Advances in Spinal Cord Stimulation
Abstract
1. Introduction
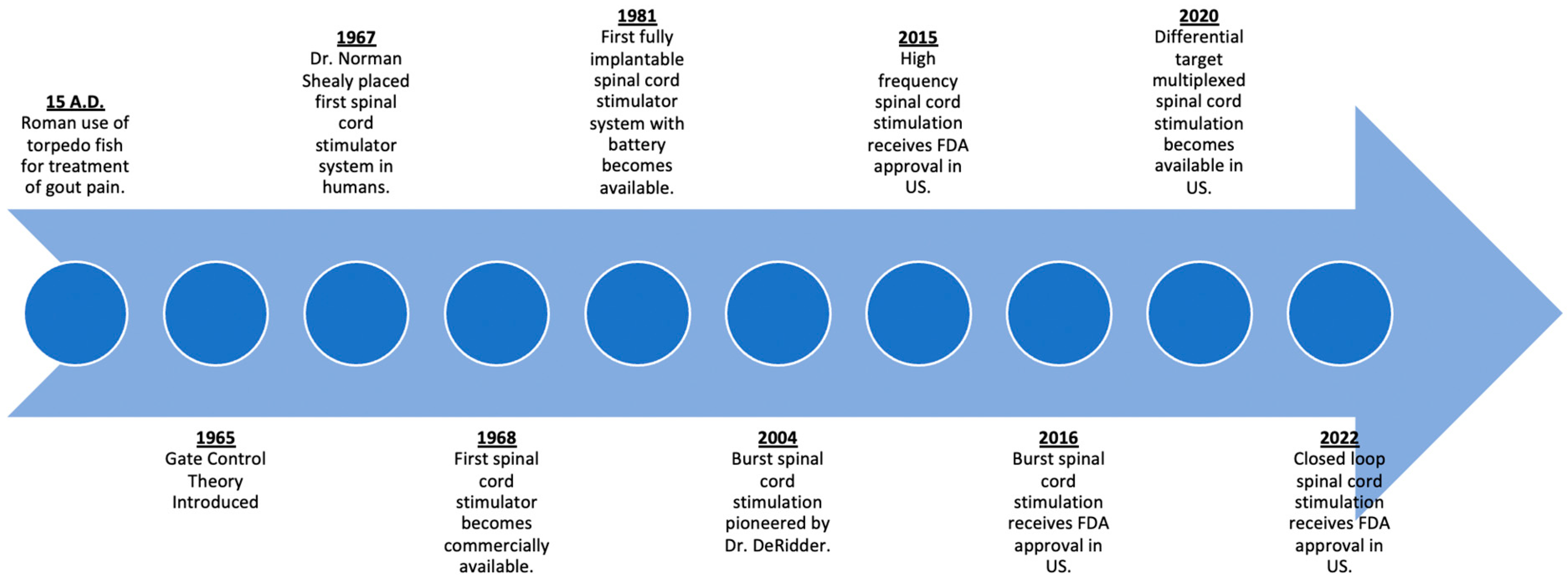
2. History of Spinal Cord Stimulation
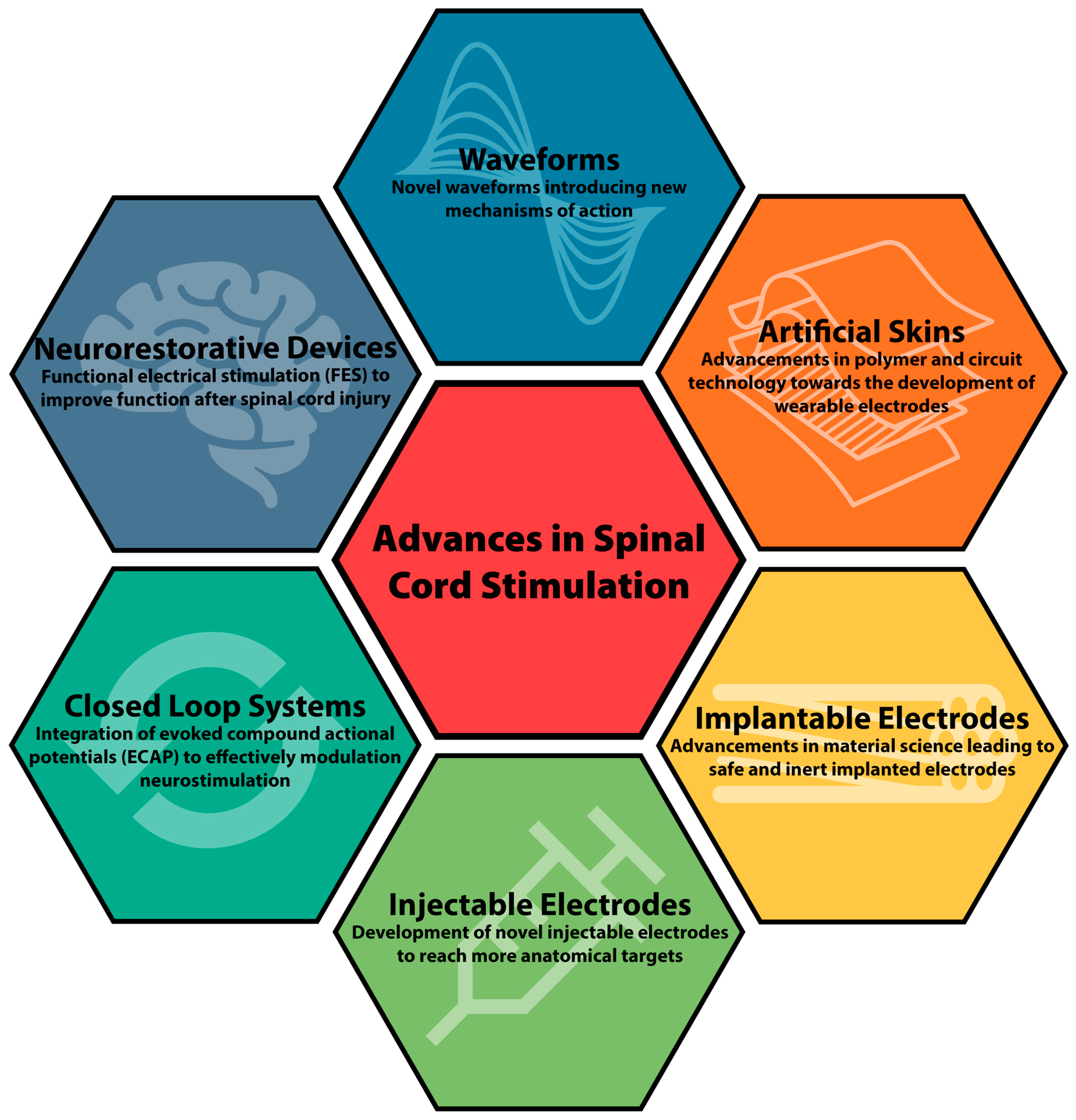
3. Waveforms
4. Artificial Skins as Electrodes
5. Implantable Electrode Material
6. Injectable Electrodes
7. Closed-Loop Systems
8. Neurorestorative Devices
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2021, 163, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Kaye, A.M.; Knezevic, N.N.; McAnally, H.; Slavin, K.; Trescot, A.M.; Blank, S.; Pampati, V.; Abdi, S.; Grider, J.S.; et al. Responsible, Safe, and Effective Prescription of Opioids for Chronic Non-Cancer Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2017, 20, S3–S92. [Google Scholar] [CrossRef]
- Lee, A.W.; Pilitsis, J.G. Spinal cord stimulation: Indications and outcomes. Neurosurg. Focus 2006, 21, E3. [Google Scholar] [CrossRef]
- Petersen, E.A.; Stauss, T.; Scowcroft, J.; Brooks, E.; White, J.; Sills, S.; Amirdelfan, K.; Guirguis, M.; Xu, J.; Yu, C.; et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients with Painful Diabetic Neuropathy: A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 687–698. [Google Scholar] [CrossRef]
- Al-Kaisy, A.; Van Buyten, J.P.; Kapural, L.; Amirdelfan, K.; Gliner, B.; Caraway, D.; Subbaroyan, J.; Edgar, D.; Rotte, A. 10 kHz spinal cord stimulation for the treatment of non-surgical refractory back pain: Subanalysis of pooled data from two prospective studies. Anaesthesia 2020, 75, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Gildenberg, P.L. Evolution of spinal cord surgery for pain. Clin. Neurosurg. 2006, 53, 11–17. [Google Scholar]
- Nahm, F.S. From the torpedo fish to the spinal cord stimulator. Korean J. Pain 2020, 33, 97–98. [Google Scholar] [CrossRef]
- Teoli, D.; An, J. Transcutaneous Electrical Nerve Stimulation; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gildenberg, P.L. History of Electrical Neuromodulation for Chronic Pain. Pain Med. 2006, 7, S7–S13. [Google Scholar] [CrossRef]
- Deer, T.R.; Lamer, T.J.; Pope, J.E.; Falowski, S.M.; Provenzano, D.A.; Slavin, K.; Golovac, S.; Arle, J.; Rosenow, J.M.; Williams, K.; et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Safety Guidelines for the Reduction of Severe Neurological Injury. Neuromodulation 2017, 20, 15–30. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Wall, P.D. Presynaptic Control of Impulses at the First Central Synapse in the Cutaneous Pathway. Prog. Brain Res. 1964, 12, 92–118. [Google Scholar] [CrossRef] [PubMed]
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth. Analg. 1967, 46, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Caylor, J.; Reddy, R.; Yin, S.; Cui, C.; Huang, M.; Huang, C.; Rao, R.; Baker, D.G.; Simmons, A.; Souza, D.; et al. Spinal cord stimulation in chronic pain: Evidence and theory for mechanisms of action. Bioelectron. Med. 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, D.; Kagan, Z.; Wang, D.; Bradley, K. Differential Modulation of Dorsal Horn Neurons by Various Spinal Cord Stimulation Strategies. Biomedicines 2021, 9, 568. [Google Scholar] [CrossRef]
- Chakravarthy, K.; Fishman, M.A.; Zuidema, X.; Hunter, C.W.; Levy, R. Mechanism of Action in Burst Spinal Cord Stimulation: Review and Recent Advances. Pain Med. 2019, 20 (Suppl. S1), S13–S22. [Google Scholar] [CrossRef]
- Vallejo, R.; Bradley, K.; Kapural, L. Spinal Cord Stimulation in Chronic Pain: Mode of Action. Spine 2017, 42 (Suppl. S14), S53–S60. [Google Scholar] [CrossRef]
- Erickson, D.L. Percutaneous trial of stimulation for patient selection for implantable stimulating devices. J. Neurosurg. 1975, 43, 440–444. [Google Scholar] [CrossRef]
- Zumpano, B.J.; Saunders, R.L. Percutaneous epidural dorsal column stimulation. Technical note. J. Neurosurg. 1976, 45, 459–460. [Google Scholar] [CrossRef]
- North, R.B.; Fischell, T.A.; Long, D.M. Chronic Dorsal Column Stimulation via Percutaneously Inserted Epidural Electrodes. Preliminary results in 31 patients. Appl. Neurophysiol. 1977, 40, 184–191. [Google Scholar] [CrossRef]
- Hussaini, S.M.Q.; Murphy, K.R.; Han, J.L.; Elsamadicy, A.A.; Yang, S.; Premji, A.; Parente, B.; Xie, J.; Pagadala, P.; Lad, S.P. Specialty-Based Variations in Spinal Cord Stimulation Success Rates for Treatment of Chronic Pain. Neuromodulation 2017, 20, 340–347. [Google Scholar] [CrossRef]
- North, R.B.; Ewend, M.; Lawton, M.; Piantadosi, S. Spinal cord stimulation for chronic, intractable pain: Superiority of “multi-channel” devices. Pain 1991, 44, 119–130. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Kidd, D.; Zahurak, M.; James, C.; Long, D. Spinal cord stimulation for chronic, intractable pain: Experience over two decades. Neurosurgery 1993, 32, 384–394; discussion 394–395. [Google Scholar] [CrossRef]
- Hosobuchi, Y.; Adams, J.E.; Weinstein, P.R. Preliminary percutaneous dorsal column stimulation prior to permanent implantation. Technical note. J. Neurosurg. 1972, 37, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Kapural, L.; Yu, C.; Doust, M.; Gliner, B.; Vallejo, R.; Sitzman, B.; Amirdelfan, K.; Morgan, D.; Brown, L.; Yearwood, T.; et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef]
- Lee, K.Y.; Bae, C.; Lee, D.; Kagan, Z.; Bradley, K.; Chung, J.M.; La, J.-H. Low-intensity, Kilohertz Frequency Spinal Cord Stimulation Differently Affects Excitatory and Inhibitory Neurons in the Rodent Superficial Dorsal Horn. Neuroscience 2020, 428, 132–139. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, D.; Wang, D.; Kagan, Z.B.; Bradley, K. Simultaneous 10 kHz and 40 Hz spinal cord stimulation increases dorsal horn inhibitory interneuron activity. Neurosci. Lett. 2022, 782, 136705. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S.; Plazier, M.; Vancamp, T. Mimicking the brain: Evaluation of St Jude Medical’s Prodigy Chronic Pain System with Burst Technology. Expert Rev. Med. Devices 2015, 12, 143–150. [Google Scholar] [CrossRef]
- De Ridder, D.; Vancamp, T.; Falowski, S.M.; Vanneste, S. All bursts are equal, but some are more equal (to burst firing): BurstDR stimulation versus Boston burst stimulation. Expert Rev. Med. Devices 2020, 17, 289–295. [Google Scholar] [CrossRef]
- Deer, T.; Slavin, K.V.; Amirdelfan, K.; North, R.B.; Burton, A.W.; Yearwood, T.L.; Tavel, E.; Staats, P.; Falowski, S.; Pope, J.; et al. Success Using Neuromodulation with BURST (SUNBURST) Study: Results from a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation 2018, 21, 56–66. [Google Scholar] [CrossRef]
- Kirketeig, T.; Schultheis, C.; Zuidema, X.; Hunter, C.W.; Deer, T. Burst Spinal Cord Stimulation: A Clinical Review. Pain Med. 2019, 20 (Suppl. S1), S31–S40. [Google Scholar] [CrossRef]
- Smith, W.J.; Cedeño, D.L.; Thomas, S.M.; Kelley, C.A.; Vetri, F.; Vallejo, R. Modulation of microglial activation states by spinal cord stimulation in an animal model of neuropathic pain: Comparing high rate, low rate, and differential target multiplexed programming. Mol. Pain 2021, 17, 1744806921999013. [Google Scholar] [CrossRef]
- Vallejo, R.; Kelley, C.A.; Gupta, A.; Smith, W.J.; Vallejo, A.; Cedeño, D.L. Modulation of neuroglial interactions using differential target multiplexed spinal cord stimulation in an animal model of neuropathic pain. Mol. Pain 2020, 16, 1744806920918057. [Google Scholar] [CrossRef]
- Vallejo, R. Method and Apparatus for Multi Modal or Multiplexed Electrical Modulation of Pain. (U.S. Patent No. US 2018/0243563). U.S. Patent and Trademark Office. 2018. Available online: https://patentimages.storage.googleapis.com/82/75/c2/3b28dc6e0fde62/US20180243563A1.pdf (accessed on 19 October 2022).
- Fishman, M.; Cordner, H.; Justiz, R.; Provenzano, D.; Merrell, C.; Shah, B.; Naranjo, J.; Kim, P.; Calodney, A.; Carlson, J.; et al. Twelve-Month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Pract. 2021, 21, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Rizvi, S.; Nguyen, R.; Abbas, M.; Bishop, S.; Murthy, V. Impact of Wait times on Spinal Cord Stimulation Therapy Outcomes. Pain Pract. 2014, 14, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Amagai, M. Toward a new generation of smart skins. Nat. Biotechnol. 2019, 37, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Liu, S.; Qu, Q.; Zhang, Y.; Zhao, M.; Liu, J. Bio-inspired flexible electronics for smart E-skin. Acta Biomater. 2022, 139, 280–295. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwödiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458–463. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Wang, W.; Wang, G.-J.N.; Rastak, R.; Molina-Lopez, F.; Chung, J.W.; Niu, S.; Feig, V.R.; Lopez, J.; et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 2018, 555, 83–88. [Google Scholar] [CrossRef]
- Miyamoto, A.; Lee, S.; Cooray, N.F.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913. [Google Scholar] [CrossRef]
- Jalili, R.; Kanneganti, A.; Romero-Ortega, M.I.; Wallace, G.G. Implantable electrodes. Curr. Opin. Electrochem. 2017, 3, 68–74. [Google Scholar] [CrossRef]
- Ochani, T.D.; Almirante, J.; Siddiqui, A.; Kaplan, R. Allergic Reaction to Spinal Cord Stimulator. Clin. J. Pain 2000, 16, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, T.; Sharma, S.; Aner, M.; Gill, J.S. The Long-Term Durability of Multilumen Concentric Percutaneous Spinal Cord Stimulator Leads. Pain Pract. 2018, 18, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Durand, D.M.; Ghovanloo, M.; Krames, E. Time to address the problems at the neural interface. J. Neural Eng. 2014, 11, 020201. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Matharu, M.S. The Use of Electroceuticals and Neuromodulation in the Treatment of Migraine and Other Headaches. In Electroceuticals: Advances in Electrostimulation Therapies; Majid, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–33. [Google Scholar]
- Luo, Y.; Sun, S.; Xie, S. Spinal cord stimulators: Implanted electrode materials, power transfer mechanism, and their applications. In Proceedings of the International Conference on Optoelectronic Materials and Devices (ICOD 2021), Guangzhou, China, 10–12 December 2021; Volume 1216412. [Google Scholar]
- Cogan, S.; Troyk, P.; Ehrlich, J.; Plante, T. In Vitro Comparison of the Charge-Injection Limits of Activated Iridium Oxide (AIROF) and Platinum-Iridium Microelectrodes. IEEE Trans. Biomed. Eng. 2005, 52, 1612–1614. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e17–e35. [Google Scholar] [CrossRef]
- Mandal, H.S.; Kastee, J.S.; McHail, D.G.; Rubinson, J.F.; Pancrazio, J.J.; Dumas, T.C. Improved Poly(3,4-Ethylenedioxythiophene) (PEDOT) for Neural Stimulation. Neuromodulation 2015, 18, 657–663. [Google Scholar] [CrossRef]
- Wallace, G.G.; Moulton, S.E.; Kapsa, R.M.I.; Higgins, M.J. Organic Conducting Polymers. In Organic Bionics; Wiley-VCH: Weinheim, Germany, 2012; pp. 81–112. [Google Scholar]
- Kozai, T.D.Y.; Catt, K.; Du, Z.; Na, K.; Srivannavit, O.; Haque, R.-U.M.; Seymour, J.; Wise, K.D.; Yoon, E.; Cui, X.T. Chronic In Vivo Evaluation of PEDOT/CNT for Stable Neural Recordings. IEEE Trans. Biomed. Eng. 2016, 63, 111–119. [Google Scholar] [CrossRef]
- Liang, Y.; Offenhäusser, A.; Ingebrandt, S.; Mayer, D. PEDOT:PSS-Based Bioelectronic Devices for Recording and Modulation of Electrophysiological and Biochemical Cell Signals. Adv. Healthc. Mater. 2021, 10, 2100061. [Google Scholar] [CrossRef]
- Ghosh, S.; Inganas, O. Electrochemical Characterization of Poly(3,4-ethylene dioxythiophene) Based Conducting Hydrogel Networks. J. Electrochem. Soc. 2000, 147, 1872. [Google Scholar] [CrossRef]
- Luo, X.; Weaver, C.L.; Zhou, D.D.; Greenberg, R.; Cui, X.T. Highly stable carbon nanotube doped poly(3,4-ethylenedioxythiophene) for chronic neural stimulation. Biomaterials 2011, 32, 5551–5557. [Google Scholar] [CrossRef] [PubMed]
- Kolarcik, C.L.; Catt, K.; Rost, E.; Albrecht, I.; Bourbeau, D.; Du, Z.; Kozai, T.; Luo, X.; Weber, D.; Cui, X. Evaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglion. J. Neural Eng. 2015, 12, 016008. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, M.; Taylor, A.; Fjorback, M.; Zachar, V.; Pennisi, C.P. Boron-Doped Nanocrystalline Diamond Electrodes for Neural Interfaces: In vivo Biocompatibility Evaluation. Front. Neurosci. 2016, 10, 87. [Google Scholar] [CrossRef]
- Ariano, P.; Giudice, A.L.; Marcantoni, A.; Vittone, E.; Carbone, E.; Lovisolo, D. A diamond-based biosensor for the recording of neuronal activity. Biosens. Bioelectron. 2009, 24, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Piret, G.; Hébert, C.; Mazellier, J.-P.; Rousseau, L.; Scorsone, E.; Cottance, M.; Lissorgues, G.; Heuschkel, M.O.; Picaud, S.; Bergonzo, P.; et al. 3D-nanostructured boron-doped diamond for microelectrode array neural interfacing. Biomaterials 2015, 53, 173–183. [Google Scholar] [CrossRef]
- Trevathan, J.K.; Baumgart, I.W.; Nicolai, E.N.; Gosink, B.A.; Asp, A.J.; Settell, M.; Polaconda, S.R.; Malerick, K.D.; Brodnick, S.K.; Zeng, W.; et al. An Injectable Neural Stimulation Electrode Made from an In-Body Curing Polymer/Metal Composite. Adv. Healthc. Mater. 2019, 8, e1900892. [Google Scholar] [CrossRef]
- Sridharan, A.; Nguyen, J.; Capadona, J.; Muthuswamy, J. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo. J. Neural Eng. 2015, 12, 036002. [Google Scholar] [CrossRef] [PubMed]
- Sohal, H.S.; Clowry, G.J.; Jackson, A.; O’Neill, A.; Baker, S.N. Mechanical Flexibility Reduces the Foreign Body Response to Long-Term Implanted Microelectrodes in Rabbit Cortex. PLoS ONE 2016, 11, e0165606. [Google Scholar] [CrossRef]
- Dalrymple, A.N.; Ting, J.E.; Bose, R.; Trevathan, J.K.; Nieuwoudt, S.; Lempka, S.F.; Franke, M.; Ludwig, K.A.; Shoffstall, A.J.; Fisher, L.E.; et al. Stimulation of the dorsal root ganglion using an Injectrode®. J. Neural Eng. 2021, 18, 056068. [Google Scholar] [CrossRef]
- Drake, P.L.; Hazelwood, K.J. Exposure-Related Health Effects of Silver and Silver Compounds: A Review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Karantonis, D.M.; Single, P.S.; Obradovic, M.; Cousins, M.J. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain 2012, 153, 593–601. [Google Scholar] [CrossRef]
- He, J.; Barolat, G.; Holsheimer, J.; Struijk, J. Perception threshold and electrode position for spinal cord stimulation. Pain 1994, 59, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, R.; Chakravarthy, K.; Will, A.; Trutnau, K.; Dinsmoor, D. A New Direction for Closed-Loop Spinal Cord Stimulation: Combining Contemporary Therapy Paradigms with Evoked Compound Action Potential Sensing. J. Pain Res. 2021, 14, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Brooker, C.; Russo, M.; Cousins, M.; Taylor, N.; Holford, L.; Martin, R.; Boesel, T.; Sullivan, R.; Hanson, E.; Gmel, G.; et al. ECAP-Controlled Closed-Loop Spinal Cord Stimulation Efficacy and Opioid Reduction Over 24-Months: Final Results of the Prospective, Multicenter, Open-Label Avalon Study. Pain Pract. 2021, 21, 680–691. [Google Scholar] [CrossRef]
- Levy, R.; Deer, T.; Poree, L.; Rosen, S.; Kapural, L.; Amirdelfan, K.; Soliday, N.; Leitner, A.; Mekhail, N. Multicenter, Randomized, Double-Blind Study Protocol Using Human Spinal Cord Recording Comparing Safety, Efficacy, and Neurophysiological Responses Between Patients Being Treated with Evoked Compound Action Potential–Controlled Closed-Loop Spinal Cord Stimulation or Open-Loop Spinal Cord Stimulation (the Evoke Study). Neuromodulation Technol. Neural Interface 2019, 22, 317–326. [Google Scholar]
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M.; et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): A double-blind, randomised, controlled trial. Lancet Neurol. 2020, 19, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Karsy, M.; Hawryluk, G. Modern Medical Management of Spinal Cord Injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 65. [Google Scholar] [CrossRef]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury. Facts and Figures at a Glance. 2017. Available online: https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%202020.pdf (accessed on 19 October 2022).
- Cao, Y.; Massaro, J.F.; Krause, J.S.; Chen, Y.; Devivo, M.J. Suicide Mortality After Spinal Cord Injury in the United States: Injury Cohorts Analysis. Arch. Phys. Med. Rehabilitation 2014, 95, 230–235. [Google Scholar] [CrossRef]
- Strauss, D.J.; DeVivo, M.J.; Paculdo, D.R.; Shavelle, R.M. Trends in Life Expectancy After Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2006, 87, 1079–1085. [Google Scholar] [CrossRef]
- Chamberlain, J.D.; Meier, S.; Mader, L.; von Groote, P.M.; Brinkhof, M.W. Mortality and Longevity after a Spinal Cord Injury: Systematic Review and Meta-Analysis. Neuroepidemiology 2015, 44, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Chin, C.; Popovic, M.R. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: A review. Biomed. Eng. Online 2020, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Duffell, L.D.; Donaldson, N.D.N. A Comparison of FES and SCS for Neuroplastic Recovery After SCI: Historical Perspectives and Future Directions. Front. Neurol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Capogrosso, M.; Milekovic, T.; Borton, D.; Wagner, F.; Moraud, E.M.; Mignardot, J.-B.; Buse, N.; Gandar, J.; Barraud, Q.; Xing, D.; et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016, 539, 284–288. [Google Scholar] [CrossRef]
- Wenger, N.; Moraud, E.M.; Gandar, J.; Musienko, P.; Capogrosso, M.; Baud, L.; Le Goff, C.G.; Barraud, Q.; Pavlova, N.; Dominici, N.; et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat. Med. 2016, 22, 138–145. [Google Scholar] [CrossRef]
- Wenger, N.; Moraud, E.M.; Raspopovic, S.; Bonizzato, M.; DiGiovanna, J.; Musienko, P.; Morari, M.; Micera, S.; Courtine, G. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci. Transl. Med. 2014, 6, 255ra133. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Gerasimenko, Y.; Pinter, M.M. Evidence for a spinal central pattern generator in humans. Ann. N. Y. Acad. Sci. 1998, 860, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
- Inc., O.M. The Up-LIFT Study of Non-Invasive ARC Therapy for Spinal Cord Injury. 2022. Available online: https://ClinicalTrials.gov/show/NCT04697472 (accessed on 19 October 2022).
- Inc, O.M. ONWARD Reports Positive Topline Results from a Pivotal Study to Restore Arm and Hand Function in People with Spinal Cord Injury. 2022. Available online: https://ir.onwd.com/static-files/0449dc19-a02d-4a81-aa0d-04051672e52d (accessed on 19 October 2022).
- Rowald, A.; Komi, S.; Demesmaeker, R.; Baaklini, E.; Hernandez-Charpak, S.D.; Paoles, E.; Montanaro, H.; Cassara, A.; Becce, F.; Lloyd, B.; et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat. Med. 2022, 28, 260–271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, C.M.; Latif, U.; Sack, A.; Govindan, S.; Sanderson, M.; Vu, D.T.; Smith, G.; Sayed, D.; Khan, T. Advances in Spinal Cord Stimulation. Bioengineering 2023, 10, 185. https://doi.org/10.3390/bioengineering10020185
Lam CM, Latif U, Sack A, Govindan S, Sanderson M, Vu DT, Smith G, Sayed D, Khan T. Advances in Spinal Cord Stimulation. Bioengineering. 2023; 10(2):185. https://doi.org/10.3390/bioengineering10020185
Chicago/Turabian StyleLam, Christopher M., Usman Latif, Andrew Sack, Susheel Govindan, Miles Sanderson, Dan T. Vu, Gabriella Smith, Dawood Sayed, and Talal Khan. 2023. "Advances in Spinal Cord Stimulation" Bioengineering 10, no. 2: 185. https://doi.org/10.3390/bioengineering10020185
APA StyleLam, C. M., Latif, U., Sack, A., Govindan, S., Sanderson, M., Vu, D. T., Smith, G., Sayed, D., & Khan, T. (2023). Advances in Spinal Cord Stimulation. Bioengineering, 10(2), 185. https://doi.org/10.3390/bioengineering10020185





