Biofabrication of Poly(glycerol sebacate) Scaffolds Functionalized with a Decellularized Bone Extracellular Matrix for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
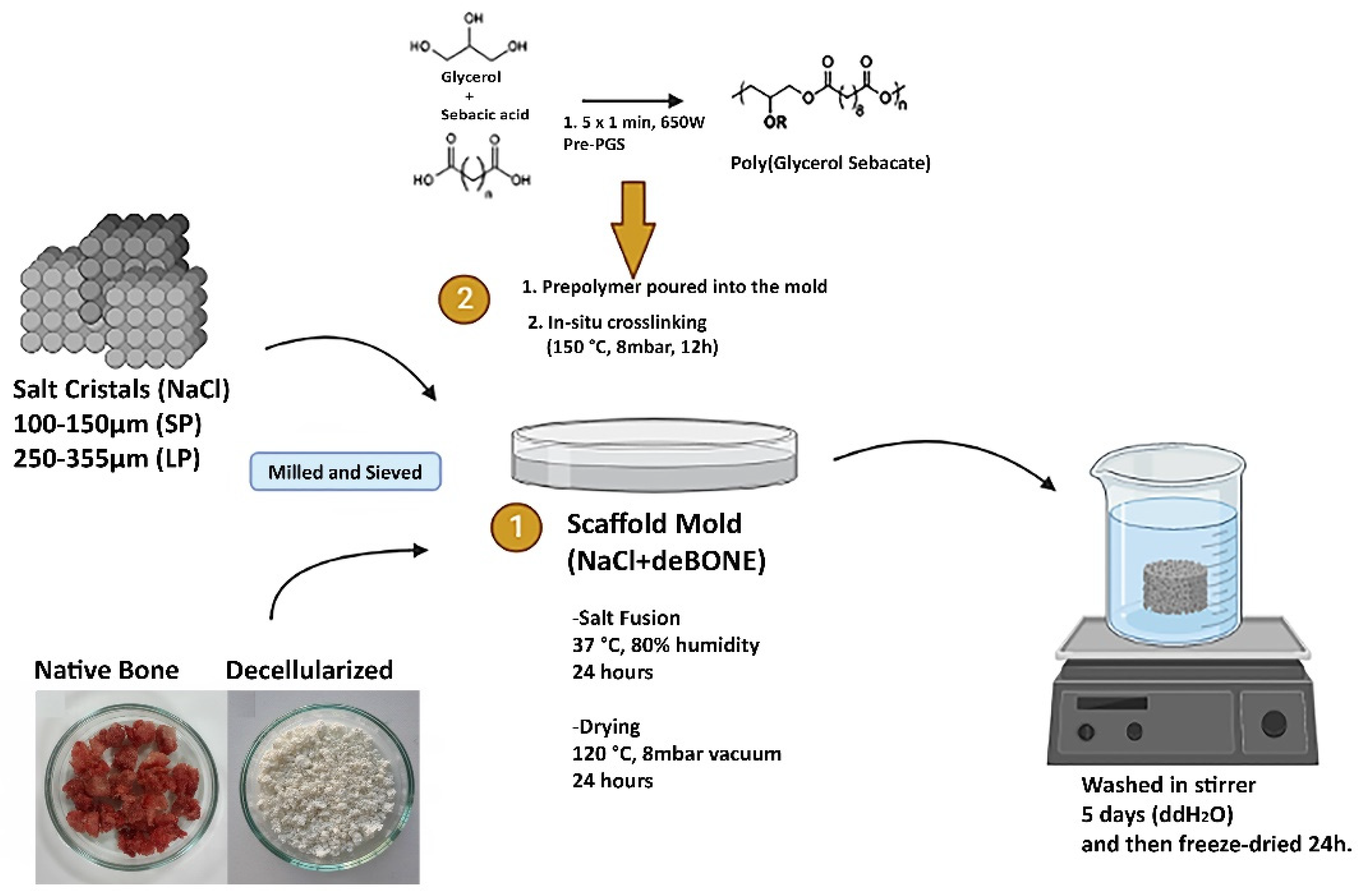
2.1. Generation of Decellularized Bone Powder
2.2. Fabrication of PGS/deB Blend Scaffolds
2.3. Characterization of PGS/deB Blend Scaffolds
2.4. Characterization of Osteogenic Differentiation
2.5. Statistical Analyses
3. Results
3.1. Characterization of the PGS-deB Blend Scaffolds
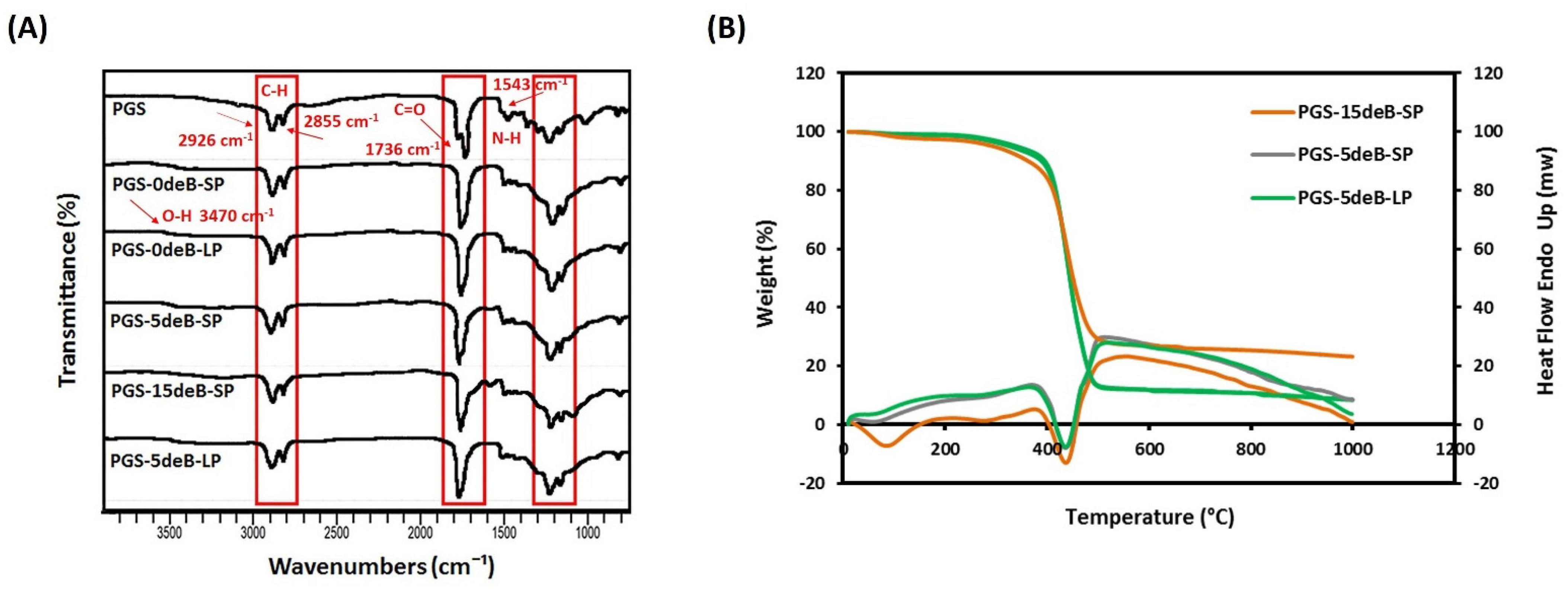
3.1.1. Physicochemical Properties
3.1.2. Scaffold Microarchitecture and Density
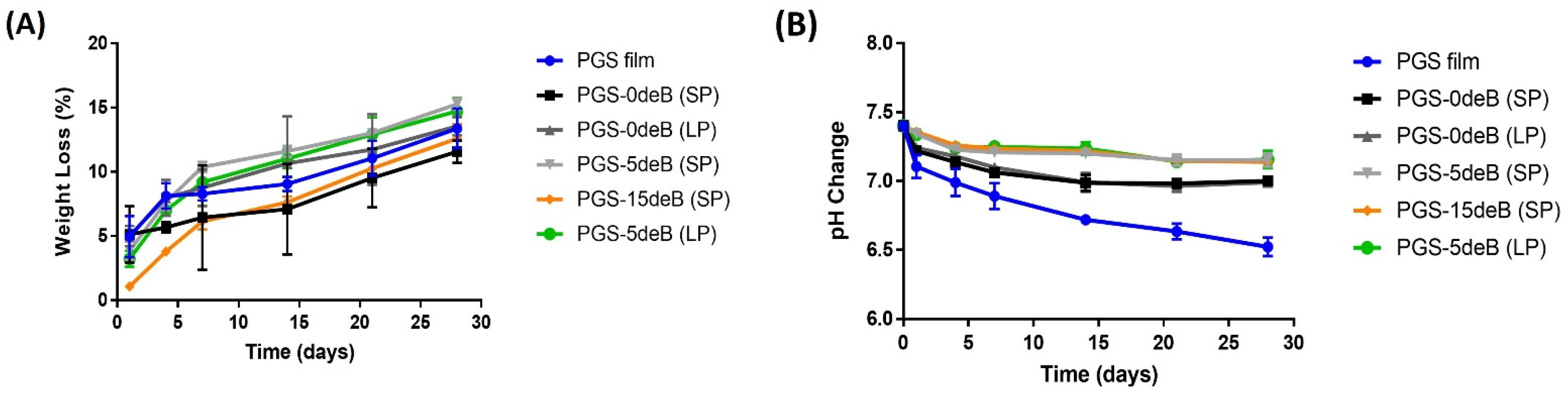
3.1.3. Degradation/pH Change of the PGS/deB Blend Scaffolds
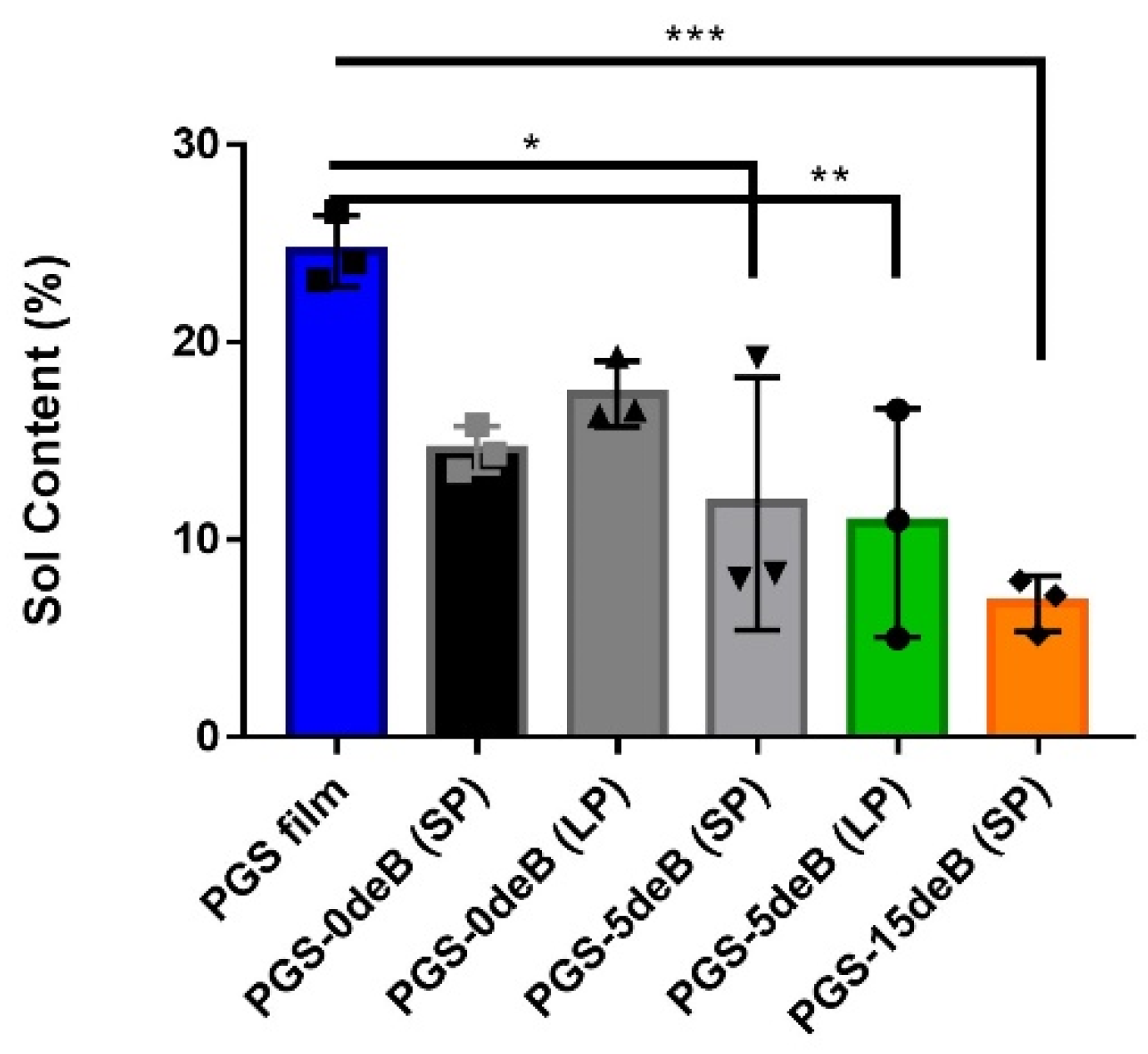
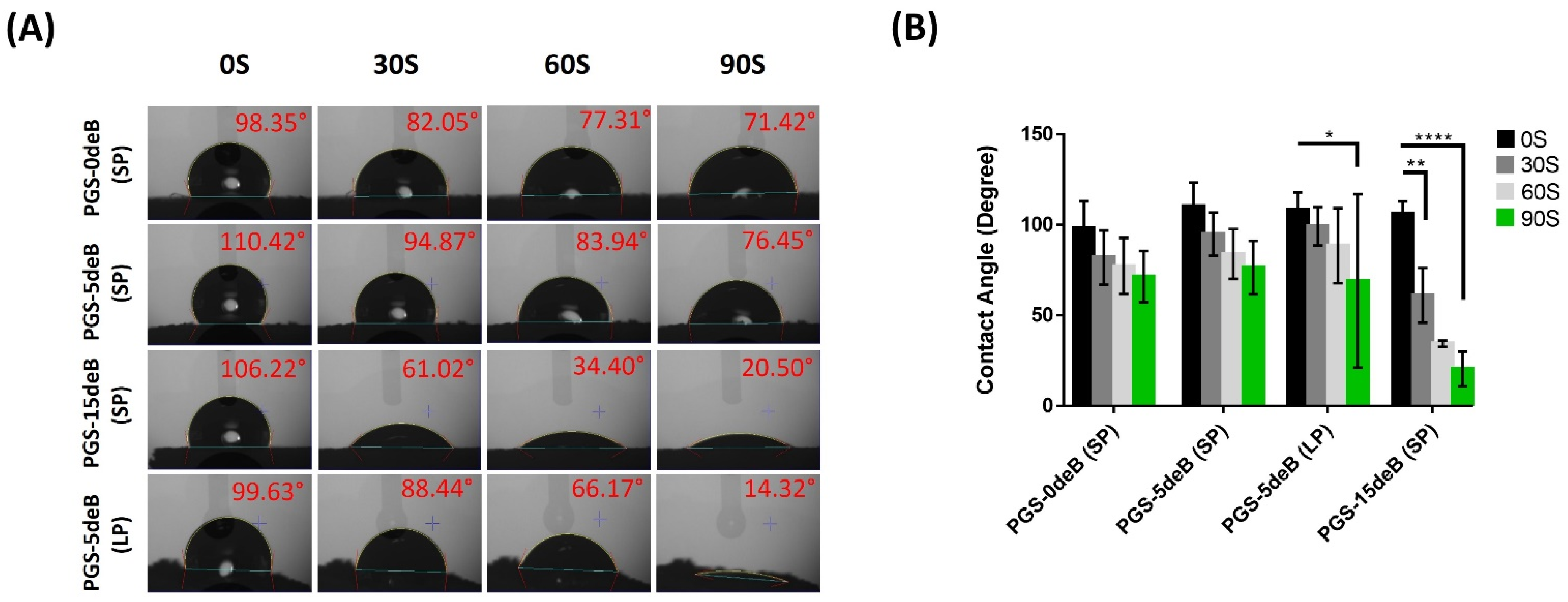
3.1.4. Bone Incorporation Enhances the Crosslinking Density and Hydrophilicity of the PGS-deB Blend Scaffolds
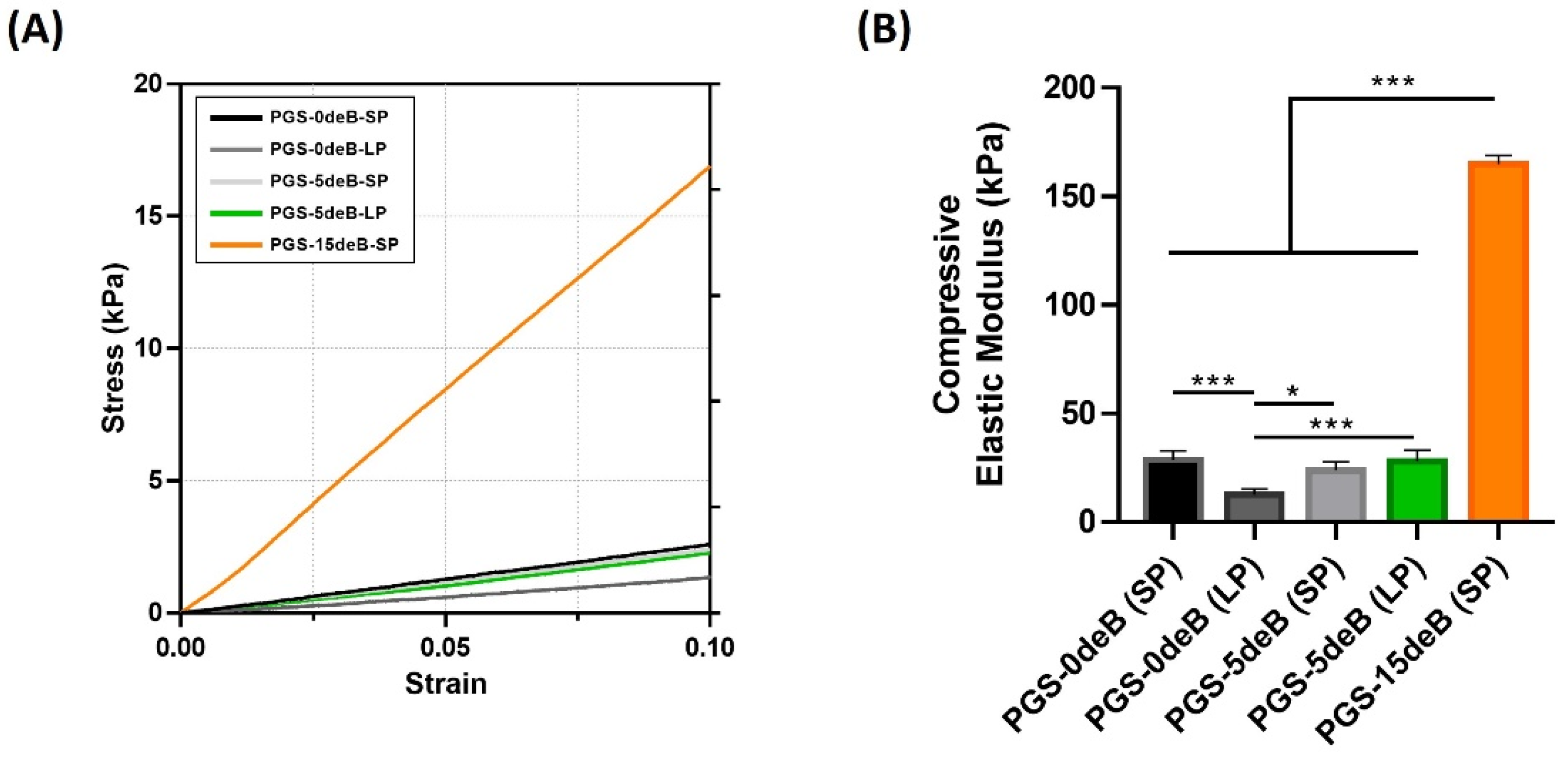
3.1.5. Bone Incorporation Enhances the Mechanical Stiffness of the PGS-deB Blend Scaffolds
3.2. Osteogenic Differentiation Potential of Isolated pBMSCs
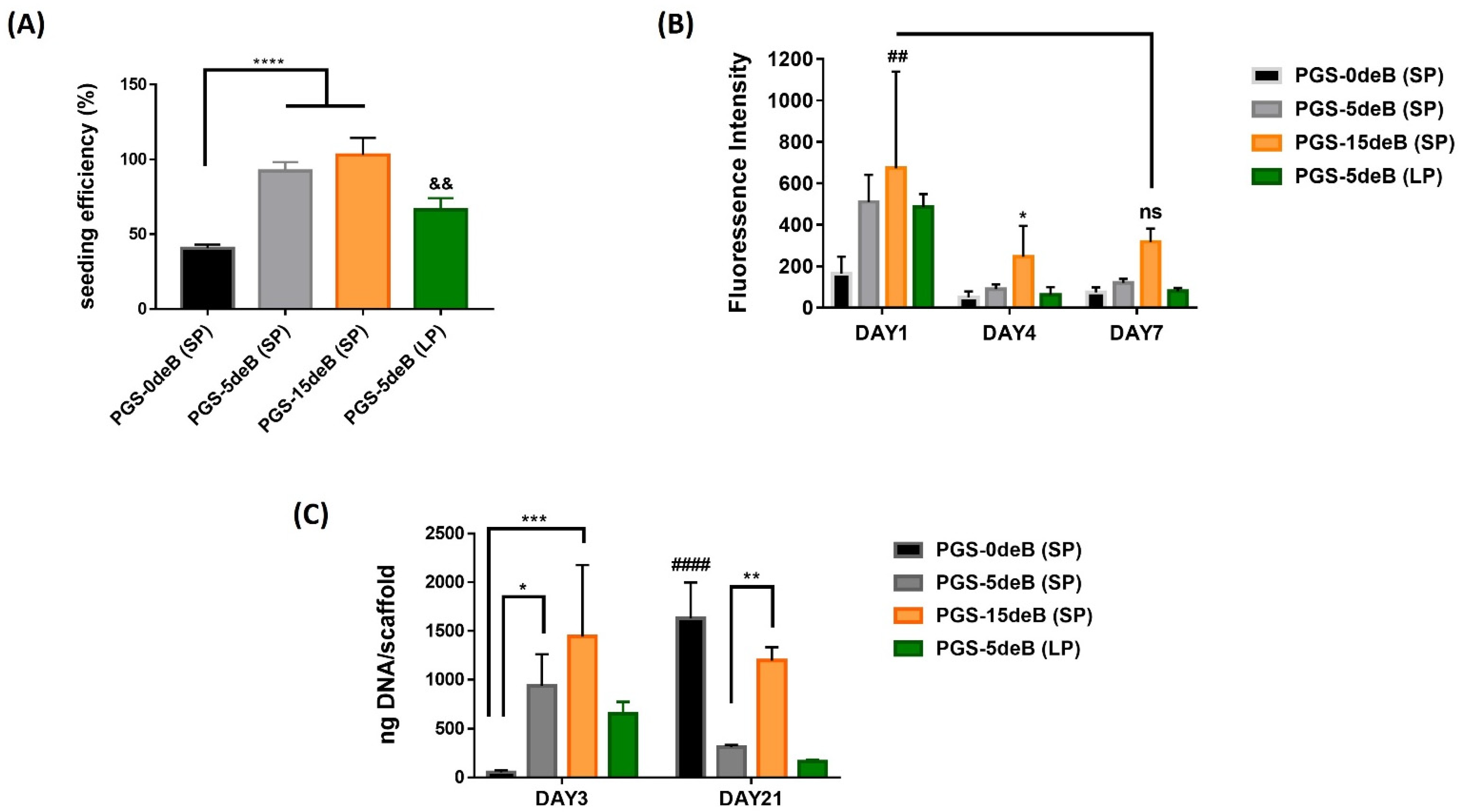
3.3. Attachment, Activity, and Proliferation of pBMSCs
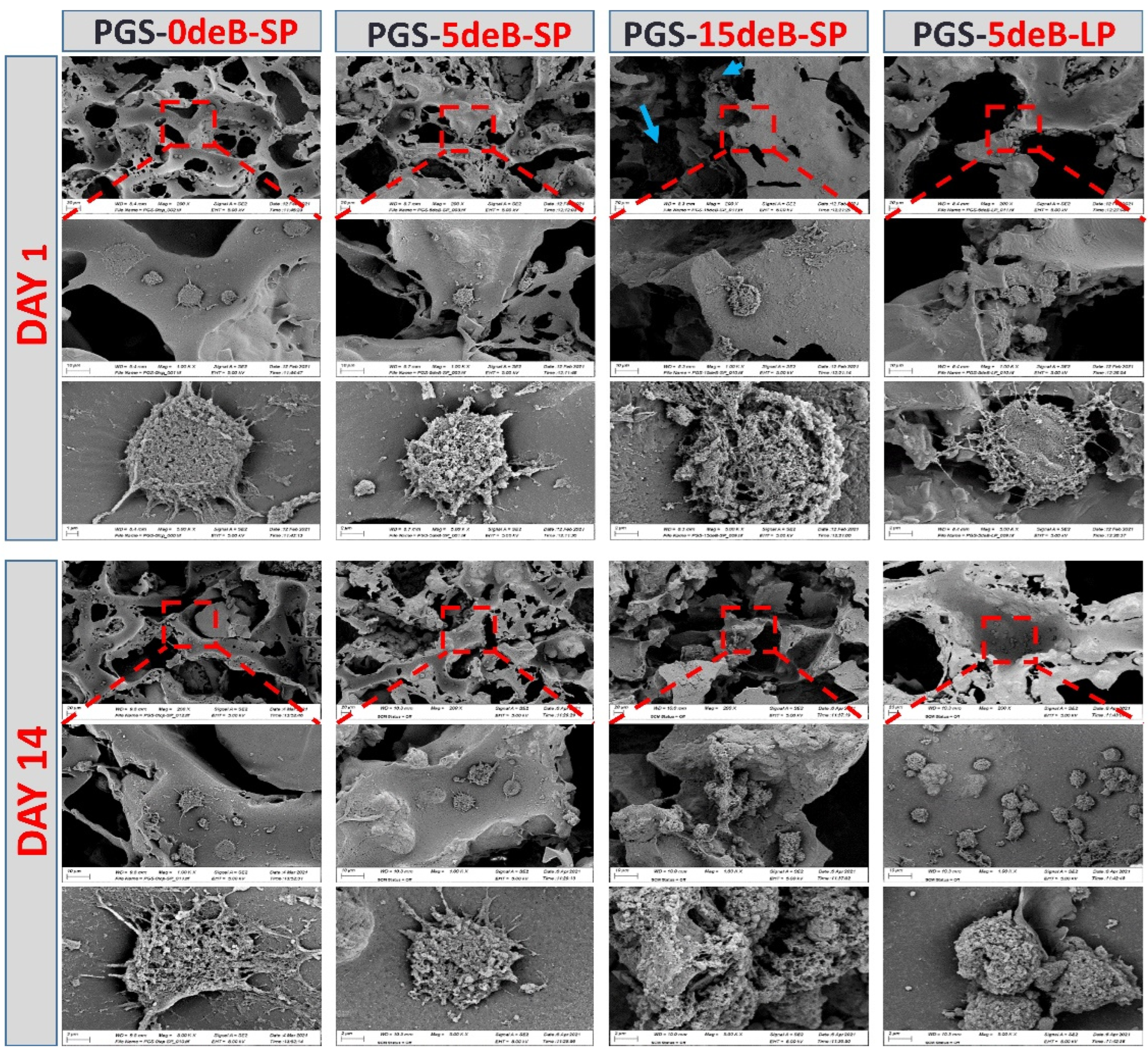
3.4. Morphological Evaluations of the Cells Seeded on the PGS-deB Blend Scaffolds
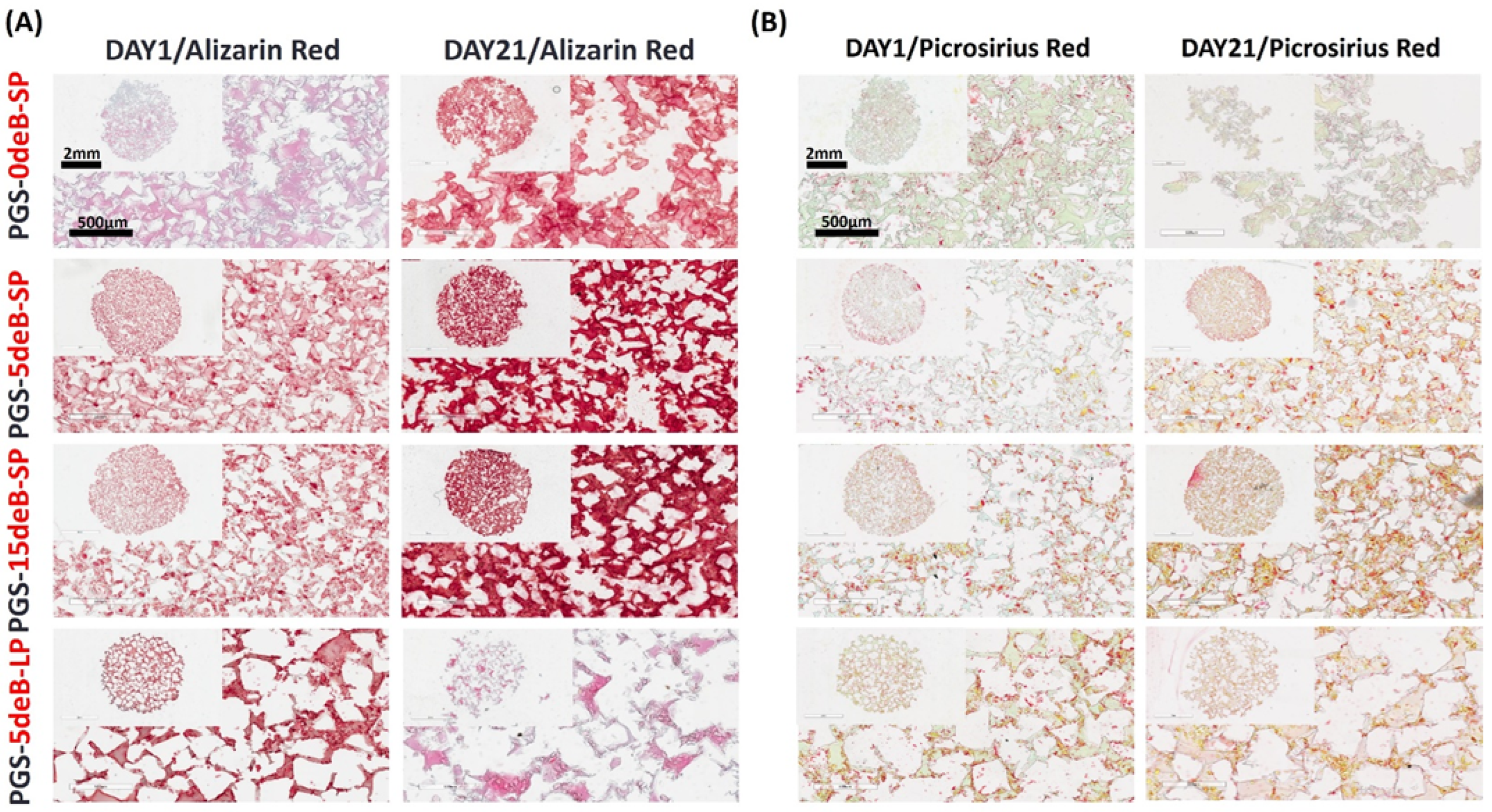
3.5. Osteogenesis of pBMSCs in the PGS-deB Blends
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mate.r Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Danzer, R. On the relationship between ceramic strength and the requirements for mechanical design. J. Eur. Ceram. Soc. 2014, 34, 3435–3460. [Google Scholar] [CrossRef]
- Klimczak, A.; Siemionow, M. Immune responses in transplantation: Application to composite tissue allograft. Semin. Plast. Surg. 2007, 21, 226–233. [Google Scholar] [CrossRef]
- Langat, D.K.; Mwenda, J.M. Potential risks of viral infections in xenotransplantation. Acta Trop. 2000, 76, 147–158. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Cunniffe, G.M.; Gonzalez-Fernandez, T.; Daly, A.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. Three-Dimensional Bioprinting of Polycaprolactone Reinforced Gene Activated Bioinks for Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 891–900. [Google Scholar] [CrossRef]
- Eichholz, K.F.; Federici, A.; Riffault, M.; Woods, I.; Mahon, O.R.; O’Driscoll, L.; Hoey, D.A. Extracellular Vesicle Functionalized Melt Electrowritten Scaffolds for Bone Tissue Engineering. Adv. NanoBiomed Res. 2021, 1, 2100037. [Google Scholar] [CrossRef]
- Atoufi, Z.; Zarrintaj, P.; Motlagh, G.H.; Amiri, A.; Bagher, Z.; Kamrava, S.K. A novel bio electro active alginate-aniline tetramer/agarose scaffold for tissue engineering: Synthesis, characterization, drug release and cell culture study. J. Biomater. Sci. Polym. Ed. 2017, 28, 1617–1638. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Phadke, A.; Hwang, Y.; Kim, S.H.; Kim, S.H.; Yamaguchi, T.; Masuda, K.; Varghese, S. Effect of scaffold microarchitecture on osteogenic differentiation of human mesenchymal stem cells. Eur. Cell Mater. 2013, 25, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, K.; Freeman, F.; Pitacco, P.; Nulty, J.; Ahern, D.; Burdis, R.; Browe, D.; Garcia, O.; Hoey, D.; Kelly, D.J. Scaffold microarchitecture regulates angiogenesis and the regeneration of large bone defects. Biofabrication 2022, 14, 45013. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Ai, C.; Liu, L.; Goh, J.C.-H. Pore size modulates in vitro osteogenesis of bone marrow mesenchymal stem cells in fibronectin/gelatin coated silk fibroin scaffolds. Mater. Sci. Eng. C 2021, 124, 112088. [Google Scholar] [CrossRef]
- Han, Y.; Lian, M.; Wu, Q.; Qiao, Z.; Sun, B.; Dai, K. Effect of Pore Size on Cell Behavior Using Melt Electrowritten Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 495. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lou, T.; Zhao, W.; Song, G.; Li, C.; Cui, G. The effect of fiber size and pore size on cell proliferation and infiltration in PLLA scaffolds on bone tissue engineering. J. Biomater. Appl. 2016, 30, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Sicchieri, L.G.; Crippa, G.E.; de Oliveira, P.T.; Beloti, M.M.; Rosa, A.L. Pore size regulates cell and tissue interactions with PLGA-CaP scaffolds used for bone engineering. J. Tissue Eng. Regen. Med. 2012, 6, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Cyster, L.A.; Grant, D.M.; Howdle, S.M.; Rose, F.; Irvine, D.J.; Freeman, D.; Scotchford, C.A.; Shakesheff, K.M. The influence of dispersant concentration on the pore morphology of hydroxyapatite ceramics for bone tissue engineering. Biomaterials 2005, 26, 697–702. [Google Scholar] [CrossRef]
- Sosnowski, S.; Wozniak, P.; Lewandowska-Szumiel, M. Polyester scaffolds with bimodal pore size distribution for tissue engineering. Macromol. Biosci. 2006, 6, 425–434. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Bismarck, A.; Hansen, U.; Junaid, S.; Tran, M.Q.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials 2008, 29, 47–57. [Google Scholar] [CrossRef]
- Zanjanizadeh Ezazi, N.; Ajdary, R.; Correia, A.; Mäkilä, E.; Salonen, J.; Kemell, M.; Hirvonen, J.; Rojas, O.J.; Ruskoaho, H.J.; Santos, H.A. Fabrication and Characterization of Drug-Loaded Conductive Poly(glycerol sebacate)/Nanoparticle-Based Composite Patch for Myocardial Infarction Applications. ACS Appl. Mater. Interfaces 2020, 12, 6899–6909. [Google Scholar] [CrossRef]
- Saudi, A.; Rafienia, M.; Zargar Kharazi, A.; Salehi, H.; Zarrabi, A.; Karevan, M. Design and fabrication of poly (glycerol sebacate)-based fibers for neural tissue engineering: Synthesis, electrospinning, and characterization. Polym. Adv. Technol. 2019, 30, 1427–1440. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, C.; Qiao, X.; Liu, T.; Sun, K. Porous poly(glycerol sebacate) (PGS) elastomer scaffolds for skin tissue engineering. Polym. Test. 2016, 54, 118–125. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, K.; Hao, J.; Yang, T.; Geng, X.; Zhang, W. Biomimetic poly(glycerol sebacate)/polycaprolactone blend scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2019, 30, 53. [Google Scholar] [CrossRef] [PubMed]
- Deniz, P.; Guler, S.; Çelik, E.; Hosseinian, P.; Aydin, H.M. Use of cyclic strain bioreactor for the upregulation of key tenocyte gene expression on Poly(glycerol-sebacate) (PGS) sheets. Mater. Sci. Eng. C 2020, 106, 110293. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.F.; Karizi, S.Z.; Samadian, H.; Nasiri, N.; Askari, H.; Asghari, M.; Frootan, F.; Bakhtiari, H.; Mahboudi, H.; Mansouri, V. Poly (glycerol sebacate) and polyhydroxybutyrate electrospun nanocomposite facilitates osteogenic differentiation of mesenchymal stem cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102796. [Google Scholar] [CrossRef]
- Zaky, S.H.; Lee, K.W.; Gao, J.; Jensen, A.; Verdelis, K.; Wang, Y.; Almarza, A.J.; Sfeir, C. Poly (glycerol sebacate) elastomer supports bone regeneration by its mechanical properties being closer to osteoid tissue rather than to mature bone. Acta Biomater. 2017, 54, 95–106. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, J.; Ma, X.Y.; Ma, Y.F.; Kan, C.; Ma, H.Y.; Li, Y.L.; Yuan, Y.; Liu, C.S. beta-Tricalcium phosphate/poly(glycerol sebacate) scaffolds with robust mechanical property for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 37–47. [Google Scholar] [CrossRef]
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Healthc. Mater. 2021, 10, 2002026. [Google Scholar] [CrossRef]
- Chi, H.; Chen, G.; He, Y.; Chen, G.; Tu, H.; Liu, X.; Yan, J.; Wang, X. 3D-HA Scaffold Functionalized by Extracellular Matrix of Stem Cells Promotes Bone Repair. Int. J. Nanomed. 2020, 15, 5825–5838. [Google Scholar] [CrossRef]
- Rindone, A.N.; Nyberg, E.; Grayson, W.L. 3D-Printing Composite Polycaprolactone-Decellularized Bone Matrix Scaffolds for Bone Tissue Engineering Applications. In Decellularized Scaffolds and Organogenesis: Methods and Protocols; Turksen, K., Ed.; Springer New York: New York, NY, USA, 2018; pp. 209–226. [Google Scholar]
- Datta, N.; Holtorf, H.L.; Sikavitsas, V.I.; Jansen, J.A.; Mikos, A.G. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials 2005, 26, 971–977. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Ahn, G.; Kim, C.; Lee, J.-S.; Lee, I.-G.; An, S.-H.; Yun, W.-S.; Kim, S.-Y.; Shim, J.-H. Synergistic Effects of Beta Tri-Calcium Phosphate and Porcine-Derived Decellularized Bone Extracellular Matrix in 3D-Printed Polycaprolactone Scaffold on Bone Regeneration. Macromol. Biosci. 2018, 18, 1800025. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, T.H.; Ryu, J.Y.; Kim, D.K.; Oh, E.J.; Kim, H.M.; Shim, J.-H.; Yun, W.-S.; Huh, J.B.; Moon, S.H.; et al. Osteogenesis of 3D-Printed PCL/TCP/bdECM Scaffold Using Adipose-Derived Stem Cells Aggregates; An Experimental Study in the Canine Mandible. Int. J. Mol. Sci. 2021, 22, 5409. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Browe, D.C.; Nulty, J.; Von Euw, S.; Grayson, W.L.; Kelly, D.J. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur. Cell Mater. 2019, 38, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Qiao, F.; Chen, G.; Lv, Y. Demineralized and decellularized bone extracellular matrix-incorporated electrospun nanofibrous scaffold for bone regeneration. J. Mater. Chem. B 2021, 9, 6881–6894. [Google Scholar] [CrossRef] [PubMed]
- Sawkins, M.J.; Bowen, W.; Dhadda, P.; Markides, H.; Sidney, L.E.; Taylor, A.J.; Rose, F.R.; Badylak, S.F.; Shakesheff, K.M.; White, L.J. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater 2013, 9, 7865–7873. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.M.; Salimi, K.; Rzayev, Z.M.O.; Pişkin, E. Microwave-assisted rapid synthesis of poly(glycerol-sebacate) elastomers. Biomater. Sci. 2013, 1, 503–509. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Q.; Pan, Y.; Yao, Y.; Tang, S.; Su, J.; Shin, J.W.; Wei, J.; Zhao, J. Nanoporosity improved water absorption, in vitro degradability, mineralization, osteoblast responses and drug release of poly(butylene succinate)-based composite scaffolds containing nanoporous magnesium silicate compared with magnesium silicate. Int. J. Nanomed. 2017, 12, 3637–3651. [Google Scholar] [CrossRef]
- Browe, D.C.; Mahon, O.R.; Díaz-Payno, P.J.; Cassidy, N.; Dudurych, I.; Dunne, A.; Buckley, C.T.; Kelly, D.J. Glyoxal cross-linking of solubilized extracellular matrix to produce highly porous, elastic, and chondro-permissive scaffolds for orthopedic tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 2222–2234. [Google Scholar] [CrossRef]
- Strobel, H.A.; Calamari, E.L.; Alphonse, B.; Hookway, T.A.; Rolle, M.W. Fabrication of Custom Agarose Wells for Cell Seeding and Tissue Ring Self-assembly Using 3D-Printed Molds. J. Vis. Exp. 2018, 134, e56618. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, K.F.; Hoey, D.A. Mediating human stem cell behaviour via defined fibrous architectures by melt electrospinning writing. Acta Biomater. 2018, 75, 140–151. [Google Scholar] [CrossRef]
- Lee, H.-p.; Gu, L.; Mooney, D.J.; Levenston, M.E.; Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017, 16, 1243–1251. [Google Scholar] [CrossRef]
- Jaafar, I.H.; Ammar, M.M.; Jedlicka, S.S.; Pearson, R.A.; Coulter, J.P. Spectroscopic evaluation, thermal, and thermomechanical characterization of poly(glycerol-sebacate) with variations in curing temperatures and durations. J. Mater. Sci. 2010, 45, 2525–2529. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Mendelsohn, R.; Boskey, A.L. Infrared assessment of bone quality: A review. Clin. Orthop. Relat. Res. 2011, 469, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Ali, S.; Khatri, Z.; Oh, K.W.; Kim, I.S.; Kim, S.H. Zein/cellulose acetate hybrid nanofibers: Electrospinning and characterization. Macromol. Res. 2014, 22, 971–977. [Google Scholar] [CrossRef]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shu, M.; Yan, J.; Liu, X.; Wang, R.; Hou, Z.; Lin, J. Luminescent net-like inorganic scaffolds with europium-doped hydroxyapatite for enhanced bone reconstruction. Nanoscale 2021, 13, 1181–1194. [Google Scholar] [CrossRef]
- Marupanthorn, K.; Tantrawatpan, C.; Kheolamai, P.; Tantikanlayaporn, D.; Manochantr, S. Bone morphogenetic protein-2 enhances the osteogenic differentiation capacity of mesenchymal stromal cells derived from human bone marrow and umbilical cord. Int. J. Mol. Med. 2017, 39, 654–662. [Google Scholar] [CrossRef]
- Król-Morkisz, K.; Pielichowska, K. 13—Thermal Decomposition of Polymer Nanocomposites With Functionalized Nanoparticles. In Polymer Composites with Functionalized Nanoparticles; Pielichowski, K., Majka, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 405–435. [Google Scholar]
- Witkowski, A.; Stec, A.A.; Hull, T.R. Thermal Decomposition of Polymeric Materials. In SFPE Handbook of Fire Protection Engineering; Hurley, M.J., Gottuk, D., Hall, J.R., Harada, K., Kuligowski, E., Puchovsky, M., Torero, J., Watts, J.M., Wieczorek, C., Eds.; Springer New York: New York, NY, USA, 2016; pp. 167–254. [Google Scholar]
- Kerativitayanan, P.; Gaharwar, A.K. Elastomeric and mechanically stiff nanocomposites from poly(glycerol sebacate) and bioactive nanosilicates. Acta Biomater. 2015, 26, 34–44. [Google Scholar] [CrossRef]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Yan, C. 3D-printed cellular structures for bone biomimetic implants. Addit. Manuf. 2017, 15, 93–101. [Google Scholar] [CrossRef]
- Frydrych, M.; Román, S.; MacNeil, S.; Chen, B. Biomimetic poly(glycerol sebacate)/poly(l-lactic acid) blend scaffolds for adipose tissue engineering. Acta Biomater. 2015, 18, 40–49. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro. Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Alamán-Díez, P.; García-Gareta, E.; Napal, P.F.; Arruebo, M.; Pérez, M. In Vitro Hydrolytic Degradation of Polyester-Based Scaffolds under Static and Dynamic Conditions in a Customized Perfusion Bioreactor. Materials 2022, 15, 2572. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, Y.M.; Langer, R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res. Part A 2003, 66, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-L.; Yang, X.-Y.; Fang, X.-Y.; Cook, W.D.; Thouas, G.A.; Chen, Q.-Z. In Vitro enzymatic degradation of poly (glycerol sebacate)-based materials. Biomaterials 2011, 32, 8486–8496. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.A.; Mikos, A.G.; Kasper, F.K. Scaffold/Extracellular matrix hybrid constructs for bone-tissue engineering. Adv. Healthc. Mater. 2013, 2, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014, 32, 462–484. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Martínez, R.; Angelo, A.P.; Pujol, F.V. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem Cell Res. Ther. 2017, 8, 265. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L.; Chen, W.; Liu, X.; Weir, M.D.; Xu, H.H. Stem Cells and Calcium Phosphate Cement Scaffolds for Bone Regeneration. J. Dent. Res. 2014, 93, 618–625. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—Facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q.; Wang, S.; Huo, B. Spreading Shape and Area Regulate the Osteogenesis of Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2019, 16, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Ivanovski, S.; Gulati, K.; Love, R.M.; Hamlet, S. Role of offset and gradient architectures of 3-D melt electrowritten scaffold on differentiation and mineralization of osteoblasts. Biomater. Res. 2020, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Bhatt, A.D. A review on design of scaffold for osteoinduction: Toward the unification of independent design variables. Biomech Model Mechanobiol 2022. [CrossRef] [PubMed]
- Di Luca, A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci. Rep. 2016, 6, 22898. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.M.; Eichholz, K.F.; Hoey, D.A. The effect of pore size within fibrous scaffolds fabricated using melt electrowriting on human bone marrow stem cell osteogenesis. Biomed. Mater. 2019, 14, 065016. [Google Scholar] [CrossRef]
- Wo, J.; Huang, S.S.; Wu, D.Y.; Zhu, J.; Li, Z.Z.; Yuan, F. The integration of pore size and porosity distribution on Ti-6A1-4V scaffolds by 3D printing in the modulation of osteo-differentation. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020934652. [Google Scholar] [CrossRef]
- Shi, F.; Xiao, D.; Zhang, C.; Zhi, W.; Liu, Y.; Weng, J. The effect of macropore size of hydroxyapatite scaffold on the osteogenic differentiation of bone mesenchymal stem cells under perfusion culture. Regen. Biomater. 2021, 8, rbab050. [Google Scholar] [CrossRef]




| Sample | Design Specification (wt%) | Decomposition Temperatures (T10%; T25%; Tf) (°C) | Residue Concentration at Tf (wt%) |
|---|---|---|---|
| PGS-0deB-SP | 0 | 362.75; 408.25; 490.50 | 2.913 |
| PGS-5deB-SP | 14 | 396.033; 422.675; 492.749 | 13.466 |
| PGS-15deB-SP | 28 | 362.745; 420.491; 505.517 | 28.822 |
| PGS-0deB-LP | 0 | 379.57; 422.21; 500.15 | 3.056 |
| PGS-5deB-LP | 14 | 389.041; 422.189; 495.293 | 13.849 |
| Sample Code | Pore Size (μm) | PGS/NaCl/deB Volume Ratio VPGS:VNaCl:VdeB | PGS/deB Weight Ratio wt%PGS:wt%deB (in Final Scaffold) | Porosity (%) |
|---|---|---|---|---|
| PGS-0deB-SP | 151.45 ± 31.32 | 30:70:0 | - | 65.67 ± 1.38 |
| PGS-0deB-LP | nd | 30:70:0 | - | 64.67 ± 4.45 |
| PGS-5deB-SP | 107.73 ± 15.93 | 30:65:5 | 87:13 | 65.37 ± 0.81 |
| PGS-5deB-LP | 246.79 ± 26.58 | 30:65:5 | 87:13 | 71.39 ± 9.22 |
| PGS-15deB-SP | 138.32 ± 19.42 | 30:55:15 | 72:28 | 71.30 ± 6.27 |
| Sample | Sol (wt%) | Gel (wt%) |
|---|---|---|
| PGS | 24.59 | 75.40 |
| PGS-0deB-SP | 14.56 | 85.43 |
| PGS-0deB-LP | 17.37 | 82.62 |
| PGS-5deB-SP | 11.82 | 88.18 |
| PGS-5deB-LP | 10.85 | 89.15 |
| PGS-15deB-SP | 6.755 | 93.25 |
| Material Type | Cell Type | Superior Osteogenesis Pore Size |
|---|---|---|
| PEOT/PBT and PCL | hMSCs | Gradient (500 μm to 1000 μm) [77] |
| PCL | hMSCs | Small (100 μm) [78] |
| PCL | hOB | Gradient + Heterogenous offset (250 μm–500 μm–750 μm) [75] |
| Ti-6Al-4V | MC3T3-E1 | Small (300 μm) [79] |
| HAp | BMSCs | Large (1300 μm-800 μm) [80] |
| PCL + DCB | BMSCs | Small (800 μm) and Large (1200 μm) * [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guler, S.; Eichholz, K.; Chariyev-Prinz, F.; Pitacco, P.; Aydin, H.M.; Kelly, D.J.; Vargel, İ. Biofabrication of Poly(glycerol sebacate) Scaffolds Functionalized with a Decellularized Bone Extracellular Matrix for Bone Tissue Engineering. Bioengineering 2023, 10, 30. https://doi.org/10.3390/bioengineering10010030
Guler S, Eichholz K, Chariyev-Prinz F, Pitacco P, Aydin HM, Kelly DJ, Vargel İ. Biofabrication of Poly(glycerol sebacate) Scaffolds Functionalized with a Decellularized Bone Extracellular Matrix for Bone Tissue Engineering. Bioengineering. 2023; 10(1):30. https://doi.org/10.3390/bioengineering10010030
Chicago/Turabian StyleGuler, Selcan, Kian Eichholz, Farhad Chariyev-Prinz, Pierluca Pitacco, Halil Murat Aydin, Daniel J. Kelly, and İbrahim Vargel. 2023. "Biofabrication of Poly(glycerol sebacate) Scaffolds Functionalized with a Decellularized Bone Extracellular Matrix for Bone Tissue Engineering" Bioengineering 10, no. 1: 30. https://doi.org/10.3390/bioengineering10010030
APA StyleGuler, S., Eichholz, K., Chariyev-Prinz, F., Pitacco, P., Aydin, H. M., Kelly, D. J., & Vargel, İ. (2023). Biofabrication of Poly(glycerol sebacate) Scaffolds Functionalized with a Decellularized Bone Extracellular Matrix for Bone Tissue Engineering. Bioengineering, 10(1), 30. https://doi.org/10.3390/bioengineering10010030






