Biomechanical Analysis of Latin Dancers’ Lower Limb during Normal Walking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol and Equipment
2.3. Experimental Procedure
2.4. Data Collection and Processing
2.5. Statistical Analysis
3. Results
3.1. Results of the Kinetics and Kinematics in the Sagittal Plane
3.2. Results of the Kinetics and Kinematics in the Coronal Plane
3.3. Results of the Kinetics and Kinematics in the Horizontal Plane
3.4. Results of the Ground Reaction Force, Patellofemoral Joint Contact Force, and Achilles Tendon Force
3.5. Results of the COP (Center of Pressure) Trajectory
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.T.; Lin, A.C.; Chen, S.F.; Shih, C.J.; Kuo, T.Y.; Wang, F.C.; Lee, P.H.; Lee, A.P. Superior gait performance and balance ability in Latin dancers. Front. Med. 2022, 9, 834497. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.M.; Alonso, A.C.; Peterson, M.; Mochizuki, L.; Greve, J.M.; Garcez-Leme, L.E. Balance and Muscle Strength in Elderly Women Who Dance Samba. Gait Posture 2022, 92, 264–270. [Google Scholar] [CrossRef]
- Kiliç, M.; Nalbant, S.S. The effect of latin dance on dynamic balance. Gait Posture 2022, 92, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Cherng, R.J.; Chen, J.J.; Su, F.C. Vestibular system in performance of standing balance of children and young adults under altered sensory conditions. Percept. Mot. Skills 2001, 92, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Dunsky, A.; Zeev, A.; Netz, Y. Balance Performance Is Task Specific in Older Adults. BioMed Res. Int. 2017, 2017, 6987017. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.I.; Houtenbos, S.; Lu, Y.H.; Mayer, F.; Wippert, P.M. The epidemiology of chronic ankle instability with perceived ankle instability- a systematic review. J. Foot Ankle Res. 2021, 14, 41. [Google Scholar] [CrossRef]
- Thompson, C.; Schabrun, S.; Romero, R.; Bialocerkowski, A.; van Dieen, J.; Marshall, P. Factors Contributing to Chronic Ankle Instability: A Systematic Review and Meta-Analysis of Systematic Reviews. Sports Med. 2018, 48, 189–205. [Google Scholar] [PubMed]
- Kobayashi, T.; Tanaka, M.; Shida, M. Intrinsic Risk Factors of Lateral Ankle Sprain: A Systematic Review and Meta-analysis. Sports Health 2016, 8, 190–193. [Google Scholar] [CrossRef]
- Houston, M.N.; Van Lunen, B.L.; Hoch, M.C. Health-related quality of life in individuals with chronic ankle instability. J. Athl. Train. 2014, 49, 758–763. [Google Scholar] [CrossRef]
- Simon, J.E.; Docherty, C.L. Health-related quality of life is decreased in middle-aged adults with chronic ankle instability. J. Sci. Med. Sports 2018, 21, 1206–1209. [Google Scholar] [CrossRef]
- Rangel, J.G.; Divino, N.; Dos Santos, W.; Viana, R.B.; Silva, M.S.; Vieira, C.A.; Campos, M.H. Studies of Classical Ballet Dancers’ Equilibrium at Different Levels of Development and Versus Non-Dancers: A Systematic Review. J. Dance Med. Sci. 2020, 24, 33–43. [Google Scholar] [CrossRef]
- de Mello, M.C.; de Sá Ferreira, A.; Ramiro Felicio, L. Postural Control During Different Unipodal Positions in Professional Ballet Dancers. J. Dance Med. Sci. 2017, 21, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, P.G.; Griffin, E.D.; Zurakowski, D. Comparison of standing balance between female collegiate dancers and soccer players. Gait Posture 2007, 26, 501–507. [Google Scholar] [CrossRef]
- Nikolaidou, M.E.; Karfis, V.; Koutsouba, M.; Schroll, A.; Arampatzis, A. Postural Balance Ability and the Effect of Visual Restriction on Older Dancers and Non-Dancers. Front. Sports 2021, 3, 707567. [Google Scholar] [CrossRef] [PubMed]
- Wanke, E.M.; Schreiter, J.; Groneberg, D.A.; Weisser, B. Muscular imbalances and balance capability in dance. J. Occup. Med. Toxicol. 2018, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.; Park, S.H.; Kim, S. Effects of DanceSport on walking balance and standing balance among the elderly. Technol. Health Care 2018, 26, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.A.; Lee, G.A.; Tuckman, A.S.; Esquenazi, A. Improving calf muscle strength in patients with spastic equinovarus deformity by transfer of the long toe flexors to the Os calcis. J. Head Trauma Rehab. 1999, 14, 163–175. [Google Scholar] [CrossRef]
- Winnicki, K.; Ochała-Kłos, A.; Rutowicz, B.; Pękala, P.A.; Tomaszewski, K.A. Functional anatomy, histology and biomechanics of the human Achilles tendon-A comprehensive review. Ann. Anat. 2020, 229, 151461. [Google Scholar] [CrossRef]
- Wearing, S.C.; Davis, I.S.; Brauner, T.; Hooper, S.L.; Horstmann, T. Do habitual foot-strike patterns in running influence functional Achilles tendon properties during gait? J. Sports Sci. 2019, 37, 2735–2743. [Google Scholar] [CrossRef]
- Joseph, M.F.; Lillie, K.R.; Bergeron, D.J.; Denegar, C.R. Measuring Achilles tendon mechanical properties: A reliable, noninvasive method. J. Strength Cond. Res. 2012, 26, 2017–2020. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, H.; Baker, J.S.; István, B.; Gu, Y. An Investigation of Differences in Lower Extremity Biomechanics During Single-Leg Landing from Height Using Bionic Shoes and Normal Shoes. Front. Bioeng. Biotech. 2021, 9, 679123. [Google Scholar] [CrossRef]
- LaPrade, M.D.; Kennedy, M.I.; Wijdicks, C.A. LaPrade RF: Anatomy and biomechanics of the medial side of the knee and their surgical implications. Sports Med. Arthrosc. 2015, 23, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhou, H.; Quan, W.; Gusztav, F.; Wang, M.; Baker, J.S.; Gu, Y. Accurately and effectively predict the ACL force: Utilizing biomechanical landing pattern before and after-fatigue. Comput. Methods Programs Biomed. 2023, 241, 107761. [Google Scholar] [CrossRef]
- Stephen, J.; Alva, A.; Lumpaopong, P.; Williams, A.; Amis, A.A. A cadaveric model to evaluate the effect of unloading the medial quadriceps on patellar tracking and patellofemoral joint pressure and stability. JEO 2018, 5, 34. [Google Scholar] [CrossRef]
- Steinmetz, S.; Brügger, A.; Chauveau, J.; Chevalley, F.; Borens, O.; Thein, E. Practical guidelines for the treatment of patellar fractures in adults. Swiss Med. Wkly. 2020, 150, 20165. [Google Scholar] [CrossRef]
- Lelas, J.L.; Merriman, G.J.; Riley, P.O.; Kerrigan, D.C. Predicting peak kinematic and kinetic parameters from gait speed. Gait Posture 2003, 17, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lemke, M.R.; Wendorff, T.; Mieth, B.; Buhl, K.; Linnemann, M. Spatiotemporal gait patterns during over ground locomotion in major depression compared with healthy controls. J. Psychiatr. Res. 2000, 34, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Quan, W.; Zhou, H.; Sun, D.; Baker, J.S.; Gu, Y. Explaining the differences of gait patterns between high and low-mileage runners with machine learning. Sci. Rep. 2022, 12, 2981. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, X.; Cen, X.; Baker, J.S.; Gu, Y. Single-Leg Landings Following a Volleyball Spike May Increase the Risk of Anterior Cruciate Ligament Injury More Than Landing on Both-Legs. Appl. Sci. 2021, 11, 130. [Google Scholar] [CrossRef]
- Xu, D.; Cen, X.; Wang, M.; Rong, M.; István, B.; Baker, J.S.; Gu, Y. Temporal Kinematic Differences between Forward and Backward Jump-Landing. Int. J. Environ. Res. Public Health 2020, 17, 6669. [Google Scholar] [CrossRef]
- Xu, D.; Lu, Z.; Shen, S.; Fekete, G.; Ugbolue, U.C.; Gu, Y. The Differences in Lower Extremity Joints Energy Dissipation Strategy during Landing between Athletes with Symptomatic Patellar Tendinopathy (PT) and without Patellar Tendinopathy (UPT). Mol. Cell Biomech. 2021, 18, 107–118. [Google Scholar] [CrossRef]
- Oberg, T.; Karsznia, A.; Oberg, K. Basic gait parameters: Reference data for normal subjects, 10–79 years of age. J. Rehabil. Res. Dev. 1993, 30, 210–223. [Google Scholar]
- Al-Obaidi, S.; Wall, J.C.; Al-Yaqoub, A.; Al-Ghanim, M. Basic gait parameters: A comparison of reference data for normal subjects 20 to 29 years of age from Kuwait and Scandinavia. J. Rehabil. Res. Dev. 2003, 40, 361–366. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, D.; Chen, C.; Ugbolue, U.; Baker, J.S.; Gu, Y. Analysis of Different Stop-Jumping Strategies on the Biomechanical Changes in the Lower Limbs. Appl. Sci. 2021, 11, 4633. [Google Scholar] [CrossRef]
- Li, F.; Zhou, H.; Xu, D.; Baker, J.; Gu, Y. Comparison of Biomechanical Characteristics during the Second Landing Phase in Female Latin Dancers: Evaluation of the Bounce and Side Chasse Step. Mol. Cell Biomech. 2022, 19, 115. [Google Scholar] [CrossRef]
- Li, X.; Lu, Z.; Sun, D.; Xuan, R.; Zheng, Z.; Gu, Y. The influence of a shoe’s heel-toe drop on gait parameters during the third trimester of pregnancy. Bioengineering 2022, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Rugg, S.G.; Gregor, R.J.; Mandelbaum, B.R.; Chiu, L. In vivo moment arm calculations at the ankle using magnetic resonance imaging (MRI). J. Biomech. 1990, 23, 495–501. [Google Scholar] [CrossRef]
- Self, B.P.; Paine, D. Ankle biomechanics during four landing techniques. Med. Sci. Sports Exerc. 2001, 33, 1338–1344. [Google Scholar] [CrossRef]
- Lu, J.; Xu, D.; Quan, W.; Baker, J.S.; Gu, Y. Effects of Forefoot Shoe on Knee and Ankle Loading during Running in Male Recreational Runners. Mol. Cell Biomech. 2022, 19, 61–75. [Google Scholar] [CrossRef]
- Ho, K.Y.; Blanchette, M.G.; Powers, C.M. The influence of heel height on patellofemoral joint kinetics during walking. Gait Posture 2012, 36, 271–275. [Google Scholar] [CrossRef]
- Kubo, K.; Miyazaki, D.; Tanaka, S.; Shimoju, S.; Tsunoda, N. Relationship between Achilles tendon properties and foot strike patterns in long-distance runners. J. Sports Sci. 2015, 33, 665–669. [Google Scholar] [CrossRef]
- van Eijden, T.M.; de Boer, W.; Weijs, W.A. The orientation of the distal part of the quadriceps femoris muscle as a function of the knee flexion-extension angle. J. Biomech. 1985, 18, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Pataky, T.C. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J. Biomech. 2010, 43, 1976–1982. [Google Scholar] [CrossRef]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Vector field statistical analysis of kinematic and force trajectories. J. Biomech. 2013, 46, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.P.; Mote, C.D., Jr.; Skinner, H.B. The effect of ankle constraint on the torsional laxity of the knee during internal-external rotation of the foot. J. Biomech. 1991, 24, 511–525. [Google Scholar] [CrossRef]
- Tao, D.; Supriya, R.; Gao, Y.; Li, F.; Liang, W.; Jiao, J. Dementia and dance: Medication or movement? Phys. Act. Health 2021, 5, 250–254. [Google Scholar] [CrossRef]
- Avdan, G.; Onal, S.; Rekabdar, B. Regression transfer learning for the prediction of three-dimensional ground reaction forces and joint moments during gait. Int. J. Biomed. Eng. Technol. 2023, 42, 317–338. [Google Scholar] [CrossRef]
- Wang, H.K.; Chen, C.H.; Shiang, T.Y.; Jan, M.H.; Lin, K.H. Risk-factor analysis of high school basketball-player ankle injuries: A prospective controlled cohort study evaluating postural sway, ankle strength, and flexibility. Arch. Phys. Med. Rehab. 2006, 87, 821–825. [Google Scholar] [CrossRef]
- Contiero, D.; Bonfigli, A.R.; Piccinini, F.; Fabbietti, P.; Gambella, E.; Giulietti, M.V.; Gagliardi, C. Improving Physical Activity and Health of Older People Involved in a Social Farming Regional Preventative Multidimensional Programme in Italy. Phys. Act. Health 2023, 7, 205–216. [Google Scholar] [CrossRef]
- Song, Y.; Cen, X.; Chen, H.; Sun, D.; Munivrana, G.; Bálint, K.; Bíró, I.; Gu, Y. The influence of running shoe with different carbon-fiber plate designs on internal foot mechanics: A pilot computational analysis. J. Biomech. 2023, 153, 111597. [Google Scholar] [CrossRef]
- Granacher, U.; Muehlbauer, T.; Gollhofer, A.; Kressig, R.W.; Zahner, L. An intergenerational approach in the promotion of balance and strength for fall prevention-a mini-review. J. Gerontol. 2011, 57, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; March, L.; Sambrook, P.N. The association of cartilage volume with knee pain. Osteoarthr. Cartil. 2003, 11, 725–729. [Google Scholar] [CrossRef] [PubMed]

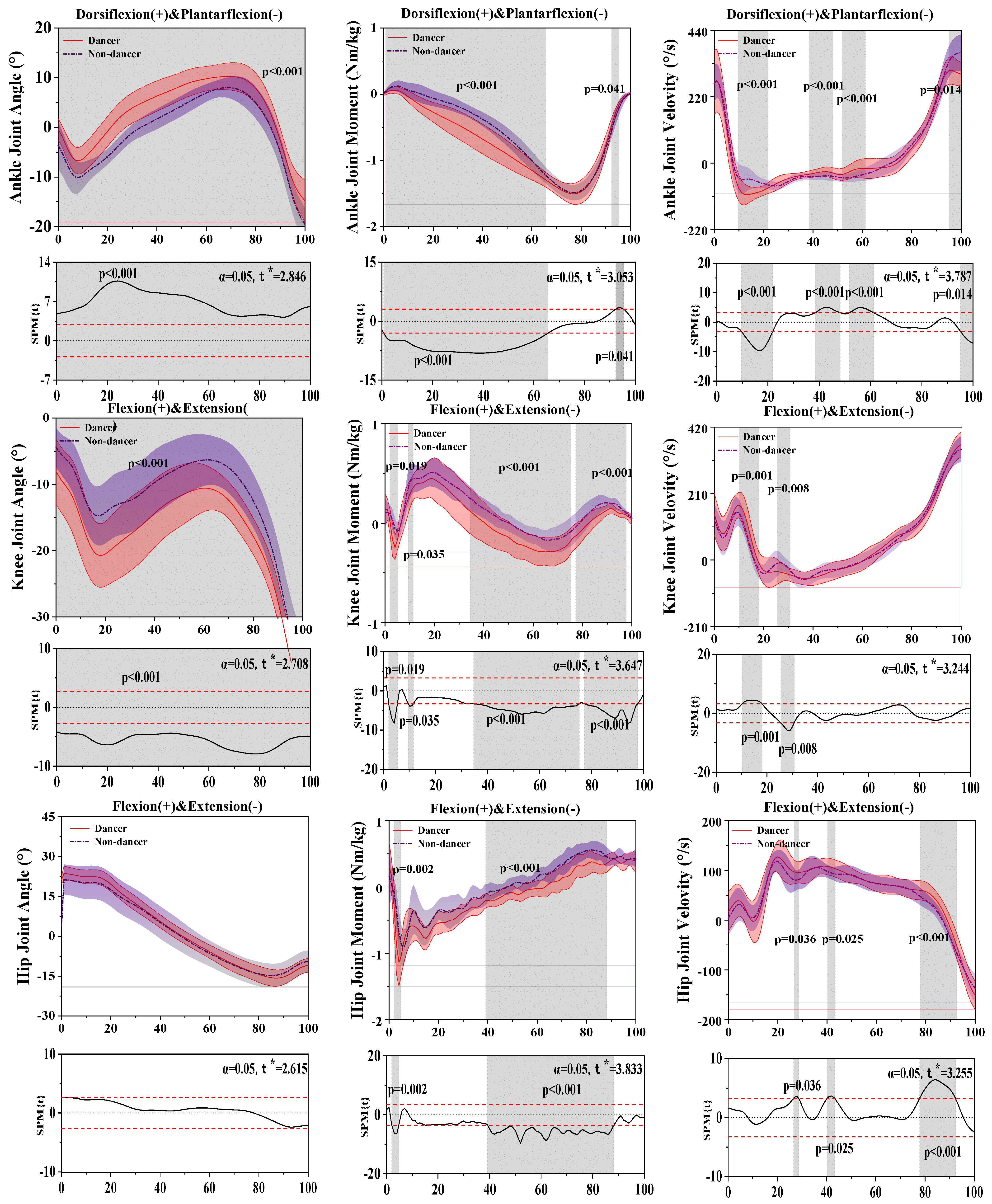
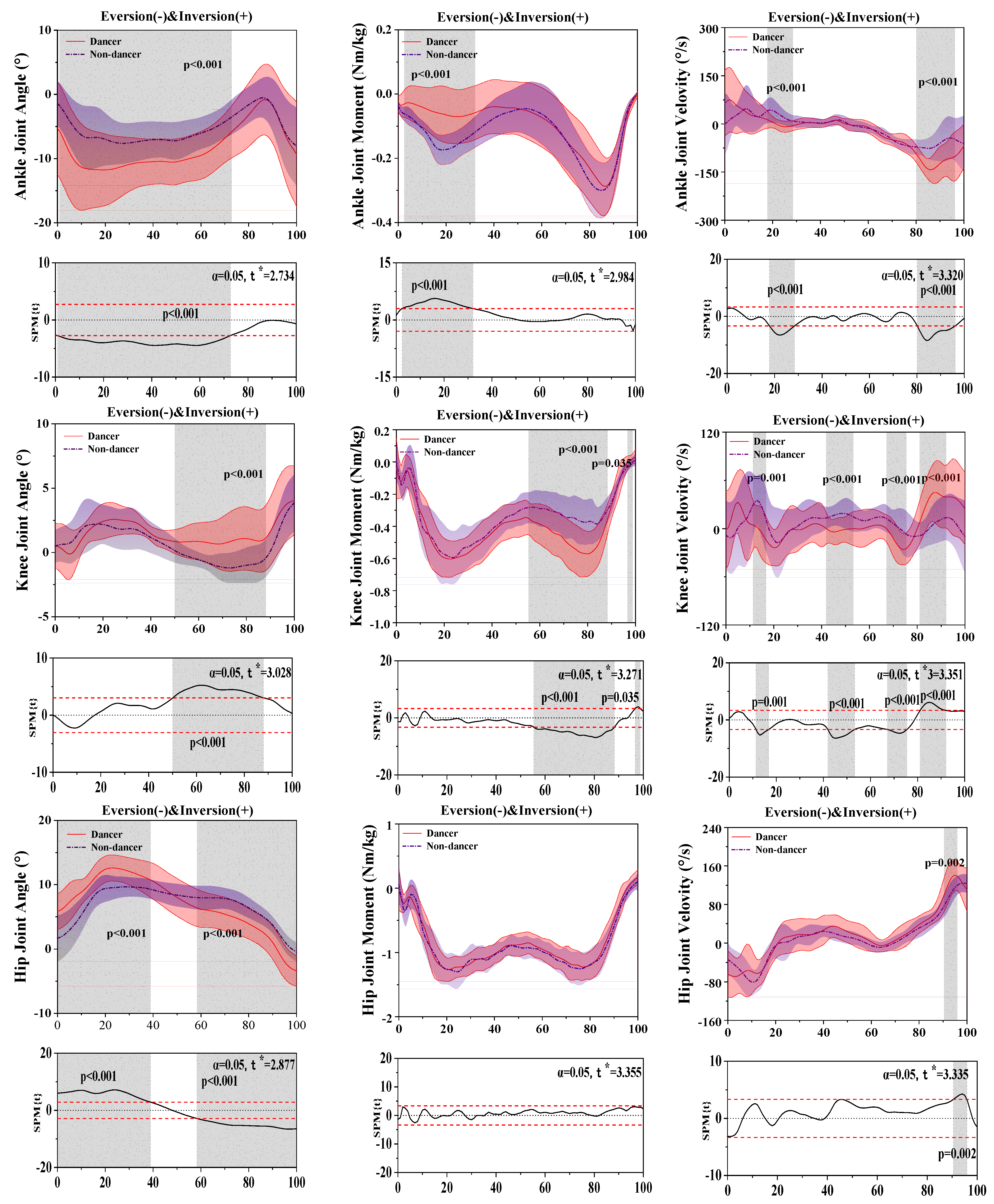
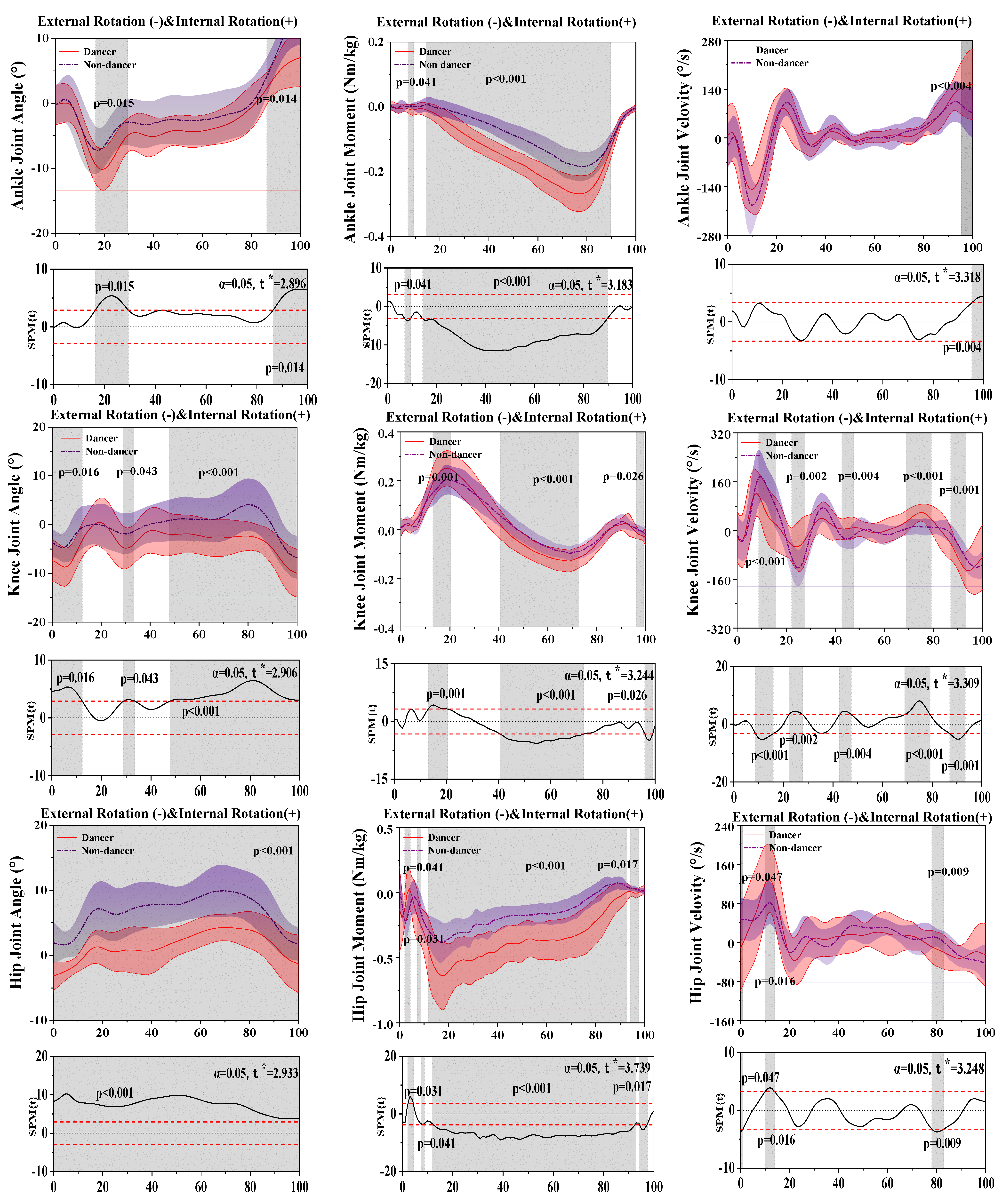
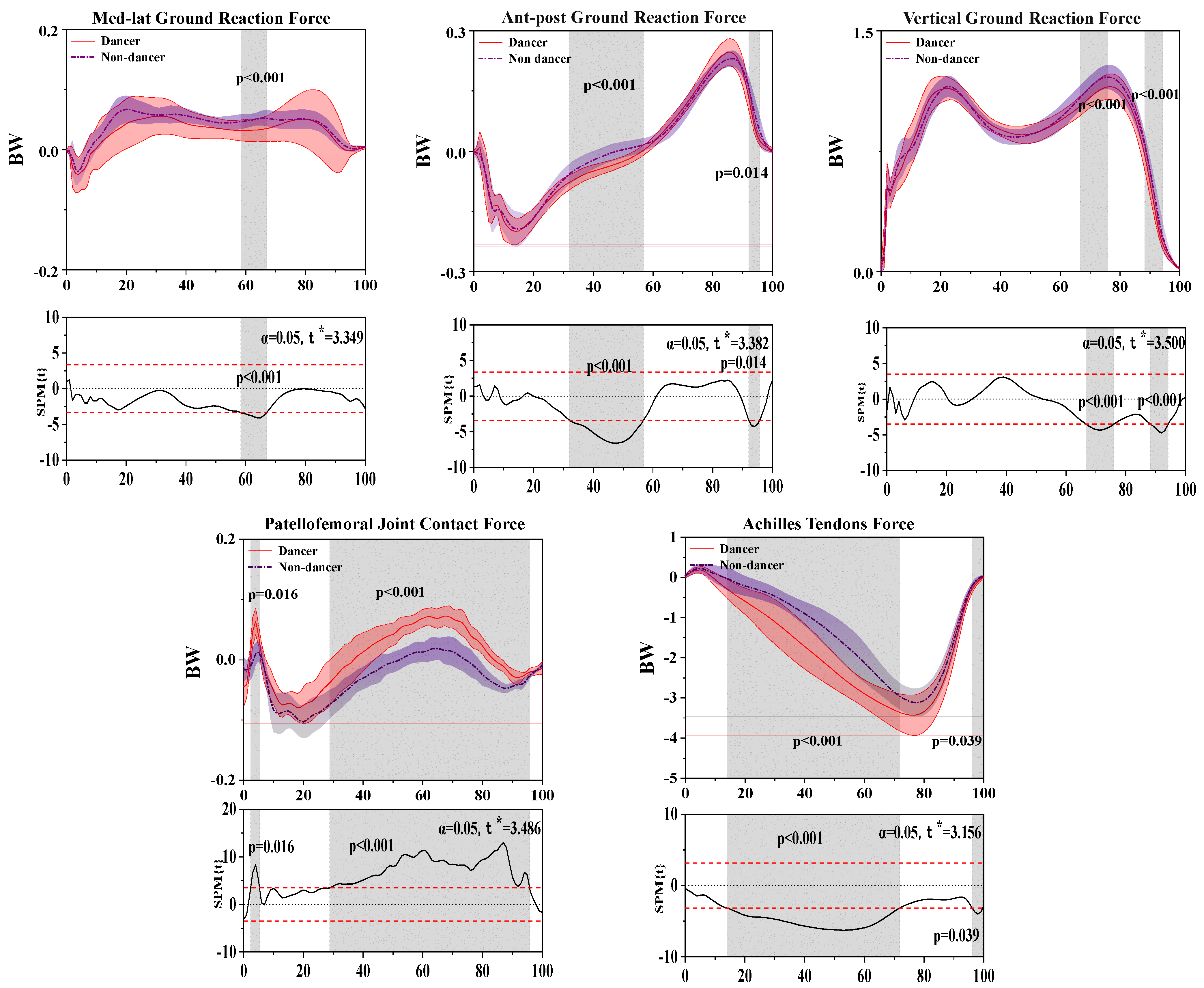

| Joint | Parameters | Peak Value | Dancer Mean ± SD | Non-Dancer Mean ± SD | p-Value |
|---|---|---|---|---|---|
| Ankle | Angle (°) | Dorsiflexion | −14.89 ± 4.18 | −19.65 ± 3.33 | <0.001 * |
| Plantarflexion | 10.99 ± 2.58 | 8.44 ± 1.92 | <0.001 * | ||
| Moment (Nm/kg) | Dorsiflexion | −1.51 ± 0.16 | −1.50 ± 0.12 | 0.63 | |
| Plantarflexion | 0.078 ± 0.042 | 0.14 ± 0.08 | <0.001 * | ||
| Velocity (°/s) | Dorsiflexion | 120.23 ± 19.67 | −92.86 ± 21.37 | <0.001 * | |
| Plantarflexion | 350.05 ± 54.05 | 375.22 ± 55.16 | 0.024 * | ||
| Knee | Angle (°) | Flexion | 24.22 ± 2.44 | 21.62 ± 5.73 | 0.003 * |
| Extension | −15.81 ± 3.09 | −14.84 ± 4.46 | 0.216 | ||
| Moment (Nm/kg) | Flexion | 0.64 ± 0.16 | 0.73 ± 0.15 | 0.006 * | |
| Extension | −1.27 ± 0.29 | −1.02 ± 0.21 | <0.001 * | ||
| Velocity (°/s) | Flexion | 143.92 ± 25.41 | 134.31 ± 17.43 | 0.039 * | |
| Extension | −150.85 ± 28.60 | −136.36 ± 29.03 | 0.016 * | ||
| Hip | Angle (°) | Flexion | −42.82 ± 3.96 | −38.15 ± 5.60 | <0.001 * |
| Extension | −7.18 ± 3.97 | −3.79 ± 2.67 | <0.001 * | ||
| Moment (Nm/kg) | Flexion | −0.37 ± 0.12 | −0.23 ± 0.09 | <0.001 * | |
| Extension | 0.51 ± 0.17 | 0.59 ± 0.15 | 0.017 * | ||
| Velocity (°/s) | Flexion | −85.65 ± 25.09 | −75.74 ± 22.83 | 0.041 * | |
| Extension | 365.32 ± 39.26 | 350.76 ± 40.57 | 0.026 * |
| Joint | Parameters | Peak Value | Dancer Mean ± SD | Non-Dancer Mean ± SD | p-Value |
|---|---|---|---|---|---|
| Ankle | Angle (°) | Eversion | −14.13 ± 6.07 | −11.06 ± 3.63 | 0.16 * |
| Inversion | 1.22 ± 4.90 | 1.19 ± 3.16 | 0.97 | ||
| Moment (Nm/kg) | Eversion | −0.29 ± 0.09 | −0.30 ± 0.09 | 0.71 | |
| Inversion | 0.03 ± 0.03 | 0.02 ± 0.02 | 0.14 * | ||
| Velocity (°/s) | Eversion | −170.49 ± 46.36 | −135.36 ± 51.84 | 0.53 | |
| Inversion | 130.11 ± 69.49 | 102.06 ± 30.66 | 0.06 | ||
| Knee | Angle (°) | Abduction | −1.64 ± 1.36 | −1.75 ± 1.00 | 0.703 |
| Adduction | 5.17 ± 1.49 | 4.03 ± 2.13 | 0.014 * | ||
| Moment (Nm/kg) | Abduction | −0.68 ± 0.13 | −0.64 ± 0.17 | 0.165 | |
| Adduction | 0.11 ± 0.09 | 0.10 ± 0.10 | 0.761 | ||
| Velocity (°) | Abduction | −61.76 ± 30.49 | −67.97 ± 28.56 | 0.340 | |
| Adduction | 86.72 ± 26.61 | 72.83 ± 29.02 | 0.026 * | ||
| Hip | Angle (°) | Abduction | 13.00 ± 2.08 | 10.27 ± 1.44 | <0.001 * |
| Adduction | −3.43 ± 2.41 | −0.82 ± 1.92 | <0.001 * | ||
| Moment (Nm/kg) | Abduction | 0.27 ± 0.13 | 0.24 ± 0.18 | 0.237 | |
| Adduction | −1.42 ± 0.20 | −1.45 ± 0.20 | 0.380 | ||
| Velocity (°) | Abduction | 153.36 ± 31.50 | 128.50 ± 18.64 | <0.001 * | |
| Adduction | −107.99 ± 21.65 | −95.60 ± 27.90 | 0.034 * |
| Joint | Parameters | Peak Value | Dancer Mean ± SD | Non-Dancer Mean ± SD | p-Value |
|---|---|---|---|---|---|
| Ankle | Angle (°) | External rotation | −7.92 ± 3.43 | −10.39 ± 3.19 | 0.001 * |
| Internal rotation | 13.62 ± 4.59 | 7.23 ± 4.34 | <0.001 * | ||
| Moment (Nm/kg) | External rotation | −0.28 ± 0.05 | −0.19 ± 0.05 | <0.001 * | |
| Internal rotation | 0.02 ± 0.10 | 0.23 ± 0.14 | 0.003 * | ||
| Velocity (°/s) | External rotation | −196.33 ± 66.33 | −221.41 ± 67.81 | 0.065 | |
| Internal rotation | 190.81 ± 66.01 | 154.27 ± 44.27 | 0.002 * | ||
| Knee | Angle (°) | External rotation | −8.42 ± 3.18 | −11.10 ± 4.45 | 0.004 * |
| Internal rotation | 5.69 ± 4.42 | 1.94 ± 3.78 | <0.001 * | ||
| Moment (Nm/kg) | External rotation | −0.14 ± 0.04 | −0.11 ± 0.34 | <0.001 * | |
| Internal rotation | 0.26 ± 0.08 | 0.21 ± 0.06 | <0.001 * | ||
| Velocity (°/s) | External rotation | −173.73 ± 57.28 | −168.32 ± 33.60 | 0.612 | |
| Internal rotation | 175.26 ± 54.77 | 215.69 ± 57.82 | 0.001 * | ||
| Hip | Angle (°) | External rotation | −0.63 ± 2.43 | −4.81 ± 1.46 | <0.001 * |
| Internal rotation | 11.77 ± 3.70 | 5.21 ± 2.28 | <0.001 * | ||
| Moment (Nm/kg) | External rotation | −0.71 ± 0.25 | −0.45 ± 0.14 | <0.001 * | |
| Internal rotation | 0.18 ± 0.11 | 0.15 ± 0.09 | 0.155 | ||
| Velocity (°/s) | External rotation | 160.27 ± 61.25 | 105.98 ± 31.50 | <0.001 * | |
| Internal rotation | −107.65 ± 36.74 | −68.44 ± 28.05 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Xu, D.; Li, F.; Baker, J.S.; Li, J.; Gu, Y. Biomechanical Analysis of Latin Dancers’ Lower Limb during Normal Walking. Bioengineering 2023, 10, 1128. https://doi.org/10.3390/bioengineering10101128
Gao X, Xu D, Li F, Baker JS, Li J, Gu Y. Biomechanical Analysis of Latin Dancers’ Lower Limb during Normal Walking. Bioengineering. 2023; 10(10):1128. https://doi.org/10.3390/bioengineering10101128
Chicago/Turabian StyleGao, Xiangli, Datao Xu, Fengfeng Li, Julien S. Baker, Jiao Li, and Yaodong Gu. 2023. "Biomechanical Analysis of Latin Dancers’ Lower Limb during Normal Walking" Bioengineering 10, no. 10: 1128. https://doi.org/10.3390/bioengineering10101128
APA StyleGao, X., Xu, D., Li, F., Baker, J. S., Li, J., & Gu, Y. (2023). Biomechanical Analysis of Latin Dancers’ Lower Limb during Normal Walking. Bioengineering, 10(10), 1128. https://doi.org/10.3390/bioengineering10101128








