Hematopoietic Stem Cell Transplantation for the Treatment of Autoimmune Neurological Diseases: An Update
Abstract
1. Introduction
2. Materials and Methods
2.1. Article Search
2.2. Statistical Analysis
3. Haematopoietic Stem Cell Transplantation in Autoimmune Diseases
3.1. Transplant Procedure
3.2. Experimental Models
3.3. Mechanism(s) of Action
3.4. Safety
4. Neuromyelitis Optica Spectrum Disorder
| Study [Reference] | Greco, R., et al., 2015 [43] | Burt, R.K., et al., 2018 [3] | Burton, J.M., et al., 2021 [44] |
|---|---|---|---|
| Baseline features | |||
| N patients; % F | 16; 81% F | 12; 92% F | 3; 67% F |
| Age, y | 37 (20–57) | 42 (19–51) | 34 (28–39) |
| Disease duration, y | 2 (<1–17) | 7 (1–19.7) | 8.3 (3–13) |
| EDSS | 6.5 (2.0–8.5) | 4.3 (2–6.5) | 4 (3.0–4.5) |
| ARR before AHSCT | N.R. | 4.3 (2–10) | 3.4 (1.3–5) in the y pre-AHSCT |
| Anti-AQP4 ab positive | 10/13 tested (62%) | 92% | 67% |
| AHSCT protocol | |||
| Mobilisation of HSCs | Cy (2–4 g/m2) + G-CSF (+ RTX 375 mg/m2 in 2 cases) | Cy 2 g/m2 + G-CSF | Cy 2 g/m2 + RTX 375 mg/m2 + G-CSF |
| Conditioning | BEAM + ATG (n = 9); thiotepa-Cy (n = 3); Cy 200 mg/Kg + ATG (n = 4) | Cy 200 mg/Kg + ATG + RTX 500 mgx2 + plasmapheresis 1 | Cy 200 mg/Kg + ATG + RTX 375 mg/m2 |
| Outcomes | |||
| Progression-free survival | 48% at y 3–5 | 90% at y 5 | 67% at last follow-up |
| EDSS | improved in 56% 2 | 3.0 (0–6.5) at y 5 | improved in 67% 2 |
| Relapse-free survival | 31% at y 3; 10% at y 5 | 80% at y 5 | 33% at last follow-up |
| IS-medications free | 19% | 83% | 33% |
| Anti-AQP4 ab positive | 8/8 tested (100%) | 17% | 67% |
| Severe adverse events and death | one death due to disease progression at month 14; one grade 4 neutropenia | no grade 4 toxicities | 1 death due to disease progression at y 3.5 |
| Secondary autoimmunity | 1 thyroiditis | 1 MG; 1 hyperthyroidism | N.R. |
| Follow-up duration, y | 4 (1.7–10.7) | 4.7 (2–5) | 7.5 (3.5–10) |
5. Stiff-Person Syndrome
| Study [Reference] | Sanders, S., et al., 2014 [54] | Kass-Iliyya, L., et al., 2021 [56] | Georges, G.E., et al., 2018 [55] | Burt, R.K., et al., 2021 [57] |
|---|---|---|---|---|
| Baseline features | ||||
| N patients; % F | 2; 100% F | 4 (3 SPS, 1 PERM); 75% F | 9; 44% F | 23; 91% F |
| Age, y | A: 53; B: 33 | 43 (36–52) | 42 (25–50) | 48 (28–60) |
| Disease duration, y | A: 5; B: 5 | 6.5 (4–9) | 5.3 (1–14.7) | 7 (2–20) |
| Need for assistance in gait | 50% (case A) | 75% wheelchair dependent; 25% restricted walking | 100% | 78% |
| Anti-GAD ab positive | 100% in serum | 100% in serum | 100% in serum | 100% in serum; 14/20 (70%) in CSF |
| EMG abnormalities | normal in A; continuous motor unit activity in B | 100% (continuous motor unit activity in 3/4, blink reflex hyperexcitability in 4/4) | 100% | 70% (continuous paraspinal muscle activity) |
| AHSCT protocol | ||||
| Mobilisation of HSCs | Cy 2.5 g/m2 + G-CSF | Cy 2 g/m2 + G-CSF | rituximab + G-CSF | Cy 2 g/m2 + G-CSF |
| Conditioning | Busulfan–Cy + ATG + CD34+ selection | Cy 200 mg/kg + ATG | BEAM + ATG | Cy 200 mg/kg + ATG + RTX 500 mg ×2 |
| Outcomes | ||||
| Clinical outcome | SPS symptoms | improvement in walking | improvement in distribution of stiffness index and functional status | discontinuation of immune medication and ≥50% decrease in antispasmodic medications |
| Rate or response | resolution in case A; improvement in case B | 100% | 100% | 48% responders; 26% partial responders |
| Anti-GAD ab | titre reduced in case A; N.A. in B | negativisation in 50% | titres decreased | titres decreased in 17% |
| EMG | N.R. | abnormal in 50% | improved or normalised in 100% | N.R. |
| Tapering/discontinuation of anti-spasmodic drugs | discontinuation in A; tapering in B | 75% (1 discontinuation; 2 tapering) | majority of the cases | 61% (3 discontinuation; 11 tapering) |
| Immunotherapy-free | 100% | 100% | N.R. | 43% |
| Severe adverse events and death | none | none | none | 1 death due to disease progression at y 1; grade 4 tox in 3 cases |
| Secondary autoimmunity | none | none | N.R. | 1 hypothyroidism at y 2 |
| Follow-up duration, y | A: 5; B: 3 | 1.7 (1–3) | 1 to 2 | 3.6 (1.5–4.5) |
6. Chronic Inflammatory Demyelinating Polyneuropathy
| Study [Reference] | Mahdi-Rogers, M. et al., 2009 [62] | Ajroud-Driss, S. et al. 2011, 1 [64] | Press, R., et al., 2013 [63] | Burt, R.K., et al., 2020 [66] | Masson-Roy, J., et al., 2021 [65] |
|---|---|---|---|---|---|
| Baseline | |||||
| N patients; % F | 3; 66% F | 15; 47% F | 11; 9% F | 66 treated, 60 analysed; 38% F | 5; 20% F |
| Age, y | 58 (29–72) | 39 (24–64) | 55 (23–68) | 43 (20–63) | 48 (28–60) |
| Disease duration, y | 13 (7–21) | N.R. | 2.5 (<1–19) | 4.7 (<1–29) | 7 (2–20) |
| AHSCT protocol | |||||
| Mobilisation of HSCs | Cy 4 g/m2 + G-CSF | N.R. | Cy 2–4 g/m2 + G-CSF (n = 9); RTX 375 mg/m2 + G-CSF (n = 2) | Cy 2 g/m2 + G-CSF | Cy 2.5 g/m2 + G-CSF |
| Conditioning | Cy 200 mg/Kg + ATG | Cy + ATG and CD34+ cells selection | Cy 35–50 mg/kg alone (n = 1) or + ATG (n = 6); melphalan (n = 1); BEAM + ATG (n = 3) | Cy 200 mg/Kg + ATG + RTX 500 mg on days −6 and +1 | busulfan + Cy + ATG (n = 3); BEAM (n = 2). + CD34+ cells selection (n = 4) |
| Outcomes | |||||
| Clinical measures | Clinical improvement 1/3 (33%), worsening 2/3 (67%). Median MRC sum score from 50.3 to 44.3 | Remission in 9/14 (64%) with significant improvement in strength; worsening in 1/14 (7%); 1 lost at follow-up | Significant improvement in the median INCAT (1) and Rankin score (1) compared with baseline (6 and 4, respectively) | Improvement in unassisted ambulation (32% → 83% at y 4–5). Immune drugs-free remission: 78% at y 4 and 83% at y 5 | Clinical improvement in 4/5 (80%); stabilisation in 1/5 (20%) |
| Electrophysiological parameters | Improvement in one case; N.R. in 2 | improvement in distal latency, and/or NCV and/or CMAP in 8/11 (73%) | Improvement in the median CMAP (1.84 mV compared to 0.88 mV) | Improvement in NCV (from 27 to 38) and CMAP (from 3.5 to 4.1) for all nerves | Trend towards improvement in most nerve conduction studies |
| Cases with relapse (time of relapse) | 1 (month 18) | 1 | 3 (months 23, 14 and 14) | 11 (19%) | 0 |
| Immune-medications | weekly IVIG and oral prednisolone (n = 1) | discontinuation in 9/14 (64%), tapering in 4 (36%) | AHSCT (n = 1); tocilizumab (n = 1); oral steroids (n = 2) | restart of IVIG, PLEX, orRTX in 11/60 (18%) | 1 (20%) hydrocortisone due to adrenal insufficiency |
| Severe adverse events and death | 1 severe pneumonia requiring intensive care | no major side effects | Klebsiella, Pseudomonas and α-Streptococci sepsis (1); pancreatitis (1) | 3 grade 4 early toxicities; two deaths considered not transplant-related. | no grade 4 toxicities |
| Follow-up, months | 19 (6–25) | 6 (3–62) | 28 (6–127) | 54 (24–60) | 41 (11–119) |
7. Myasthenia Gravis
| Study [Reference] | Bryant, A., et al., 2016 [71] | Sossa Melo, C.L. et al., 2019 [72] | Inan, B., et al., 2022 [75] | Håkansson, I. et al., 2016 [73] | Mitsumune, S. et al., 2018 [76] | Strober, J. et al., 2009 [74] |
|---|---|---|---|---|---|---|
| N cases (gender) | 7 (6F, 1M) | 1 (M) | 1 (F) | 1 (F) | 1 (M) | 1 (M) |
| Age, y | 24–55 | 56 | 26 | 64 | 54 | 17 |
| HSCT | autologous | autologous | autologous | autologous | autologous | allogenic |
| Mobilisation of HSCs | Cy + G-CSF | Cy + G-CSF | Cy + G-CSF | RTX + G-CSF | N.R. | N.R. |
| Conditioning | Cy + TBI + ATG (n = 2); busulfan-Cy + ATG (n = 4); etoposide, melphalan + TBI (n = 1) | Cy + ATG | Cy + ATG | BEAM + ATG | N.R. | Busulfan + fludarabine + alemtuzumab |
| Outcome | remission | remission | partial response | partial response | remission | partial response |
| Follow-up, m | N.R. | 65 | 30 | 24 | N.R. | 40 |
8. Inflammatory Myopathies
9. Rare Neurological Disorders and Systemic Autoimmune Diseases with Neurological Involvement
| Study [Reference] | N cases (Gender) | Age | Mobilisation of HSCs | Conditioning | Outcome | Follow-Up |
|---|---|---|---|---|---|---|
| Behcet Disease | ||||||
| Statkute, L. et al. [93] | 2 (F) | 25, 36 | Cy + G-CSF | Cy + ATG | (1) remission, (2) no response | 28 m |
| De Cata, A. et al., 2007 [91] | 2 (M) | 22, 23 | Cy + G-CSF | BEAM | partial response | 48 m |
| Marmont, A.M. et al., 2006 [92] | 1 (F) | 34 | Cy | BEAM | severe relapse < 3 m → allogenic HSCT with relapse at y 2 | |
| Daikeler, T. et al., 2007 [90] | 1 (M) | 49 | Cy + G-CSF | Melphalan | partial response | |
| Sjogren syndrome | ||||||
| Statkute, L. et al., 2008 [93] | 1 (F) | 42 | Cy + G-CSF | Cy + ATG | remission | 28 m |
| Wegener granulomatosis | ||||||
| Statkute, L. et al., 2008 [93] | 1 (F) | 27 | Cy + G-CSF | Cy + ATG | remission | 28 m |
| Systemic lupus erythematosus | ||||||
| Burt, R.K. et al., 2006 [94] | 18 | Cy + G-CSF | Cy + ATG | response | 5 y | |
| Lehnhardt, F.G. et al., 2006 [95] | 1 (F) | 19 | Cy + G-CSF | Cy + ATG | remission | 18 m |
| Trysberg, E. et al., 2000 [96] | 1 (F) | 19 | Cy + G-CSF | Cy + TBI | remission | 18 m |
| Goklemez, S. et al., 2022 [97] | 3 (1M, 2F) | 33, 20, 15 | Cy + G-CSF + RTX | Cy + fludarabine + RTX | (1,2) remission, (3) relapse at 18 m | 162 m |
| Lisukov, I.A. et al., 2004 [98] | 2 (F) | 21, 25 | (1) collected from BM, (2) mobilised with Cy + G-CSF | (1) etoposide + melphalan, (2) Cy | remission | 45 m, 6 m |
| Autoimmune encephalitis | ||||||
| Froehlich, M. et al., 2020 [99] | 1 (F) | 35 | Cy | Cy + ATG | remission | 18 m |
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharrack, B.; Saccardi, R.; Alexander, T.; Badoglio, M.; Burman, J.; Farge, D.; Greco, R.; Jessop, H.; Kazmi, M.; Kirgizov, K.; et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: Updated guidelines and recommendations from the ebmt autoimmune diseases working party (adwp) and the joint accreditation committee of ebmt and isct (jacie). Bone Marrow Transplant. 2019, 55, 283–306. [Google Scholar] [CrossRef]
- Saccardi, R. (Cell Therapy and Transfusion Medicine Unit, Careggi University Hospital, Florence, Italy). Personal communication, 2022.
- Burt, R.K.; Balabanov, R.; Han, X.; Morgan, A.; Clendenan, A.; Calvario, M.A.; Henry, J.; Quigley, K.; Gastala, J.; Jovanovic, B.; et al. Autologous non-myeloablative hematopoietic stem cell transplantation in patients with neuromyelitis optica spectrum disorder (nmosd): An open-label pilot study. Neurology 2018. Preprint. [Google Scholar]
- Cohen, J.A.; Baldassari, L.E.; Atkins, H.L.; Bowen, J.D.; Bredeson, C.; Carpenter, P.A.; Corboy, J.R.; Freedman, M.S.; Griffith, L.M.; Lowsky, R.; et al. Autologous hematopoietic cell transplantation for treatment-refractory relapsing multiple sclerosis: Position statement from the american society for blood and marrow transplantation. Biol. Blood Marrow Transplant. 2019, 25, 845–854. [Google Scholar] [CrossRef]
- Greco, R.; Labopin, M.; Badoglio, M.; Veys, P.; Silva, J.M.F.; Abinun, M.; Gualandi, F.; Bornhauser, M.; Ciceri, F.; Saccardi, R. Allogeneic hsct for autoimmune diseases: A retrospective study from the ebmt adwp, iewp, and pdwp working parties. Front. Immunol. 2019, 10, 1570. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Corbacioglu, S.; de la Cámara, R.; Dolstra, H.; Glass, B.; Greco, R.; Mohty, M.; Neven, B.; et al. Impact of the sars-cov-2 pandemic on hematopoietic cell transplantation and cellular therapies in europe 2020: A report from the ebmt activity survey. Bone Marrow Transplant. 2022, 57, 742–752. [Google Scholar] [CrossRef]
- Greco, R.; Blood and Marrow Transplant (BMT) Unit, IRCCS San Raffaele Hospital, Milan, Italy. Personal communication, 2022.
- Ikehara, S.; Good, R.A.; Nakamura, T.; Sekita, K.I.; Inoue, S.; Oo, M.M.; Muso, E.; Ogawa, K.; Hamashima, Y. Rationale for bone marrow transplantation in the treatment of autoimmune diseases. Proc. Natl. Acad. Sci. USA 1985, 82, 2483–2487. [Google Scholar] [CrossRef]
- Van Bekkum, D.W.; Bohre, E.; Houben, P.; Knaan-Shanzer, S. Regression of adjuvant-induced arthritis in rats following bone marrow transplantation. Proc. Natl. Acad. Sci. USA 1989, 86, 10090–10094. [Google Scholar] [CrossRef]
- van Bekkum, D.W. New opportunities for the treatment of severe autoimmune diseases: Bone marrow transplantation. Clin. Immunol. Immunopathol. 1998, 89, 1–10. [Google Scholar] [CrossRef]
- Knaan-Shanzer, S.; Houben, P.; Kinwel-Bohré, E.P.; van Bekkum, D.W. Remission induction of adjuvant arthritis in rats by total body irradiation and autologous bone marrow transplantation. Bone Marrow Transplant. 1991, 8, 333–338. [Google Scholar]
- Karussis, D.; Slavin, S.; Lehmann, D.; Mizrachi-Koll, R.; Abramsky, O.; Ben-Nun, A. Prevention of experimental autoimmune encephalomyelitis and induction of tolerance with acute immunosuppression followed by syngeneic bone marrow transplantation. J. Immunol. 1992, 148, 1693–1698. [Google Scholar] [CrossRef]
- van Gelder, M.; Kinwel-Bohre, E.P.; van Bekkum, D.W. Treatment of experimental allergic encephalomyelitis in rats with total body irradiation and syngeneic bmt. Bone Marrow Transplant. 1993, 11, 233–241. [Google Scholar]
- Pestronk, A.; Drachman, D.B.; Teoh, R.; Adams, R.N. Combined short-term immunotherapy for experimental autoimmune myasthenia gravis. Ann. Neurol. 1983, 14, 235–241. [Google Scholar] [CrossRef]
- Alexander, T.; Thiel, A.; Rosen, O.; Massenkeil, G.; Sattler, A.; Kohler, S.; Mei, H.; Radtke, H.; Gromnica-Ihle, E.; Burmester, G.R.; et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory sle induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 2009, 113, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.M.; de Kleer, I.M.; Cate, R.; van Rossum, M.A.; Bekkering, W.P.; Fasth, A.; van Tol, M.J.; Kuis, W.; Wulffraat, N.M.; Vossen, J.M. Autologous stem cell transplantation in children with severe progressive systemic or polyarticular juvenile idiopathic arthritis: Long-term follow-up of a prospective clinical trial. Arthritis Rheum. 2007, 56, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Arruda, L.C.; Brigant, F.; Clave, E.; Douay, C.; Marjanovic, Z.; Deligny, C.; Maki, G.; Gluckman, E.; Toubert, A. Long-term immune reconstitution and t cell repertoire analysis after autologous hematopoietic stem cell transplantation in systemic sclerosis patients. J. Hematol. Oncol. 2017, 10, 21. [Google Scholar] [CrossRef]
- Harris, K.M.; Lim, N.; Lindau, P.; Robins, H.; Griffith, L.M.; Nash, R.A.; Turka, L.A.; Muraro, P.A. Extensive intrathecal t cell renewal following hematopoietic transplantation for multiple sclerosis. JCI Insight 2020, 5, e127655. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.A.; Douek, D.C.; Packer, A.; Chung, K.; Guenaga, F.J.; Cassiani-Ingoni, R.; Campbell, C.; Memon, S.; Nagle, J.W.; Hakim, F.T.; et al. Thymic output generates a new and diverse tcr repertoire after autologous stem cell transplantation in multiple sclerosis patients. J. Exp. Med. 2005, 201, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.A.; Robins, H.; Malhotra, S.; Howell, M.; Phippard, D.; Desmarais, C.; de Paula Alves Sousa, A.; Griffith, L.M.; Lim, N.; Nash, R.A.; et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J. Clin. Investig. 2014, 124, 1168–1172. [Google Scholar] [CrossRef]
- Abrahamsson, S.V.; Angelini, D.F.; Dubinsky, A.N.; Morel, E.; Oh, U.; Jones, J.L.; Carassiti, D.; Reynolds, R.; Salvetti, M.; Calabresi, P.A.; et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes il-17 producing mucosal-associated invariant t cells in multiple sclerosis. Brain 2013, 136, 2888–2903. [Google Scholar] [CrossRef]
- Arruda, L.C.; Lorenzi, J.C.; Sousa, A.P.; Zanette, D.L.; Palma, P.V.; Panepucci, R.A.; Brum, D.S.; Barreira, A.A.; Covas, D.T.; Simoes, B.P.; et al. Autologous hematopoietic sct normalizes mir-16, -155 and -142-3p expression in multiple sclerosis patients. Bone Marrow Transplant. 2015, 50, 380–389. [Google Scholar] [CrossRef]
- Delemarre, E.M.; van den Broek, T.; Mijnheer, G.; Meerding, J.; Wehrens, E.J.; Olek, S.; Boes, M.; van Herwijnen, M.J.; Broere, F.; van Royen, A.; et al. Autologous stem cell transplantation aids autoimmune patients by functional renewal and tcr diversification of regulatory t cells. Blood 2016, 127, 91–101. [Google Scholar] [CrossRef]
- de Kleer, I.; Vastert, B.; Klein, M.; Teklenburg, G.; Arkesteijn, G.; Yung, G.P.; Albani, S.; Kuis, W.; Wulffraat, N.; Prakken, B. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive t cells and restoring the cd4+cd25+ immune regulatory network. Blood 2006, 107, 1696–1702. [Google Scholar] [CrossRef]
- Burt, R.K.; Balabanov, R.; Han, X.; Burns, C.; Gastala, J.; Jovanovic, B.; Helenowski, I.; Jitprapaikulsan, J.; Fryer, J.P.; Pittock, S.J. Autologous nonmyeloablative hematopoietic stem cell transplantation for neuromyelitis optica. Neurology 2019, 93, e1732–e1741. [Google Scholar] [CrossRef] [PubMed]
- Karnell, F.G.; Lin, D.; Motley, S.; Duhen, T.; Lim, N.; Campbell, D.J.; Turka, L.A.; Maecker, H.T.; Harris, K.M. Reconstitution of immune cell populations in multiple sclerosis patients after autologous stem cell transplantation. Clin. Exp. Immunol. 2017, 189, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Zagrivnaja, M.; Schwartz, S.; Badbaran, A.; Zabelina, T.; Lioznov, M.; Ayuk, F.; Zander, A.; Fehse, B. Kinetics of plasma-cell chimerism after allogeneic stem cell transplantation by highly sensitive real-time pcr based on sequence polymorphism and its value to quantify minimal residual disease in patients with multiple myeloma. Exp. Hematol. 2006, 34, 688–694. [Google Scholar] [CrossRef]
- Suzuki, I.; Milner, E.C.; Glas, A.M.; Hufnagle, W.O.; Rao, S.P.; Pfister, L.; Nottenburg, C. Immunoglobulin heavy chain variable region gene usage in bone marrow transplant recipients: Lack of somatic mutation indicates a maturational arrest. Blood 1996, 87, 1873–1880. [Google Scholar] [CrossRef]
- Avanzini, M.A.; Locatelli, F.; Santos, C.D.; Maccario, R.; Lenta, E.; Oliveri, M.; Giebel, S.; De Stefano, P.; Rossi, F.; Giorgiani, G.; et al. B lymphocyte reconstitution after hematopoietic stem cell transplantation: Functional immaturity and slow recovery of memory cd27+ b cells. Exp. Hematol. 2005, 33, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Daikeler, T.; Labopin, M.; Di Gioia, M.; Abinun, M.; Alexander, T.; Miniati, I.; Gualandi, F.; Fassas, A.; Martin, T.; Schwarze, C.P.; et al. Secondary autoimmune diseases occurring after hsct for an autoimmune disease: A retrospective study of the ebmt autoimmune disease working party. Blood 2011, 118, 1693–1698. [Google Scholar] [CrossRef]
- Loh, Y.; Oyama, Y.; Statkute, L.; Quigley, K.; Yaung, K.; Gonda, E.; Barr, W.; Jovanovic, B.; Craig, R.; Stefoski, D. Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases: Role of conditioning regimen used. Blood 2007, 109, 2548–2643. [Google Scholar] [CrossRef]
- Massarotti, C.; Sbragia, E.; Boffa, G.; Vercelli, C.; Zimatore, G.B.; Cottone, S.; Frau, J.; Raiola, A.; Varaldo, R.; Mancardi, G.; et al. Menstrual cycle resumption and female fertility after autologous hematopoietic stem cell transplantation for multiple sclerosis. Mult. Scler. J. 2021, 27, 2103–2107. [Google Scholar] [CrossRef]
- Massenkeil, G.; Alexander, T.; Rosen, O.; Dorken, B.; Burmester, G.; Radbruch, A.; Hiepe, F.; Arnold, R. Long-term follow-up of fertility and pregnancy in autoimmune diseases after autologous haematopoietic stem cell transplantation. Rheumatol. Int. 2016, 36, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Snarski, E.; Snowden, J.A.; Oliveira, M.C.; Simoes, B.; Badoglio, M.; Carlson, K.; Burman, J.; Moore, J.; Rovira, M.; Clark, R.E.; et al. Onset and outcome of pregnancy after autologous haematopoietic sct (ahsct) for autoimmune diseases: A retrospective study of the ebmt autoimmune diseases working party (adwp). Bone Marrow Transplant. 2015, 50, 216–220. [Google Scholar] [CrossRef]
- Muraro, P.A.; Pasquini, M.; Atkins, H.L.; Bowen, J.D.; Farge, D.; Fassas, A.; Freedman, M.S.; Georges, G.E.; Gualandi, F.; Hamerschlak, N.; et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 2017, 74, 459–469. [Google Scholar] [CrossRef]
- Snowden, J.A.; Badoglio, M.; Labopin, M.; Giebel, S.; McGrath, E.; Marjanovic, Z.; Burman, J.; Moore, J.; Rovira, M.; Wulffraat, N.M.; et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017, 1, 2742–2755. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.A.; Martin, R.; Mancardi, G.L.; Nicholas, R.; Sormani, M.P.; Saccardi, R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 391–405. [Google Scholar] [CrossRef]
- Sormani, M.P.; Muraro, P.A.; Schiavetti, I.; Signori, A.; Laroni, A.; Saccardi, R.; Mancardi, G.L. Autologous hematopoietic stem cell transplantation in multiple sclerosis: A meta-analysis. Neurology 2017, 88, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Qiu, W.; Li, J.; Hu, X.; Huang, R.; Lin, D.; Bao, J.; Jiang, Y.; Bian, L. A preliminary result of treatment of neuromyelitis optica with autologous peripheral hematopoietic stem cell transplantation. Neurologist 2010, 16, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Matiello, M.; Pittock, S.J.; Porrata, L.; Weinshenker, B.G. Failure of autologous hematopoietic stem cell transplantation to prevent relapse of neuromyelitis optica. Arch. Neurol. 2011, 68, 953–955. [Google Scholar] [CrossRef]
- Aouad, P.; Li, J.; Arthur, C.; Burt, R.; Fernando, S.; Parratt, J. Resolution of aquaporin-4 antibodies in a woman with neuromyelitis optica treated with human autologous stem cell transplant. J. Clin. Neurosci. 2015, 22, 1215–1217. [Google Scholar] [CrossRef]
- Hoay, K.Y.; Ratnagopal, P. Autologous hematopoietic stem cell transplantation for the treatment of neuromyelitis optica in singapore. Acta Neurol. Taiwan 2018, 27, 26–32. [Google Scholar]
- Greco, R.; Bondanza, A.; Oliveira, M.C.; Badoglio, M.; Burman, J.; Piehl, F.; Hagglund, H.; Krasulova, E.; Simoes, B.P.; Carlson, K.; et al. Autologous hematopoietic stem cell transplantation in neuromyelitis optica: A registry study of the ebmt autoimmune diseases working party. Mult. Scler. 2015, 21, 189–197. [Google Scholar] [CrossRef]
- Burton, J.M.; Duggan, P.; Costello, F.; Metz, L.; Storek, J. A pilot trial of autologous hematopoietic stem cell transplant in neuromyelitis optic spectrum disorder. Mult. Scler. Relat. Disord. 2021, 53, 102990. [Google Scholar] [CrossRef] [PubMed]
- Ceglie, G.; Papetti, L.; Valeriani, M.; Merli, P. Hematopoietic stem cell transplantation in neuromyelitis optica-spectrum disorders (nmo-sd): State-of-the-art and future perspectives. Int. J. Mol. Sci. 2020, 21, 5304. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, F.; Masrouri, S.; Sharifkazemi, H.; Azami, M.; Nikfarjam, M.; Moghadasi, A.N. Autologous hematopoietic stem cell transplantation in neuromyelitis optica spectrum disorder: A systematic review and meta-analysis. J. Clin. Neurosci. 2022, 105, 37–44. [Google Scholar] [CrossRef]
- Hadavi, S.; Noyce, A.J.; Leslie, R.D.; Giovannoni, G. Stiff person syndrome. Pract. Neurol. 2011, 11, 272–282. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Arino, H.; McKeon, A.; Iizuka, T.; Titulaer, M.J.; Simabukuro, M.M.; Lancaster, E.; Petit-Pedrol, M.; Planaguma, J.; Blanco, Y.; et al. Clinical and immunologic investigations in patients with stiff-person spectrum disorder. JAMA Neurol. 2016, 73, 714–720. [Google Scholar] [CrossRef]
- Newsome, S.D.; Johnson, T. Stiff person syndrome spectrum disorders; more than meets the eye. J. Neuroimmunol. 2022, 369, 577915. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Jankovic, J. Stiff-person syndrome: Insights into a complex autoimmune disorder. J. Neurol. Neurosurg. Psychiatry 2015, 86, 840–848. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Fujii, M.; Li, M.; Lutfi, B.; Kyhos, J.; McElroy, B. High-dose intravenous immune globulin for stiff-person syndrome. N. Engl. J. Med. 2001, 345, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.R.; Das, M.; Isaacs, J.; Fawcett, P.R.; Bates, D. Treatment of stiff person syndrome with rituximab. J. Neurol. Neurosurg. Psychiatry 2005, 76, 999–1001. [Google Scholar] [CrossRef]
- McKeon, A.; Robinson, M.T.; McEvoy, K.M.; Matsumoto, J.Y.; Lennon, V.A.; Ahlskog, J.E.; Pittock, S.J. Stiff-man syndrome and variants: Clinical course, treatments, and outcomes. Arch. Neurol. 2012, 69, 230–238. [Google Scholar] [CrossRef]
- Sanders, S.; Bredeson, C.; Pringle, C.E.; Martin, L.; Allan, D.; Bence-Bruckler, I.; Hamelin, L.; Hopkins, H.S.; Sabloff, M.; Sheppard, D.; et al. Autologous stem cell transplantation for stiff person syndrome: Two cases from the ottawa blood and marrow transplant program. JAMA Neurol. 2014, 71, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Georges, G.E.; Bowen, J.D.; Pearlman, M.; Wundes, A.; von Geldern, G.; Kraft, G.H.; Weiss, M.D.; McLaughlin, B.; Sytsma, J.; Nash, R. Autologous hematopoietic stem cell transplantation may be highly effective treatment for severe stiff person syndrome. Biol. Blood Marrow Transplant. 2018, 24, S120. [Google Scholar] [CrossRef]
- Kass-Iliyya, L.; Snowden, J.A.; Thorpe, A.; Jessop, H.; Chantry, A.D.; Sarrigiannis, P.G.; Hadjivassiliou, M.; Sharrack, B. Autologous haematopoietic stem cell transplantation for refractory stiff-person syndrome: The uk experience. J. Neurol. 2021, 268, 265–275. [Google Scholar] [CrossRef]
- Burt, R.K.; Balabanov, R.; Han, X.; Quigley, K.; Arnautovic, I.; Helenowski, I.; Rose, J.; Siddique, T. Autologous hematopoietic stem cell transplantation for stiff-person spectrum disorder: A clinical trial. Neurology 2021, 96, e817–e830. [Google Scholar] [CrossRef]
- Dalakas, M.C. Limited benefits halt enrollment in hematopoietic stem cell transplantation trial for stiff-person syndrome: Should there be more to come? Neurology 2021, 96, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.C.; Burke, D.; Kuwabara, S. Chronic inflammatory demyelinating polyneuropathy: Update on diagnosis, immunopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2019, 90, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Van Oers, M. Successful autologous stem cell transplantation in a patient with chronic inflammatory demyelinating polyneuropathy. J. Neurol. Neurosurg. Psychiatry 2002, 72, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Van Oers, M. Relapse of chronic inflammatory demyelinating polyneuropathy 5 years after autologous stem cell transplantation. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1154. [Google Scholar] [CrossRef] [PubMed]
- Mahdi-Rogers, M.; Kazmi, M.; Ferner, R.; Hughes, R.A.; Renaud, S.; Steck, A.J.; Fuhr, P.; Halter, J.; Gratwohl, A.; Tyndall, A. Autologous peripheral blood stem cell transplantation for chronic acquired demyelinating neuropathy. J. Peripher. Nerv. Syst. 2009, 14, 118–124. [Google Scholar] [CrossRef]
- Press, R.; Askmark, H.; Svenningsson, A.; Andersen, O.; Axelson, H.W.; Strömberg, U.; Wahlin, A.; Isaksson, C.; Johansson, J.J.; Hägglund, H. Autologous haematopoietic stem cell transplantation: A viable treatment option for cidp. J. Neurol. Neurosurg. Psychiatry 2014, 85, 618–624. [Google Scholar] [CrossRef]
- Ajroud-Driss, S.; Gozdziak, P.; Sufit, R.; Burt, R. Non-myeloablative autologous hematopoietic stem cell transplantation for the treatment of refractory cidp. J. Peripher. Nerv. Syst. 2011, 16, 1–160. [Google Scholar]
- Masson-Roy, J.; Breiner, A.; Warman-Chardon, J.; Pringle, C.E.; Allan, D.; Bredeson, C.; Huebsch, L.; Kekre, N.; Kennah, M.L. Martin. Autologous hematopoietic stem cell transplantation for chronic inflammatory demyelinating polyradiculoneuropathy. Can. J. Neurol. Sci. 2021, 48, 760–766. [Google Scholar] [PubMed]
- Burt, R.K.; Balabanov, R.; Tavee, J.; Han, X.; Sufit, R.; Ajroud-Driss, S.; Jovanovic, B.; Quigley, K. Arnautovic and I. Helenowski. Hematopoietic stem cell transplantation for chronic inflammatory demyelinating polyradiculoneuropathy. J. Neurol. 2020, 267, 3378–3391. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Sufit, R.; Ajroud-Driss, S.; Burt, R. Nonmyeloablative autologous hematopoetic stem cell transplant for the treatment of cidp: An interim report. J. Peripher. Nerv. Syst. 2013, 18, 5. [Google Scholar]
- Burt, R.K.; Tappenden, P.; Balabanov, R.; Han, X.; Quigley, K.; Snowden, J.A.; Sharrack, B. The cost effectiveness of immunoglobulin vs. Hematopoietic stem cell transplantation for cidp. Front. Neurol. 2021, 12, 645263. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E. Myasthenia gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Bril, V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs 2022, 82, 865–887. [Google Scholar] [CrossRef]
- Bryant, A.; Atkins, H.; Pringle, C.E.; Allan, D.; Anstee, G.; Bence-Bruckler, I.; Hamelin, L.; Hodgins, M.; Hopkins, H.; Huebsch, L.; et al. Myasthenia gravis treated with autologous hematopoietic stem cell transplantation. JAMA Neurol. 2016, 73, 652–658. [Google Scholar] [CrossRef]
- Sossa Melo, C.L.; Peña, A.M.; Salazar, L.A.; Jiménez, S.I.; Gómez, E.D.; Chalela, C.M.; Ayala-Castillo, M.; Peña, I.M. Autologous hematopoietic stem cell transplantation in a patient with refractory seropositive myasthenia gravis: A case report. Neuromuscul. Disord. 2019, 29, 142–145. [Google Scholar] [CrossRef]
- Håkansson, I.; Sandstedt, A.; Lundin, F.; Askmark, H.; Pirskanen, R.; Carlson, K.; Piehl, F.; Hägglund, H. Successful autologous haematopoietic stem cell transplantation for refractory myasthenia gravis-a case report. Neuromuscul. Disord. 2017, 27, 90–93. [Google Scholar] [CrossRef]
- Strober, J.; Cowan, M.J.; Horn, B.N. Allogeneic hematopoietic cell transplantation for refractory myasthenia gravis. Arch. Neurol. 2009, 66, 659–661. [Google Scholar] [CrossRef]
- Inan, B.; Bekircan-Kurt, C.E.; Demiroğlu, H.; Göker, H.; Erdem-Özdamar, S.; Tan, E. Autologous stem cell transplantation in a patient with refractory anti-musk-positive myasthenia gravis and familial mediterranean fever. Neurol. Sci. Neurophysiol. 2022, 39, 115. [Google Scholar] [CrossRef]
- Mitsumune, S.; Manabe, Y.; Yunoki, T.; Kono, S.; Aoyama, K.; Shinno, Y.; Narai, H.; Abe, K. Autologous bone marrow transplantation for polymyositis combined with myasthenia gravis and aplastic anemia: A case report. Case Rep. Neurol. 2018, 10, 108–111. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Hematopoietic Stem Cell Therapy for Patients with Refractory Myasthenia Gravis. 2022. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00424489 (accessed on 23 November 2022).
- Findlay, A.R.; Goyal, N.A.; Mozaffar, T. An overview of polymyositis and dermatomyositis. Muscle Nerve 2015, 51, 638–656. [Google Scholar] [CrossRef]
- Baron, F.; Ribbens, C.; Kaye, O.; Fillet, G.; Malaise, M.; Beguin, Y. Effective treatment of jo-1-associated polymyositis with t-cell-depleted autologous peripheral blood stem cell transplantation. Br. J. Haematol. 2000, 110, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Holzer, U.; van Royen-Kerkhof, A.; Van Der Torre, P.; Kuemmerle-Deschner, J.; Well, C.; Handgretinger, R.; Mueller, I.; Wulffraat, N. Successful autologous stem cell transplantation in two patients with juvenile dermatomyositis. Scand. J. Rheumatol. 2010, 39, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Su, G.; Lai, J.; Dong, B.; Kang, M.; Li, S.; Zhou, Z.; Wu, F. Long-term follow-up of autologous hematopoietic stem cell transplantation for refractory juvenile dermatomyositis: A case-series study. Pediatr. Rheumatol. 2018, 16, 72. [Google Scholar] [CrossRef]
- Chahin, N.; Selcen, D.; Engel, A.G. Sporadic late onset nemaline myopathy. Neurology 2005, 65, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Belkhribchia, M.R.; Tazi, I.; Louhab, N.; Kissani, N.; Mahmal, L.; Pereon, Y. Autologous stem cell transplantation in a patient with sporadic late-onset nemaline myopathy and monoclonal gammopathy: First moroccan experience. Presse Médicale 2017, 46, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Voermans, N.C.; Benveniste, O.; Minnema, M.C.; Lokhorst, H.; Lammens, M.; Meersseman, W.; Delforge, M.; Kuntzer, T.; Novy, J.; Pabst, T.; et al. Sporadic late-onset nemaline myopathy with mgus: Long-term follow-up after melphalan and sct. Neurology 2014, 83, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Hanbali, A.; Rasheed, W.; Peedikayil, M.C.; Boholega, S.; Alzahrani, H.A. Mitochondrial neurogastrointestinal encephalomyopathy syndrome treated with stem cell transplant: A case series and literature review. Exp. Clin. Transplant. 2018, 16, 773–778. [Google Scholar] [PubMed]
- Halter, J.P.; Michael, W.; Schüpbach, M.; Mandel, H.; Casali, C.; Orchard, K.; Collin, M.; Valcarcel, D.; Rovelli, A.; Filosto, M. Allogeneic haematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Brain 2015, 138, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Marti, R.; Casali, C.; Tadesse, S.; Uldrick, T.; Fine, B.; Escolar, D.; Valentino, M.; Nishino, I.; Hesdorffer, C. Allogeneic stem cell transplantation corrects biochemical derangements in mngie. Neurology 2006, 67, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Born, A.P.; Müller, K.; Marquart, H.V.; Heilmann, C.; Schejbel, L.; Vissing, J. Myositis in griscelli syndrome type 2 treated with hematopoietic cell transplantation. Neuromuscul. Disord. 2010, 20, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Pavlakis, P.P. Rheumatologic disorders and the nervous system. Continuum 2020, 26, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Daikeler, T.; Kötter, I.; Tyndall, C.B.; Apperley, J.; Attarbaschi, A.; Guardiola, P.; Gratwohl, A.; Jantunen, E.; Marmont, A.; Porretto, F.; et al. Haematopoietic stem cell transplantation for vasculitis including behcet’s disease and polychondritis: A retrospective analysis of patients recorded in the european bone marrow transplantation and european league against rheumatism databases and a review of the literature. Ann. Rheum. Dis. 2007, 66, 202–207. [Google Scholar] [CrossRef]
- De Cata, A.; Intiso, D.; Bernal, M.; Molinaro, F.; Mazzoccoli, G.; D’Alessandro, V.; Greco, A.; Curci, S.; Sperandeo, M.; Frusciante, V.; et al. Prolonged remission of neuro-behcet disease following autologous transplantation. Int. J. Immunopathol. Pharmacol. 2007, 20, 91–96. [Google Scholar] [CrossRef]
- Marmont, A.M.; Gualandi, F.; Piaggio, G.; Podestà, M.; van Lint, M.T.; Bacigalupo, A.; Nobili, F. Allogeneic bone marrow transplantation (bmt) for refractory behçet’s disease with severe cns involvement. Bone Marrow Transplant. 2006, 37, 1061–1063. [Google Scholar] [CrossRef]
- Statkute, L.; Oyama, Y.; Barr, W.G.; Sufit, R.; Ho, S.; Verda, L.; Loh, Y.; Yaung, K.; Quigley, K.; Burt, R.K. Autologous non-myeloablative haematopoietic stem cell transplantation for refractory systemic vasculitis. Ann. Rheum. Dis. 2008, 67, 991–997. [Google Scholar] [CrossRef]
- Burt, R.K.; Traynor, A.; Statkute, L.; Barr, W.G.; Rosa, R.; Schroeder, J.; Verda, L.; Krosnjar, N.; Quigley, K.; Yaung, K.; et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA 2006, 295, 527–535. [Google Scholar] [CrossRef]
- Lehnhardt, F.G.; Scheid, C.; Holtik, U.; Burghaus, L.; Neveling, M.; Impekoven, P.; Rüger, A.; Hallek, M.; Jacobs, A.H.; Rubbert, A. Autologous blood stem cell transplantation in refractory systemic lupus erythematodes with recurrent longitudinal myelitis and cerebral infarction. Lupus 2006, 15, 240–243. [Google Scholar] [CrossRef]
- Trysberg, E.; Lindgren, I.; Tarkowski, A. Autologous stem cell transplantation in a case of treatment resistant central nervous system lupus. Ann. Rheum. Dis. 2000, 59, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Goklemez, S.; Hasni, S.; Hakim, F.T.; Muraro, P.A.; Pirsl, F.; Rose, J.; Memon, S.; Fowler, D.F.; Steinberg, S.M.; Baker, E.H.; et al. Long-term follow-up after lymphodepleting autologous haematopoietic cell transplantation for treatment-resistant systemic lupus erythematosus. Rheumatology 2022, 61, 3317–3328. [Google Scholar] [CrossRef] [PubMed]
- Lisukov, I.A.; Sizikova, S.A.; Kulagin, A.D.; Kruchkova, I.V.; Gilevich, A.V.; Konenkova, L.P.; Zonova, E.V.; Chernykh, E.R.; Leplina, O.Y.; Sentyakova, T.N.; et al. High-dose immunosuppression with autologous stem cell transplantation in severe refractory systemic lupus erythematosus. Lupus 2004, 13, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, M.; Schwaneck, E.C.; Gernert, M.; Gadeholt, O.; Strunz, P.-P.; Morbach, H.; Tony, H.-P.; Schmalzing, M. Autologous stem cell transplantation in common variable immunodeficiency: A case of successful treatment of severe refractory autoimmune encephalitis. Front. Immunol. 2020, 11, 1317. [Google Scholar] [CrossRef]
- Snowden, J.A.; Sanchez-Ortega, I.; Corbacioglu, S.; Basak, G.W.; Chabannon, C.; de la Camara, R.; Dolstra, H.; Duarte, R.F.; Glass, B.; Greco, R.; et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in europe, 2022. Bone Marrow Transplant. 2022, 57, 1217–1239. [Google Scholar] [CrossRef]
- Burt, R.K.; Han, X.; Gozdziak, P.; Yaung, K.; Morgan, A.; Clendenan, A.M.; Henry, J.; Calvario, M.A.; Datta, S.K.; Helenowski, I.; et al. Five year follow-up after autologous peripheral blood hematopoietic stem cell transplantation for refractory, chronic, corticosteroid-dependent systemic lupus erythematosus: Effect of conditioning regimen on outcome. Bone Marrow Transplant. 2018, 53, 692–700. [Google Scholar] [CrossRef]
- Greco, R.; Alexander, T.; Burman, J.; Del Papa, N.; de Vries-Bouwstra, J.; Farge, D.; Henes, J.; Kazmi, M.; Kirgizov, K.; Muraro, P.A. Hematopoietic stem cell transplantation for autoimmune diseases in the time of covid-19: Ebmt guidelines and recommendations. Bone Marrow Transplant. 2021, 56, 1493–1508. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Autologous Peripheral Blood Stem Cell Transplant for Neurologic Autoimmune Diseases. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00716066?term=hematopoietic+stem+cell&cond=CIDP&draw=2&rank=2 (accessed on 28 August 2021).
- Clinicaltrials.gov. Stem Cell Transplantation in Idiopathic Inflammatory Myopathy Diseases. 2022. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00278564?term=hematopoietic+stem+cell&cond=Neurological+Disease&draw=2&rank=5 (accessed on 30 November 2022).

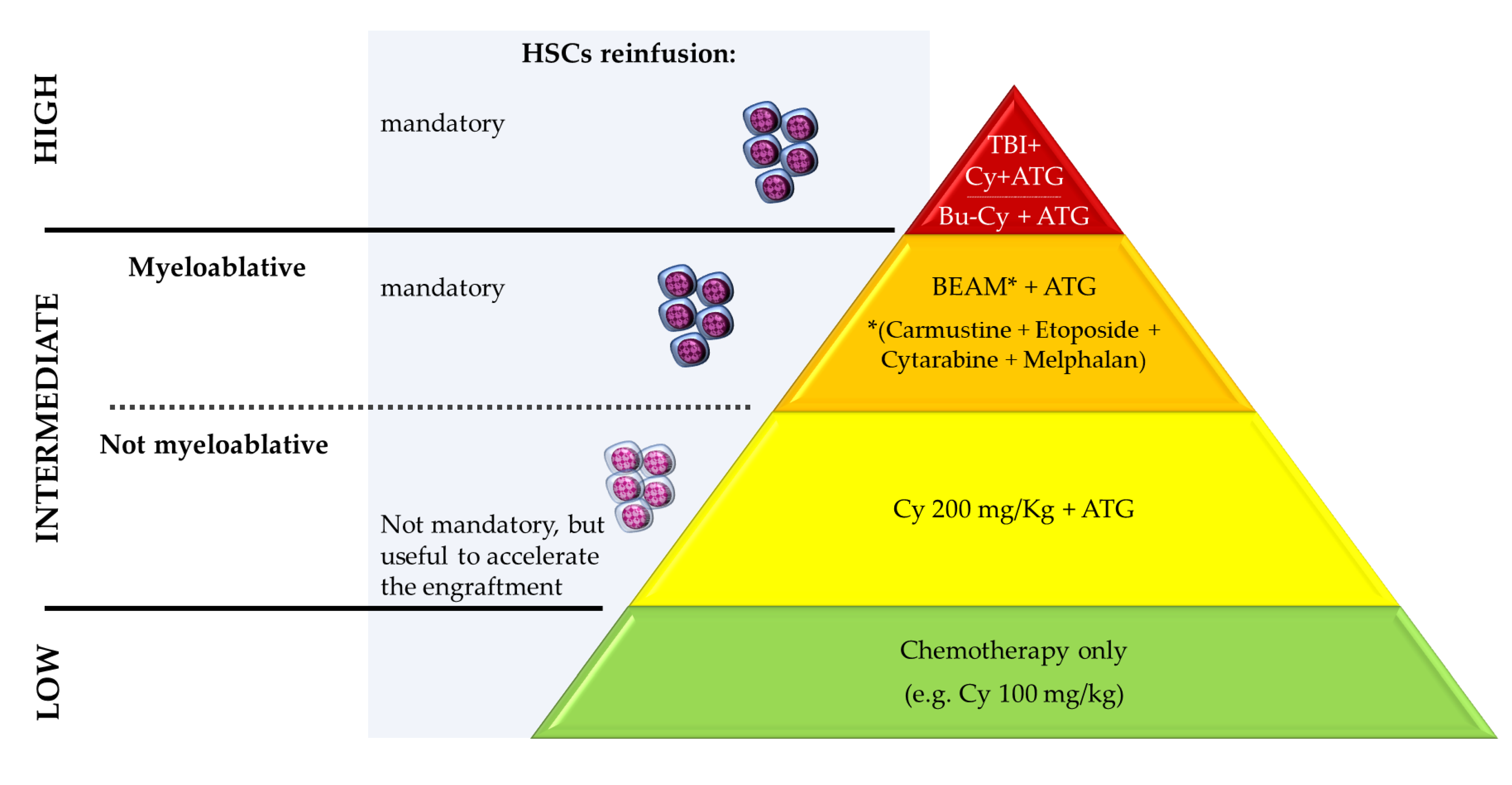
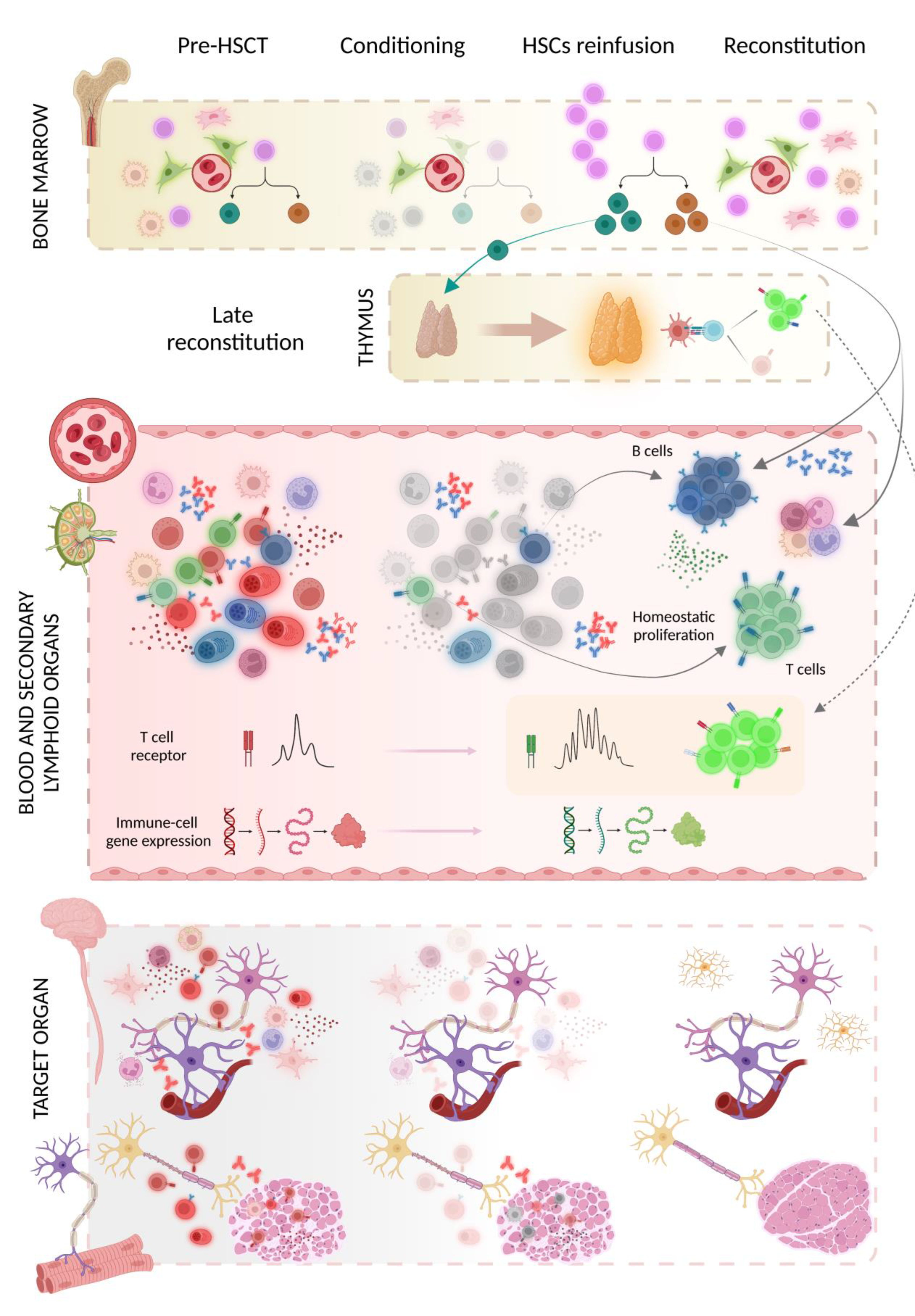
| Study [Reference] | Animal Model | HSCT Protocol | Evaluation Time after BMT | Clinical Evaluation and Outcomes | Histopathological/Laboratory Assessment |
|---|---|---|---|---|---|
| Ikehara, S. et al., 1985 [8] | Mice; strains with spontaneous SLE-like disease | Conditioning: TBI (850–950 rads from a cobalt-60 source) Reconstitution: IV injection of 2 × 107 allogenic BM cells from young healthy mice BMT either (1) before or (2) after AD onset | 3 and 5 months | (1) Mice in pre-AD state (BMT before disease onset): no development of thymic abnormalities and AD up to month 5; (2) Mice with evidence of AD and lymphadenopathy at BMT: survival beyond month 3 after BMT. | (1) no development of thymic abnormalities (histological examination); (2) disappearance of lymphadenopathy and marked amelioration of lymphoid cell infiltrations into the kidney and liver; marked reduction of glomerular deposits of IgG, IgA, C3, and gp70 (immunofluorescence); reduced levels of CICs and anti-dsDNA ab. |
| van Bekkum, D. W. et al., 1989 [9] | Rats; inbred strains Buffalo and Wag/Rij, with induced AA a | Conditioning: TBI (850 cGy) Reconstitution: IV injection of 5 × 107 BM cells from syngeneic (Buffalo) or allogeneic (Wag/Rij) sex-matched donors BM cells collection: suspension by flushing of femoral bones cavities with Hanks’ salt solution | Weekly observation (arthritic score) up to: −14 w after syngeneic BMT −9 w after allogenic BMT. | Clear-cut complete regression of arthritis (expressed as a gradual decrease of paw thickness) in BMT-treated groups compared to controls. | Absence of inflammatory reaction in treated rats at 4 weeks after BMT versus classical severe inflammation and destruction process in control rats (histological examination). |
| Karussis, D. M. et al., 1992 [12] | SJL/J mice with induced EAE b | Conditioning regimens (three groups): (1) TBI (900 cGy) (2) TBI (1100 cGy) (3) Cy (300 mg/kg) Reconstitution: syngeneic BM cells | 3 months | (1) delayed onset and reduction in incidence and severity of EAE; (2) reduced incidence of EAE (developed in 1/7 treated mice); (3) no development of EAE, and resistance to rechallenge with the same encephalitogenic inoculum. | Lymphocytes obtained from treated mice did not proliferate in vitro in response to myelin basic protein or tuberculin-purified protein derivative, contrary to controls. |
| van Gelder, M. et al., 1993 [13] | Buffalo rats with induced EAE c | Conditioning: TBI (900 cGy) Reconstitution: syngeneic BM cells from healthy donors. | - | In mice with already developed severe paresis before BMT, greatly accelerated recovery of paresis compared with untreated controls. | N.R. |
| Pestronk, A. et al., 1983 [14] | Female Lewis rats with induced EAM d | Conditioning: TBI (600 rads from a dual-source cesium 137 irradiator) + Cy (200 mg/kg) Reconstitution: IV reinfusion of 6 × 107 autologous BM cells BM cells collection: autologous femur, tibia, spleen, and lymph nodes, washed twice and suspended in RPMI. | 8 weeks | N.R. | Prompt and sustained fall in the levels of serum ab against both foreign (Torpedo), and self (rat) AChR: −titre of ab against Torpedo AChR reduced to 2% of pre-treatment levels at 8 weeks; −titre of auto-ab against rat AChR fell to undetectable levels within 2–3 weeks and did not rise subsequently. |
| Study (NCT); Phase [Reference] | Diseases Included | Conditioning Regimen | Estimated Enrolment (n Participants) | Start Date | Estimated Completion Date | Status |
|---|---|---|---|---|---|---|
| Autologous Peripheral Blood Stem Cell Transplant for Neurologic Autoimmune Diseases (NCT00716066); phase II [103] | Primary CNS vasculitis Rasmussen’s encephalitis CIDP; Autoimmune peripheral neuropathy Autoimmune cerebellar degeneration Gait Ataxia with Late age Onset Polyneuropathy SPS MG; Lambert-Eaton myasthenic syndrome HTLV-1-associated myelopathy/tropical spastic paraparesis Opsoclonus/myoclonus MS; Neuromyelitis optica Other central or peripheral nervous system autoimmune diseases as approved by study neurologists and faculty | BEAM + ATG followed by autologous or syngeneic stem cell transplantation | 80 | June 2008 | June 2023 | Recruiting |
| Stem Cell Transplantation in Idiopathic Inflammatory Myopathy Diseases (NCT00278564); phase I [104] | Polymyositis, Dermatomyositis, Juvenile polymyositis/dermatomyositis, Myositis associated with other collagen diseases | Cy + ATG + rituximab | 7 | September 2005 | July 2016 | Terminated (high relapse rate) |
| Hematopoietic Stem Cell Therapy for Patients With Refractory Myasthenia Gravis (NCT00424489); phase I [77] | MG | Cy + ATG | 9 | February 2002 | June 2016 | Terminated (No plan to continue study) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariottini, A.; Bulgarini, G.; Cornacchini, S.; Damato, V.; Saccardi, R.; Massacesi, L. Hematopoietic Stem Cell Transplantation for the Treatment of Autoimmune Neurological Diseases: An Update. Bioengineering 2023, 10, 176. https://doi.org/10.3390/bioengineering10020176
Mariottini A, Bulgarini G, Cornacchini S, Damato V, Saccardi R, Massacesi L. Hematopoietic Stem Cell Transplantation for the Treatment of Autoimmune Neurological Diseases: An Update. Bioengineering. 2023; 10(2):176. https://doi.org/10.3390/bioengineering10020176
Chicago/Turabian StyleMariottini, Alice, Giovanni Bulgarini, Sara Cornacchini, Valentina Damato, Riccardo Saccardi, and Luca Massacesi. 2023. "Hematopoietic Stem Cell Transplantation for the Treatment of Autoimmune Neurological Diseases: An Update" Bioengineering 10, no. 2: 176. https://doi.org/10.3390/bioengineering10020176
APA StyleMariottini, A., Bulgarini, G., Cornacchini, S., Damato, V., Saccardi, R., & Massacesi, L. (2023). Hematopoietic Stem Cell Transplantation for the Treatment of Autoimmune Neurological Diseases: An Update. Bioengineering, 10(2), 176. https://doi.org/10.3390/bioengineering10020176







