Indicates Limited Long-Term Engraftment and No Ectopic Tissue Formation. Stem Cells 2012, 30, 1575–1578. [Google Scholar] [CrossRef]
- Acharya, C.; Adesida, A.; Zajac, P.; Mumme, M.; Riesle, J.; Martin, I.; Barbero, A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J. Cell. Physiol. 2011, 227, 88–97. [Google Scholar] [CrossRef]
- Wu, L.; Prins, H.-J.; Helder, M.N.; van Blitterswijk, C.A.; Karperien, H.B.J. Trophic Effects of Mesenchymal Stem Cells in Chondrocyte Co-Cultures are Independent of Culture Conditions and Cell Sources. Tissue Eng. Part A 2012, 18, 1542–1551. [Google Scholar] [CrossRef]
- Saulnier, N.; Viguier, E.; Perrier-Groult, E.; Chenu, C.; Pillet-Michelland, E.; Roger, T.; Maddens, S.; Boulocher, C. Intra-articular administration of xenogeneic neonatal Mesenchymal Stromal Cells early after meniscal injury down-regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthr. Cartil. 2015, 23, 122–133. [Google Scholar] [CrossRef]
- Desando, G.; Cavallo, C.; Sartoni, F.; Martini, L.; Parrilli, A.; Veronesi, F.; Fini, M.; Giardino, R.; Facchini, A.; Grigolo, B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Thromb. Haemost. 2013, 15, R22. [Google Scholar] [CrossRef]
- Lee, C.H.; Cook, J.L.; Mendelson, A.; Moioli, E.K.; Yao, H.; Mao, J.J. Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet 2010, 376, 440–448. [Google Scholar] [CrossRef]
- Ozeki, N.; Muneta, T.; Koga, H.; Nakagawa, Y.; Mizuno, M.; Tsuji, K.; Mabuchi, Y.; Akazawa, C.; Kobayashi, E.; Matsumoto, K.; et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthr. Cartil. 2016, 24, 1061–1070. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Davatchi, F.; Abdollahi, B.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef]
- Emadedin, M.; Aghdami, N.; Taghiyar, L.; Fazeli, R.; Moghadasali, R.; Jahangir, S.; Farjad, R.; Eslaminejad, M.B. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch. Iran. Med. 2012, 15, 422–428. [Google Scholar]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Koh, Y. Mesenchymal stem cell implantation in knee osteoarthritis: An assessment of the factors influencing clinical outcomes. Am. J. Sports Med. 2015, 43, 2293–2301. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kwon, O.R.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am. J. Sports Med. 2015, 43, 2738–2746. [Google Scholar] [CrossRef]
- Zellner, J.; Pattappa, G.; Koch, M.; Lang, S.; Weber, J.; Pfeifer, C.G.; Mueller, M.B.; Kujat, R.; Nerlich, M.; Angele, P. Autologous mesenchymal stem cells or meniscal cells: What is the best cell source for regenerative meniscus treatment in an early osteoarthritis situation? Stem Cell Res. Ther. 2017, 8, 225. [Google Scholar] [CrossRef]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef]
- Wong, K.; Lee, K.; Tai, B.; Law, P.; Lee, E.; Hui, J. Injectable Cultured Bone Marrow-Derived Mesenchymal Stem Cells in Varus Knees With Cartilage Defects Undergoing High Tibial Osteotomy: A Prospective, Randomized Controlled Clinical Trial With 2 Years’ Follow-up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Jevotovsky, D.S.; Alfonso, A.R.; Einhorn, T.; Chiu, E.S. Osteoarthritis and stem cell therapy in humans: A systematic review. Osteoarthr. Cartil. 2018, 26, 711–729. [Google Scholar] [CrossRef] [PubMed]
- Afizah, H.; Hui, J.H.P. Mesenchymal stem cell therapy for osteoarthritis. J. Clin. Orthop. Trauma 2016, 7, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 2015, 19, 219–225. [Google Scholar] [CrossRef]
- Horie, M.; Sekiya, I.; Muneta, T.; Ichinose, S.; Matsumoto, K.; Saito, H.; Murakami, T.; Kobayashi, E. Intra-articular Injected Synovial Stem Cells Differentiate into Meniscal Cells Directly and Promote Meniscal Regeneration Without Mobilization to Distant Organs in Rat Massive Meniscal Defect. Stem Cells 2009, 27, 878–887. [Google Scholar] [CrossRef]
- Horie, M.; Choi, H.; Lee, R.; Reger, R.; Ylostalo, J.; Muneta, T.; Sekiya, I.; Prockop, D. Intra-articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Osteoarthr. Cartil. 2012, 20, 1197–1207. [Google Scholar] [CrossRef]
- Cui, Y.P.; Cao, Y.P.; Liu, H.; Yang, X.; Meng, Z.C.; Wang, R. Bone marrow mesenchymal stem cells in Sprague-Dawley rat model of osteoarthritis. J. Peking Univ. Health Sci. 2015, 47, 211–218. [Google Scholar]
- Van Buul, G.M.; Siebelt, M.; Leijs, M.J.C.; Bos, P.K.; Waarsing, J.H.; Kops, N.; Weinans, H.; Verhaar, J.A.N.; Bernsen, M.R.; van Osch, G.J.V.M. Mesenchymal stem cells reduce pain but not degenerative changes in a mono-iodoacetate rat model of osteoarthritis. J. Orthop. Res. 2014, 32, 1167–1174. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Xing, D.; Wang, K.; Wu, J.; Zhao, Y.; Liu, W.; Li, J.; Gao, T.; Yan, D.; Wang, L.; Hao, J.; et al. Clinical-Grade Human Embryonic Stem Cell-Derived Mesenchymal Stromal Cells Ameliorate the Progression of Osteoarthritis in a Rat Model. Molecules 2021, 26, 604. [Google Scholar] [CrossRef]
- Yang, W.-T.; Ke, C.-Y.; Yeh, K.-T.; Huang, S.-G.; Lin, Z.-Y.; Wu, W.-T.; Lee, R.-P. Stromal-vascular fraction and adipose-derived stem cell therapies improve cartilage regeneration in osteoarthritis-induced rats. Sci. Rep. 2022, 12, 2828. [Google Scholar] [CrossRef]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; de Llano, J.J.M.; Carda, C. In Vivo Articular Cartilage Regeneration Using Human Dental Pulp Stem Cells Cultured in an Alginate Scaffold: A Preliminary Study. Stem Cells Int. 2017, 2017, 8309256. [Google Scholar] [CrossRef]
- Riester, S.M.; Denbeigh, J.M.; Lin, Y.; Jones, D.L.; de Mooij, T.; Lewallen, E.A.; Nie, H.; Paradise, C.R.; Radel, D.J.; Dudakovic, A.; et al. Safety Studies for Use of Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells in a Rabbit Model for Osteoarthritis to Support a Phase I Clinical Trial. Stem Cells Transl. Med. 2016, 6, 910–922. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Yoon, K.-A.; An, E.S.; Kang, T.-W.; Sim, Y.-B.; Ahn, J.; Choi, E.-K.; Lee, S.; Seo, K.-W.; Kim, Y.-B.; et al. Therapeutic Effects of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Combined with Cartilage Acellular Matrix Mediated Via Bone Morphogenic Protein 6 in a Rabbit Model of Articular Cruciate Ligament Transection. Stem Cell Rev. Rep. 2020, 16, 596–611. [Google Scholar] [CrossRef]
- Pei, M.; He, F.; Boyce, B.; Kish, V. Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthr. Cartil. 2009, 17, 714–722. [Google Scholar] [CrossRef]
- Lee, J.-C.; Min, H.J.; Park, H.J.; Lee, S.; Seong, S.C.; Lee, M.C. Synovial Membrane–Derived Mesenchymal Stem Cells Supported by Platelet-Rich Plasma Can Repair Osteochondral Defects in a Rabbit Model. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1034–1046. [Google Scholar] [CrossRef]
- Shimomura, K.; Moriguchi, Y.; Ando, W.; Nansai, R.; Fujie, H.; Hart, D.A.; Gobbi, A.; Kita, K.; Horibe, S.; Shino, K.; et al. Osteochondral Repair Using a Scaffold-Free Tissue-Engineered Construct Derived from Synovial Mesenchymal Stem Cells and a Hydroxyapatite-Based Artificial Bone. Tissue Eng. Part A 2014, 20, 2291–2304. [Google Scholar] [CrossRef]
- Li, H.; Qian, J.; Chen, J.; Zhong, K.; Chen, S. Osteochondral repair with synovial membrane-derived mesenchymal stem cells. Mol. Med. Rep. 2016, 13, 2071–2077. [Google Scholar] [CrossRef]
- Schmal, H.; Kowal, J.M.; Kassem, M.; Seidenstuecker, M.; Bernstein, A.; Böttiger, K.; Xiong, T.; Südkamp, N.P.; Kubosch, E.J. Comparison of Regenerative Tissue Quality following Matrix-Associated Cell Implantation Using Amplified Chondrocytes Compared to Synovium-Derived Stem Cells in a Rabbit Model for Cartilage Lesions. Stem Cells Int. 2018, 2018, 4142031. [Google Scholar] [CrossRef]
- Murphy, J.M.; Fink, D.J.; Hunziker, E.B.; Barry, F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheumatol. 2003, 48, 3464–3474. [Google Scholar] [CrossRef] [PubMed]
- Saw, K.; Hussin, P.; Loke, S.; Azam, M.; Chen, H.; Tay, Y.; Low, S.; Wallin, K.; Ragavanaidu, K. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic Acid: An experimental study in a goat model. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. North Am. Int. Arthrosc. Assoc. 2009, 25, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Luo, X.; He, N.; Xia, H.; Lv, X.; Zhang, X.; Li, D.; Wang, F.; He, J.; Zhang, L.; et al. Efficacy and Persistence of Allogeneic Adipose-Derived Mesenchymal Stem Cells Combined with Hyaluronic Acid in Osteoarthritis After Intra-articular Injection in a Sheep Model. Tissue Eng. Part A 2018, 24, 219–233. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.; Frisbie, D.; Rodkey, W.; Kisiday, J.; Werpy, N.; Kawcak, C.; Steadman, J. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. North Am. Int. Arthrosc. Assoc. 2011, 27, 1552–1561. [Google Scholar] [CrossRef]
- Black, L.L.; Gaynor, J.; Gahring, D.; Adams, C.; Aron, D.; Harman, S.; Gingerich, D.A.; Harman, R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double-blinded, multicenter, controlled trial. Vet. Ther. 2007, 8, 272. [Google Scholar]
- Black, L.L.; Gaynor, J.; Adams, C.; Dhupa, S.; Sams, A.E.; Taylor, R.; Harman, S.; Gingerich, D.A.; Harman, R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet. Ther. 2008, 9, 192–200. [Google Scholar]
- Huňáková, K.; Hluchý, M.; Špaková, T.; Matejová, J.; Mudroňová, D.; Kuricová, M.; Rosocha, J.; Ledecký, V. Study of bilateral elbow joint osteoarthritis treatment using conditioned medium from allogeneic adipose tissue-derived MSCs in Labrador retrievers. Res. Vet. Sci. 2020, 132, 513–520. [Google Scholar] [CrossRef]
- Lee, K.B.; Hui, J.H.; Song, I.C.; Ardany, L.; Lee, E.H. Injectable Mesenchymal Stem Cell Therapy for Large Cartilage Defects—A Porcine Model. Stem Cells 2007, 25, 2964–2971. [Google Scholar] [CrossRef]
- Kubosch, E.J.; Lang, G.; Furst, D.; Kubosch, D.; Izadpanah, K.; Rolauffs, B.; Sudkamp, N.P.; Schmal, H. The Potential for Synovium-derived Stem Cells in Cartilage Repair. Curr. Stem Cell Res. Ther. 2018, 13, 174–184. [Google Scholar] [CrossRef]
- Kohno, Y.; Mizuno, M.; Ozeki, N.; Katano, H.; Komori, K.; Fujii, S.; Otabe, K.; Horie, M.; Koga, H.; Tsuji, K.; et al. Yields and chondrogenic potential of primary synovial mesenchymal stem cells are comparable between rheumatoid arthritis and osteoarthritis patients. Stem Cell Res. Ther. 2017, 8, 115. [Google Scholar] [CrossRef]
- Kubosch, E.; Heidt, E.; Niemeyer, P.; Bernstein, A.; Südkamp, N.; Schmal, H. In-vitro chondrogenic potential of synovial stem cells and chondrocytes allocated for autologous chondrocyte implantation—A comparison: Synovial stem cells as an alternative cell source for autologous chondrocyte implantation. Int. Orthop. 2017, 41, 991–998. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef]
- Wakitani, S.; Nawata, M.; Tensho, K.; Okabe, T.; Machida, H.; Ohgushi, H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: Three case reports involving nine defects in five knees. J. Tissue Eng. Regen. Med. 2007, 1, 74–79. [Google Scholar] [CrossRef]
- Centeno, C.J.; Busse, D.; Kisiday, J.; Keohan, C.; Freeman, M.; Karli, D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 2008, 11, 343–353. [Google Scholar]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: Two-year follow-up results. Transplantation 2014, 97, e66–e68. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.; Mora, G.; Blanco, J.; Granero-Moltó, F.; Nuñez-Córdoba, J.; Sánchez-Echenique, C.; Bondía, J.; Aquerreta, J.; Andreu, E.; Ornilla, E. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2016, 14, 246. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Blanco, J.F.; Sánchez, M.; Moreno, V.; Granero-Moltó, F.; Sánchez-Guijo, F.; Crespo-Cullel, I.; Mora, G.; Vicente, D.D.S.; Pompei-Fernández, O.; et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J. Transl. Med. 2020, 18, 356. [Google Scholar] [CrossRef]
- Vangsness, C., Jr.; Farr, J., 2nd; Boyd, J.; Dellaero, D.; Mills, C.; LeRoux-Williams, M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: A randomized, double-blind, controlled study. J. Bone Jt. Surg. Am. 2014, 96, 90–98. [Google Scholar] [CrossRef]
- Vega, A.; Martin-Ferrero, M.; Del Canto, F.; Alberca, M.; Garcia, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis with Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Gupta, P.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.; Vellotare, P.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef]
- Koh, Y.-G.; Choi, Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, H.; Kim, K.; Kim, G.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. Ther. 2019, 10, 143. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Du, H.; Li, S.; Ye, P.; Zhang, L.; Wang, X.; Song, Y.; Togashi, R.; Vangsness, C.T.; et al. Intra-articular injections of allogeneic human adipose-derived mesenchymal progenitor cells in patients with symptomatic bilateral knee osteoarthritis: A Phase I pilot study. Regen. Med. 2020, 15, 1625–1636. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, W.; Liu, H.; Cui, Y.; Mao, Q.; Fei, Z.; Xiang, C. Curative Effect of Human Umbilical Cord Mesenchymal Stem Cells by Intra-Articular Injection for Degenerative Knee Osteoarthritis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2016, 30, 1472–1477. [Google Scholar]
- Park, Y.; Ha, C.; Lee, C.; Yoon, Y.; Park, Y. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Prósper, F.; Blanco, J.F.; Sánchez-Guijo, F.; Alberca, M.; García, V.; González-Vallinas, M.; García-Sancho, J. Long-term efficacy of autologous bone marrow mesenchymal stromal cells for treatment of knee osteoarthritis. J. Transl. Med. 2021, 19, 506. [Google Scholar] [CrossRef]
- Sadlik, B.; Jaroslawski, G.; Gladysz, D.; Puszkarz, M.; Markowska, M.; Pawelec, K.; Boruczkowski, D.; Oldak, T. Knee Cartilage Regeneration with Umbilical Cord Mesenchymal Stem Cells Embedded in Collagen Scaffold Using Dry Arthroscopy Technique. Adv. Exp. Med. Biol. 2017, 1020, 113–122. [Google Scholar] [CrossRef]
- Sadlik, B.; Jaroslawski, G.; Puszkarz, M.; Blasiak, A.; Oldak, T.; Gladysz, D.; Whyte, G. Cartilage Repair in the Knee Using Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells Embedded onto Collagen Scaffolding and Implanted Under Dry Arthroscopy. Arthrosc. Tech. 2018, 7, e57–e63. [Google Scholar] [CrossRef]
- McIntyre, J.A.; Jones, I.; Danilkovich, A.; Vangsness, C.T. The Placenta: Applications in Orthopaedic Sports Medicine. Am. J. Sports Med. 2017, 46, 234–247. [Google Scholar] [CrossRef]
- Peeters, C.; Leijs, M.; Reijman, M.; van Osch, G.; Bos, P. Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: A systematic literature review. Osteoarthr. Cartil. 2013, 21, 1465–1473. [Google Scholar] [CrossRef]
- Cui, G.-H.; Wang, Y.Y.; Li, C.-J.; Shi, C.H.; Wang, W.-S. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp. Ther. Med. 2016, 12, 3390–3400. [Google Scholar] [CrossRef]
- Xing, D.; Wang, Q.; Yang, Z.; Hou, Y.; Zhang, W.; Chen, Y.; Lin, J. Mesenchymal stem cells injections for knee osteoarthritis: A systematic overview. Rheumatol. Int. 2017, 38, 1399–1411. [Google Scholar] [CrossRef]
- Pas, H.I.; Winters, M.; Haisma, H.J.; Koenis, M.J.; Tol, J.L.; Moen, M.H. Stem cell injections in knee osteoarthritis: A systematic review of the literature. Br. J. Sports Med. 2017, 51, 1125–1133. [Google Scholar] [CrossRef]
- Xia, P.; Wang, X.; Lin, Q.; Li, X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: A systematic review and meta-analysis. Int. Orthop. 2015, 39, 2363–2372. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Li, L.; Sun, T.; Lin, B.; Chen, L.; Hills, R. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS ONE 2017, 14, e0175449. [Google Scholar] [CrossRef]
- Agarwal, N.; Mak, C.; Bojanic, C.; To, K.; Khan, W. Meta-Analysis of Adipose Tissue Derived Cell-Based Therapy for the Treatment of Knee Osteoarthritis. Cells 2021, 10, 1365. [Google Scholar] [CrossRef]
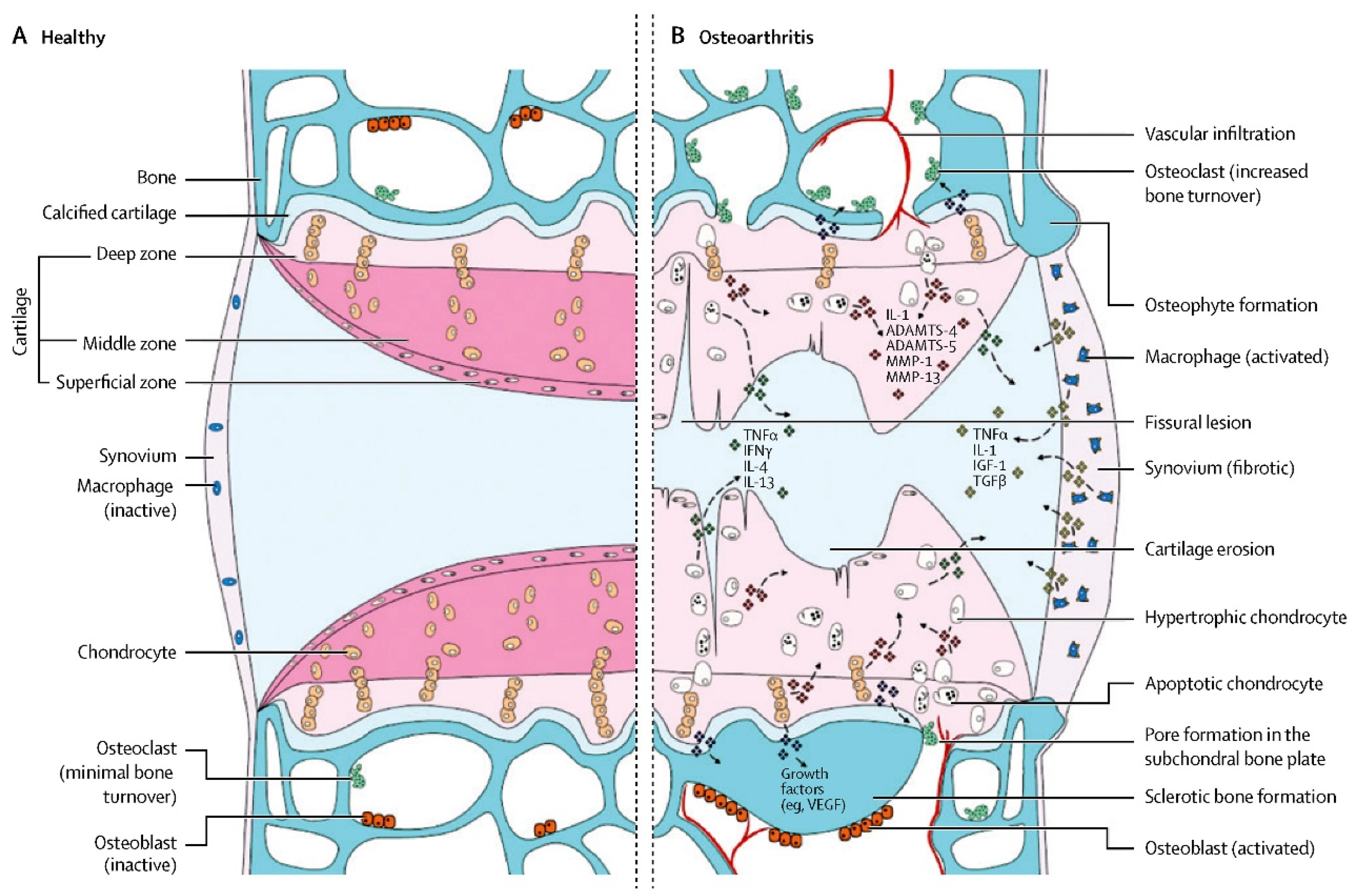
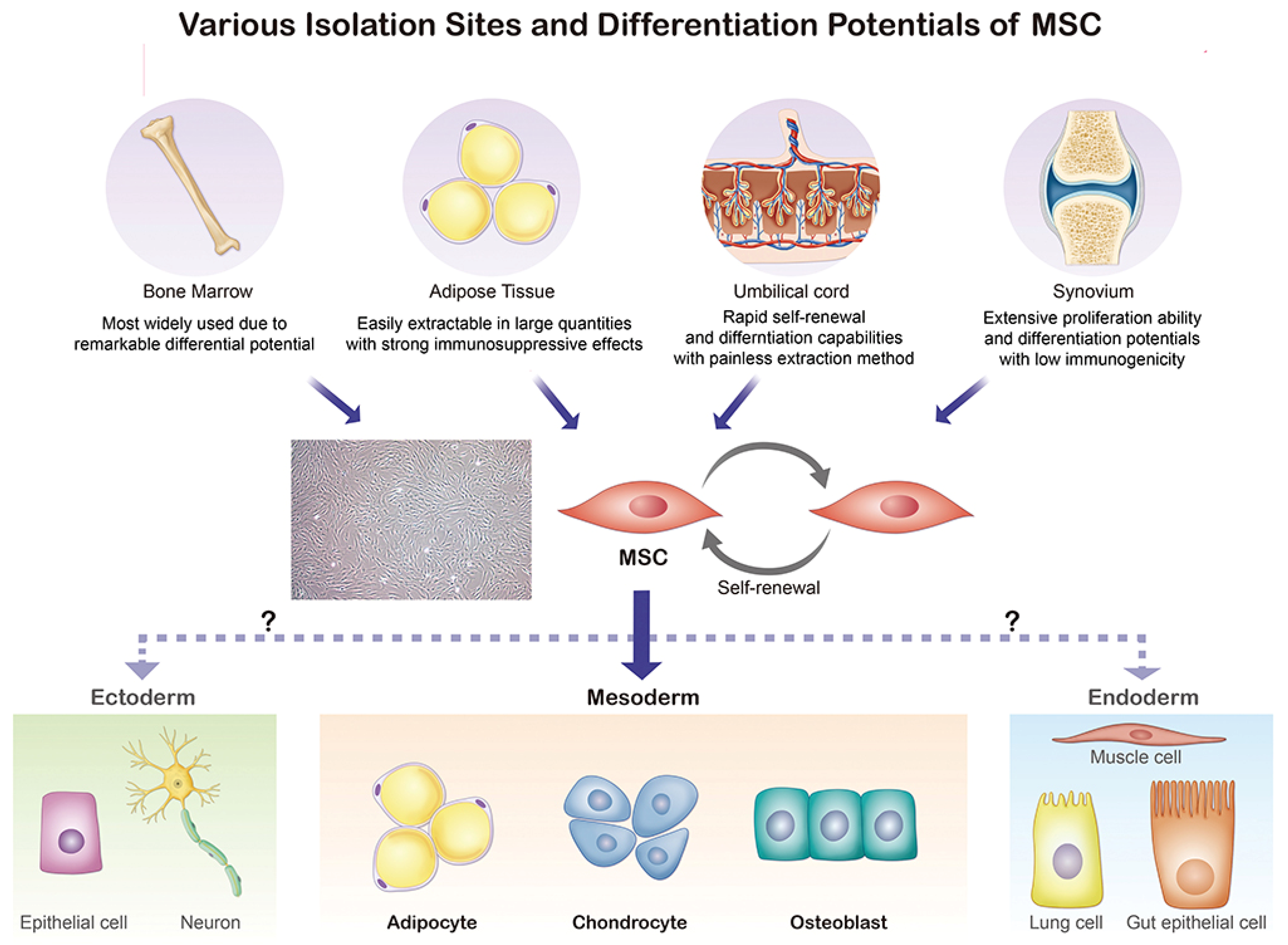
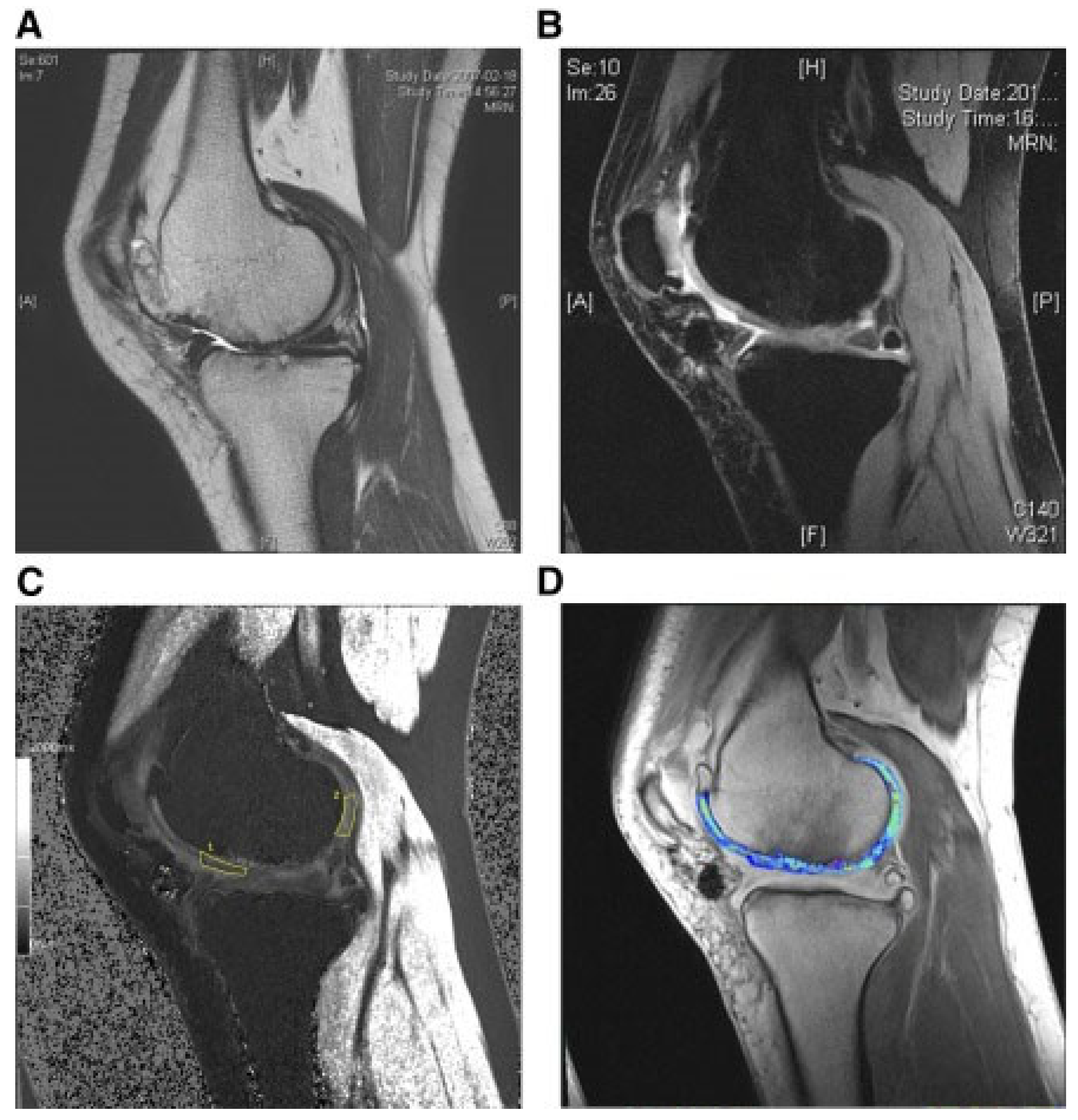
| References | Year | Animal Model | Source of MSCs | Lesion Preparation | Outcomes |
|---|---|---|---|---|---|
| Horie et al. [76] | 2009 | Rat | Autologous synovium | Meniscectomy | MSCs clung to meniscus lesions and directly developed into meniscal cells to facilitate meniscus repair and regeneration. |
| Horie et al. [77] | 2012 | Rat | Xenogeneic (human) bone marrow | Hemi-meniscectomy | Type II collagen expression levels rose and the progression of OA was dramatically slowed. |
| Cui et al. [78] | 2015 | Rat | Allogeneic bone marrow | ACLT/medial meniscus excising | The Mankin score was significantly improved, and the mRNA expression of type II collagen increased. |
| van Buul et al. [79] | 2014 | Rat | Allogeneic bone marrow | Inducted by MIA injection | The local injection of MSCs significantly improved joint function, but there was no statistical difference in cartilage improvement, subchondral bone pathology, and synovitis. |
| Ozeki et al. [61] | 2016 | Rat | Xenogeneic (human) synovium | ACLT | Injected Sy-MSCs increased the expression of genes associated with chondroprotection such as PRG-4 and BMP-2 by more than 50 fold. |
| He et al. [80] | 2020 | Rat | Allogeneic bone marrow | Inducted by sodium iodoacetate injection | The COL2A1 protein was significantly upregulated and the MMP13 protein was significantly downregulated in cartilage tissue after exosome therapy. |
| Xing et al. [81] | 2021 | Rat | Xenogeneic (human) embryonic stem cell | ACLT | The better therapeutic benefits of many injections of embryonic MSCs were maintained in both the short- and long-term after treatment. |
| Yang et al. [82] | 2022 | Rat | Autologous adipose tissue | Inducted by sodium iodoacetate injection | Treatments using adipose-derived stem cells aided articular cartilage repair. |
| Zellner et al. [68] | 2017 | Rabbit | Autologous and xenogeneic (human) bone marrow | Punch defects on the lateral meniscus | The human MSCs demonstrated the considerably increased expression of the collagen type II gene and the synthesis of collagen. |
| Mata et al. [83] | 2017 | Rabbit | Xenogeneic (human) dental pulp | Defects in femoral trochlear groove | Obvious cartilage regeneration was observed 3 months after operation. |
| Riester et al. [84] | 2017 | Rabbit | Xenogeneic (human) adipose tissue | Bilateral medial anterior hemi-meniscectomy | The tolerance was good, and no evidence of intra-articular joint tissue damage was found. |
| Jeon et al. [85] | 2020 | Rabbit | Xenogeneic (human) umbilical cord blood | ACLT | Rabbit synovial fluid and joints treated with HUCB-MSCs showed reduced inflammation and improved proteoglycan and collagen type 2 production and structure. |
| Pei et al. [86] | 2009 | Rabbit | Allogeneic synovium | Full-thickness femoral condyle cartilage defects | The regenerated cartilage appeared as smooth hyaline cartilage at a 6-month follow-up. |
| Lee et al. [87] | 2013 | Rabbit | Autologous synovium | Osteochondral defects on trochlear groove of femur | The treated group showed significantly improved microscopic and macroscopic scores at a 6-month follow-up. |
| Shimomura et al. [88] | 2014 | Rabbit | Allogeneic synovium | Osteochondral defects on the femoral groove | Subjects using Sy-MSCs and HA exhibited faster integration and the improved appearance of osteochondral bone compared with controls using only HA. |
| Li et al. [89] | 2016 | Rabbit | Autologous synovium | Osteochondral defects on right knee trochlea | The treated animals had a higher quality of tissue. |
| Schmal et al. [90] | 2018 | Rabbit | Allogeneic synovium | Osteochondral defects on medial femoral condyle | Macroscopic regenerative capacity increased. |
| Murphy et al. [91] | 2003 | Goat | Autologous bone marrow | Surgical removal of the medial meniscus and anterior cruciate ligament reconstruction | Cartilage tissue regeneration was observed, but a relative lack of labeled MSCs was found in the regenerated cartilage area. |
| Saw et al. [92] | 2009 | Goat | Autologous bone marrow | Arthroscopic subchondral drilling | Tissue integration and tissue repair could be improved with the use of a bone marrow aspiration primer combined with hyaluronic acid. |
| Feng et al. [93] | 2018 | Goat | Allogeneic adipose tissue | Anterior cruciate ligament resection/medial meniscectomy | An examination using MRI, macroscopic and microcomputer tomography, and cartilage-specific staining showed that the AD-MSCs + HA treatment group retained the typical characteristics of articular cartilage, effectively blocked the progress of OA, and boosted cartilage regeneration. |
| McIlwraith et al. [94] | 2011 | Horse | Allogeneic bone marrow | Subchondral bone microfracture | The histological analysis of the intra-articular BM-MSC injection group revealed improved proteoglycan and tissue stiffness in the restored cartilage. |
| Black et al. [95] | 2007 | Dog | Autologous adipose tissue | Functional disabilities | The claudication index, pain score, and range of motion were substantially increased. |
| Black et al. [96] | 2008 | Dog | Autologous adipose tissue | Functional disabilities | The claudication and range of movement of dogs were significantly improved. |
| Huňáková et al. [97] | 2020 | Dog | Allogeneic adipose tissue | Untreated elbow dysplasia | The double intra-articular administration of canine adipose tissue derived from Labrador retrievers improved the functional ability of dogs. |
| LEE et al. [98] | 2007 | Pig | Autologous bone marrow | Cartilage defect in themedial femoral condyle | Cartilage defect cartilage healing improved. |
| References | Years | Condition | Sample Size | Source of MSCs | Mode of Administration | Follow-Up | Outcomes |
|---|---|---|---|---|---|---|---|
| Wakitani et al. [103] | 2007 | Full-thickness articular cartilage defects of the patellofemoral joints | N = 3; Females = 1; Males = 2; Mean age = 40 years | Autologous BM-MSCs | Surgical implantation in the form of collagen gel wrapped around BM-MSCs (5 × 106 cells/mL) | 1 year | IKDC scores all improved to more than 60. MRI showed defects that were repaired with the fibrocartilaginous tissue. |
| Centeno et al. [104] | 2008 | Degenerative knee osteoarthritis | N = 1; Females = 0; Males = 1; Mean age = 46 years | Autologous BM-MSCs | Percutaneously injection (2.24 × 108) | 6 months | After 3 months, the VAS dropped from 4 to 0.38, a 95% reduction. The joint range of motion increased from −2 degrees to +3 degrees when stretching. |
| Orozco et al. [69] | 2013 | K–L grade II–IV knee osteoarthritis | N = 12; Females = 6; Males = 6; Mean age = 49 ± 5 years | Autologous BM-MSCs | Intra-articular injection (40 × 10 cells) | 1 year | Quantification of cartilage quality by T2relaxation measurements demonstrated a 27% decrease in poor cartilage areas on average; the mean VASvalues of 45 and 47 were recorded. |
| Orozco et al. [105] | 2014 | K–L grade II–IV knee osteoarthritis | N = 12; Females = 6; Males = 6; Mean age = 49 ± 5 years | Autologous BM-MSCs | Intra-articular injection (40 × 10 cells) | 2 years | The therapeutic efficiency was 0.71 for VAS and 0.66 for the Lequesne severity index; the WOMAC score varied between 0.44 and 0.78. |
| Lamo-Espinosa et al. [106] | 2016 | K–L grade II–IV knee osteoarthritis | N = 30; Females = 11; Males = 19; Mean age = 61 years | Autologous BM-MSCs | Intra-articular administration (10 or 100 × 106) | 1 year | The median VAS scores in the control, low-dose, and high-dose groups changed from 5, 7, and 6 to 4, 2, and 2, respectively, after 1 year. The WOMAC scores in the high-dose group showed a 16.5-point improvement after 1 year. |
| Lamo-Espinosa et al. [107] | 2020 | K–L grade II–IV knee osteoarthritis | N = 60; Females = 27; Males = 3; Mean age = 55 years | Autologous BM-MSCs | Lateral patellar administration (100 × 106) | 1 year | The mean VAS values in the PRGF® and BM-MSC with PRGF® groups changed from 5 and 5.3 to 4.5 and 3.5, respectively, after 1 year. The WOMAC scores changed from 31.9 and 33.4 to 22.3 and 23.0, respectively. |
| Vangsness et al. [108] | 2014 | After partial meniscectomy | N = 55; Females = 20; Males = 35; Mean age = 46 years | Allogeneic BM-MSCs | Superolateral knee injection (50 or 150 × 106) | 2 years | Meniscal volumes (24% of patients in the group injected with 50 × 106 BM-MSCs and 6% in group injected with 150 × 106 BM-MSCs) considerably increased. |
| Vega et al. [109] | 2015 | K–L grade II–IV chronic knee osteoarthritis | N = 30; Females = 17; Males = 13; Mean age = 57 ± 9 years | Allogeneic BM-MSCs | Intra-articular injection (40 × 106) | 1 year | The mean VAS scores in the experimental group and the control group increased from 54 and 64 to 33 and 51, respectively. The WOMAC pain scores increased from 46 and 50 to 30 and 44, respectively. |
| Gupta et al. [110] | 2016 | K–L grade II–III knee osteoarthritis | N = 60; Females = 45; Males = 15; Mean age = 56 ± 7.43 years | Allogeneic BM-MSCs | Intra-articular injection (25, 50, 75, or 150 × 106) | 1 year | The WOMAC and total ICOAP scores decreased in all treatment groups, the VAS score decreased in all but the 150 × 106 group, and the 25 × 106 group had the largest decreases (64.8%, 34.6%, and 67.4%). |
| Jo et al. [65] | 2014 | K–L grade III–IV knee osteoarthritis | N = 18; Females = 15; Males = 3; Mean age = 62 years | Autologous AD-MSCs | Intra-articular injection (1, 5, or 10 × 107) | 6 months | The WOMAC score in the 10 × 107 group decreased by 39%, and the knee score of KSS in the 1 and 10 × 107 groups increased by 91% and 50%, respectively. |
| Koh et al. [111] | 2012 | K–L grade II–IV knee osteoarthritis | N = 25; Females = 17; Males = 8; Mean age = 54.1 years | Autologous AD-MSCs | Percutaneous injection combined with arthroscopic debridement (1.89 × 106) | 16.4 months | The mean Lysholm and Tegner activity scales in the studygroup improved by 26.9 and 1.3 points, respectively; the VAS score decreased by 2.2 points. |
| Pers et al. [112] | 2016 | K–L grade III–IV knee osteoarthritis | N = 18; Females = 10; Males = 8; Mean age = 64.6 years | Autologous AD-MSCs | Intra-articular injection (2, 10, or 50 × 106) | 6 months | No serious adverse events were reported, and the WOMACpain score decreased by 30.7 ± 10.7 mm in the 2 × 106 group. |
| Freitag et al. [113] | 2019 | K–L grade II–III knee osteoarthritis | N = 30; Females = 14; Males = 16; Mean age = 53.6 years | Autologous AD-MSCs | Intra-articular injection (100 × 106) | 1 year | NPRS was improved by 69% in the treatment group. The mean WOMAC score changed from 57 to 85.7. |
| Lee et al. [114] | 2019 | K–L grade II–IV knee osteoarthritis | N = 24; Females = 18; Males = 6; Mean age = 62.7 years | Autologous AD-MSCs | Intra-articular injection (1 × 108) | 6 months | The WOMAC and VAS scores in the AD-MSC group changed from 60 and 6.8 to 26.7 and 3.4, respectively. |
| Lu et al. [115] | 2019 | K–L grade I–III knee osteoarthritis | N = 52; Females = 46; Males = 6; Mean age = 55 years | Autologous AD-MSCs | Intra-articular injection (5 × 107) | 1 year | The total volume of articular cartilage in the treatment group increased by 193.36 ± 282.80 mm3 compared with the baseline for the left knee and 108.70 ± 220.13 mm3 for the right knee in 1 year. |
| Lu et al. [116] | 2020 | K–L grade II–III knee osteoarthritis | N = 22; Females = 19; Males = 3; Mean age = 57.93 years | Allogeneic AD-MSCs | Intra-articular injection (1, 2, or 5 × 107) | 48 weeks | A joint assessment of VAS, SF-36, and WOMAC scores improved, with averages of 2.03, 15.3, and 16.97, respectively, in three experimental groups. |
| Wang et al. [117] | 2016 | Moderate or severe degenerative knee osteoarthritis | N = 36; Females = 15; Males = 21; Mean age = 53.33 years | Allogeneic HUC-MSCs | Intra-articular injection ((2–3) × 107) | 6 months | The Lysholm and WOMAC at 1–6 months and the SF-36 scale score at 2–6 months were significantly better than before treatment in the cell treatment group. |
| Park et al. [118] | 2017 | K–L grade III knee osteoarthritis and ICRS grade IV lesions | N = 7; Females = 5; Males = 2; Mean age = 58.7 years | Allogeneic HUCB-MSCs | Surgical implantation of a complex containing stem cells and hyaluronic acid hydrogel (0.5 × 107) | 7 years | Maturing repair tissue was observed at the 12-week arthroscopic examination. The 100 mm VAS and IKDC scores changed from 49.1 and 39.1 to 19.3 and 63.2, respectively, at 24 weeks. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Z.; Cai, X.; Bian, Y.; Wei, Z.; Zhu, W.; Zhao, X.; Weng, X. Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives. Bioengineering 2023, 10, 195. https://doi.org/10.3390/bioengineering10020195
Lv Z, Cai X, Bian Y, Wei Z, Zhu W, Zhao X, Weng X. Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives. Bioengineering. 2023; 10(2):195. https://doi.org/10.3390/bioengineering10020195
Chicago/Turabian StyleLv, Zehui, Xuejie Cai, Yixin Bian, Zhanqi Wei, Wei Zhu, Xiuli Zhao, and Xisheng Weng. 2023. "Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives" Bioengineering 10, no. 2: 195. https://doi.org/10.3390/bioengineering10020195
APA StyleLv, Z., Cai, X., Bian, Y., Wei, Z., Zhu, W., Zhao, X., & Weng, X. (2023). Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives. Bioengineering, 10(2), 195. https://doi.org/10.3390/bioengineering10020195









