Mesoporous Bioactive Glass-Incorporated Injectable Strontium-Containing Calcium Phosphate Cement Enhanced Osteoconductivity in a Critical-Sized Metaphyseal Defect in Osteoporotic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations
2.2. Preparation of pS100 and pS100G10
In Vitro Investigations
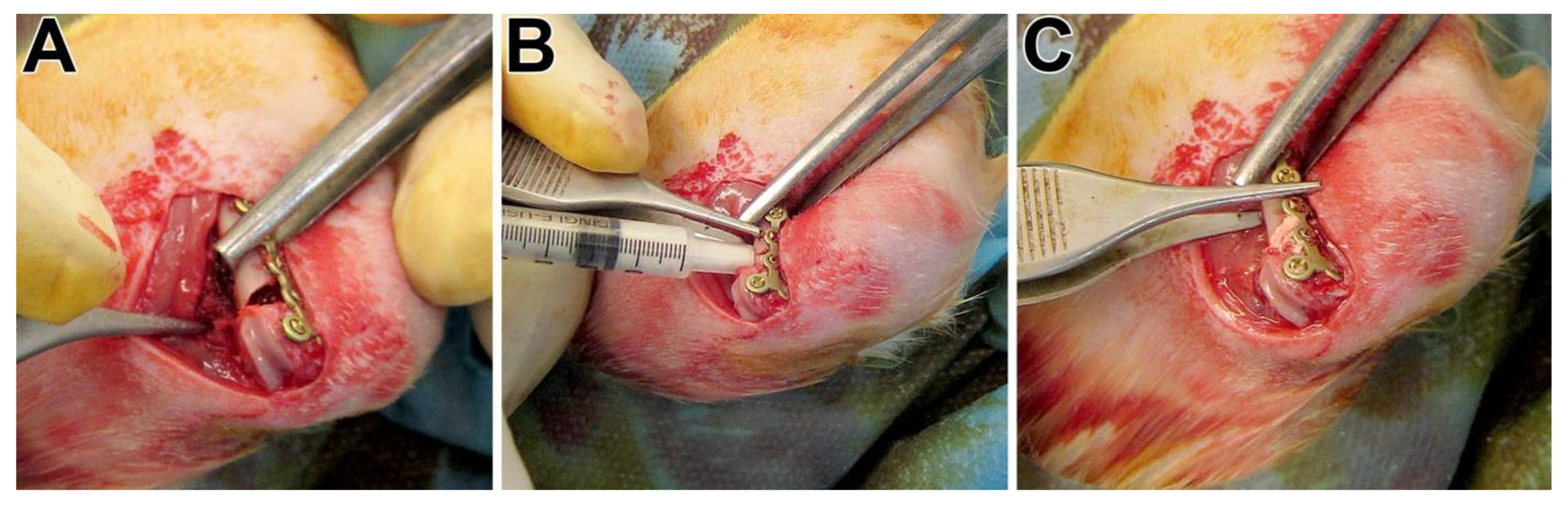
2.3. Animal Surgery
2.4. Sample Processing, Staining Procedures, and Histomorphometry
2.5. Immunohistochemistry
2.6. ToF-SIMS Measurements
2.7. mRNA Preparation and Gene Expression Analysis
2.8. Statistics
3. Results
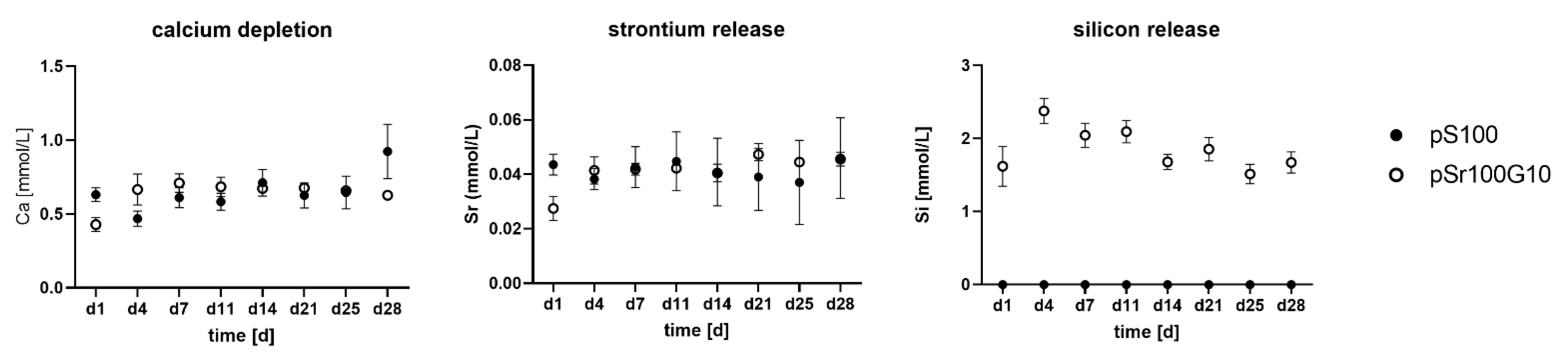
3.1. In Vitro Characterization of pS100 and pS100G10
3.2. In Vivo Characterization of pS100 and pS100G10–General Information
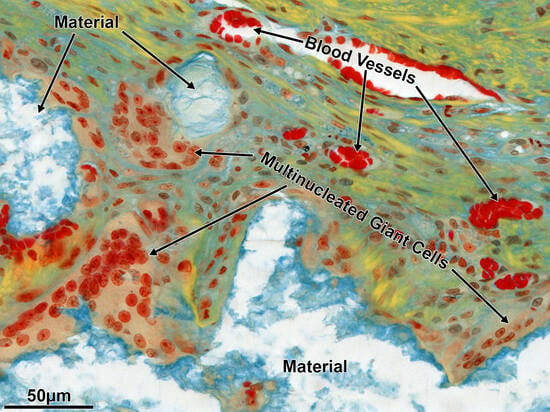
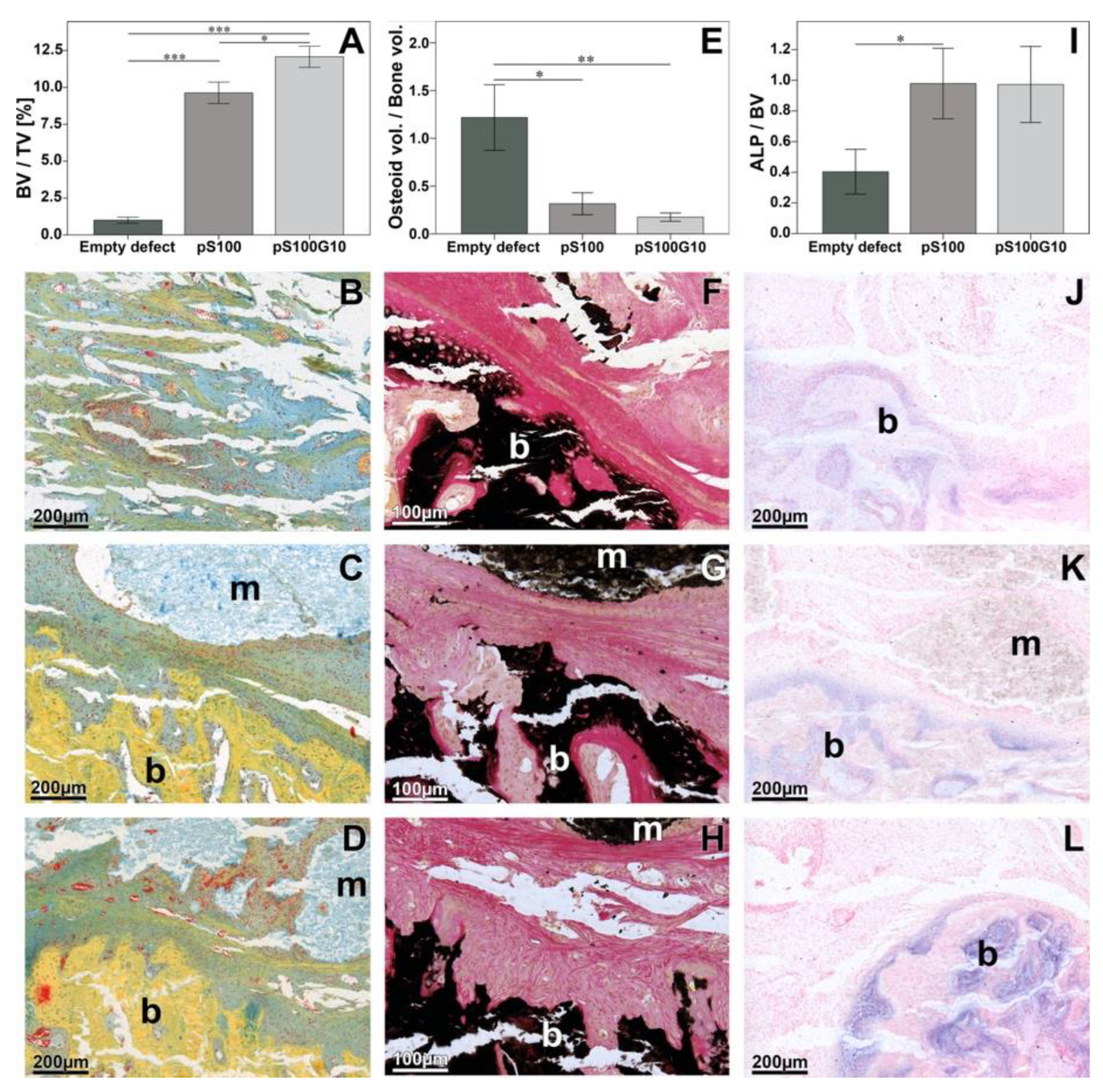
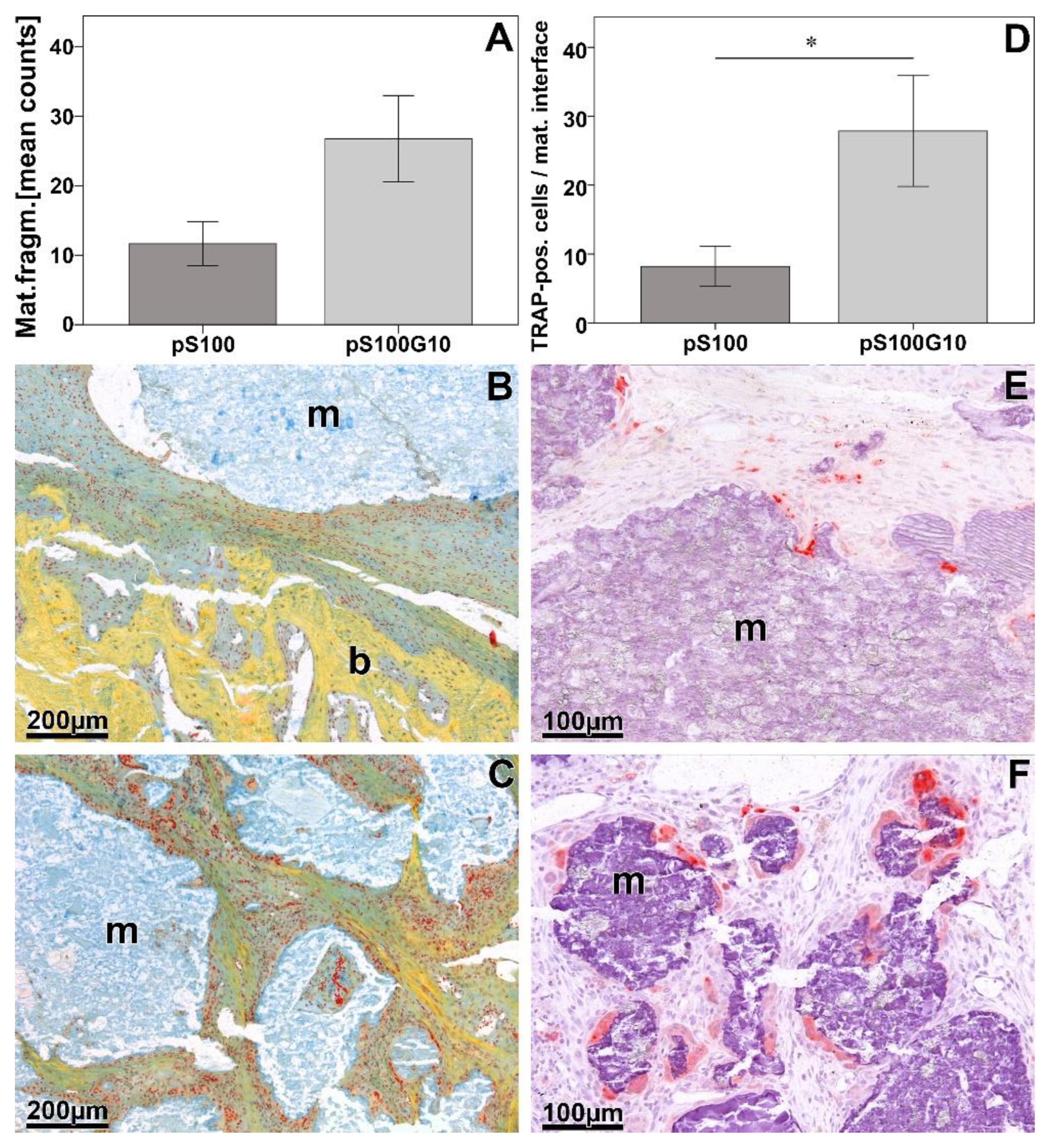
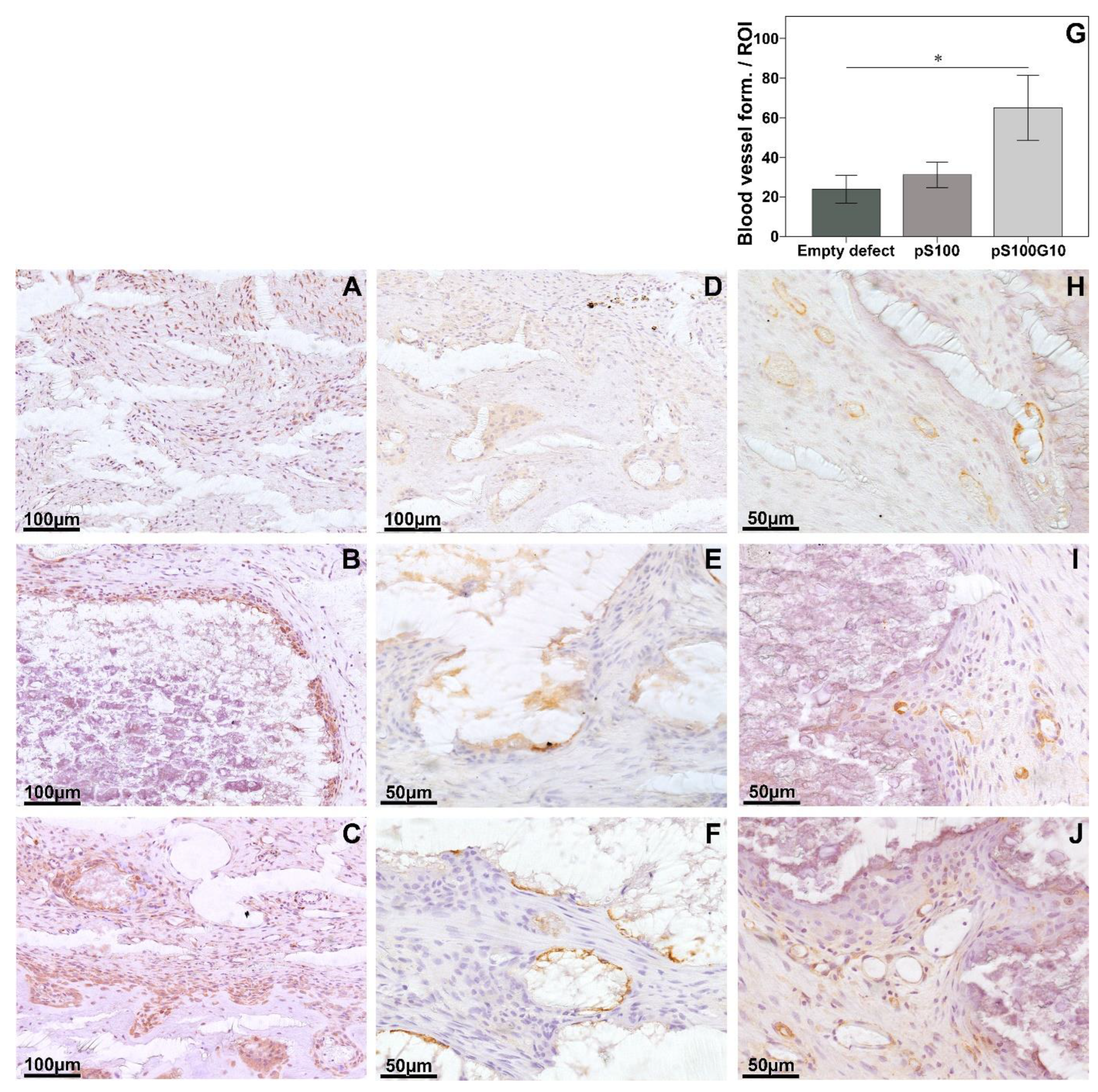
3.3. Histomorphometry, Histology, and Immunohistochemistry
3.4. Molecular Biology
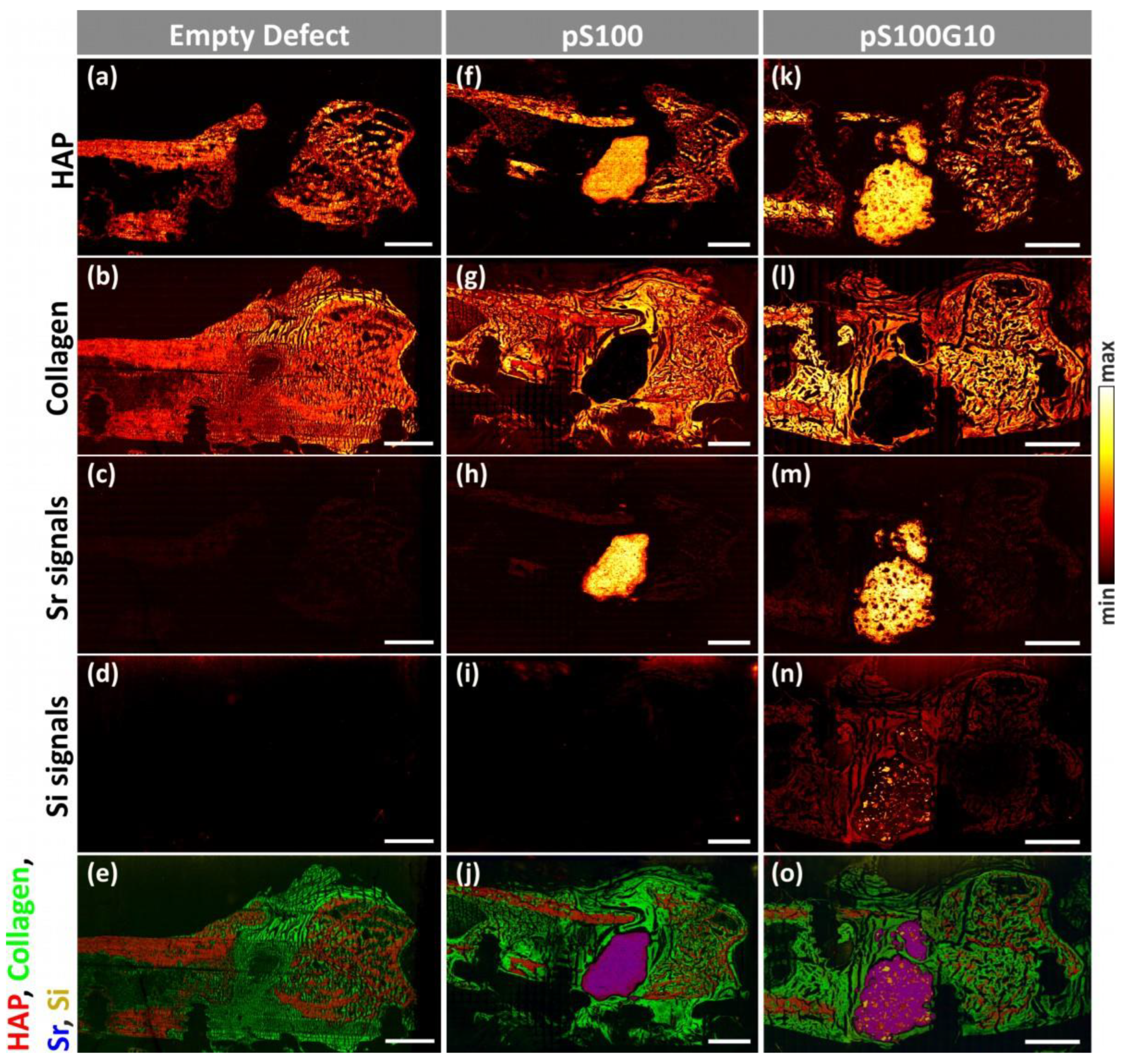
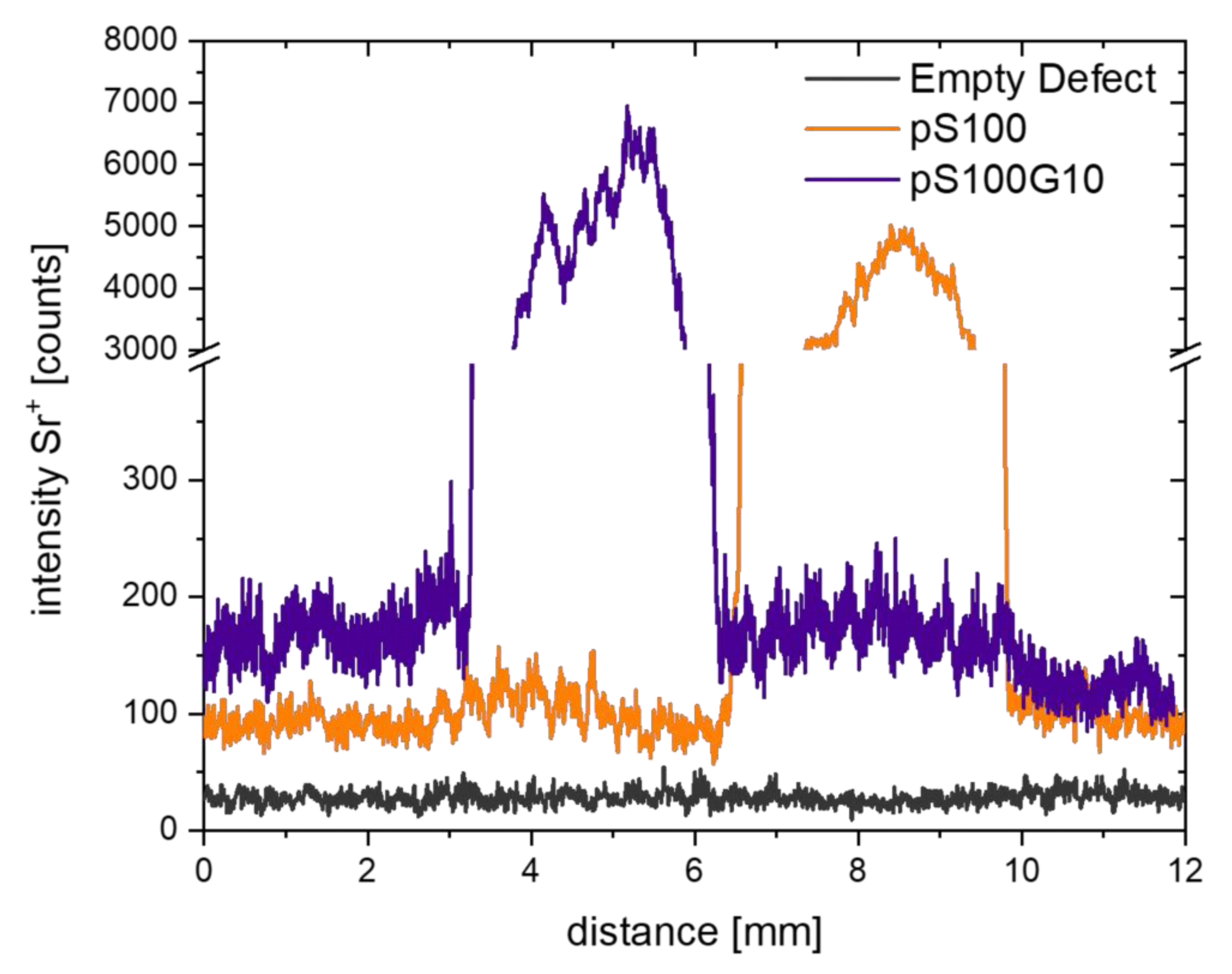
3.5. ToF-SIMS Analysis
| Mineralized Bone Signals (HAP) | Collagen Signals | Strontium Signals | |||||
|---|---|---|---|---|---|---|---|
| m/z | Peak Label | m/z | Peak Label | m/z | Peak Label | m/z | Peak Label |
| 39.96 | Ca+ | 230.78 | Ca3PO5+ | 30.04 | CH4N+ | 85.89 | 86Sr+ |
| 55.97 | CaO+ | 286.71 | Ca4PO6+ | 44.05 | C2H6N+ | 86.91 | 87Sr+ |
| 56.96 | CaOH+ | 70.06 | C4H8N+ | 87.89 | Sr+ | ||
| 95.92 | Ca2O+ | 86.06 | C4H8NO+ | 88.90 | SrH+ | ||
| 102.92 | CaPO2+ | 103.90 | SrO+ | ||||
| 111.90 | Ca2O2+ | 104.90 | SrOH+ | ||||
| 112.91 | Ca2O2H+ | 150.85 | SrPO2+ | ||||
| 118.92 | CaPO3+ | 166.85 | SrPO3+ | ||||
| 134.88 | CaPO4+ | 182.86 | SrPO4+ | ||||
| 151.84 | Ca3O2+ | Silicon signals | 191.77 | Sr2O+ | |||
| 158.85 | Ca2PO3+ | m/z | Peak label | 207.80 | Sr2O2+ | ||
| 168.84 | Ca3O3H+ | 27.97 | Si+ | 208.80 | Sr2O2H+ | ||
| 174.84 | Ca2PO4+ | 28.98 | SiH+ | 254.80 | Sr2PO3+ | ||
| 214.79 | Ca3PO4+ | 44.98 | SiOH+ | 270.76 | Sr2PO4+ | ||
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Zhao, L.; Chen, W.; Liu, X.; Weir, M.D.; Xu, H.H. Stem Cells and Calcium Phosphate Cement Scaffolds for Bone Regeneration. J. Dent. Res. 2014, 93, 618–625. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Self-Setting Calcium Orthophosphate Formulations: Cements, Concretes, Pastes and Putties. Int. J. Mater. Chem. 2011, 1, 1–48. [Google Scholar]
- Heinemann, S.; Rossler, S.; Lemm, M.; Ruhnow, M.; Nies, B. Properties of injectable ready-to-use calcium phosphate cement based on water-immiscible liquid. Acta Biomater. 2013, 9, 6199–6207. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Tiainen, H.; Michel, P.; Döbelin, N. Design of an inorganic dual-paste apatite cement using cation exchange. J. Mater. Sci. Mater. Med. 2015, 26, 63. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Barralet, J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011, 7, 3013–3026. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Barbara, A.; Delannoy, P.; Denis, B.G.; Marie, P.J. Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism 2004, 53, 532–537. [Google Scholar] [CrossRef]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef]
- Schumacher, M.; Gelinsky, M. Strontium modified calcium phosphate cements—Approaches towards targeted stimulation of bone turnover. J. Mater. Chem. B 2015, 3, 4626–4640. [Google Scholar] [CrossRef]
- Schumacher, M.; Henß, A.; Rohnke, M.; Gelinsky, M. A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef]
- Schumacher, M.; Lode, A.; Helth, A.; Gelinsky, M. A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomater. 2013, 9, 9547–9557. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Wagner, A.S.; Kokesch-Himmelreich, J.; Bernhardt, A.; Rohnke, M.; Wenisch, S.; Gelinsky, M. Strontium substitution in apatitic CaP cements effectively attenuates osteoclastic resorption but does not inhibit osteoclastogenesis. Acta Biomater. 2016, 37, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Thormann, U.; Ray, S.; Sommer, U.; Elkhassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef] [PubMed]
- Lode, A.; Heiss, C.; Knapp, G.; Thomas, J.; Nies, B.; Gelinsky, M.; Schumacher, M. Strontium-modified premixed calcium phosphate cements for the therapy of osteoporotic bone defects. Acta Biomater. 2018, 65, 475–485. [Google Scholar] [CrossRef]
- Bernhardt, A.; Schumacher, M.; Gelinsky, M. Formation of osteoclasts on calcium phosphate bone cements and polystyrene depends on monocyte isolation conditions. Tissue Eng. Part C Methods 2015, 21, 160–170. [Google Scholar] [CrossRef]
- Felix Lanao, R.P.; Leeuwenburgh, S.C.; Wolke, J.G.; Jansen, J.A. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomater. 2011, 7, 3459–3468. [Google Scholar] [CrossRef]
- Felix Lanao, R.P.; Leeuwenburgh, S.C.; Wolke, J.G.; Jansen, J.A. Bone response to fast-degrading, injectable calcium phosphate cements containing PLGA microparticles. Biomaterials 2011, 32, 8839–8847. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; Stumpel, F.; Jansen, J.; van den Beucken, J. Early-stage macroporosity enhancement in calcium phosphate cements by inclusion of poly(N-vinylpyrrolidone) particles as a porogen. Mater. Today Commun. 2020, 23, 100901. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23 (Suppl. 1), S53–S82. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef]
- Bosetti, M.; Cannas, M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials 2005, 26, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Guldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, C.; Ramaswamy, Y.; Kockrick, E.; Simon, P.; Kaskel, S.; Zreiqat, H. Preparation, characterization and in vitro bioactivity of mesoporous bioactive glasses (MBGs) scaffolds for bone tissue engineering. Microporous Mesoporous Mater. 2008, 112, 494–503. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef]
- Schumacher, M.; Reither, L.; Thomas, J.; Kampschulte, M.; Gbureck, U.; Lode, A.; Gelinsky, M. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Biomater. Sci. 2017, 5, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Schumacher, M.; Rohnke, M.; Glenske, K.; Gelinsky, M.; Arnhold, S.; Mazurek, S.; Wenisch, S. Incorporation of silicon into strontium modified calcium phosphate bone cements promotes osteoclastogenesis of human peripheral mononuclear blood cells. Biomed. Mater. 2019, 14, 025004. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, P.; Schlewitz, G.; Schliefke, N.; Weisweiler, D.; Alt, V.; Thormann, U.; Lips, K.S.; Wenisch, S.; Langheinrich, A.C.; Zahner, D.; et al. Implications of combined ovariectomy/multi-deficiency diet on rat bone with age-related variation in bone parameters and bone loss at multiple skeletal sites by DEXA. Med. Sci. Monit. Basic Res. 2013, 19, 76–86. [Google Scholar] [CrossRef]
- Heiss, C.; Govindarajan, P.; Schlewitz, G.; Hemdan, N.Y.A.; Schliefke, N.; Alt, V.; Thormann, U.; Lips, K.S.; Wenisch, S.; Langheinrich, A.C.; et al. Induction of osteoporosis with its influence on osteoporotic determinants and their interrelationships in rats by DEXA. Med. Sci. Monit. 2012, 18, BR199–BR207. [Google Scholar] [CrossRef]
- Schlewitz, G.; Govindarajan, P.; Schliefke, N.; Alt, V.; Böcker, W.; Elkhassawna, T.; Thormann, U.; Lips, K.S.; Hemdan, N.Y.; Zahner, D.; et al. Ovariectomy and calcium/vitamin D2/D3 deficient diet as a model of osteoporosis in the spine of Sprague-Dawley rats. Z. Orthop. Unf. 2013, 151, 14–19. [Google Scholar]
- Alt, V.; Thormann, U.; Ray, S.; Zahner, D.; Dürselen, L.; Lips, K.; El Khassawna, T.; Heiss, C.; Riedrich, A.; Schlewitz, G.; et al. A new metaphyseal bone defect model in osteoporotic rats to study biomaterials for the enhancement of bone healing in osteoporotic fractures. Acta Biomater. 2013, 9, 7035–7042. [Google Scholar] [CrossRef]
- Bernhardt, A.; Wolf, S.; Weiser, E.; Vater, C.; Gelinsky, M. An improved method to isolate primary human osteocytes from bone. Biomed. Eng. 2020, 65, 107–111. [Google Scholar] [CrossRef]
- Albers, J.; Schulze, J.; Beil, F.T.; Gebauer, M.; Baranowsky, A.; Keller, J.; Marshall, R.P.; Wintges, K.; Friedrich, F.W.; Priemel, M.; et al. Control of bone formation by the serpentine receptor Frizzled-9. J. Cell Biol. 2011, 192, 1057–1072. [Google Scholar] [CrossRef]
- Peters, A.; Toben, D.; Lienau, J.; Schell, H.; Bail, H.J.; Matziolis, G.; Duda, G.N.; Kaspar, K. Locally applied osteogenic predifferentiated progenitor cells are more effective than undifferentiated mesenchymal stem cells in the treatment of delayed bone healing. Tissue Eng. Part A 2009, 15, 2947–2954. [Google Scholar] [CrossRef]
- Henss, A.; Rohnke, M.; El Khassawna, T.; Govindarajan, P.; Schlewitz, G.; Heiss, C.; Janek, J. Applicability of ToF-SIMS for monitoring compositional changes in bone in a long-term animal model. J. R. Soc. Interface 2013, 10, 20130332. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.; Ray, S.; Gelinsky, M.; Bellew, A.; Pirkl, A.; Rohnke, M. New insights into ToF-SIMS imaging in osteoporotic bone research. Biointerphases 2020, 15, 031005. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.; Kern, S.; Henss, A.; Rohnke, M. Secondary ion mass spectrometry for bone research. Biointerphases 2023, 18, 041203. [Google Scholar] [CrossRef]
- Henss, A.; Hild, A.; Rohnke, M.; Wenisch, S.; Janek, J. Time of flight secondary ion mass spectrometry of bone—Impact of sample preparation and measurement conditions. Biointerphases 2016, 11, 02A302. [Google Scholar] [CrossRef]
- Kokesch-Himmelreich, J.; Schumacher, M.; Rohnke, M.; Gelinsky, M.; Janek, J. ToF-SIMS analysis of osteoblast-like cells and their mineralized extracellular matrix on strontium enriched bone cements. Biointerphases 2013, 8, 17. [Google Scholar] [CrossRef]
- Valerio, P.; Pereira, M.M.; Goes, A.M.; Leite, M.F. The effect of ionic products from bioactive glass dissolution on osteoblast proliferation and collagen production. Biomaterials 2004, 25, 2941–2948. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, X.; Wei, J.; Lu, X.; Zhang, S.; Shi, J.; Liu, C. Stimulated osteoblastic proliferation by mesoporous silica xerogel with high specific surface area. J. Mater. Sci. Mater. Med. 2011, 22, 731–739. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- Richter, R.F.; Ahlfeld, T.; Gelinsky, M.; Lode, A. Composites consisting of calcium phosphate cements and mesoporous bioactive glasses as a 3D plottable drug delivery system. Acta Biomater. 2022, 156, 146–157. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: An essential element for the chick. Science 1972, 178, 619–621. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: A requirement in bone formation independent of vitamin D1. Calcif. Tissue Int. 1981, 33, 27–34. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Tucker, K.; Cupples, N.; Kiel, D.; Powell, J. Dietary Silicon Intake is Positively Associated with Bone Mineral Density in Men and Premenopausal Women of the Framingham Offspring Cohort. JBMR 2004, 19, 297–307. [Google Scholar] [CrossRef]
- Hing, K.A.; Revell, P.A.; Smith, N.; Buckland, T. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 2006, 27, 5014–5026. [Google Scholar] [CrossRef]
- Patel, N.; Best, S.M.; Bonfield, W.; Gibson, I.R.; Hing, K.A.; Damien, E.; Revell, P.A. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med. 2002, 13, 1199–1206. [Google Scholar] [CrossRef]
- Ranmuthu, C.D.S.; Ranmuthu, C.K.I.; Russell, J.C.; Singhania, D.; Khan, W.S. Evaluating the Effect of Non-cellular Bioactive Glass-Containing Scaffolds on Osteogenesis and Angiogenesis in in vivo Animal Bone Defect Models. Front. Bioeng. Biotechnol. 2020, 8, 430. [Google Scholar] [CrossRef]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Effect of sintered silicate-substituted hydroxyapatite on remodelling processes at the bone-implant interface. Biomaterials 2004, 25, 3303–3314. [Google Scholar] [CrossRef]
- Botelho, C.M.; Brooks, R.A.; Spence, G.; McFarlane, I.; Lopes, M.A.; Best, S.M.; Santos, J.D.; Rushton, N.; Bonfield, W. Differentiation of mononuclear precursors into osteoclasts on the surface of Si-substituted hydroxyapatite. J. Biomed. Mater. Res. A 2006, 78, 709–720. [Google Scholar] [CrossRef]
- Botelho, C.M.; Brooks, R.A.; Best, S.M.; Lopes, M.A.; Santos, J.D.; Rushton, N.; Bonfield, W. Human osteoblast response to silicon-substituted hydroxyapatite. J. Biomed. Mater. Res. A 2006, 79, 723–730. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Hoppe, A.; Boccaccini, A.R. Biological Impact of Bioactive Glasses and Their Dissolution Products. Front. Oral Biol. 2015, 17, 22–32. [Google Scholar]
- Li, H.; Chang, J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic. Acta Biomater. 2013, 9, 5379–5389. [Google Scholar] [CrossRef]
- Li, H.; Chang, J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater. 2013, 9, 6981–6991. [Google Scholar] [CrossRef]
- Yan, R.; Li, J.; Wu, Q.; Zhang, X.; Hu, L.; Deng, Y.; Jiang, R.; Wen, J.; Jiang, X. Trace Element-Augmented Titanium Implant With Targeted Angiogenesis and Enhanced Osseointegration in Osteoporotic Rats. Front. Chem. 2022, 10, 839062. [Google Scholar] [CrossRef]
- Kern, C.; Quade, M.; Ray, S.; Thomas, J.; Schumacher, M.; Gemming, T.; Gelinsky, M.; Alt, V.; Rohnke, M. Investigation of strontium transport and strontium quantification in cortical rat bone by time-of-flight secondary ion mass spectrometry. J. R. Soc. Interface 2019, 16, 20180638. [Google Scholar] [CrossRef]
- Rohnke, M.; Pfitzenreuter, S.; Mogwitz, B.; Henss, A.; Thomas, J.; Bieberstein, D.; Gemming, T.; Otto, S.K.; Ray, S.; Schumacher, M.; et al. Strontium release from Sr(2+)-loaded bone cements and dispersion in healthy and osteoporotic rat bone. J. Control Release 2017, 262, 159–169. [Google Scholar] [CrossRef]
- Nardone, V.; Zonefrati, R.; Mavilia, C.; Romagnoli, C.; Ciuffi, S.; Fabbri, S.; Palmini, G.; Galli, G.; Tanini, A.; Brandi, M.L. In Vitro Effects of Strontium on Proliferation and Osteoinduction of Human Preadipocytes. Stem Cells Int. 2015, 2015, 871863. [Google Scholar] [CrossRef]
- Reitmaier, S.; Kovtun, A.; Schuelke, J.; Kanter, B.; Lemm, M.; Hoess, A.; Heinemann, S.; Nies, B.; Ignatius, A. Strontium(II) and mechanical loading additively augment bone formation in calcium phosphate scaffolds. J. Orthop. Res. 2018, 36, 106–117. [Google Scholar] [CrossRef]
- Richter, R.F.; Ahlfeld, T.; Gelinsky, M.; Lode, A. Development and Characterization of Composites Consisting of Calcium Phosphate Cements and Mesoporous Bioactive Glass for Extrusion-Based Fabrication. Materials 2019, 12, 2022. [Google Scholar] [CrossRef]
- Hasan, M.L.; Kim, B.; Padalhin, A.R.; Faruq, O.; Sultana, T.; Lee, B.T. In vitro and in vivo evaluation of bioglass microspheres incorporated brushite cement for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109775. [Google Scholar] [CrossRef]



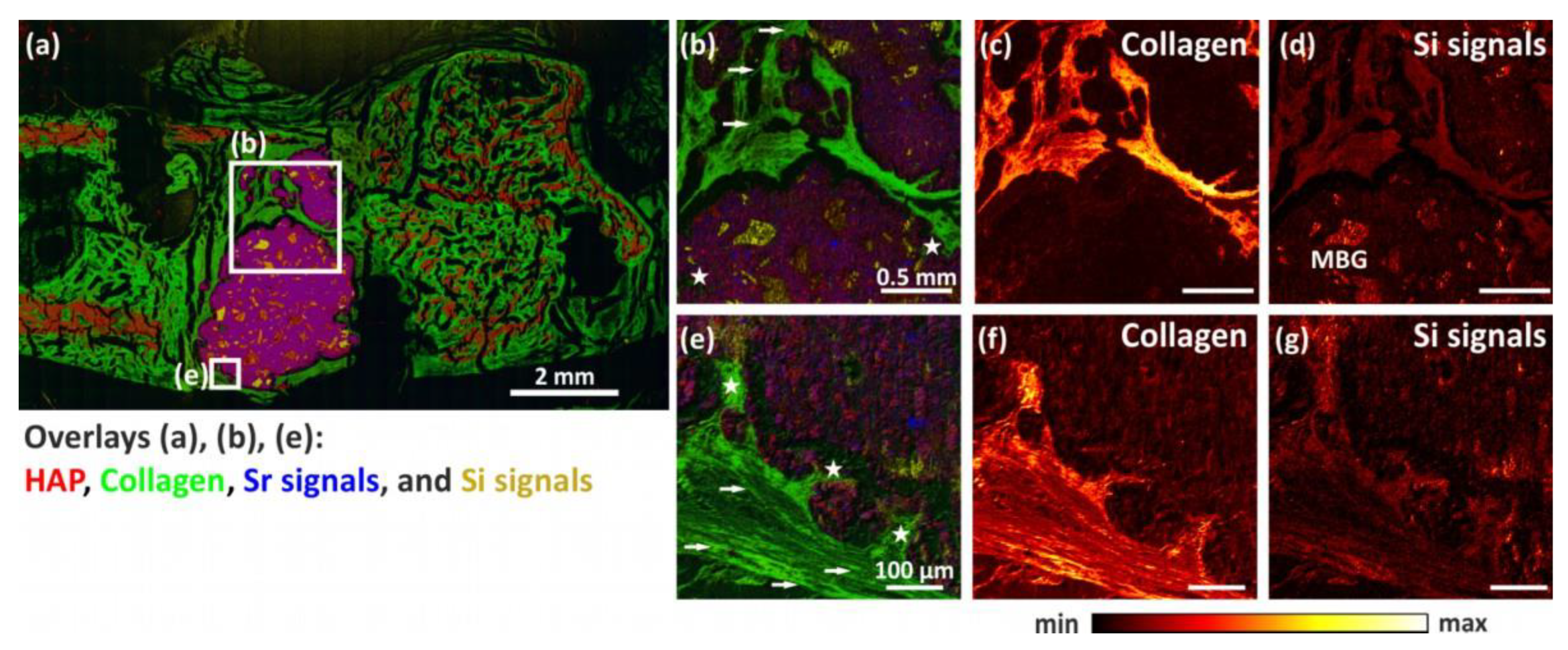
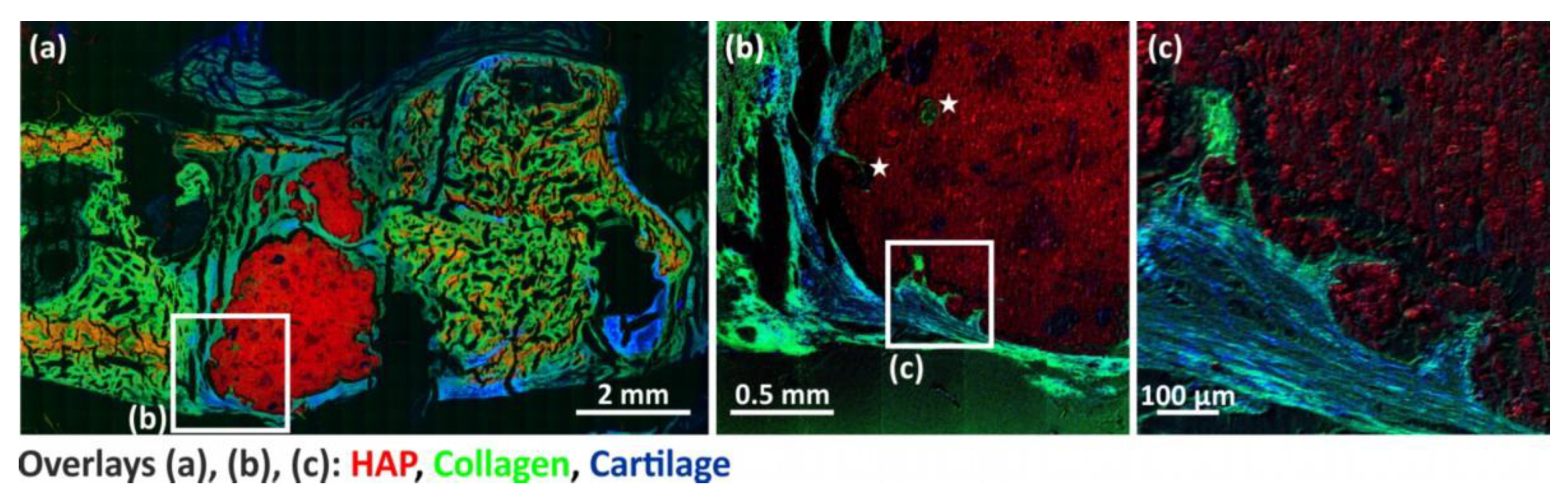
| Figure | 11b–d | 11e–g, 12c | 12b |
| Analysis options | |||
| Cycle time | 65 µs | 65 µs | 65 µs |
| Analysis area | 2 × 2 mm2 | 500 × 500 µm2 | 2 × 2 mm2 |
| Raster mode | sawtooth | sawtooth | sawtooth |
| Primary ion current | 0.05 pA | 0.04 pA | 0.05 pA |
| Pixel resolution | 1600 × 1600 | 1024 × 1024 | 2000 × 2000 |
| Frames per patch | 3 | 1 | 3 |
| Patch size | 0.4 mm | 0.5 mm | 0.4 mm |
| Primary ion Shots/frame/pixel | 3 | 2 | 3 |
| Number of scans | 10 | 150 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, S.; Thormann, U.; Kramer, I.; Sommer, U.; Budak, M.; Schumacher, M.; Bernhardt, A.; Lode, A.; Kern, C.; Rohnke, M.; et al. Mesoporous Bioactive Glass-Incorporated Injectable Strontium-Containing Calcium Phosphate Cement Enhanced Osteoconductivity in a Critical-Sized Metaphyseal Defect in Osteoporotic Rats. Bioengineering 2023, 10, 1203. https://doi.org/10.3390/bioengineering10101203
Ray S, Thormann U, Kramer I, Sommer U, Budak M, Schumacher M, Bernhardt A, Lode A, Kern C, Rohnke M, et al. Mesoporous Bioactive Glass-Incorporated Injectable Strontium-Containing Calcium Phosphate Cement Enhanced Osteoconductivity in a Critical-Sized Metaphyseal Defect in Osteoporotic Rats. Bioengineering. 2023; 10(10):1203. https://doi.org/10.3390/bioengineering10101203
Chicago/Turabian StyleRay, Seemun, Ulrich Thormann, Inga Kramer, Ursula Sommer, Matthäus Budak, Matthias Schumacher, Anne Bernhardt, Anja Lode, Christine Kern, Marcus Rohnke, and et al. 2023. "Mesoporous Bioactive Glass-Incorporated Injectable Strontium-Containing Calcium Phosphate Cement Enhanced Osteoconductivity in a Critical-Sized Metaphyseal Defect in Osteoporotic Rats" Bioengineering 10, no. 10: 1203. https://doi.org/10.3390/bioengineering10101203
APA StyleRay, S., Thormann, U., Kramer, I., Sommer, U., Budak, M., Schumacher, M., Bernhardt, A., Lode, A., Kern, C., Rohnke, M., Heiss, C., Lips, K. S., Gelinsky, M., & Alt, V. (2023). Mesoporous Bioactive Glass-Incorporated Injectable Strontium-Containing Calcium Phosphate Cement Enhanced Osteoconductivity in a Critical-Sized Metaphyseal Defect in Osteoporotic Rats. Bioengineering, 10(10), 1203. https://doi.org/10.3390/bioengineering10101203