Limitations of Reconstructing Pentacam Rabbit Corneal Tomography by Zernike Polynomials
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Subjects
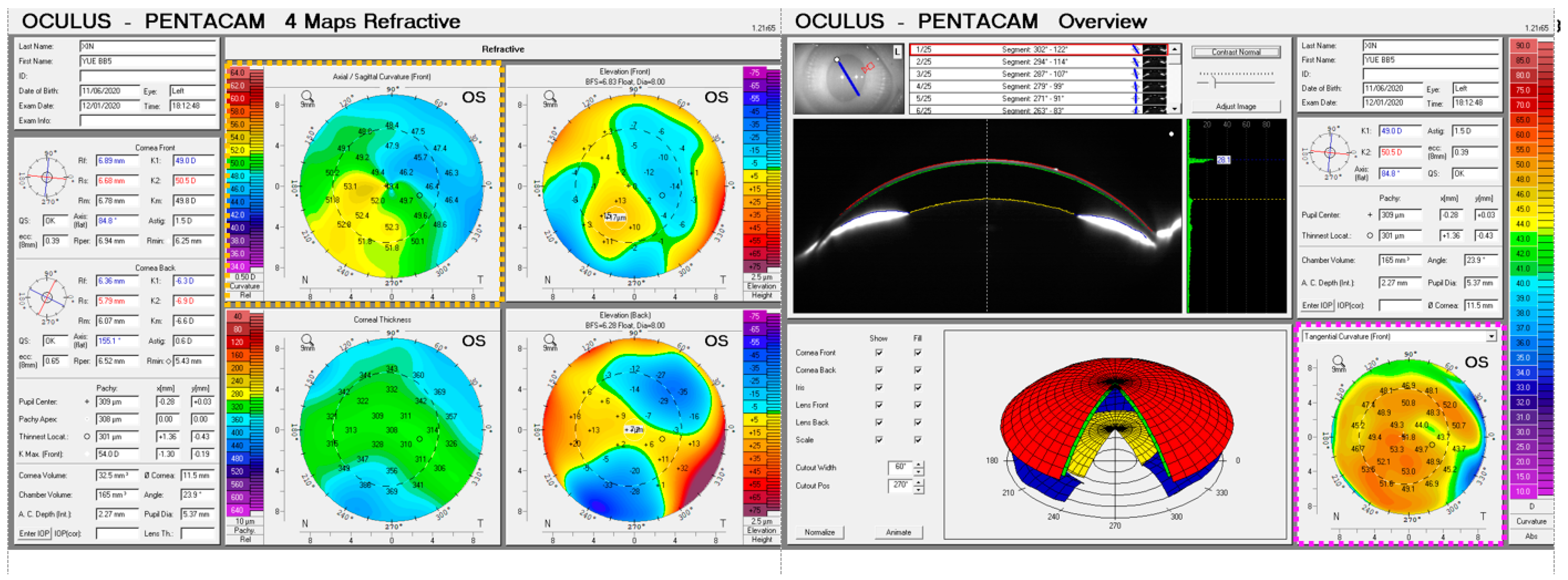
2.2. Data Collection
2.3. Corneal Surface Fitting
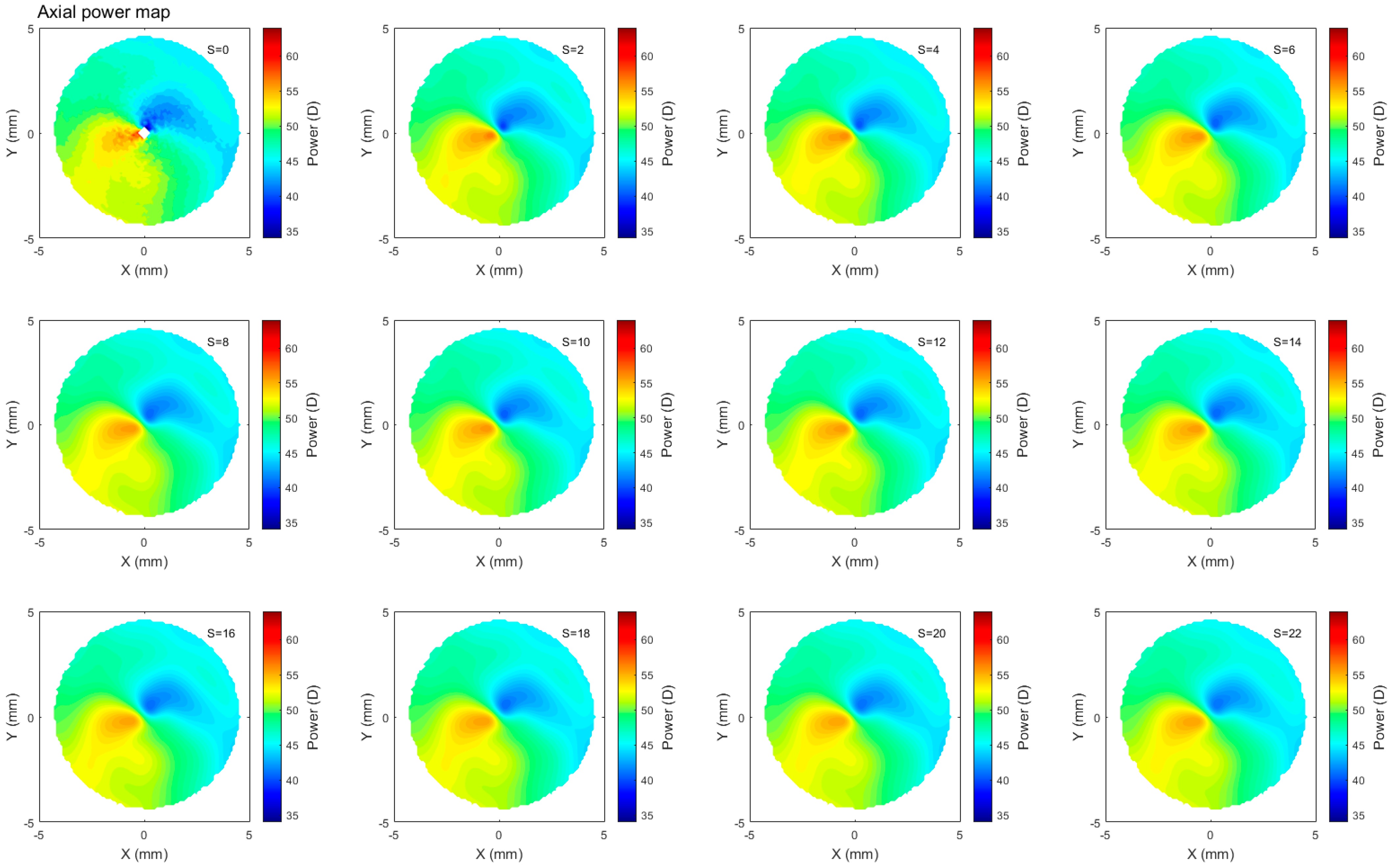
2.4. Corneal Refractive Power Estimates
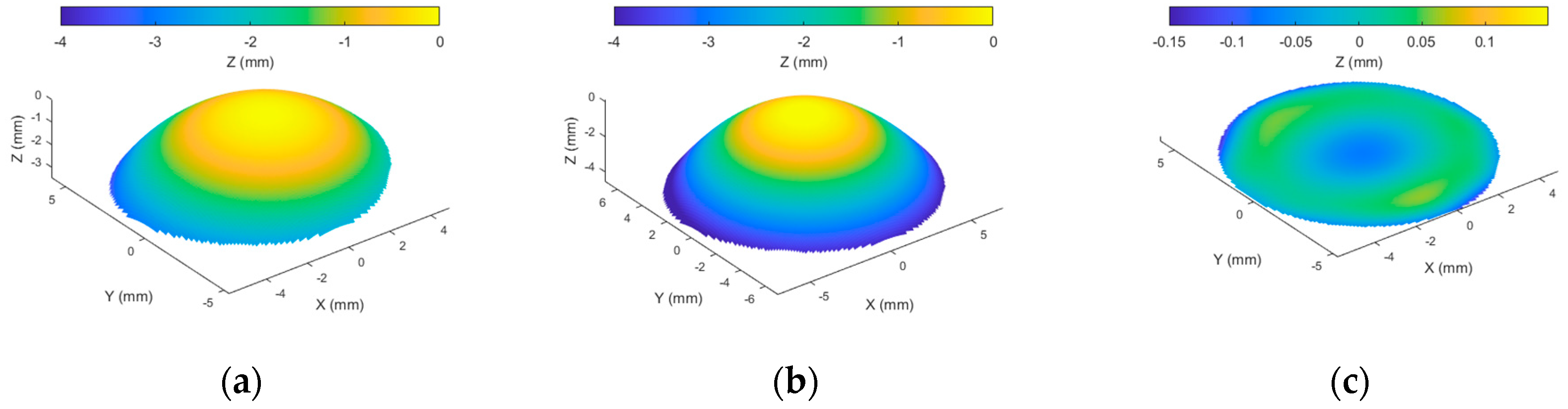
2.5. Smoothing
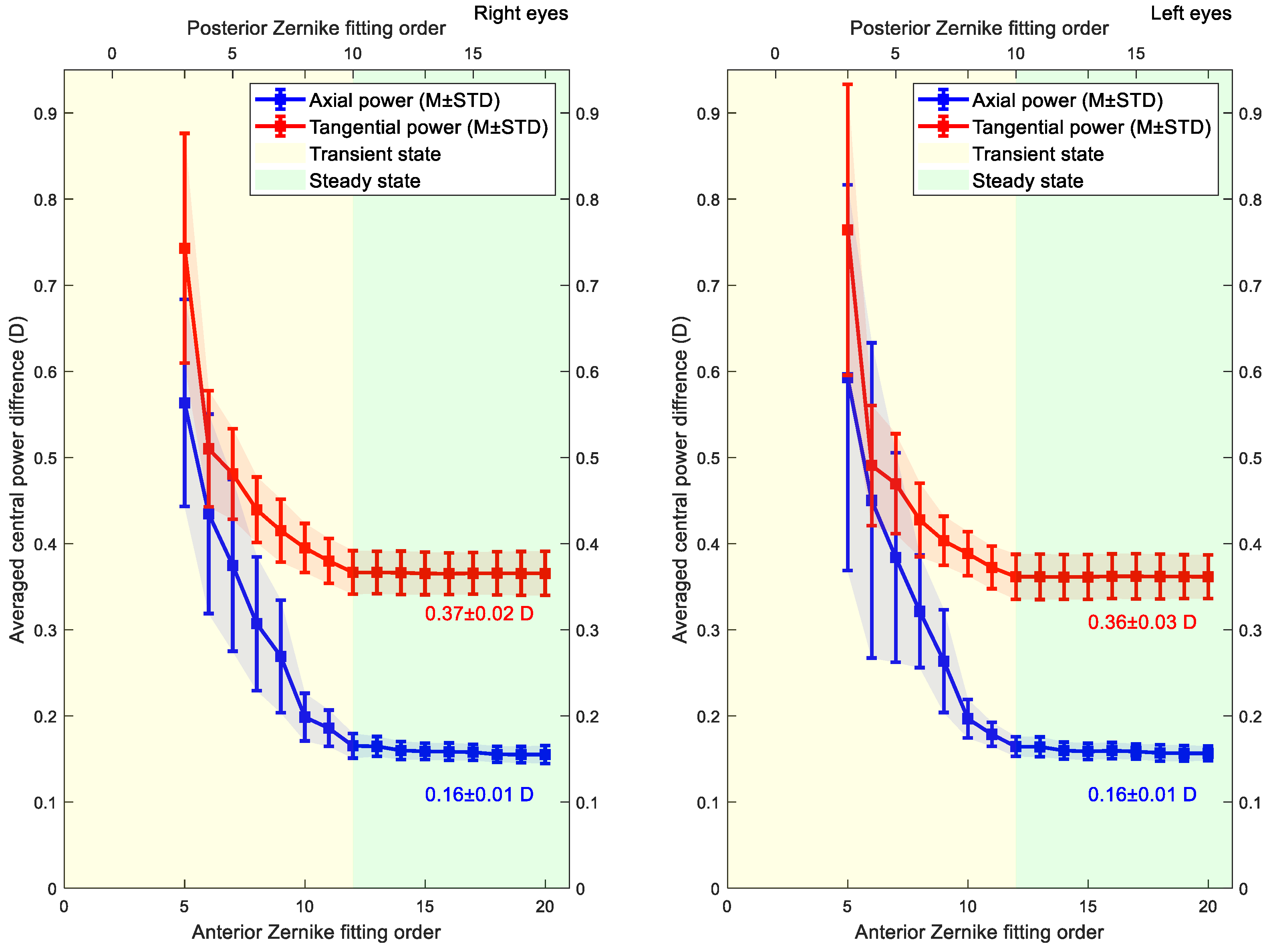
3. Statistical Analysis
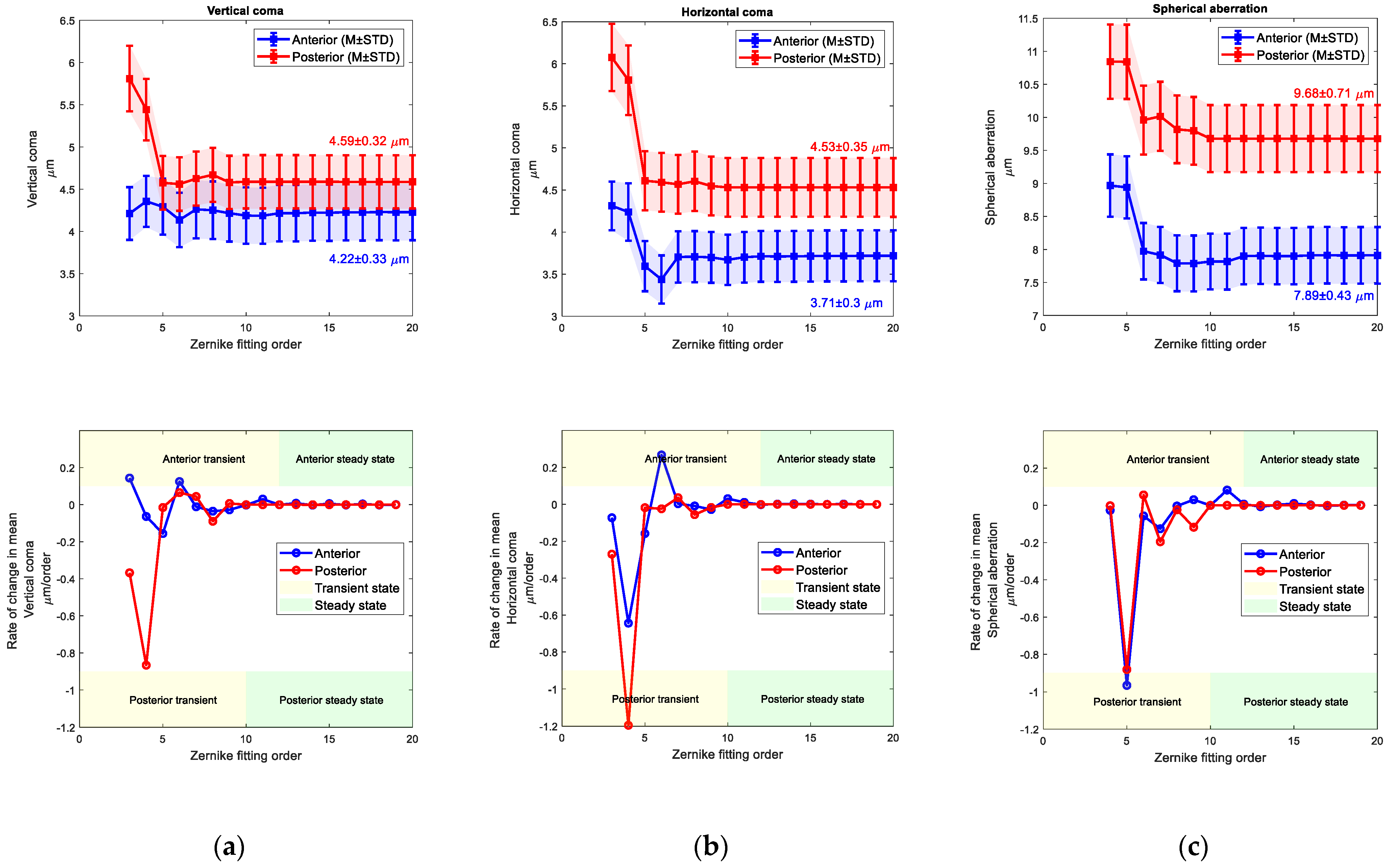
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ceylan, O.M.; Turk, A.; Erdurman, C.; Mumcuoglu, T.; Erdem, U.; Gokce, G.; Dagli, S. Comparison of Oculus Pentacam and Stratus Optical Coherence Tomography for Measurement of Central Corneal Thickness. Cornea 2011, 30, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, M.; Heidari, Z. Pentacam. In Diagnostics in Ocular Imaging: Cornea, Retina, Glaucoma and Orbit; Mohammadpour, M., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 65–162. [Google Scholar] [CrossRef]
- Fan, R.; Chan, T.C.; Prakash, G.; Jhanji, V. Applications of corneal topography and tomography: A review. Clin. Exp. Ophthalmol. 2018, 46, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lopes, B.T.; Eliasy, A.; Wu, R.; Fathy, A.; Elsheikh, A.; Abass, A. Performance of Zernike polynomials in reconstructing raw-elevation data captured by Pentacam HR, Medmont E300 and Eye Surface Profiler. Heliyon 2021, 7, e08623. [Google Scholar] [CrossRef] [PubMed]
- Zernike, V.F. Beugungstheorie des schneidenver-fahrens und seiner verbesserten form, der phasenkontrastmethode. Physica 1934, 1, 689–704. [Google Scholar] [CrossRef]
- Harvey, H.B.; Sotardi, S.T. The Pareto Principle. J. Am. Coll. Radiol. 2018, 15, 931. [Google Scholar] [CrossRef]
- Fathy, A.; Lopes, B.T.; Ambrósio, R.; Wu, R.; Abass, A. The Efficiency of Using Mirror Imaged Topography in Fellow Eyes Analyses of Pentacam HR Data. Symmetry 2021, 13, 2132. [Google Scholar] [CrossRef]
- Consejo, A.; Fathy, A.; Lopes, B.T.; Ambrósio, R.; Abass, A. Effect of Corneal Tilt on the Determination of Asphericity. Sensors 2021, 21, 7636. [Google Scholar] [CrossRef]
- Kang, M.-J.; Byun, Y.-S.; Yoo, Y.-S.; Whang, W.-J.; Joo, C.-K. Long-term outcome of intrastromal corneal ring segments in keratoconus: Five-year follow up. Sci. Rep. 2019, 9, 315. [Google Scholar] [CrossRef]
- de Sanctis, U.; Vinai, L.; Bartoli, E.; Donna, P.; Grignolo, F. Total spherical aberration of the cornea in patients with cataract. Optom. Vis. Sci. 2014, 91, 1251–1258. [Google Scholar] [CrossRef]
- Olsen, T. On the calculation of power from curvature of the cornea. Br. J. Ophthalmol. 1986, 70, 152–154. [Google Scholar] [CrossRef]
- Ho, J.-D.; Tsai, C.-Y.; Tsai, R.J.-F.; Kuo, L.-L.; Tsai, I.L.; Liou, S.-W. Validity of the keratometric index: Evaluation by the Pentacam rotating Scheimpflug camera. J. Cataract Refract. Surg. 2008, 34, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Smit, G.; Atchison, D.A. The Eye and Visual Optical Instruments; Cambridge University Press: Cambridge, UK, 1970. [Google Scholar]
- Vojnikovi, B.o.; Tamajo, E. Gullstrand’s Optical Schematic System of the Eye Modified by Vojnikovi & Tamajo. Coll. Antropol. 2013, 37, 41–45. [Google Scholar]
- KLEIN, S.A. Axial Curvature and the Skew Ray Error in Corneal Topography. Optom. Vis. Sci. 1997, 74, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.L.; Kruse, J.A. Clinical Methods: The History, Physical. JAMA 1990, 264, 2808–2809. [Google Scholar]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. Clinical Methods: The History, Physical, and Laboratory Examinations; Butterworths: Portland, OR, USA, 1990. [Google Scholar]
- Garcia, D. Robust smoothing of gridded data in one and higher dimensions with missing values. Comput. Stat. Data Anal. 2010, 54, 1167–1178. [Google Scholar] [CrossRef]
- Abass, A.; Clamp, J.; Bao, F.; Ambrosio, R., Jr.; Elsheikh, A. Non-Orthogonal Corneal Astigmatism among Normal and Keratoconic Brazilian and Chinese populations. Curr. Eye Res. 2018, 43, 717–724. [Google Scholar] [CrossRef]
- Doll, T.; Moore, J.; Shihab, A.H.; Lopes, B.T.; Eliasy, A.; Maklad, O.; Wu, R.; White, L.; Jones, S.; Elsheikh, A.; et al. Which feature influences on-eye power change of soft toric contact lenses: Design or corneal shape? PLoS ONE 2020, 15, e0242243. [Google Scholar] [CrossRef]
- Everitt, B.S.; Skrondal, A. The Cambridge Dictionary of Statistics, 4th ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Marcos, S. Aberrometry: Basic science and clinical applications. Bull. Soc. Belg. Ophtalmol. 2006, 3, 197–213. [Google Scholar]
- Yüksel, H.; Türkcü, F.M.; Ari, Ş.; Çinar, Y.; Cingü, A.K.; Şahin, M.; Şahin, A.; Özkurt, Z.; Çaça, İ. Anterior segment parameters of rabbits with rotating Scheimpflug camera. Vet. Ophthalmol. 2015, 18, 210–213. [Google Scholar] [CrossRef]
- Robinson, M.L. Animal Models in Eye Research. Hum. Genom. 2009, 3, 381–382. [Google Scholar] [CrossRef]
- Kim, E.; Ehrmann, K.; Choo, J.; Franz, S.; Moilanen, J. The Effect of Inlay Implantation on Corneal Thickness and Radius of Curvature in Rabbit Eyes. Cornea 2013, 32, e106-12. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.P.; Vaishnavi, K.S.; Ojha, S.K.; Singh, V.; Basu, S. A reliable animal model of corneal stromal opacity: Development and validation using in vivo imaging. Ocul. Surf. 2020, 18, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Peyman, G.A.; Badaro, R.M.; Khoobehi, B. Corneal ablation in rabbits using an infrared (2.9-microns) erbium: YAG laser. Ophthalmology 1989, 96, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Pallikaris, I.G.; Papatzanaki, M.E.; Stathi, E.Z.; Frenschock, O.; Georgiadis, A. Laser in situ keratomileusis. Lasers Surg. Med. 1990, 10, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Maloney, R.K. Diffuse lamellar keratitis: A new syndrome in lamellar refractive surgery11The authors have no proprietary interest related to this article. Ophthalmology 1998, 105, 1721–1726. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Chu, R.-Y.; Zhou, X.-T.; Dai, J.-H.; Sun, X.-H.; Hoffman, M.R.; Zhang, X.-R. Morphologic and Histopathologic Changes in the Rabbit Cornea Produced by Femtosecond Laser–Assisted Multilayer Intrastromal Ablation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Nishi, O.; Hara, T.; Hara, T.; Sakka, Y.; Hayashi, F.; Nakamae, K.; Yamada, Y. Refilling the lens with a inflatable endocapsular balloon: Surgical procedure in animal eyes. Graefes Arch. Clin. Exp. Ophthalmol. 1992, 230, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Khorin, P.; Ilyasova, N.; Paringer, R.A. Informative feature selection based on the Zernike polynomial coefficients for various pathologies of the human eye cornea. Comput. Opt. 2018, 42, 159–166. [Google Scholar] [CrossRef]
- McAlinden, C.; Schwiegerling, J.; Khadka, J.; Pesudovs, K. Corneal aberrations measured with a high-resolution Scheimpflug tomographer: Repeatability and reproducibility. J. Cataract Refract. Surg. 2020, 46, 581–590. [Google Scholar] [CrossRef]
- Schröder, S.; Mäurer, S.; Eppig, T.; Seitz, B.; Rubly, K.; Langenbucher, A. Comparison of Corneal Tomography: Repeatability, Precision, Misalignment, Mean Elevation, and Mean Pachymetry. Curr. Eye Res. 2018, 43, 709–716. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.C.; Gray, B.; Chernyak, D.A. Comparison of wavefront aberrations in rabbit and human eyes. Clin. Exp. Optom. 2014, 97, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Bouazizi, H.; Brunette, I.; Meunier, J. Are There Categories of Corneal Shapes? In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2719–2723. [Google Scholar]
- Read, S.A.; Collins, M.J.; Carney, L.G.; Franklin, R.J. The topography of the central and peripheral cornea. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.; Morelande, M.R.; Iskander, D.R.; Collins, M.J.; Davis, B.A. Combining Central and Peripheral Videokeratoscope Maps to Investigate Total Corneal Topography. Eye Contact Lens Sci. Clin. Pract. 2006, 32, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.J.; Gokul, A.; Vellara, H.R.; Prime, Z.; McGhee, C.N.J. Repeatability and Agreement of Orbscan II, Pentacam HR, and Galilei Tomography Systems in Corneas with Keratoconus. Am. J. Ophthalmol. 2017, 175, 122–128. [Google Scholar] [CrossRef]
- Kosekahya, P.; Koc, M.; Caglayan, M.; Kiziltoprak, H.; Atilgan, C.U.; Yilmazbas, P. Repeatability and reliability of ectasia display and topometric indices with the Scheimpflug system in normal and keratoconic eyes. J. Cataract Refract. Surg. 2018, 44, 63–70. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baraya, M.; Moore, J.; Lopes, B.T.; Wu, R.; Bao, F.; Zheng, X.; Consejo, A.; Abass, A. Limitations of Reconstructing Pentacam Rabbit Corneal Tomography by Zernike Polynomials. Bioengineering 2023, 10, 39. https://doi.org/10.3390/bioengineering10010039
Baraya M, Moore J, Lopes BT, Wu R, Bao F, Zheng X, Consejo A, Abass A. Limitations of Reconstructing Pentacam Rabbit Corneal Tomography by Zernike Polynomials. Bioengineering. 2023; 10(1):39. https://doi.org/10.3390/bioengineering10010039
Chicago/Turabian StyleBaraya, Mohamed, Jessica Moore, Bernardo T. Lopes, Richard Wu, FangJun Bao, XiaoBo Zheng, Alejandra Consejo, and Ahmed Abass. 2023. "Limitations of Reconstructing Pentacam Rabbit Corneal Tomography by Zernike Polynomials" Bioengineering 10, no. 1: 39. https://doi.org/10.3390/bioengineering10010039
APA StyleBaraya, M., Moore, J., Lopes, B. T., Wu, R., Bao, F., Zheng, X., Consejo, A., & Abass, A. (2023). Limitations of Reconstructing Pentacam Rabbit Corneal Tomography by Zernike Polynomials. Bioengineering, 10(1), 39. https://doi.org/10.3390/bioengineering10010039








