Exosome-Coated tPA/Catalase Nanoformulation for Thrombolytic Therapy
Abstract
1. Introduction
2. Materials and Methods
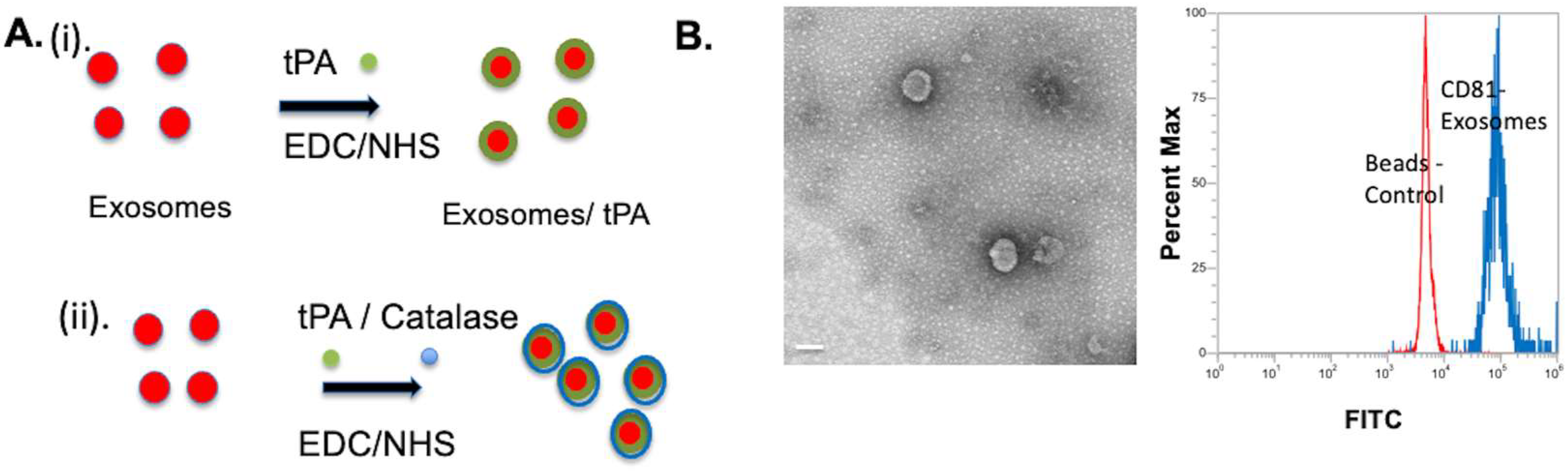
2.1. Exosomes tPA Conjugation
2.2. Exosomes tPA/Catalase Conjugation
2.3. TEM and DLS
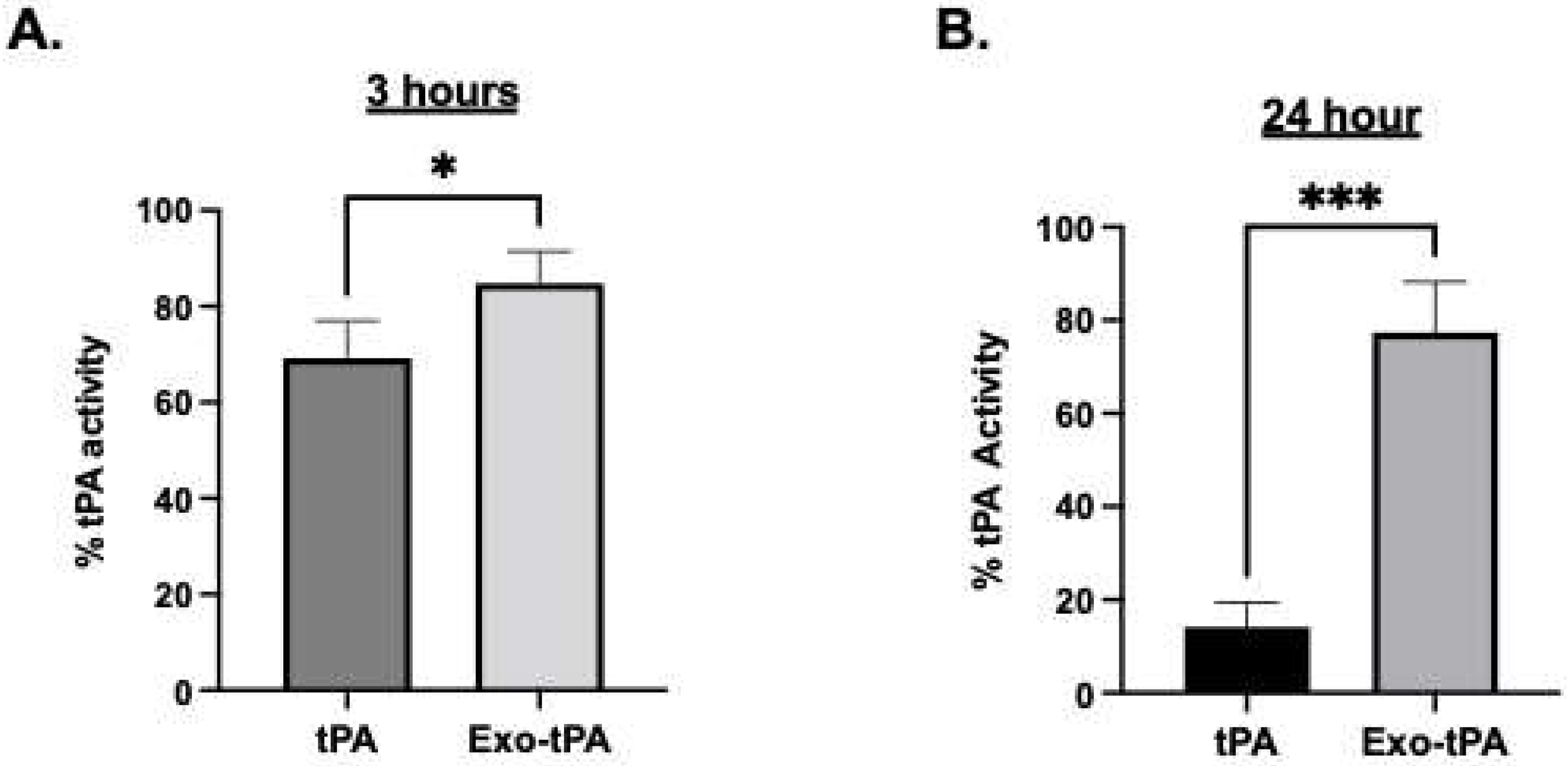
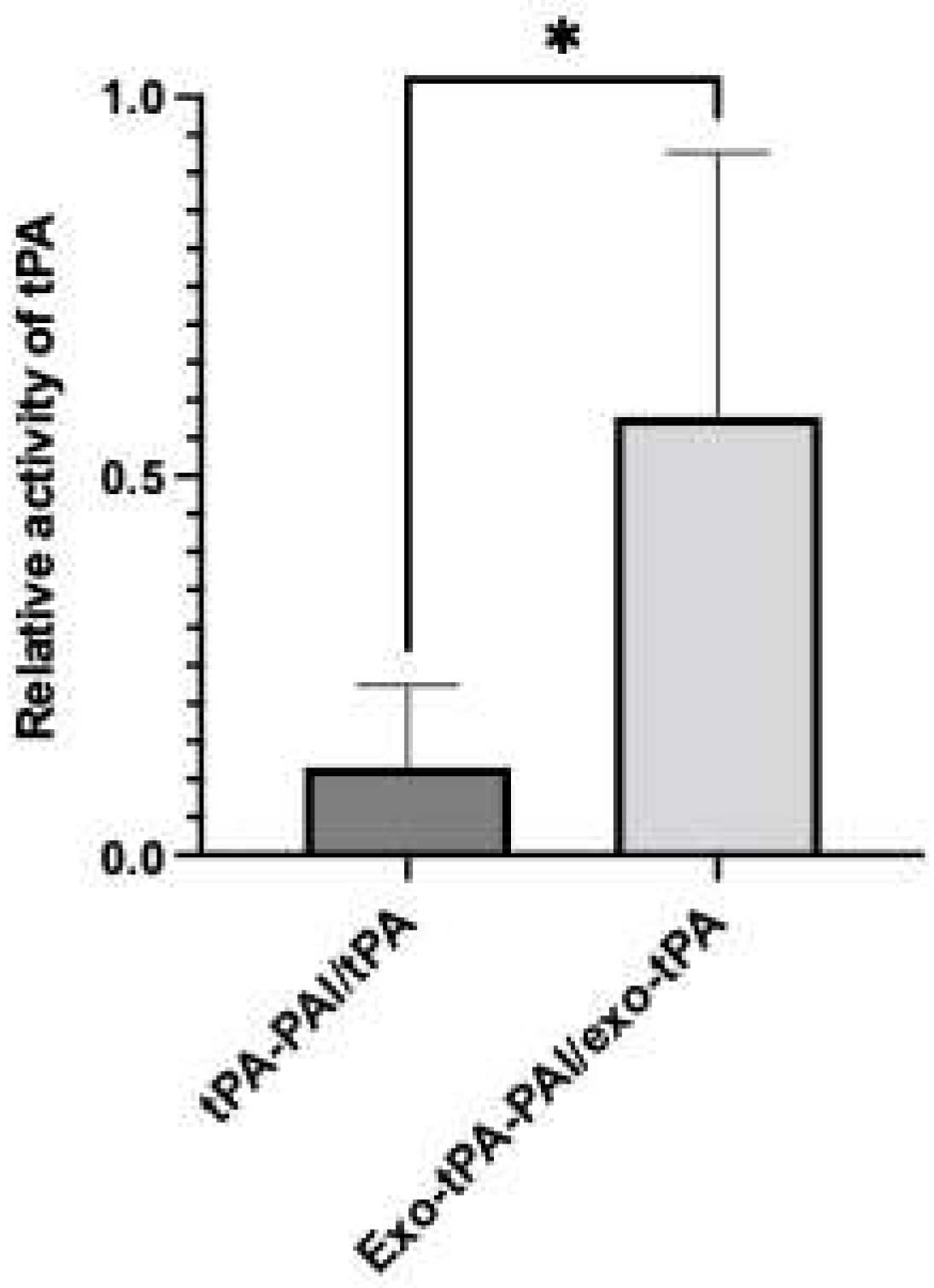
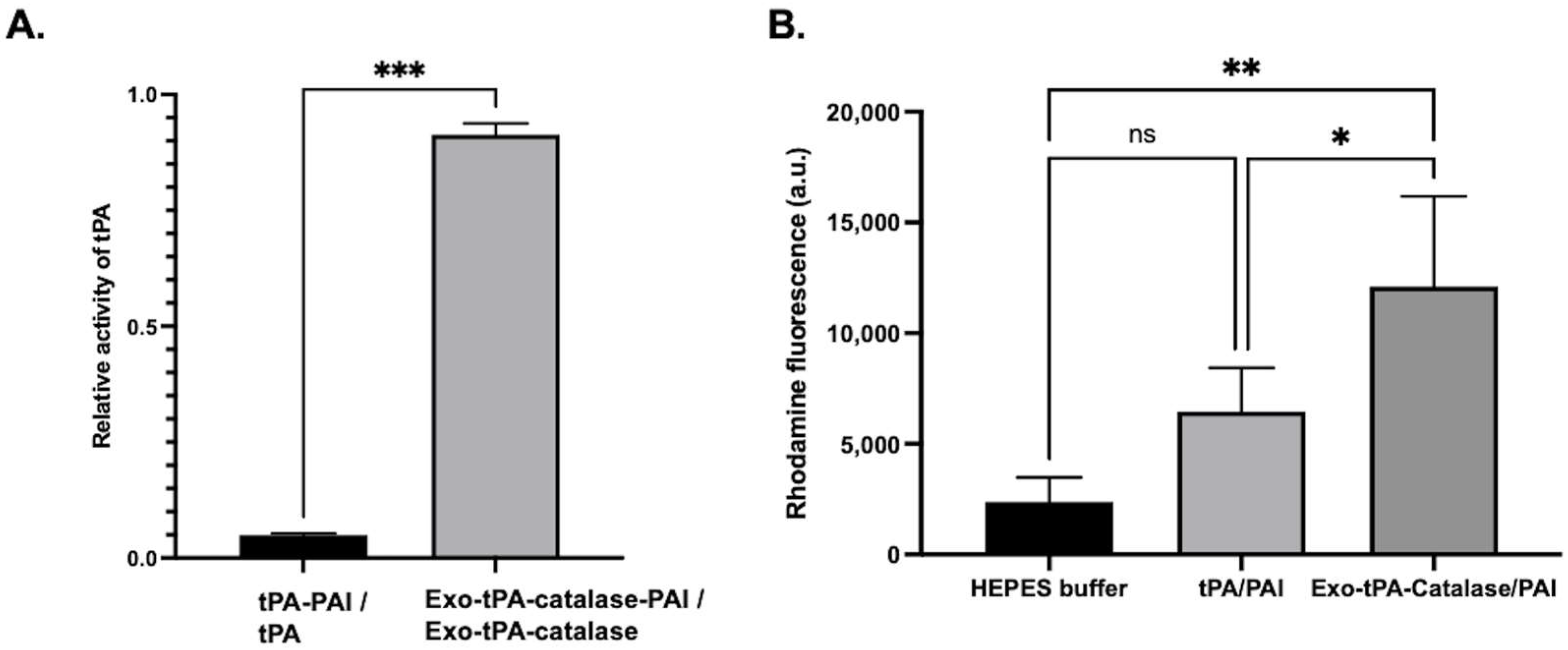
2.4. tPA Activity Assay and Protein Assay
2.5. Fibrin Clot Lysis Assay
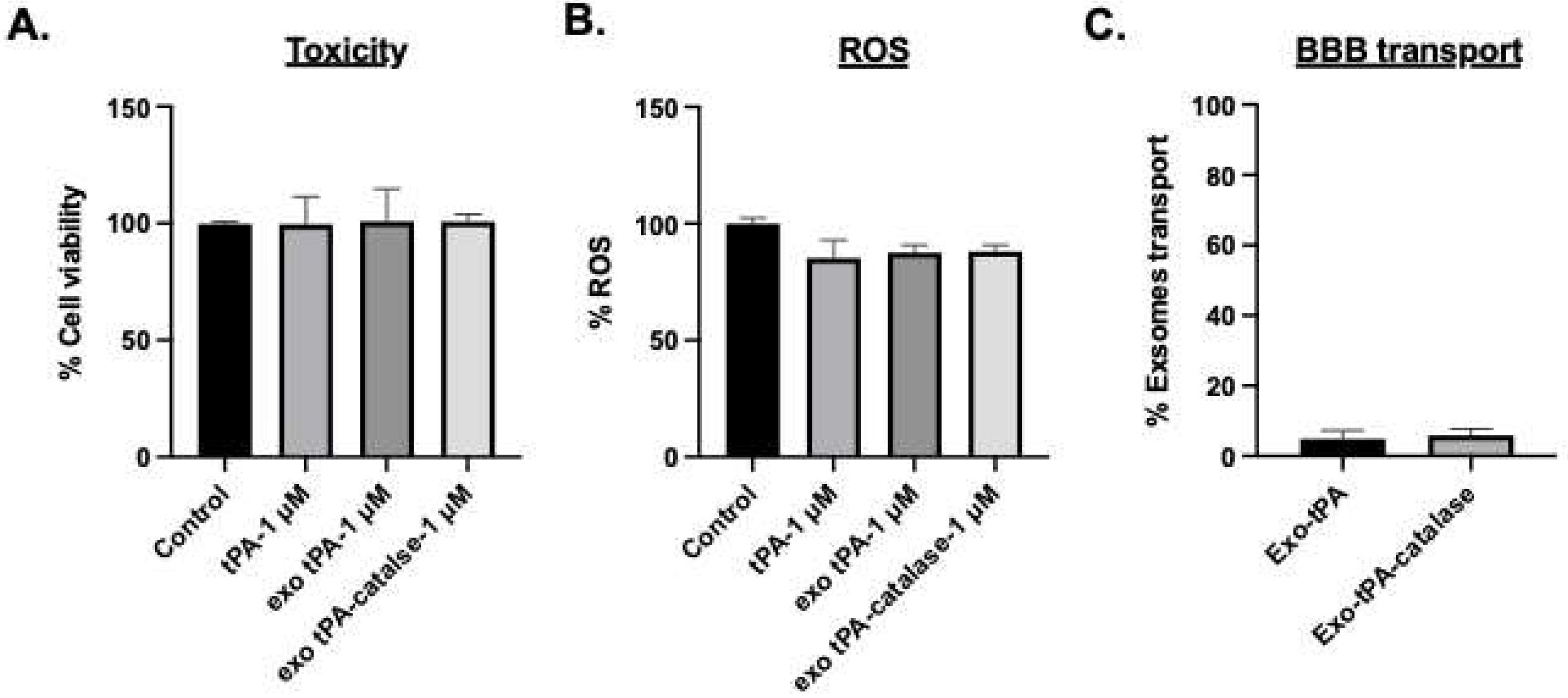
2.6. Toxicity
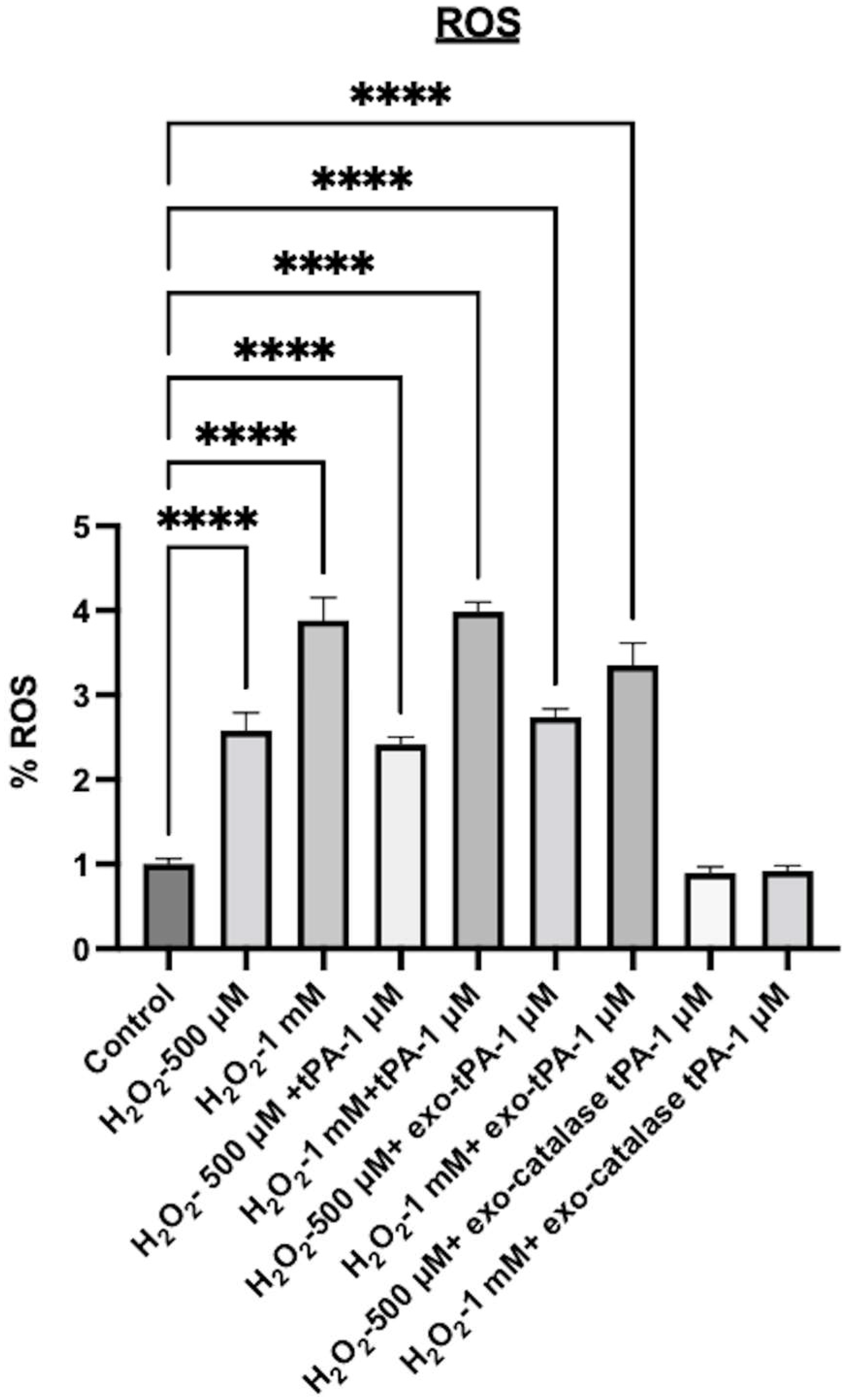
2.7. ROS
2.8. Permeability
2.9. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Wong, A.A.; Read, S.J. Early changes in physiological variables after stroke. Ann. Indian Acad. Neurol. 2008, 11, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S. Cerebral perfusion and stroke. J. Neurol. Neurosurg. Psychiatry 2004, 75, 353–361. [Google Scholar] [CrossRef]
- The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997, 28, 2109–2118. [Google Scholar] [CrossRef]
- Tanne, D.; Kasner, S.E.; Demchuk, A.M.; Koren-Morag, N.; Hanson, S.; Grond, M.; Levine, S.R. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: The Multicenter rt-PA Stroke Survey. Circulation 2002, 105, 1679–1685. [Google Scholar] [CrossRef]
- Hacke, W.; Donnan, G.; Fieschi, C.; Kaste, M.; von Kummer, R.; Broderick, J.P.; Brott, T.; Frankel, M.; Grotta, J.C.; Haley, E.C., Jr.; et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004, 363, 768–774. [Google Scholar] [CrossRef]
- Hill, M.D.; Buchan, A.M.; Canadian Alteplase for Stroke Effectiveness Study, I. Thrombolysis for acute ischemic stroke: Results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ 2005, 172, 1307–1312. [Google Scholar] [CrossRef]
- Lansberg, M.G.; Albers, G.W.; Wijman, C.A. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: A review of the risk factors. Cerebrovasc. Dis. 2007, 24, 1–10. [Google Scholar] [CrossRef]
- Su, E.J.; Fredriksson, L.; Geyer, M.; Folestad, E.; Cale, J.; Andrae, J.; Gao, Y.; Pietras, K.; Mann, K.; Yepes, M.; et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat. Med. 2008, 14, 731–737. [Google Scholar] [CrossRef]
- Kleindorfer, D.; de los Rios La Rosa, F.; Khatri, P.; Kissela, B.; Mackey, J.; Adeoye, O. Temporal trends in acute stroke management. Stroke 2013, 44, S129–S131. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, S.; Ruff, I.; Bernstein, R.A. Acute stroke intervention: A systematic review. JAMA 2015, 313, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wen, Z.; Shen, H.; Shen, M.; Chen, G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 1203285. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Jaffer, H.; Morris, V.B.; Stewart, D.; Labhasetwar, V. Advances in stroke therapy. Drug Deliv. Transl. Res. 2011, 1, 409–419. [Google Scholar] [CrossRef]
- Han, J.; Shuvaev, V.V.; Muzykantov, V.R. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J. Pharmacol. Exp. Ther. 2011, 338, 82–91. [Google Scholar] [CrossRef]
- Li, W.; Yang, S. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016, 2, 153–163. [Google Scholar] [CrossRef]
- Petro, M.; Jaffer, H.; Yang, J.; Kabu, S.; Morris, V.B.; Labhasetwar, V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials 2016, 81, 169–180. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Aamir, R.; Fyffe, C.; Korin, N.; Lawrence, D.A.; Su, E.J.; Kanapathipillai, M. Heparin and Arginine Based Plasmin Nanoformulation for Ischemic Stroke Therapy. Int. J. Mol. Sci. 2021, 22, 11477. [Google Scholar] [CrossRef] [PubMed]
- Korin, N.; Kanapathipillai, M.; Matthews, B.D.; Crescente, M.; Brill, A.; Mammoto, T.; Ghosh, K.; Jurek, S.; Bencherif, S.A.; Bhatta, D.; et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 2012, 337, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Marosfoi, M.G.; Korin, N.; Gounis, M.J.; Uzun, O.; Vedantham, S.; Langan, E.T.; Papa, A.L.; Brooks, O.W.; Johnson, C.; Puri, A.S.; et al. Shear-Activated Nanoparticle Aggregates Combined With Temporary Endovascular Bypass to Treat Large Vessel Occlusion. Stroke 2015, 46, 3507–3513. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, Y.; Kawata, H.; Saito, Y.; Tabata, Y. Ultrasound-responsive thrombus treatment with zinc-stabilized gelatin nano-complexes of tissue-type plasminogen activator. J. Drug Target. 2012, 20, 224–234. [Google Scholar] [CrossRef]
- Murciano, J.C.; Medinilla, S.; Eslin, D.; Atochina, E.; Cines, D.B.; Muzykantov, V.R. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat. Biotechnol. 2003, 21, 891–896. [Google Scholar] [CrossRef]
- Danielyan, K.; Ganguly, K.; Ding, B.S.; Atochin, D.; Zaitsev, S.; Murciano, J.C.; Huang, P.L.; Kasner, S.E.; Cines, D.B.; Muzykantov, V.R. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation 2008, 118, 1442–1449. [Google Scholar] [CrossRef]
- Jun, J.; Shang-Yi, J.; Xia, H.; Wen-Ping, L. Preparation of ultrasound microbubbles crosslinked to albumin nanoparticles packaged with tissue-type plasminogen activator gene plasmid and method of in vivo transfection. J. Exp. Pharmacol. 2011, 3, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- de Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Jiang, C.; Wang, Y.; Wang, K.; Marsh, J.; Zhang, D.; Chen, X.; Nie, L. Engineered extracellular vesicles as intelligent nanosystems for next-generation nanomedicine. Nanoscale Horiz. 2022, 7, 682–714. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Beiter, T.; Schluesener, H.J. Nanovesicular vaccines: Exosomes. Arch. Immunol. Ther. Exp. 2005, 53, 329–335. [Google Scholar]
- Karamanidou, T.; Tsouknidas, A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int. J. Mol. Sci. 2021, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Huda, M.N.; Nafiujjaman, M.; Deaguero, I.G.; Okonkwo, J.; Hill, M.L.; Kim, T.; Nurunnabi, M. Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications. ACS Biomater. Sci. Eng. 2021, 7, 2106–2149. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; Andre, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef]
- Chen, J.; Chopp, M. Exosome Therapy for Stroke. Stroke 2018, 49, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, Y.; Yang, G.Y. Therapeutic application of exosomes in ischaemic stroke. Stroke Vasc. Neurol. 2021, 6, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, W.; Ye, J.; Wang, Y. Potential Role of Exosomes in Ischemic Stroke Treatment. Biomolecules 2022, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Otero-Ortega, L.; Laso-Garcia, F.; Gomez-de Frutos, M.; Fuentes, B.; Diekhorst, L.; Diez-Tejedor, E.; Gutierrez-Fernandez, M. Role of Exosomes as a Treatment and Potential Biomarker for Stroke. Transl. Stroke Res. 2019, 10, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Doeppner, T.R.; Herz, J.; Gorgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem. Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Seyedaghamiri, F.; Salimi, L.; Ghaznavi, D.; Sokullu, E.; Rahbarghazi, R. Exosomes-based therapy of stroke, an emerging approach toward recovery. Cell Commun. Signal. 2022, 20, 110. [Google Scholar] [CrossRef]
- Khan, H.; Pan, J.J.; Li, Y.; Zhang, Z.; Yang, G.Y. Native and Bioengineered Exosomes for Ischemic Stroke Therapy. Front. Cell Dev. Biol. 2021, 9, 619565. [Google Scholar] [CrossRef]
- Lee, E.C.; Ha, T.W.; Lee, D.H.; Hong, D.Y.; Park, S.W.; Lee, J.Y.; Lee, M.R.; Oh, J.S. Utility of Exosomes in Ischemic and Hemorrhagic Stroke Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 8637. [Google Scholar] [CrossRef]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging. Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef]
- Du, T.; Yang, C.L.; Ge, M.R.; Liu, Y.; Zhang, P.; Li, H.; Li, X.L.; Li, T.; Liu, Y.D.; Dou, Y.C.; et al. M1 Macrophage Derived Exosomes Aggravate Experimental Autoimmune Neuritis via Modulating Th1 Response. Front. Immunol. 2020, 11, 1603. [Google Scholar] [CrossRef]
- Schuldt, B.R.; Kalagara, R.; Chennareddy, S.; Odland, I.C.; Downes, M.H.; Reford, E.; Vicari, J.M.; Ali, M.; Bhimani, A.D.; Putrino, D.; et al. Exosome-Based Therapy for Ischemic Stroke: A Bibliometric Analysis of Current Trends and Future Directions. World Neurosurg. 2022, in press. [CrossRef]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Heeremans, J.L.; Gerritsen, H.R.; Meusen, S.P.; Mijnheer, F.W.; Gangaram Panday, R.S.; Prevost, R.; Kluft, C.; Crommelin, D.J. The preparation of tissue-type Plasminogen Activator (t-PA) containing liposomes: Entrapment efficiency and ultracentrifugation damage. J. Drug Target. 1995, 3, 301–310. [Google Scholar] [CrossRef]
- Hedou, E.; Douceau, S.; Chevilley, A.; Varangot, A.; Thiebaut, A.M.; Triniac, H.; Bardou, I.; Ali, C.; Maillasson, M.; Crepaldi, T.; et al. Two-Chains Tissue Plasminogen Activator Unifies Met and NMDA Receptor Signalling to Control Neuronal Survival. Int. J. Mol. Sci. 2021, 22, 13438. [Google Scholar] [CrossRef]
- Bohm, T.; Geiger, M.; Binder, B.R. Isolation and characterization of tissue-type plasminogen activator- binding proteoglycans from human umbilical vein endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, R.; Hamood, M.A.; Gomez Zubieta, D.M.; Kondapalli, K.C. Na(+)/H(+) Exchanger 9 Regulates Iron Mobilization at the Blood-Brain Barrier in Response to Iron Starvation. J. Biol. Chem. 2017, 292, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Nizet, V.; Kim, K.S.; Stins, M.; Jonas, M.; Chi, E.Y.; Nguyen, D.; Rubens, C.E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 1997, 65, 5074–5081. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yotnda, P. Production and detection of reactive oxygen species (ROS) in cancers. J. Vis. Exp. 2011, 57, 3357. [Google Scholar] [CrossRef]
- Ashfaq, M.; Talreja, N.; Chauhan, D.; Viswanathan, M.R. Synthesis of Cu-doped 2D-WS2 nanosheet-based nano-antibiotic materials for inhibiting E. Coli and S. aureus bacterial strains. New J. Chem. 2022, 46, 5581–5587. [Google Scholar] [CrossRef]
- Akram, A.M.; Omar, R.A.; Ashfaq, M. Chitosan/calcium phosphate-nanoflakes-based biomaterial: A potential hemostatic wound dressing material. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, Z.; Xu, J.; Liu, J.; Guo, Z.N. Targeted nano-delivery strategies for facilitating thrombolysis treatment in ischemic stroke. Drug Deliv. 2021, 28, 357–371. [Google Scholar] [CrossRef] [PubMed]


| Nanoformulation | Size |
|---|---|
| Exosomes | 179 ± 25.875 |
| Exosomes + tPA (exo-tPA) | 317 ± 76.782 |
| Exosomes + tPA + catalase | 438.9 ± 10.712 |
| (exo-tPA-catalase) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, S.; Kanapathipillai, M. Exosome-Coated tPA/Catalase Nanoformulation for Thrombolytic Therapy. Bioengineering 2023, 10, 177. https://doi.org/10.3390/bioengineering10020177
Khalil S, Kanapathipillai M. Exosome-Coated tPA/Catalase Nanoformulation for Thrombolytic Therapy. Bioengineering. 2023; 10(2):177. https://doi.org/10.3390/bioengineering10020177
Chicago/Turabian StyleKhalil, Sara, and Mathumai Kanapathipillai. 2023. "Exosome-Coated tPA/Catalase Nanoformulation for Thrombolytic Therapy" Bioengineering 10, no. 2: 177. https://doi.org/10.3390/bioengineering10020177
APA StyleKhalil, S., & Kanapathipillai, M. (2023). Exosome-Coated tPA/Catalase Nanoformulation for Thrombolytic Therapy. Bioengineering, 10(2), 177. https://doi.org/10.3390/bioengineering10020177





