Treatment of Severe Pulmonary Regurgitation in Enlarged Native Right Ventricular Outflow Tracts: Transcatheter Pulmonary Valve Replacement with Three-Dimensional Printing Guidance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Preprocedural Imaging
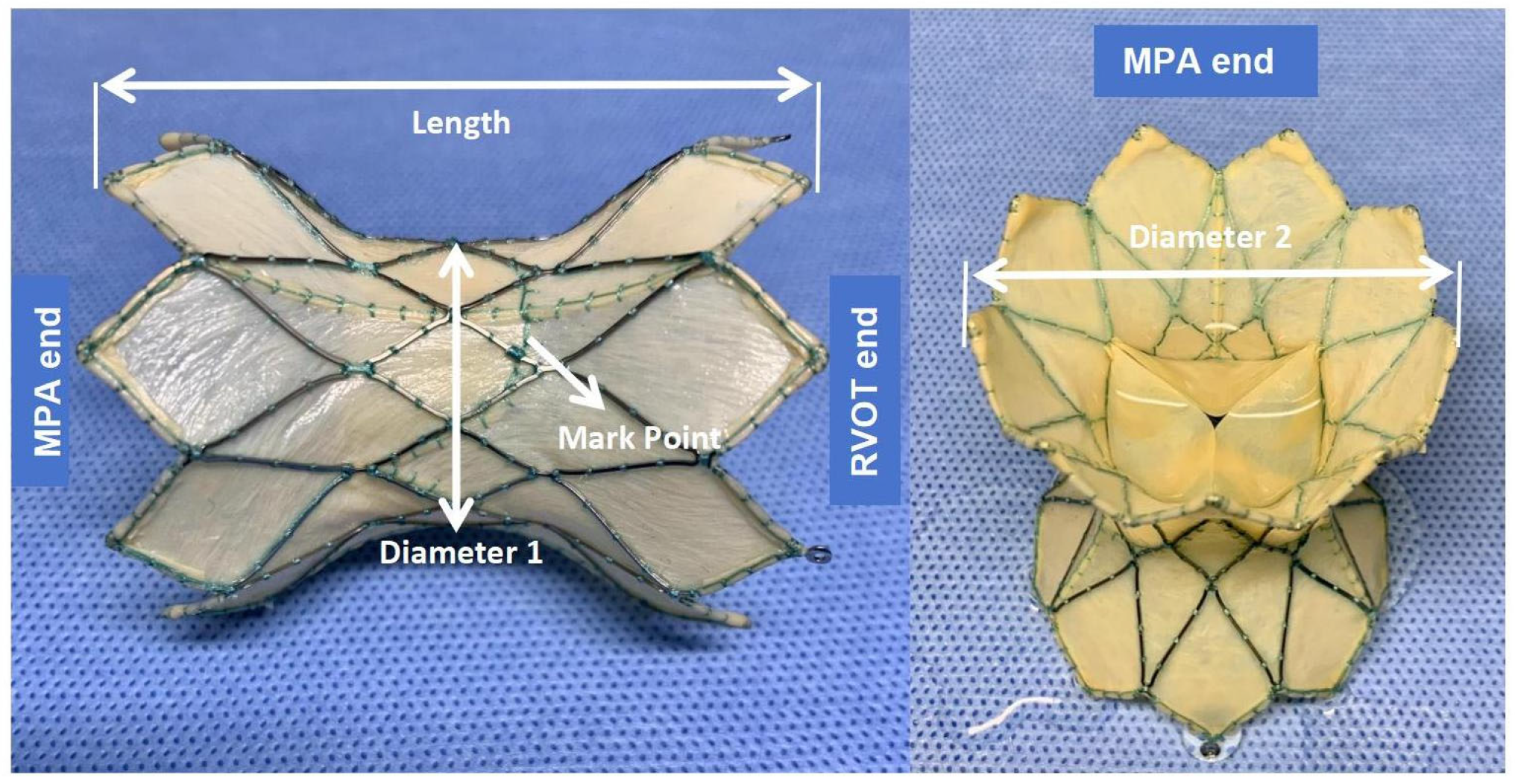
2.3. Device Description
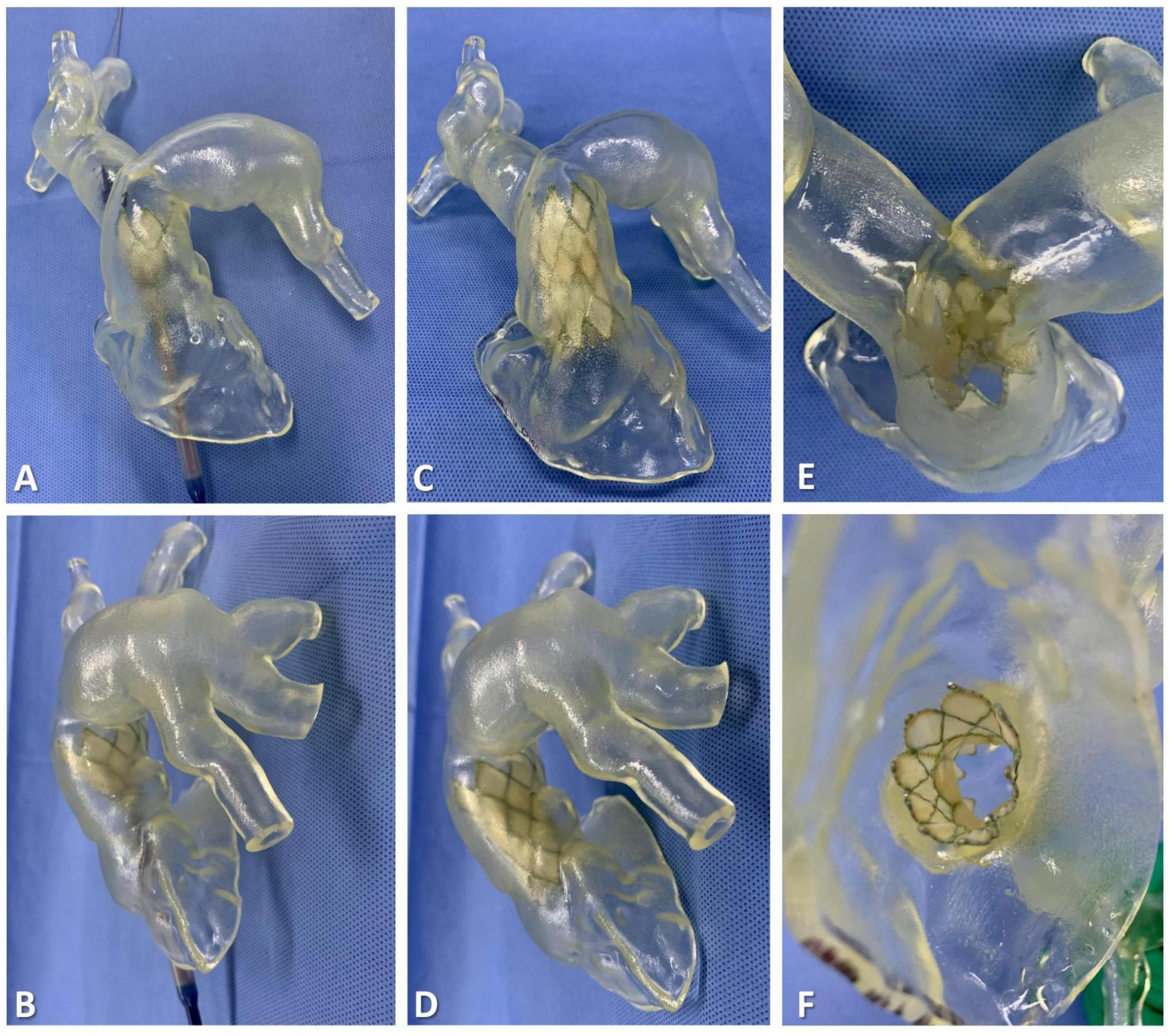
2.4. 3D Reconstruction and Preprocedural 3D Testing In Vitro
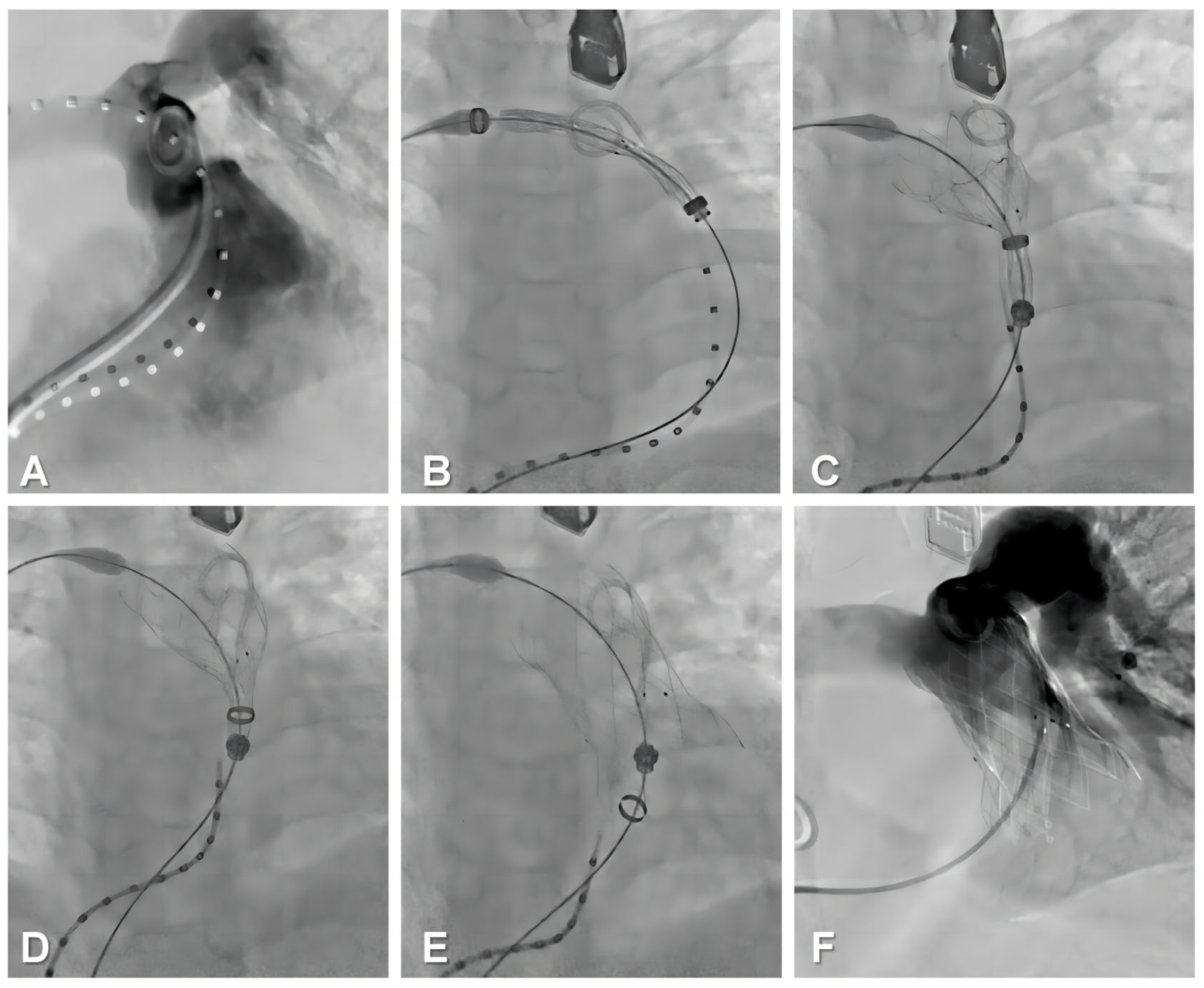
2.5. Procedural Steps
2.6. Data Collection
2.7. Follow-Up
2.8. Statistical Analyses
3. Results
3.1. Baseline Data
3.2. Perioperative and In-Hospital Outcomes
3.3. One-Year Follow-Up Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tatewaki, H.; Shiose, A. Pulmonary valve replacement after repaired Tetralogy of Fallot. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 509–515. [Google Scholar] [CrossRef]
- Latus, H.; Stammermann, J.; Voges, I.; Waschulzik, B.; Gutberlet, M.; Diller, G.; Schranz, D.; Ewert, P.; Beerbaum, P.; Kühne, T.; et al. Impact of Right Ventricular Pressure Load After Repair of Tetralogy of Fallot. J. Am. Heart Assoc. 2022, 11, e022694. [Google Scholar] [CrossRef] [PubMed]
- Basquin, A.; Pineau, E.; Galmiche, L.; Bonnet, D.; Sidi, D.; Boudjemline, Y. Transcatheter valve insertion in a model of enlarged right ventricular outflow tracts. J. Thorac. Cardiovasc. Surg. 2010, 139, 198–208. [Google Scholar] [CrossRef]
- Güzeltaş, A.; Tanıdır, I.C.; Gökalp, S.; Topkarcı, M.A.; Şahin, M.; Ergül, Y. Implantation of the Edwards SAPIEN XT and SAPIEN 3 valves for pulmonary position in enlarged native right ventricular outflow tract. Anatol. J. Cardiol. 2021, 25, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.H.; Meadows, J.; McElhinney, D.B.; Goldstein, B.H.; Bergersen, L.; Qureshi, A.M.; Shahanavaz, S.; Aboulhosn, J.; Berman, D.; Peng, L.; et al. Safety and Feasibility of Melody Transcatheter Pulmonary Valve Replacement in the Native Right Ventricular Outflow Tract: A Multicenter Pediatric Heart Network Scholar Study. JACC Cardiovasc. Interv. 2018, 11, 1642–1650. [Google Scholar] [CrossRef]
- Georgiev, S.; Tanase, D.; Ewert, P.; Meierhofer, C.; Hager, A.; von Ohain, J.P.; Eicken, A. Percutaneous pulmonary valve implantation in patients with dysfunction of a “native” right ventricular outflow tract—Mid-term results. Int. J. Cardiol. 2018, 258, 31–35. [Google Scholar] [CrossRef]
- Jalal, Z.; Valdeolmillos, E.; Malekzadeh-Milani, S.; Eicken, A.; Georgiev, S.; Hofbeck, M.; Sieverding, L.; Gewillig, M.; Ovaert, C.; Bouvaist, H.; et al. Mid-Term Outcomes Following Percutaneous Pulmonary Valve Implantation Using the “Folded Melody Valve” Technique. Circ. Cardiovasc. Interv. 2021, 14, e009707. [Google Scholar] [CrossRef]
- Álvarez-Fuente, M.; Toledano, M.; Garrido-Lestache, E.; Sánchez, I.; Molina, I.; Rivero, N.; García-Ormazábal, I.; del Cerro, M.J. Balloon-Expandable Pulmonary Valves for Patched or Native Right Ventricular Outflow Tracts. Pediatr. Cardiol. 2023, 44, 1285–1292. [Google Scholar] [CrossRef]
- Tannous, P.; Nugent, A. Transcatheter pulmonary valve replacement in native and nonconduit right ventricle outflow tracts. J. Thorac. Cardiovasc. Surg. 2021, 162, 967–970. [Google Scholar] [CrossRef]
- Gillespie, M.J.; McElhinney, D.B.; Jones, T.K.; Levi, D.S.; Asnes, J.; Gray, R.G.; Cabalka, A.K.; Fujimoto, K.; Qureshi, A.M.; Justino, H.; et al. One-Year Outcomes in a Pooled Cohort of Harmony Transcatheter Pulmonary Valve Clinical Trial Participants. JACC Cardiovasc. Interv. 2023, 16, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, W.; Jilaihawi, H.; Zhang, G.; Feng, Y.; Pan, X.; Liu, J.; Yu, S.; Cao, Q.; Ge, J. A self-expanding percutaneous valve for patients with pulmonary regurgitation and an enlarged native right ventricular outflow tract: One-year results. EuroIntervention 2019, 14, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Pluchinotta, F.R.; Sturla, F.; Caimi, A.; Giugno, L.; Chessa, M.; Giamberti, A.; Votta, E.; Redaelli, A.; Carminati, M. 3-Dimensional personalized planning for transcatheter pulmonary valve implantation in a dysfunctional right ventricular outflow tract. Int. J. Cardiol. 2020, 309, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Singh, G.K.; Miller, J.; Sharma, M.; Manning, P.; Billadello, J.J.; Eghtesady, P.; Woodard, P.K. 3D Printing is a Transformative Technology in Congenital Heart Disease. JACC Basic Transl. Sci. 2018, 3, 294–312. [Google Scholar] [CrossRef]
- Schievano, S.; Migliavacca, F.; Coats, L.; Khambadkone, S.; Carminati, M.; Wilson, N.; Deanfield, J.E.; Bonhoeffer, P.; Taylor, A.M. Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data. Radiology 2007, 242, 490–497. [Google Scholar] [CrossRef]
- Valverde, I.; Sarnago, F.; Prieto, R.; Zunzunegui, J.L. Three-dimensional printing in vitro simulation of percutaneous pulmonary valve implantation in large right ventricular outflow tract. Eur. Heart J. 2017, 38, 1262–1263. [Google Scholar] [CrossRef]
- Jivanji, S.G.M.; Qureshi, S.A.; Rosenthal, E. Novel use of a 3D printed heart model to guide simultaneous percutaneous repair of severe pulmonary regurgitation and right ventricular outflow tract aneurysm. Cardiol. Young 2019, 29, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Uebing, A.; Fischer, G.; Bethge, M.; Scheewe, J.; Schmiel, F.; Stieh, J.; Brossmann, J.; Kramer, H.H. Influence of the pulmonary annulus diameter on pulmonary regurgitation and right ventricular pressure load after repair of tetralogy of Fallot. Heart 2002, 88, 510–514. [Google Scholar] [CrossRef]
- Schievano, S.; Coats, L.; Migliavacca, F.; Norman, W.; Frigiola, A.; Deanfield, J.; Bonhoeffer, P.; Taylor, A.M. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: Implications for percutaneous pulmonary valve implantation. J. Cardiovasc. Magn. Reson. 2007, 9, 687–695. [Google Scholar] [CrossRef]
- Zahn, E.M. Self-Expanding Pulmonary Valves for Large Diameter Right Ventricular Outflow Tracts. Interv. Cardiol. Clin. 2019, 8, 73–80. [Google Scholar] [CrossRef]
- Tretter, J.T.; Friedberg, M.K.; Wald, R.M.; McElhinney, D.B. Defining and refining indications for transcatheter pulmonary valve replacement in patients with repaired tetralogy of Fallot: Contributions from anatomical and functional imaging. Int. J. Cardiol. 2016, 221, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Pluchinotta, F.R.; Caimi, A.; Pilati, M.; Carminati, M.; Sturla, F. Computer-based prediction of coronary artery compression in the planning of transcatheter pulmonary valve implantation. EuroIntervention 2021, 17, 584–585. [Google Scholar] [CrossRef]
- Ruiz, C.E.; Pasala, T.K.R. Are We Ready for Transcatheter Pulmonary Valve Replacement in Native Right Ventricular Outflow Tract? JACC Cardiovasc. Interv. 2018, 11, 1651–1653. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P.; Kister, T. Why we need another percutaneous pulmonary valve: If size matters. EuroIntervention 2019, 14, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Chen, C.A.; Chen, S.J.; Huang, J.-H.; Chang, Y.-H.; Chiu, S.-N.; Lu, C.-W.; Wu, M.-H.; Wang, J.-K. Self-Expanding Pulmonary Valves in 53 Patients with Native Repaired Right Ventricular Outflow Tracts. Can. J. Cardiol. 2023, 39, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Odemis, E.; Yenidogan, I.; Kizilkaya, M.H. Early results of Pulsta® transcatheter heart valve in patients with enlarged right ventricular outflow tract and severe pulmonary regurgitation due to transannular patch. Cardiol. Young 2022, 16, 1–9. [Google Scholar]
- Gao, Y.; Xie, M.; Wang, B.; Shang, X.; Zhang, L.; Xie, Y.; Li, Y. First-in-human transcatheter pulmonic valve implantation of Med-Zenith PT-Valve in a stenotic pulmonary conduit. QJM 2020, 113, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Dong, N.; Zhang, C.; Wang, Y. The Clinical Trial Outcomes of Med-Zenith PT-Valve in the Treatment of Patients with Severe Pulmonary Regurgitation. Front. Cardiovasc. Med. 2022, 9, 887886. [Google Scholar] [CrossRef]
- Georgiev, S.; Ewert, P.; Tanase, D.; Hess, J.; Hager, A.; Cleuziou, J.; Meierhofer, C.; Eicken, A. A Low Residual Pressure Gradient Yields Excellent Long-Term Outcome after Percutaneous Pulmonary Valve Implantation. JACC Cardiovasc. Interv. 2019, 12, 1594–1603. [Google Scholar] [CrossRef]
- Marro, M.; Kossar, A.P.; Xue, Y.; Frasca, A.; Levy, R.J.; Ferrari, G. Noncalcific Mechanisms of Bioprosthetic Structural Valve Degeneration. J. Am. Heart Assoc. 2021, 10, e018921. [Google Scholar] [CrossRef]



| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Age, years/sex | 48/F | 31/F | 17/M | 13/F | 17/F | 52/M | 12/M | 17/M |
| Weight (kg) | 65 | 61 | 70 | 55 | 75 | 50 | 56 | 56 |
| Height (cm) | 155 | 168 | 175 | 154 | 164 | 168 | 150 | 170 |
| Symptoms | Chest tightness, edema, | Palpitation, edema | Edema, dyspnea | Palpitation, edema, dyspnea | Chest tightness, edema | Chest tightness, edema, dyspnea | Edema, dyspnea | Chest tightness, edema |
| QRS duration (ms) | 116 | 127 | 119 | 109 | 147 | 134 | 131 | 139 |
| 6-MWT (m) | 290 | 350 | 390 | 420 | 310 | 300 | 390 | 340 |
| NYHA functional class | IV | III | III | II | III | IV | II | II |
| Diagnosis | PR, rPA(TOF) | PR, rTOF | PR, rPA(TOF) | PR, rTOF + rVSD | PR, rTOF | PR, rTOF | PR, rTOF | PR, rTOF + AVR |
| Atrial fibrillation/atrial flutter | Yes | Yes | No | No | No | Yes | No | No |
| Peak O2 | 15 | 19 | 24 | 23 | 26 | 14 | 19 | 21 |
| NT-proBNP | 1961 | 1672 | 961 | 541 | 1316 | 1782 | 782 | 1542 |
| RVOT repair age, year | 22 | 22 | 1 | 1 | 3 | 30 | 1 | 2 |
| RVOT type | nRVOT | TAP | nRVOT | TAP | TAP | TAP | TAP | TAP |
| Echocardiographic Parameters | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| PR severity grade | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ |
| Peak transpulmonary valve gradient | 21 | 7 | 11 | 13 | 15 | 14 | 9 | 9 |
| TR severity grade | 3+ | 3+ | 2+ | 2+ | 1+ | 0 | 2+ | 1+ |
| TASPE (mm) | 15 | 16 | 17 | 16 | 18 | 14 | 15 | 16 |
| RV–FAC (%) | 34 | 33 | 32 | 34 | 36 | 32 | 34 | 31 |
| Computed tomography parameters | ||||||||
| RV–PA conduit length (mm) | 57 | 63 | 48 | 56 | 55 | 46 | 43 | 52 |
| nRVOT diameter (mm) | 46 | 42 | 35 | 30 | 30 | 33 | 31 | 33 |
| The narrowest plane/diameter (mm) | Mid MPA/34 | PA/ 37 | Distal MPA/23 | Distal MPA/24 | Mid MPA/24 | PA/ 26 | Distal MPA/22 | Mid MPA/23 |
| MRI parameters | ||||||||
| PR fraction (%) | 55 | 52 | 48 | 41 | 40 | 46 | 59 | 54 |
| RVEDVI (ml/m2) | 189 | 176 | 163 | 158 | 165 | 156 | 171 | 156 |
| RVEF (%) | 26 | 21 | 18 | 26 | 24 | 19 | 27 | 21 |
| 3D Printing Guidance | ||||||||
| Valve size (mm) | 44–26 | 44–26 | 36–26 | 32–23 | 32–23 | 32–23 | 36–26 | 36–26 |
| Proximal landing zone | RVOT | PA | RVOT | PA | PA | RVOT | RVOT | RVOT |
| Distal landing zone | Distal MPA | MPA sinus | Mid MPA | MPA sinus | Mid MPA | Mid MPA | Mid MPA | Mid MPA |
| Perioperative Outcomes | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Right Heart Catheterization Parameters | |||||||||
| Pre-/Post-devices | PASP (mmHg) | 32/36 | 36/39 | 39/35 | 34/37 | 36/38 | 35/37 | 32/35 | 38/37 |
| PADP (mmHg) | 6/11 | 5/12 | 3/11 | 4/9 | 3/8 | 7/12 | 2/7 | 3/9 | |
| mRAP (mmHg) | 10/4 | 9/7 | 6/5 | 5/4 | 7/4 | 8/5 | 8/6 | 10/8 | |
| RVEDP (mmHg) | 8/5 | 7/4 | 11/6 | 10/4 | 9/5 | 6/3 | 8/6 | 6/5 | |
| PA–RV gradient (mmHg) | 10/6 | 8/2 | −4/−2 | −1/−3 | 9/6 | 2/−2 | −3/−5 | 2/−1 | |
| Intraoperative, post-devices TEE | |||||||||
| Residual PR | None | None | Trace | None | None | None | Trace | None | |
| Peak transpulmonary valve gradient | 11 | 10 | 6 | 8 | 9 | 8 | 10 | 7 | |
| Operative outcomes | |||||||||
| Procedure time (minutes) | 52 | 57 | 68 | 62 | 58 | 63 | 71 | 63 | |
| Fluoroscopy time (minutes) | 26 | 14 | 16 | 17 | 21 | 15 | 24 | 19 | |
| Balloon inflation test | No | No | No | No | No | No | Yes | No | |
| Coronary compression | No | No | No | No | No | No | No | No | |
| In-hospital outcomes | |||||||||
| ICU time (days) | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | |
| Postoperative hospitalization time (days) | 5 | 4 | 5 | 4 | 4 | 5 | 4 | 4 | |
| Procedural success | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Device-related adverse events | None | None | None | None | None | None | None | None | |
| Complications | None | None | Hemoptysis | None | None | None | Hemoptysis | None | |
| Baseline | 30 Days | 6 Months | 1 Year | |||||
|---|---|---|---|---|---|---|---|---|
| Clinical Characteristic | Results | p-Value | Results | p-Value | Results | p-Value | ||
| Peak O2 | 20 (15, 14) | 21 (17, 26) | 0.466 | 25 (22, 29) | 0.014 | 29 (24, 33) | 0.002 | |
| 6-MWT (m) | 345 (300, 390) | 420 (390, 460) | 0.003 | 445 (415,480) | <0.001 | 460 (420, 495) | <0.001 | |
| QRS duration (ms) | 129 (116, 139) | 110 (101, 139) | 0.072 | 114 (96, 142) | 0.053 | 112 (98, 138) | 0.106 | |
| Echocardiographic parameters | ||||||||
| PR severity | None, n (%) | 0 (0.0) | 8 (100.0%) | <0.001 | 7 (87.5%) | <0.001 | 6 (75.0%) | <0.001 |
| Trace, n (%) | 0 (0.0) | 0 (0.0) | —— | 1 (12.5%) | 0.231 | 2 (25.0%) | <0.001 | |
| Mild, n (%) | 0 (0.0) | 0 (0.0) | —— | 0 (0.0) | —— | 0 (0.0) | —— | |
| Moderate, n (%) | 0 (0.0) | 0 (0.0) | —— | 0 (0.0) | —— | 0 (0.0) | —— | |
| Severe, n (%) | 8 (100.0%) | 0 (0.0) | <0.001 | 0 (0.0) | <0.001 | 0 (0.0) | <0.001 | |
| Peak transpulmonary valve gradient (mmHg) | 13.5 (9, 14) | 9 (8, 15) | 0.183 | 10(8, 11) | 0.148 | 11 (9, 13) | 0.550 | |
| TASPE (mm) | 16 (15, 17) | 16 (14, 17) | 0.998 | 16 (15, 17) | 0.364 | 17 (16, 19) | 0.023 | |
| RV–FAC (%) | 33.5 (32, 34) | 34 (33, 36) | 0.138 | 37 (34, 39) | 0.003 | 38 (36,41) | <0.001 | |
| MRI parameters | ||||||||
| PR fraction (%) | 50 (41, 55) | —— | —— | 2.5 (2, 3.5) | <0.001 | 1.5 (1, 2) | <0.001 | |
| RVEDVI (ml/m2) | 164 (156, 176) | —— | —— | 137 (126, 168) | 0.004 | 119 (107, 148) | <0.001 | |
| RVEF (%) | 22.5 (19, 26) | —— | —— | 31 (28, 34) | <0.001 | 33 (31, 39) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jin, P.; Meng, X.; Li, L.; Mao, Y.; Zheng, M.; Liu, L.; Liu, Y.; Yang, J. Treatment of Severe Pulmonary Regurgitation in Enlarged Native Right Ventricular Outflow Tracts: Transcatheter Pulmonary Valve Replacement with Three-Dimensional Printing Guidance. Bioengineering 2023, 10, 1136. https://doi.org/10.3390/bioengineering10101136
Wang Y, Jin P, Meng X, Li L, Mao Y, Zheng M, Liu L, Liu Y, Yang J. Treatment of Severe Pulmonary Regurgitation in Enlarged Native Right Ventricular Outflow Tracts: Transcatheter Pulmonary Valve Replacement with Three-Dimensional Printing Guidance. Bioengineering. 2023; 10(10):1136. https://doi.org/10.3390/bioengineering10101136
Chicago/Turabian StyleWang, Yiwei, Ping Jin, Xin Meng, Lanlan Li, Yu Mao, Minwen Zheng, Liwen Liu, Yang Liu, and Jian Yang. 2023. "Treatment of Severe Pulmonary Regurgitation in Enlarged Native Right Ventricular Outflow Tracts: Transcatheter Pulmonary Valve Replacement with Three-Dimensional Printing Guidance" Bioengineering 10, no. 10: 1136. https://doi.org/10.3390/bioengineering10101136
APA StyleWang, Y., Jin, P., Meng, X., Li, L., Mao, Y., Zheng, M., Liu, L., Liu, Y., & Yang, J. (2023). Treatment of Severe Pulmonary Regurgitation in Enlarged Native Right Ventricular Outflow Tracts: Transcatheter Pulmonary Valve Replacement with Three-Dimensional Printing Guidance. Bioengineering, 10(10), 1136. https://doi.org/10.3390/bioengineering10101136









