Optimized Conditions for the Long-Term Maintenance of Precision-Cut Murine Myocardium in Biomimetic Tissue Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Surgical Procedure
2.3. Organ Bath
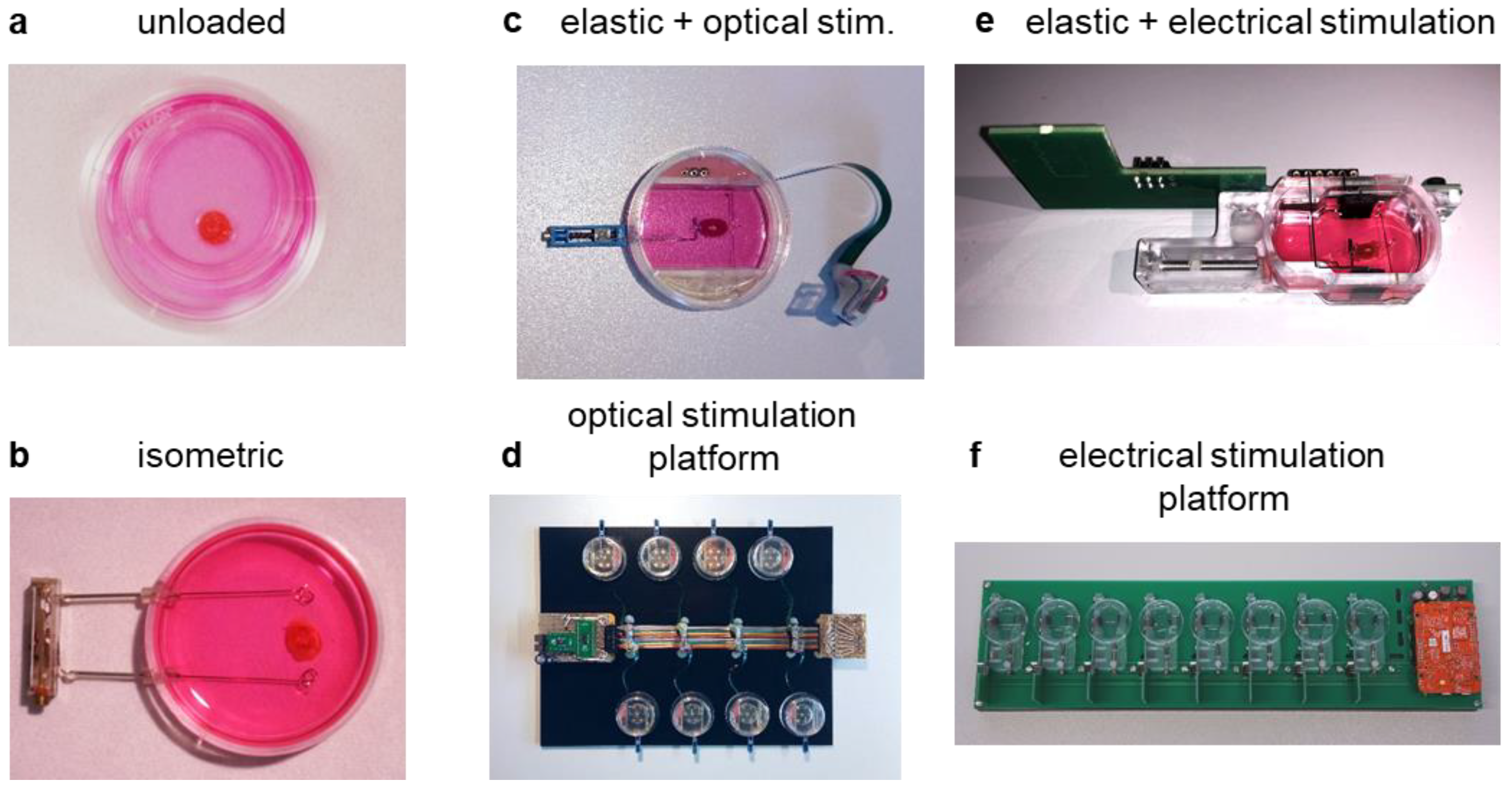
2.4. Unloaded, Unstirred Culture
2.5. Submerged, Isometric Culture
2.6. Optically Stimulated Culture with Preload
2.7. Electrically Stimulated Culture with Preload
2.8. Force Measurement and Analysis
2.9. Viability Determination
2.10. Confocal Microscopy
2.11. Real-Time qPCR
2.12. Statistical Analysis
3. Results
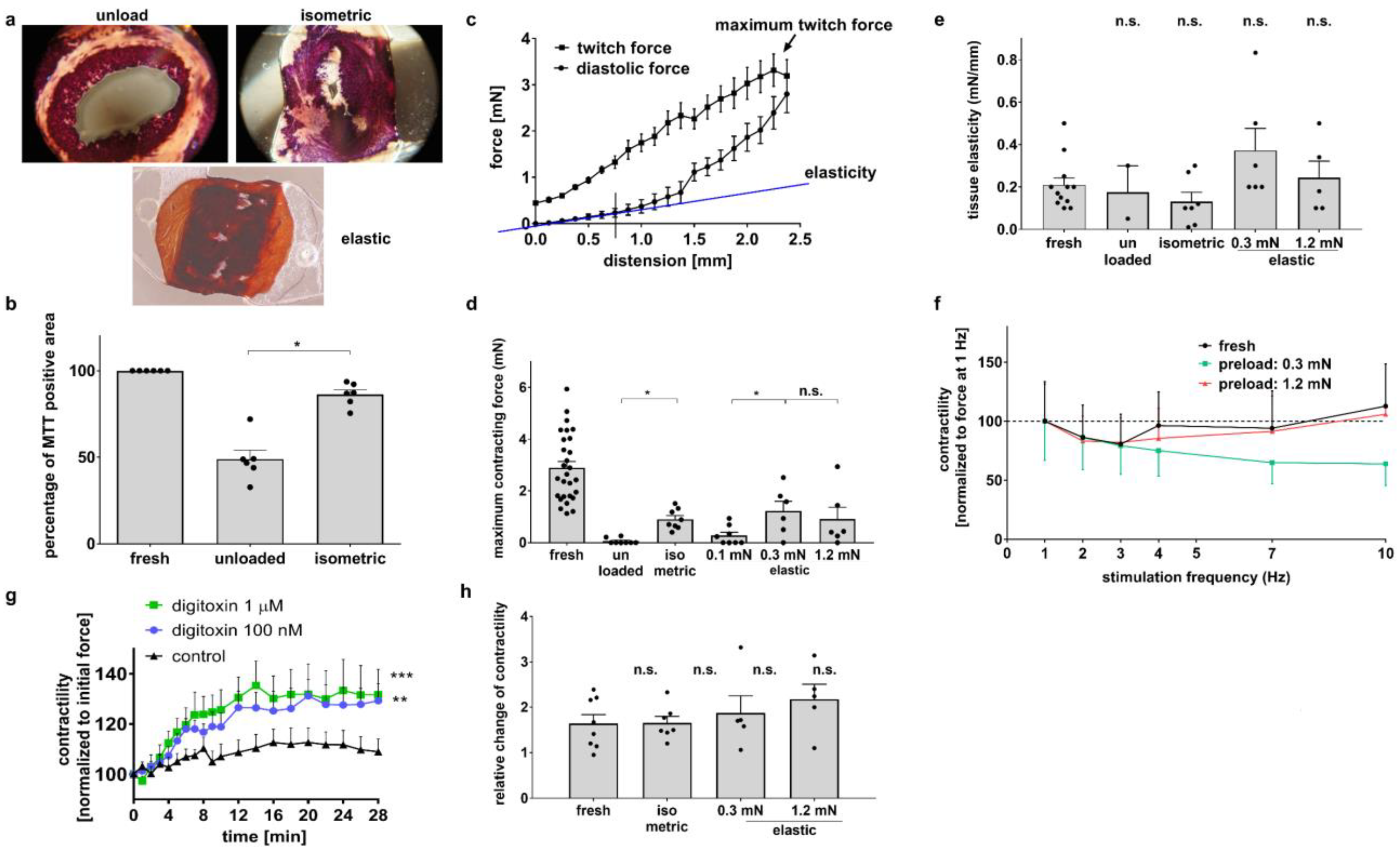
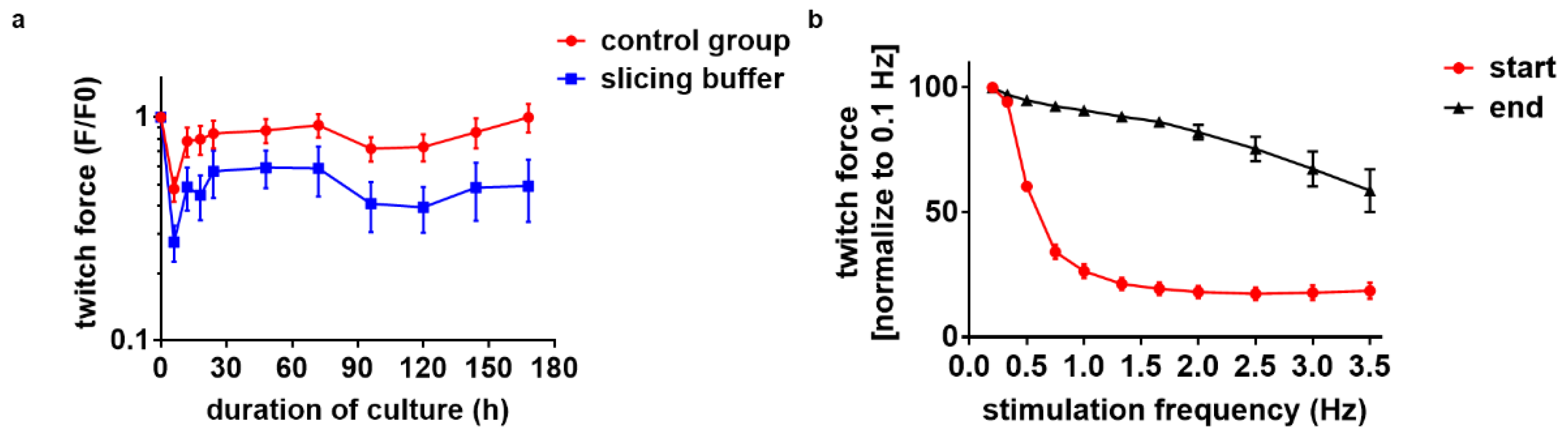
3.1. Diastolic Tension Is Critical for Tissue Integrity
3.2. Optical Stimulation Impairs the Preservation of Myocardium after 48 h Culture
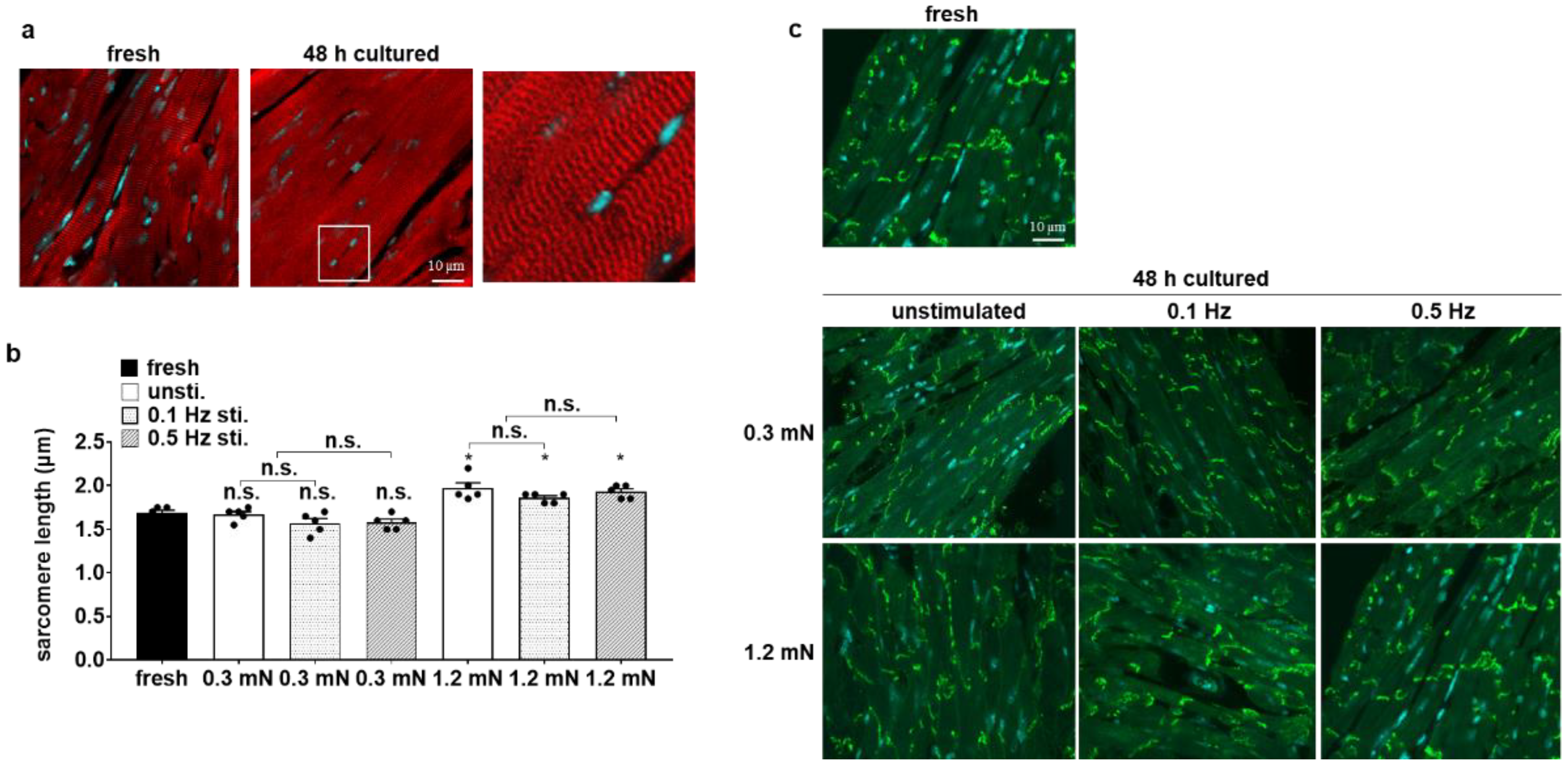
3.3. Cellular Structure Is Preserved in Biomimetic Culture under Optical Stimulation
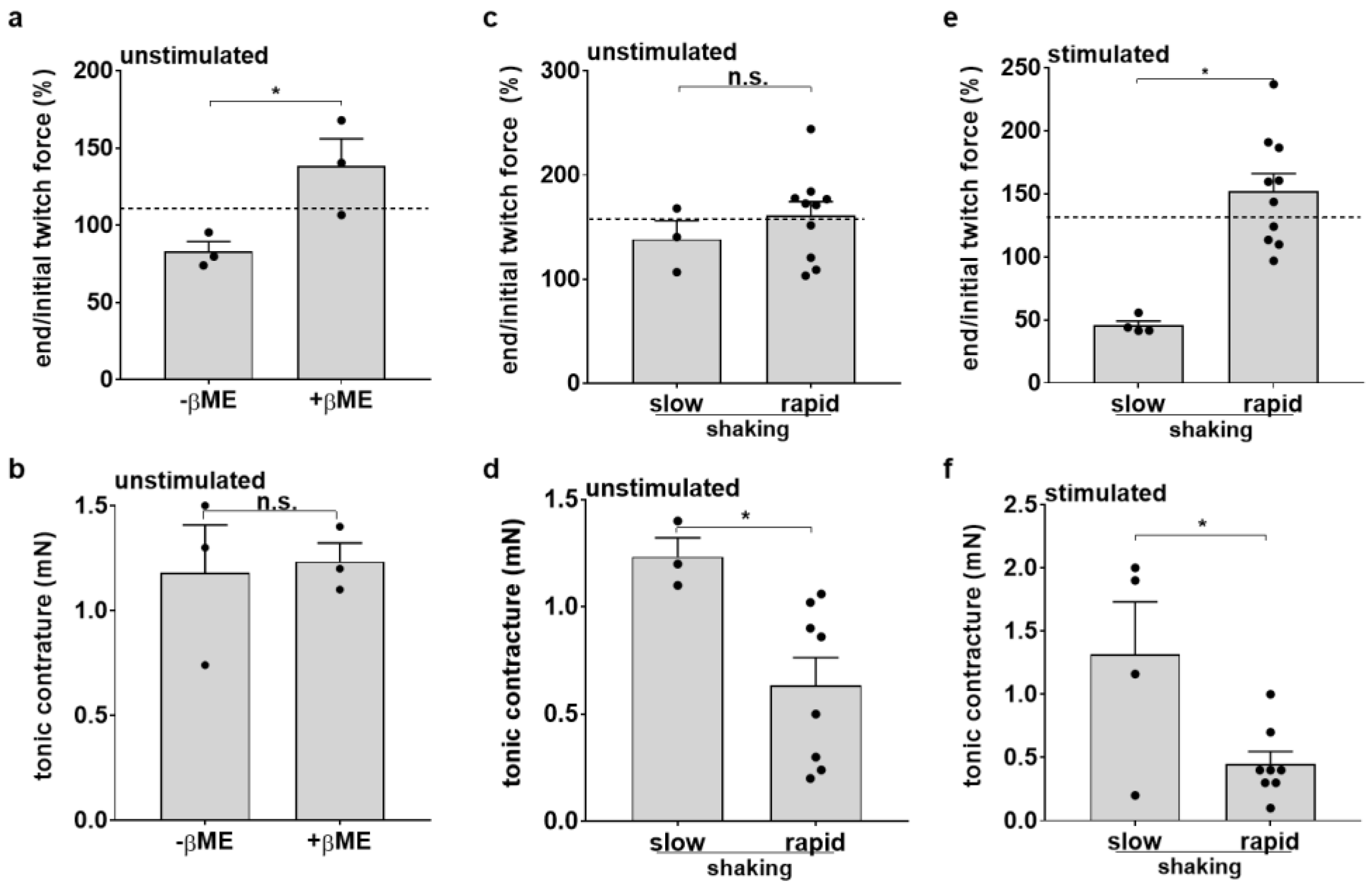
3.4. Medium Agitation and Antioxidant Supplement Improve Contractility under Optical Stimulation
3.5. RNA Expression Indicates Downregulation of Sarcomeric Proteins and Presence of Hypoxia
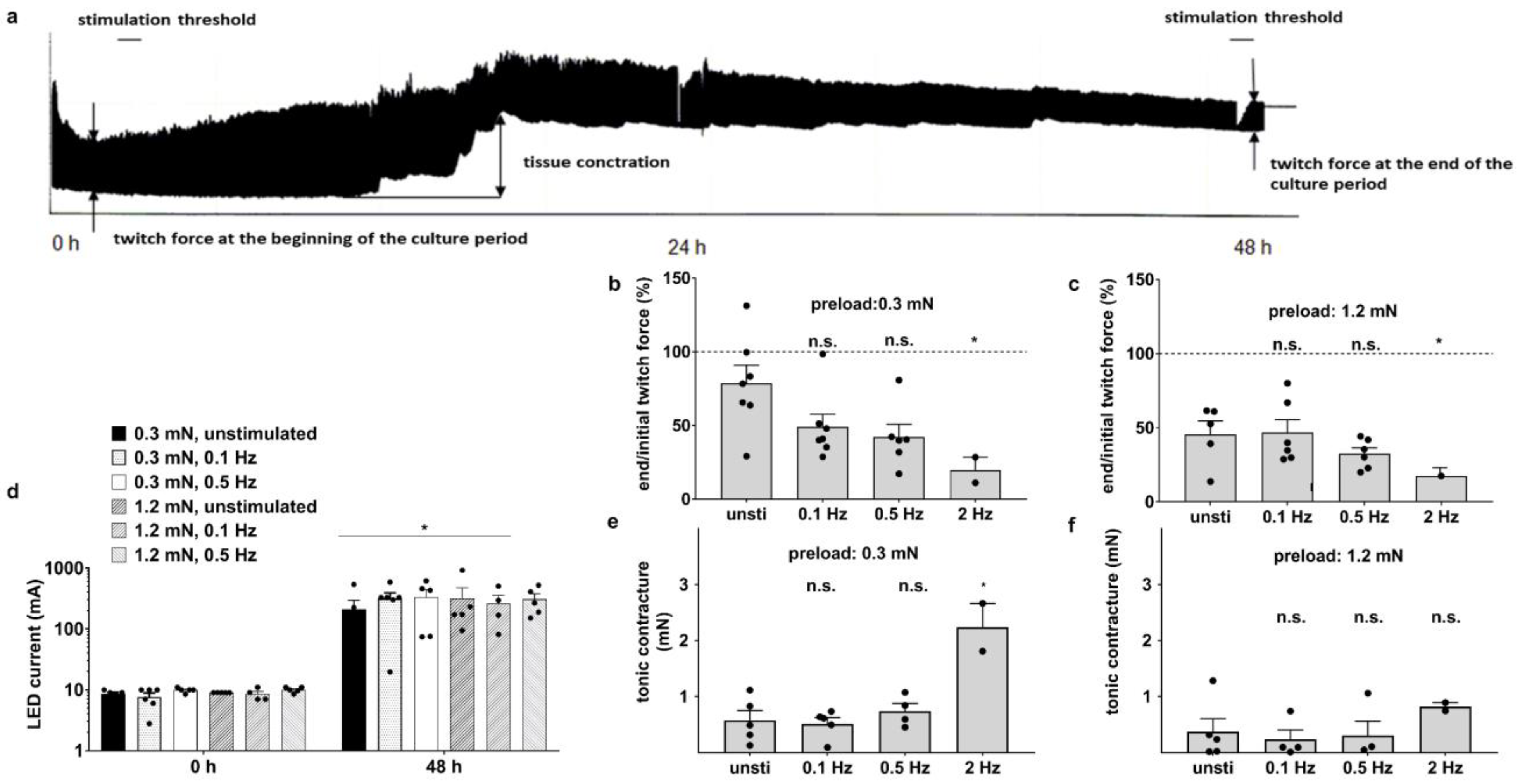
3.6. Electrical Stimulation Preserves Full Contractility for Days
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopalan, S.M.; Flaim, C.; Bhatia, S.N.; Hoshijima, M.; Knoell, R.; Chien, K.R.; Omens, J.H.; McCulloch, A.D. Anisotropic Stretch-Induced Hypertrophy in Neonatal Ventricular Myocytes Micropatterned on Deformable Elastomers. Biotechnol. Bioeng. 2003, 81, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.E.; Ali, N.N.; Brito-Martins, M.; Gorelik, J. The Human Embryonic Stem Cell-Derived Cardiomyocyte as a Pharmacological Model. Pharmacol. Ther. 2007, 113, 341–353. [Google Scholar] [CrossRef]
- Jost, N.; Virág, L.; Bitay, M.; Takács, J.; Lengyel, C.; Biliczki, P.; Nagy, Z.; Bogáts, G.; Lathrop, D.A.; Papp, J.G.; et al. Restricting Excessive Cardiac Action Potential and QT Prolongation. Circulation 2005, 112, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Ohler, A.; Amos, G.J.; Wettwer, E.; Ravens, U. Frequency-Dependent Effects of E-4031, Almokalant, Dofetilide and Tedisamil on Action Potential Duration: No Evidence for “reverse Use Dependent” Block. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1994, 349, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Glukhov, A.V.; Fedorov, V.V.; Lou, Q.; Ravikumar, V.K.; Kalish, P.W.; Schuessler, R.B.; Moazami, N.; Efimov, I.R. Transmural Dispersion of Repolarization in Failing and Nonfailing Human Ventricle. Circ. Res. 2010, 106, 981–991. [Google Scholar] [CrossRef]
- Camelliti, P.; Al-Saud, S.A.; Smolenski, R.T.; Al-Ayoubi, S.; Bussek, A.; Wettwer, E.; Banner, N.R.; Bowles, C.T.; Yacoub, M.H.; Terracciano, C.M. Adult Human Heart Slices Are a Multicellular System Suitable for Electrophysiological and Pharmacological Studies. J. Mol. Cell. Cardiol. 2011, 51, 390–398. [Google Scholar] [CrossRef]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human Embryonic-Stem-Cell-Derived Cardiomyocytes Regenerate Non-Human Primate Hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef]
- Hirt, M.N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Börnchen, C.; Müller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stoehr, A.; et al. Functional Improvement and Maturation of Rat and Human Engineered Heart Tissue by Chronic Electrical Stimulation. J. Mol. Cell. Cardiol. 2014, 74, 151–161. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Mummery, C.; Knollmann, B.C. Modelling Sarcomeric Cardiomyopathies in the Dish: From Human Heart Samples to iPSC Cardiomyocytes. Cardiovasc. Res. 2015, 105, 424–438. [Google Scholar] [CrossRef]
- Kang, C.; Qiao, Y.; Li, G.; Baechle, K.; Camelliti, P.; Rentschler, S.; Efimov, I.R. Human Organotypic Cultured Cardiac Slices: New Platform For High Throughput Preclinical Human Trials. Sci. Rep. 2016, 6, 28798. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Montagutelli, X. Animal Models Are Essential to Biological Research: Issues and Perspectives. Future Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef]
- Meyer, T.; Stuerz, K.; Guenther, E.; Edamura, M.; Kraushaar, U. Cardiac Slices as a Predictive Tool for Arrhythmogenic Potential of Drugs and Chemicals. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1461–1475. [Google Scholar] [CrossRef]
- Wang, K.; Lee, P.; Mirams, G.R.; Sarathchandra, P.; Borg, T.K.; Gavaghan, D.J.; Kohl, P.; Bollensdorff, C. Cardiac Tissue Slices: Preparation, Handling, and Successful Optical Mapping. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1112–H1125. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Scigliano, M.; Bardi, I.; Ascione, R.; Terracciano, C.M.; Perbellini, F. Preparation of Viable Adult Ventricular Myocardial Slices from Large and Small Mammals. Nat. Protoc. 2017, 12, 2623–2639. [Google Scholar] [CrossRef] [PubMed]
- Perbellini, F.; Watson, S.A.; Scigliano, M.; Alayoubi, S.; Tkach, S.; Bardi, I.; Quaife, N.; Kane, C.; Dufton, N.P.; Simon, A.; et al. Investigation of Cardiac Fibroblasts Using Myocardial Slices. Cardiovasc. Res. 2018, 114, 77–89. [Google Scholar] [CrossRef]
- Schneider-Warme, F. The Power of Optogenetics: Potential in Cardiac Experimental and Clinical Electrophysiology. Herzschrittmacherther. Elektrophysiol. 2018, 29, 24–29. [Google Scholar] [CrossRef]
- Watson, S.A.; Terracciano, C.M.; Perbellini, F. Myocardial Slices: An Intermediate Complexity Platform for Translational Cardiovascular Research. Cardiovasc. Drugs Ther. 2019, 33, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Barclay, C.J. Modelling Diffusive O2 Supply to Isolated Preparations of Mammalian Skeletal and Cardiac Muscle. J. Muscle Res. Cell Motil. 2005, 26, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Kelley, M.A.; Janssen, P.M.L. Effect of Muscle Dimensions on Trabecular Contractile Performance under Physiological Conditions. Pflügers Arch. Eur. J. Physiol. 2006, 451, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Brandenburger, M.; Wenzel, J.; Bogdan, R.; Richardt, D.; Nguemo, F.; Reppel, M.; Hescheler, J.; Terlau, H.; Dendorfer, A. Organotypic Slice Culture from Human Adult Ventricular Myocardium. Cardiovasc. Res. 2012, 93, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Milting, H.; Fein, E.; Reiser, E.; Lu, K.; Seidel, T.; Schinner, C.; Schwarzmayr, T.; Schramm, R.; Tomasi, R.; et al. Long-Term Functional and Structural Preservation of Precision-Cut Human Myocardium under Continuous Electromechanical Stimulation in Vitro. Nat. Commun. 2019, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Halbach, M.; Pillekamp, F.; Brockmeier, K.; Hescheler, J.; Müller-Ehmsen, J.; Reppel, M. Ventricular Slices of Adult Mouse Hearts—A New Multicellular in Vitro Model for Electrophysiological Studies. Cell. Physiol. Biochem. 2006, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.; Schiatti, T.; Kaltenbacher, W.; Dente, M.; Semenjakin, A.; Kok, T.; Fiegle, D.J.; Seidel, T.; Ravens, U.; Kohl, P.; et al. Consecutive-Day Ventricular and Atrial Cardiomyocyte Isolations from the Same Heart: Shifting the Cost-Benefit Balance of Cardiac Primary Cell Research. Cells 2022, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-Timescale, Genetically Targeted Optical Control of Neural Activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Bruegmann, T.; Boyle, P.M.; Vogt, C.C.; Karathanos, T.V.; Arevalo, H.J.; Fleischmann, B.K.; Trayanova, N.A.; Sasse, P. Optogenetic Defibrillation Terminates Ventricular Arrhythmia in Mouse Hearts and Human Simulations. J. Clin. Investig. 2016, 126, 3894–3904. [Google Scholar] [CrossRef]
- Bruegmann, T.; Malan, D.; Hesse, M.; Beiert, T.; Fuegemann, C.J.; Fleischmann, B.K.; Sasse, P. Optogenetic Control of Heart Muscle in Vitro and in Vivo. Nat. Methods 2010, 7, 897–900. [Google Scholar] [CrossRef]
- Nagel, G.; Brauner, M.; Liewald, J.F.; Adeishvili, N.; Bamberg, E.; Gottschalk, A. Light Activation of Channelrhodopsin-2 in Excitable Cells of Caenorhabditis Elegans Triggers Rapid Behavioral Responses. Curr. Biol. 2005, 15, 2279–2284. [Google Scholar] [CrossRef]
- Olinga, P.; Groen, K.; Hof, I.H.; De Kanter, R.; Koster, H.J.; Leeman, W.R.; Rutten, A.A.; Van Twillert, K.; Groothuis, G.M. Comparison of Five Incubation Systems for Rat Liver Slices Using Functional and Viability Parameters. J. Pharmacol. Toxicol. Methods 1997, 38, 59–69. [Google Scholar] [CrossRef]
- Kelly, J.J.; Simek, J.; Laird, D.W. Mechanisms Linking Connexin Mutations to Human Diseases. Cell Tissue Res. 2015, 360, 701–721. [Google Scholar] [CrossRef]
- Leybaert, L.; Lampe, P.D.; Dhein, S.; Kwak, B.R.; Ferdinandy, P.; Beyer, E.C.; Laird, D.W.; Naus, C.C.; Green, C.R.; Schulz, R. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol. Rev. 2017, 69, 396–478. [Google Scholar] [CrossRef]
- Chopra, A.; Tabdanov, E.; Patel, H.; Janmey, P.A.; Yasha Kresh, J. Cardiac Myocyte Remodeling Mediated by N-Cadherin-Dependent Mechanosensing. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1252–H1266. [Google Scholar] [CrossRef]
- Kobirumaki-Shimozawa, F.; Oyama, K.; Shimozawa, T.; Mizuno, A.; Ohki, T.; Terui, T.; Minamisawa, S.; Ishiwata, S.; Fukuda, N. Nano-Imaging of the Beating Mouse Heart in Vivo: Importance of Sarcomere Dynamics, as Opposed to Sarcomere Length per Se, in the Regulation of Cardiac Function. J. Gen. Physiol. 2016, 147, 53–62. [Google Scholar] [CrossRef]
- Pacher, P.; Nagayama, T.; Mukhopadhyay, P.; Bátkai, S.; Kass, D.A. Measurement of Cardiac Function Using Pressure-Volume Conductance Catheter Technique in Mice and Rats. Nat. Protoc. 2008, 3, 1422–1434. [Google Scholar] [CrossRef]
- Saito, S.; Masuda, K.; Mori, Y.; Nakatani, S.; Yoshioka, Y.; Murase, K. Mapping of Left Ventricle Wall Thickness in Mice Using 11.7-T Magnetic Resonance Imaging. Magn. Reson. Imaging 2017, 36, 128–134. [Google Scholar] [CrossRef]
- Casha, A.R.; Camilleri, L.; Manché, A.; Gatt, R.; Gauci, M.; Camilleri-Podesta, M.-T.; Grima, J.N.; Scarci, M.; Chetcuti, S. Physiological Rules for the Heart, Lungs and Other Pressure-Based Organs. J. Thorac. Dis. 2017, 9, 3793–3801. [Google Scholar] [CrossRef]
- Fuchs, F.; Martyn, D.A. Length-Dependent Ca(2+) Activation in Cardiac Muscle: Some Remaining Questions. J. Muscle Res. Cell Motil. 2005, 26, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Linke, W.A.; Popov, V.I.; Pollack, G.H. Passive and Active Tension in Single Cardiac Myofibrils. Biophys. J. 1994, 67, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Berendson, J.; Simonsson, D. Electrochemical Aspects of Treatment of Tissue with Direct Current. Eur. J. Surg. Suppl. 1994, 574, 111–115. [Google Scholar]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G.R. Electrical Stimulation of Excitable Tissue: Design of Efficacious and Safe Protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Beardslee, M.A.; Lerner, D.L.; Tadros, P.N.; Laing, J.G.; Beyer, E.C.; Yamada, K.A.; Kléber, A.G.; Schuessler, R.B.; Saffitz, J.E. Dephosphorylation and Intracellular Redistribution of Ventricular connexin43 during Electrical Uncoupling Induced by Ischemia. Circ. Res. 2000, 87, 656–662. [Google Scholar] [CrossRef]
- Doevendans, P.A.; Daemen, M.J.; de Muinck, E.D.; Smits, J.F. Cardiovascular Phenotyping in Mice. Cardiovasc. Res. 1998, 39, 34–49. [Google Scholar] [CrossRef]
- Shao, D.; Tian, R. Glucose Transporters in Cardiac Metabolism and Hypertrophy. Compr. Physiol. 2015, 6, 331–351. [Google Scholar]
- Lafleur, M.V.; Hoorweg, J.J.; Joenje, H.; Westmijze, E.J.; Retèl, J. The Ambivalent Role of Glutathione in the Protection of DNA against Singlet Oxygen. Free Radic. Res. 1994, 21, 9–17. [Google Scholar] [CrossRef]
- Takahashi, M.; Nagai, T.; Hamano, S.; Kuwayama, M.; Okamura, N.; Okano, A. Effect of Thiol Compounds on in Vitro Development and Intracellular Glutathione Content of Bovine Embryos. Biol. Reprod. 1993, 49, 228–232. [Google Scholar] [CrossRef]
- Abdallah, Y.; Gkatzoflia, A.; Pieper, H.; Zoga, E.; Walther, S.; Kasseckert, S.; Schäfer, M.; Schlüter, K.D.; Piper, H.M.; Schäfer, C. Mechanism of cGMP-Mediated Protection in a Cellular Model of Myocardial Reperfusion Injury. Cardiovasc. Res. 2005, 66, 123–131. [Google Scholar] [CrossRef]
- Schäfer, C.; Ladilov, Y.; Inserte, J.; Schäfer, M.; Haffner, S.; Garcia-Dorado, D.; Piper, H.M. Role of the Reverse Mode of the Na+/Ca2+ Exchanger in Reoxygenation-Induced Cardiomyocyte Injury. Cardiovasc. Res. 2001, 51, 241–250. [Google Scholar] [CrossRef]
- Woodcock, E.A.; Matkovich, S.J. Cardiomyocytes Structure, Function and Associated Pathologies. Int. J. Biochem. Cell Biol. 2005, 37, 1746–1751. [Google Scholar] [CrossRef]
- Ruiz-Meana, M.; Abellán, A.; Miró-Casas, E.; Agulló, E.; Garcia-Dorado, D. Role of Sarcoplasmic Reticulum in Mitochondrial Permeability Transition and Cardiomyocyte Death during Reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1281–H1289. [Google Scholar] [CrossRef]
- Bers, D.M.; Barry, W.H.; Despa, S. Intracellular Na+ Regulation in Cardiac Myocytes. Cardiovasc. Res. 2003, 57, 897–912. [Google Scholar] [CrossRef]
- Siegmund, B.; Schlack, W.; Ladilov, Y.V.; Balser, C.; Piper, H.M. Halothane Protects Cardiomyocytes against Reoxygenation-Induced Hypercontracture. Circulation 1997, 96, 4372–4379. [Google Scholar] [CrossRef]
- Roth, D.M.; Swaney, J.S.; Dalton, N.D.; Gilpin, E.A.; Ross, J., Jr. Impact of Anesthesia on Cardiac Function during Echocardiography in Mice. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2134–H2140. [Google Scholar] [CrossRef] [PubMed]
- Endoh, M. Force-Frequency Relationship in Intact Mammalian Ventricular Myocardium: Physiological and Pathophysiological Relevance. Eur. J. Pharmacol. 2004, 500, 73–86. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| MLC-2V | ATCGACAAGAATGACCTAAGGGA | ATTTTTCACGTTCACTCGTCCT |
| GJA1 | ACAAGGTCCAAGCCTACTCCA | CCGGGTTGTTGAGTGTTACAG |
| N2A | GGCATCTCCAGGACGTTACTC | TTCACTCTGCCTTGAGGTTTAAG |
| N2B | GCACAGAAGGAAGATCCTGA | ACCTGCTTTTCCTCAAGTGCT |
| Glut1 | CAGTTCGGCTATAACACTGGTG | GCCCCCGACAGAGAAGATG |
| A: Gene Expression Referenced to β-actin mRNA | |||||
|---|---|---|---|---|---|
| Culture condition | MLC-2V | N2A | N2B | GJA1 | Glut1 |
| 0.3 mN unstim. slices/fresh | 0.068 * | 0.207 * | 0.063 * | 0.404 * | 3.149 * |
| 0.3 mN stim. slices/fresh | 0.055 * | 0.19 * | 0.055 * | 0.402 * | 3.574 * |
| 1.2 mN/0.3 mN (stim. slices) | 1.090 | 1.111 | 1.276 | NA | 0.785 |
| rapid shaking/ slow shaking (stim.+unstim. slices) | 1.022 | 0.858 | 0.663 | 1.176 | 0.426 * |
| +ß-ME/-ß-ME (stim.+unstim. slices) | 1.043 | 1.106 | 1.011 | 1.001 | 1.057 |
| B: Gene expression referenced to total RNA | |||||
| Culture condition | MLC-2V | N2A | N2B | GJA1 | Glut1 |
| 0.3 mN unstim. slices/fresh | 0.37 * | 1.12 | 0.341 * | 2.184 | 16.994 * |
| 0.3 mN stim. slices/fresh | 0.324 * | 1.108 | 0.324 * | 2.341 | 20.78 * |
| 1.2 mN/0.3 mN (stim. slices) | 0.928 | 0.946 | 1.087 | N/A | 0.668 |
| rapid shaking/ slow shaking (stim.+unstim. slices) | 1.059 | 0.889 | 0.687 | 1.218 | 0.442 * |
| +ß-ME/-ß-ME (stim.+unstim. slices) | 1.054 | 0.995 | 1.088 | 1.098 | 1.040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao-Ehlker, X.; Fischer, C.; Lu, K.; Bruegmann, T.; Sasse, P.; Dendorfer, A.; Tomasi, R. Optimized Conditions for the Long-Term Maintenance of Precision-Cut Murine Myocardium in Biomimetic Tissue Culture. Bioengineering 2023, 10, 171. https://doi.org/10.3390/bioengineering10020171
Cao-Ehlker X, Fischer C, Lu K, Bruegmann T, Sasse P, Dendorfer A, Tomasi R. Optimized Conditions for the Long-Term Maintenance of Precision-Cut Murine Myocardium in Biomimetic Tissue Culture. Bioengineering. 2023; 10(2):171. https://doi.org/10.3390/bioengineering10020171
Chicago/Turabian StyleCao-Ehlker, Xiaochun, Carola Fischer, Kun Lu, Tobias Bruegmann, Philipp Sasse, Andreas Dendorfer, and Roland Tomasi. 2023. "Optimized Conditions for the Long-Term Maintenance of Precision-Cut Murine Myocardium in Biomimetic Tissue Culture" Bioengineering 10, no. 2: 171. https://doi.org/10.3390/bioengineering10020171
APA StyleCao-Ehlker, X., Fischer, C., Lu, K., Bruegmann, T., Sasse, P., Dendorfer, A., & Tomasi, R. (2023). Optimized Conditions for the Long-Term Maintenance of Precision-Cut Murine Myocardium in Biomimetic Tissue Culture. Bioengineering, 10(2), 171. https://doi.org/10.3390/bioengineering10020171






