Autotrophic Production of the Sesquiterpene α-Humulene with Cupriavidus necator in a Controlled Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid and Strains
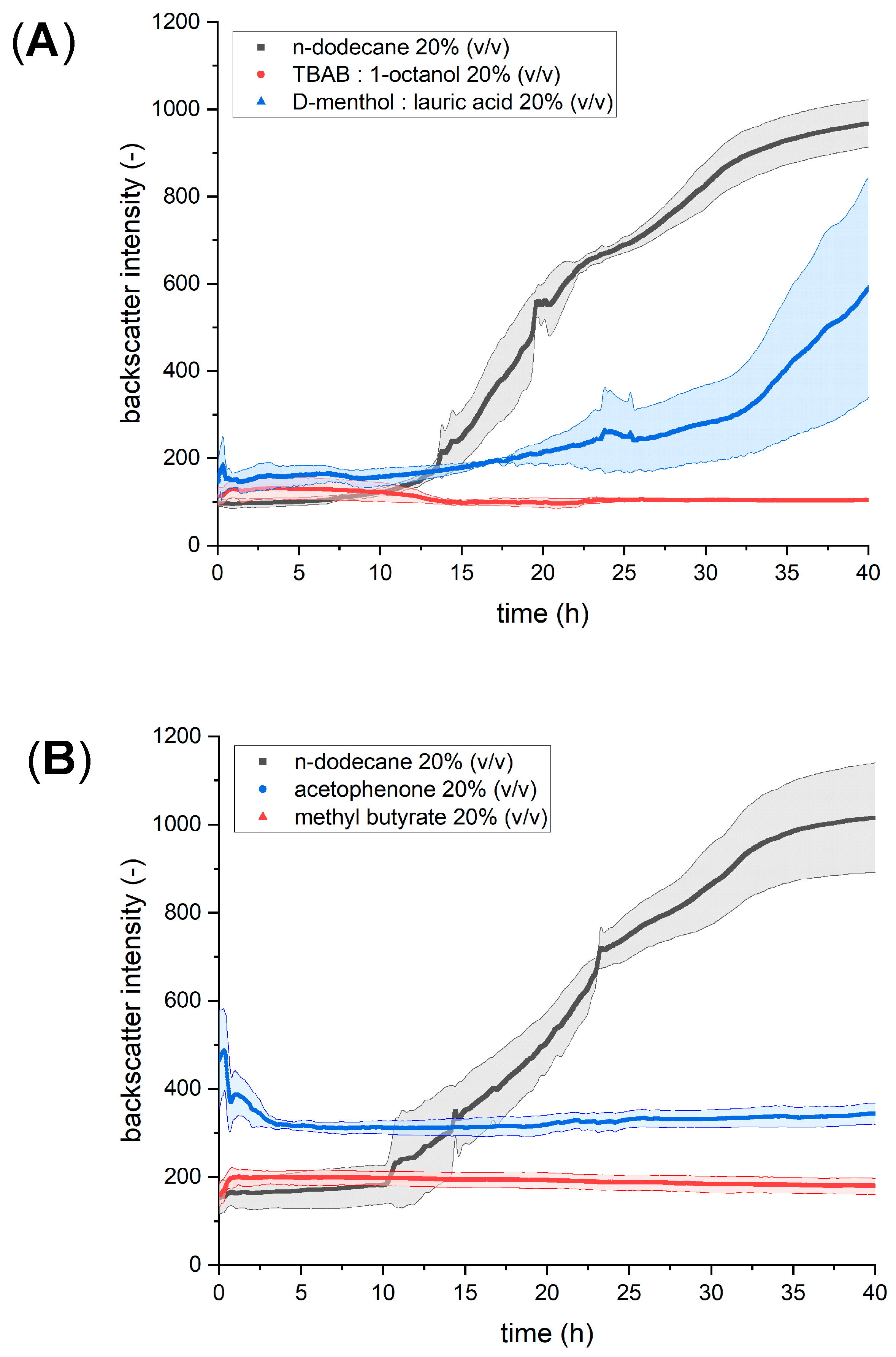
2.2. Solvent Selection and Testing
2.3. Preparation of Deep Eutectic Solvents
2.4. Heterotrophic Cultivation for Solvent Selection
2.5. Media Compositions for the Seed Train of the Bioreactor Process
2.6. Cultivation Conditions in the Seed Train of the Bioreactor Process
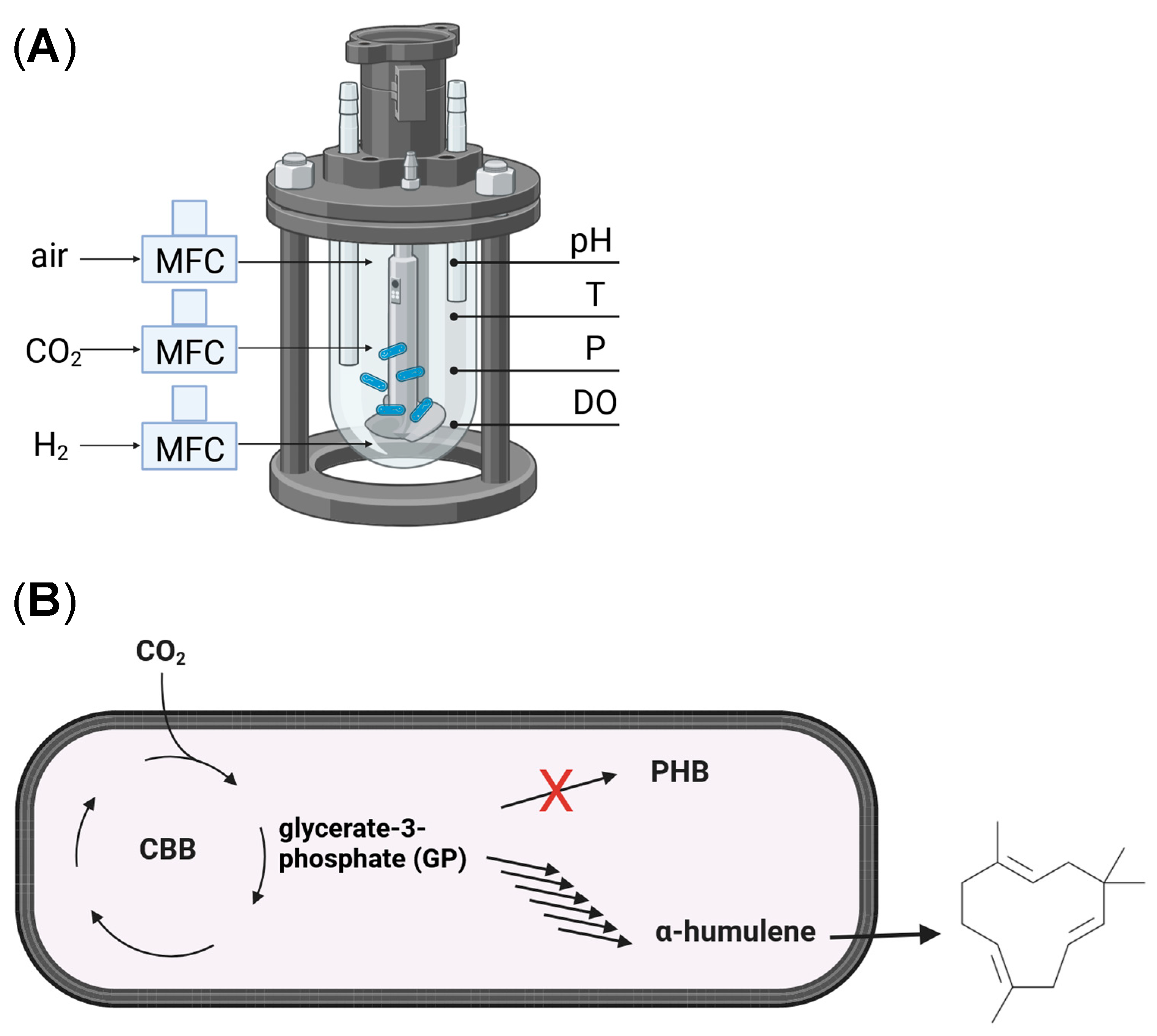
2.7. Autotrophic Culture Conditions and Bioreactor System
2.8. Characterization of the Fermentation Process
2.9. Quantification of α-Humulene with GC-MS
2.10. Statistical Analyses
3. Results
3.1. Identification of the Most Promising Solvent for In Situ Product Removal
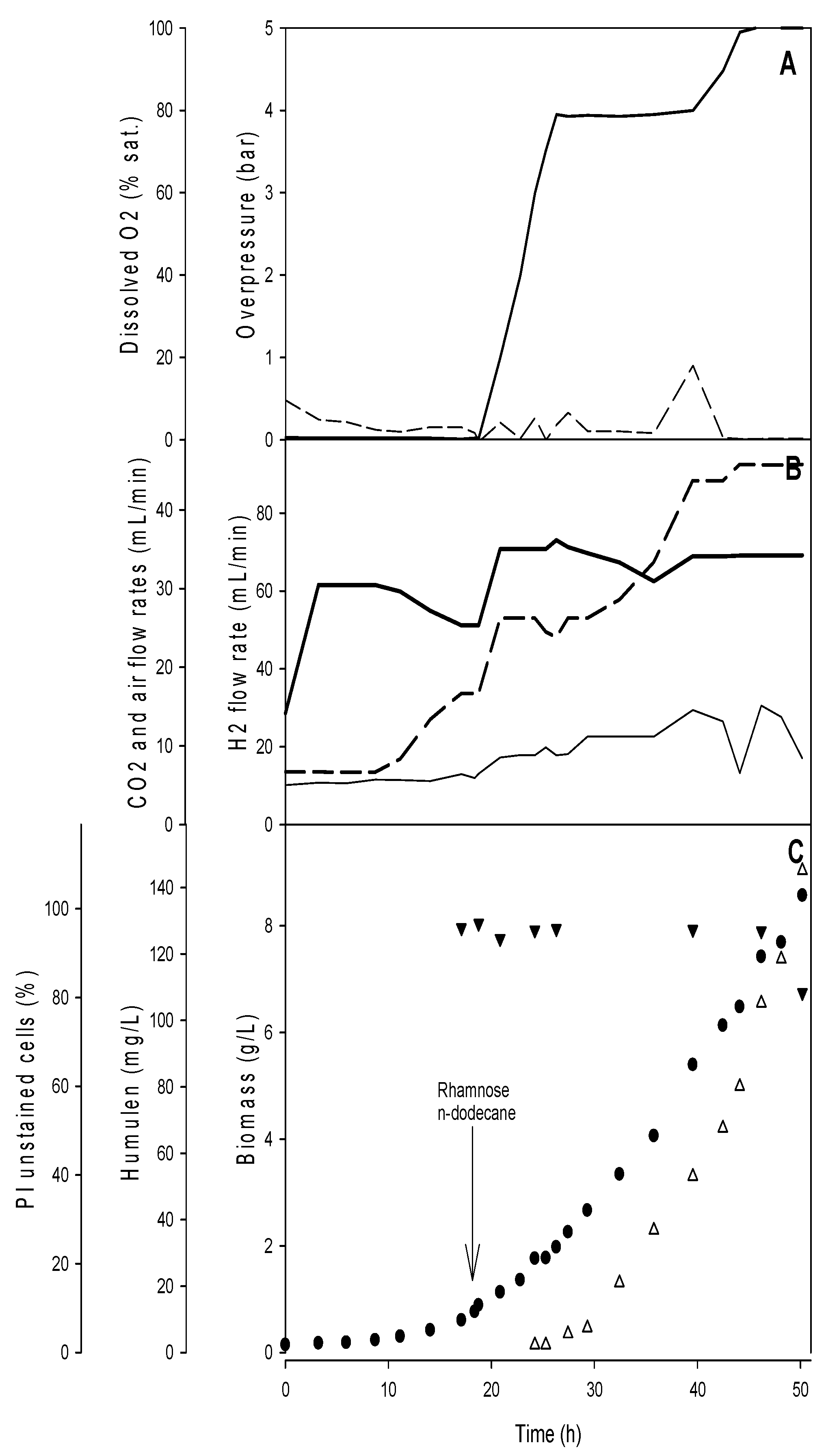
3.2. Optimization of the Autotrophic α-Humulene in a Controlled Gas Bioreactor
4. Discussion and Outlook
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vandamme, P.; Coenye, T. Taxonomy of the genus Cupriavidus: A tale of lost and found. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 6, 2285–2289. [Google Scholar] [CrossRef] [PubMed]
- Wohlers, H.; Assil-Companioni, L.; Holtmann, D. Cupriavidus necator—A broadly applicable aerobic hydrogen-oxidizing bacterium. In The Autotrophic Biorefinery; Robert, K., Sandy, S., Eds.; De Gruyter: Berlin, Germany; Boston, CA, USA, 2021; pp. 297–318. [Google Scholar]
- Salinas, A.; McGregor, C.; Irorere, V.; Arenas-López, C.; Bommareddy, R.R.; Winzer, K.; Minton, N.P.; Kovács, K. Metabolic engineering of Cupriavidus necator H16 for heterotrophic and autotrophic production of 3-hydroxypropionic acid. Metab. Eng. 2022, 74, 178–190. [Google Scholar] [CrossRef]
- Sydow, A.; Krieg, T.; Ulber, R.; Holtmann, D. Growth medium and electrolyte—How to combine the different requirements on the reaction solution in bioelectrochemical systems using Cupriavidus necator. Eng. Life Sci. 2017, 17, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pan, H.; Li, Z.; Liu, T.; Liu, F.; Xiu, S.; Wang, J.; Wang, H.; Hou, Y.; Yang, B.; et al. Efficient production of lycopene from CO2 via microbial electrosynthesis. Chem. Eng. J. 2022, 430, 132943. [Google Scholar] [CrossRef]
- Liu, C.; Colón, B.C.; Ziesack, M.; Silver, P.A.; Nocera, D.G. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 2016, 352, 1210–1213. [Google Scholar] [CrossRef]
- Sohn, Y.J.; Son, J.; Jo, S.Y.; Park, S.Y.; Yoo, J.I.; Baritugo, K.-A.; Na, J.G.; Choi, J.-I.; Kim, H.T.; Joo, J.C.; et al. Chemoautotroph Cupriavidus necator as a potential game-changer for global warming and plastic waste problem: A review. Bioresour. Technol. 2021, 340, 125693. [Google Scholar] [CrossRef]
- Langsdorf, A.; Volkmar, M.; Ulber, R.; Hollmann, F.; Holtmann, D. Peroxidases from grass clippings for the removal of phenolic compounds from wastewater. Bioresour. Technol. Rep. 2023, 22, 101471. [Google Scholar] [CrossRef]
- Langsdorf, A.; Drommershausen, A.-L.; Volkmar, M.; Ulber, R.; Holtmann, D. Fermentative α-Humulene Production from Homogenized Grass Clippings as a Growth Medium. Molecules 2022, 27, 8684. [Google Scholar] [CrossRef] [PubMed]
- Lambauer, V.; Kratzer, R. Lab-Scale Cultivation of Cupriavidus necator on Explosive Gas Mixtures: Carbon Dioxide Fixation into Polyhydroxybutyrate. Bioengineering 2022, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Krieg, T.; Sydow, A.; Faust, S.; Huth, I.; Holtmann, D. CO2 to Terpenes: Autotrophic and Electroautotrophic α-Humulene Production with Cupriavidus necator. Angew. Chem. Int. Ed. 2018, 57, 1879–1882. [Google Scholar] [CrossRef]
- Milker, S.; Holtmann, D. First time β-farnesene production by the versatile bacterium Cupriavidus necator. Microb. Cell Factories 2021, 20, 89. [Google Scholar] [CrossRef]
- Crépin, L.; Lombard, E.; Guillouet, S.E. Metabolic engineering of Cupriavidus necator for heterotrophic and autotrophic alka(e)ne production. Metab. Eng. 2016, 37, 92–101. [Google Scholar] [CrossRef]
- Crépin, L.; Barthe, M.; Leray, F.; Guillouet, S.E. Alka(e)ne synthesis in Cupriavidus necator boosted by the expression of endogenous and heterologous ferredoxin–ferredoxin reductase systems. Biotechnol. Bioeng. 2018, 115, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; MacEachran, D.; Burd, H.; Sathitsuksanoh, N.; Bi, C.; Yeh, Y.-C.; Lee, T.S.; Hillson, N.J.; Chhabra, S.R.; Singer, S.W.; et al. Engineering of Ralstonia eutropha H16 for autotrophic and heterotrophic production of methyl ketones. Appl. Environ. Microbiol. 2013, 79, 4433–4439. [Google Scholar] [CrossRef] [PubMed]
- Grousseau, E.; Lu, J.; Gorret, N.; Guillouet, S.E.; Sinskey, A.J. Isopropanol production with engineered Cupriavidus necator as bioproduction platform. Appl. Microbiol. Biotechnol. 2014, 98, 4277–4290. [Google Scholar] [CrossRef]
- Li, H.; Opgenorth, P.H.; Wernick, D.G.; Rogers, S.; Wu, T.-Y.; Higashide, W.; Malati, P.; Huo, Y.-X.; Cho, K.M.; Liao, J.C. Integrated electromicrobial conversion of CO2 to higher alcohols. Science 2012, 335, 1596. [Google Scholar] [CrossRef]
- Sydow, A.; Pannek, A.; Krieg, T.; Huth, I.; Guillouet, S.E.; Holtmann, D. Expanding the genetic tool box for Cupriavidus necator by a stabilized L-rhamnose inducible plasmid system. J. Biotechnol. 2017, 263, 1–10. [Google Scholar] [CrossRef]
- Gruber, S.; Hagen, J.; Schwab, H.; Koefinger, P. Versatile and stable vectors for efficient gene expression in Ralstonia eutropha H16. J. Biotechnol. 2014, 186, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Schwab, H.; Koefinger, P. Versatile plasmid-based expression systems for Gram-negative bacteria--General essentials exemplified with the bacterium Ralstonia eutropha H16. New Biotechnol. 2015, 32, 552–558. [Google Scholar] [CrossRef]
- Bi, C.; Su, P.; Müller, J.; Yeh, Y.-C.; Chhabra, S.R.; Beller, H.R.; Singer, S.W.; Hillson, N.J. Development of a broad-host synthetic biology toolbox for Ralstonia eutropha and its application to engineering hydrocarbon biofuel production. Microb. Cell Fact. 2013, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Panich, J.; Fong, B.; Singer, S.W. Metabolic Engineering of Cupriavidus necator H16 for Sustainable Biofuels from CO(2). Trends Biotechnol. 2021, 39, 412–424. [Google Scholar] [CrossRef]
- Pan, H.; Wang, J.; Wu, H.; Li, Z.; Lian, J. Synthetic biology toolkit for engineering Cupriviadus necator H16 as a platform for CO2 valorization. Biotechnol. Biofuels 2021, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.P.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene alpha-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Jang, H.-I.; Rhee, K.-J.; Eom, Y.-B. Antibacterial and antibiofilm effects of α-humulene against Bacteroides fragilis. Can. J. Microbiol. 2020, 66, 389–399. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Eyres, G.; Dufour, J.-P. 22-Hop Essential Oil: Analysis, Chemical Composition and Odor Characteristics. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 239–254. [Google Scholar]
- Dietz, C.; Cook, D.; Huismann, M.; Wilson, C.; Ford, R. The multisensory perception of hop essential oil: A review. J. Inst. Brew. 2020, 126, 320–342. [Google Scholar] [CrossRef]
- Schempp, F.M.; Drummond, L.; Buchhaupt, M.; Schrader, J. Microbial Cell Factories for the Production of Terpenoid Flavor and Fragrance Compounds. J. Agric. Food Chem. 2018, 66, 2247–2258. [Google Scholar] [CrossRef]
- Putra, I.M.H.; Pratama, I.P.A.A.C.; Putra, K.D.A.; Pradnyaswari, G.A.D.; Laksmiani, N.P.L. The potency of alpha-humulene as HER-2 inhibitor by molecular docking. Pharm. Rep. 2022, 2, 19. [Google Scholar] [CrossRef]
- Chaves, J.S.; Leal, P.C.; Pianowisky, L.; Calixto, J.B. Pharmacokinetics and tissue distribution of the sesquiterpene alpha-humulene in mice. Planta Med. 2008, 74, 1678–1683. [Google Scholar] [CrossRef]
- Milker, S.; Sydow, A.; Torres-Monroy, I.; Jach, G.; Faust, F.; Kranz, L.; Tkatschuk, L.; Holtmann, D. Gram-scale production of the sesquiterpene α-humulene with Cupriavidus necator. Biotechnol. Bioeng. 2021, 118, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Triaux, Z.; Petitjean, H.; Marchioni, E.; Boltoeva, M.; Marcic, C. Deep eutectic solvent–based headspace single-drop microextraction for the quantification of terpenes in spices. Anal. Bioanal. Chem. 2020, 412, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Manurung, R.; Siregar, A.G.A. Performance of menthol based deep eutectic solvents in the extraction of carotenoids from crude palm oil. Int. J. Geomate 2020, 19, 131–137. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Wang, J.; Yang, S.; Li, B.; Xu, N. Aqueous ionic liquid based ultrasonic assisted extraction of four acetophenones from the Chinese medicinal plant Cynanchum bungei Decne. Ultrason. Sonochem. 2013, 20, 180–186. [Google Scholar] [CrossRef]
- De Brabander, P.; Uitterhaegen, E.; Verhoeven, E.; Cruyssen, C.V.; De Winter, K.; Soetaert, W. In Situ Product Recovery of Bio-Based Industrial Platform Chemicals: A Guideline to Solvent Selection. Fermentation 2021, 7, 26. [Google Scholar] [CrossRef]
- Lima, P.S.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.S. Inclusion of terpenes in cyclodextrins: Preparation, characterization and pharmacological approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef]
- Garrigues, L.; Maignien, L.; Lombard, E.; Singh, J.; Guillouet, S.E. Isopropanol production from carbon dioxide in Cupriavidus necator in a pressurized bioreactor. New Biotechnol. 2020, 56, 16–20. [Google Scholar] [CrossRef]
- Laane, C.; Boeren, S.; Vos, K. On optimizing organic solvents in multi-liquid-phase biocatalysis. Trends Biotechnol. 1985, 3, 251–252. [Google Scholar] [CrossRef]
- Reslow, M.; Adlercreutz, P.; Mattiasson, B. Organic solvents for bioorganic synthesis. Appl. Microbiol. Biotechnol. 1987, 26, 1–8. [Google Scholar] [CrossRef]
- Bruce, L.J.; Daugulis, A.J. Solvent Selection Strategies for Extractive Biocatalysis. Biotechnol. Prog. 1991, 7, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Horikoshi, K. Estimation of solvent-tolerance of bacteria by the solvent parameter log P. J. Ferment. Bioeng. 1991, 71, 194–196. [Google Scholar] [CrossRef]
- Kollerup, F.; Daugulis, A.J. Ethanol production by extractive fermentation–solvent identification and prototype development. Can. J. Chem. Eng. 1986, 64, 598–606. [Google Scholar] [CrossRef]
- Brennan, T.C.; Turner, C.D.; Krömer, J.O.; Nielsen, L.K. Alleviating monoterpene toxicity using a two-phase extractive fermentation for the bioproduction of jet fuel mixtures in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Lambauer, V.; Permann, A.; Petrášek, Z.; Subotić, V.; Hochenauer, C.; Kratzer, R.; Reichhartinger, M. Automatic Control of Chemolithotrophic Cultivation of Cupriavidus necator: Optimization of Oxygen Supply for Enhanced Bioplastic Production. Fermentation 2023, 9, 619. [Google Scholar] [CrossRef]

| Medium Component MM | Concentration (g L−1) | Trace Element Stock | Concentration (g L−1) |
|---|---|---|---|
| Na2HPO4 | 2.895 | FeSO4 · 7 H2O | 15.0 |
| NaH2PO4 · H2O | 2.707 | MnSO4 · H2O | 2.4 |
| CaSO4 · 2 H2O | 0.097 | ZnSO4 · 7 H2O | 2.4 |
| K2SO4 | 0.170 | Na2MoO4 · 2 H2O | 1.8 |
| (NH4)2SO4 | 0.943 | CuSO4 · 5 H2O | 0.48 |
| MgSO4 · 7 H2O | 0.8 | NiSO4 · 6 H2O | 1.5 |
| D-Fructose | 4.0 | CoSO4 · 7 H2O | 0.04 |
| Trace elements | 1:20,000 from stock |
| Temperature (°C) | Time (min) |
|---|---|
| 70 | 0 |
| 70 | 1.5 |
| 200 | 9.625 |
| 200 | 10.125 |
| Septum Flask, n = 3 [12] | Controlled Gas Bioreactor [This Work] | |
|---|---|---|
| Substrate feed | (Fed)-batch | Fed-batch |
| Externally provided gases | H2, CO2, O2 | H2, CO2, Air |
| Minimal medium at t = 0 (mL) | 20 | 300 |
| n-Dodecane (v/v) | 20% | 20% |
| n-Dodecane added | At the beginning of cultivation | At the induction time point |
| Inducer (mM) | 11 | 11 |
| Mixing type | Incubating shaker | Magnetic stirring |
| Mixing frequency (rpm) | 180 | 795 ± 15 |
| pH control | No | Yes (≥6.6) |
| CDW (g L−1) | 2.69 ± 0.05 | 8.57 |
| µ (h−1) | 0.12 ± 0.00 | 0.13 |
| α-humulene titer (mg L−1) | 22.0 ± 2.2 | 146 |
| Space-time yield (mg L−1 h−1) | 0.35 ± 0.02 | 4.63 |
| Specific productivity (mg gCDW−1) | 8.62 ± 1.13 | 17.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sydow, A.; Becker, L.; Lombard, E.; Ulber, R.; Guillouet, S.E.; Holtmann, D. Autotrophic Production of the Sesquiterpene α-Humulene with Cupriavidus necator in a Controlled Bioreactor. Bioengineering 2023, 10, 1194. https://doi.org/10.3390/bioengineering10101194
Sydow A, Becker L, Lombard E, Ulber R, Guillouet SE, Holtmann D. Autotrophic Production of the Sesquiterpene α-Humulene with Cupriavidus necator in a Controlled Bioreactor. Bioengineering. 2023; 10(10):1194. https://doi.org/10.3390/bioengineering10101194
Chicago/Turabian StyleSydow, Anne, Lucas Becker, Eric Lombard, Roland Ulber, Stephane E. Guillouet, and Dirk Holtmann. 2023. "Autotrophic Production of the Sesquiterpene α-Humulene with Cupriavidus necator in a Controlled Bioreactor" Bioengineering 10, no. 10: 1194. https://doi.org/10.3390/bioengineering10101194
APA StyleSydow, A., Becker, L., Lombard, E., Ulber, R., Guillouet, S. E., & Holtmann, D. (2023). Autotrophic Production of the Sesquiterpene α-Humulene with Cupriavidus necator in a Controlled Bioreactor. Bioengineering, 10(10), 1194. https://doi.org/10.3390/bioengineering10101194








