Recent Advances in Phage-Based Therapeutics for Multi-Drug Resistant Acinetobacter baumannii
Abstract
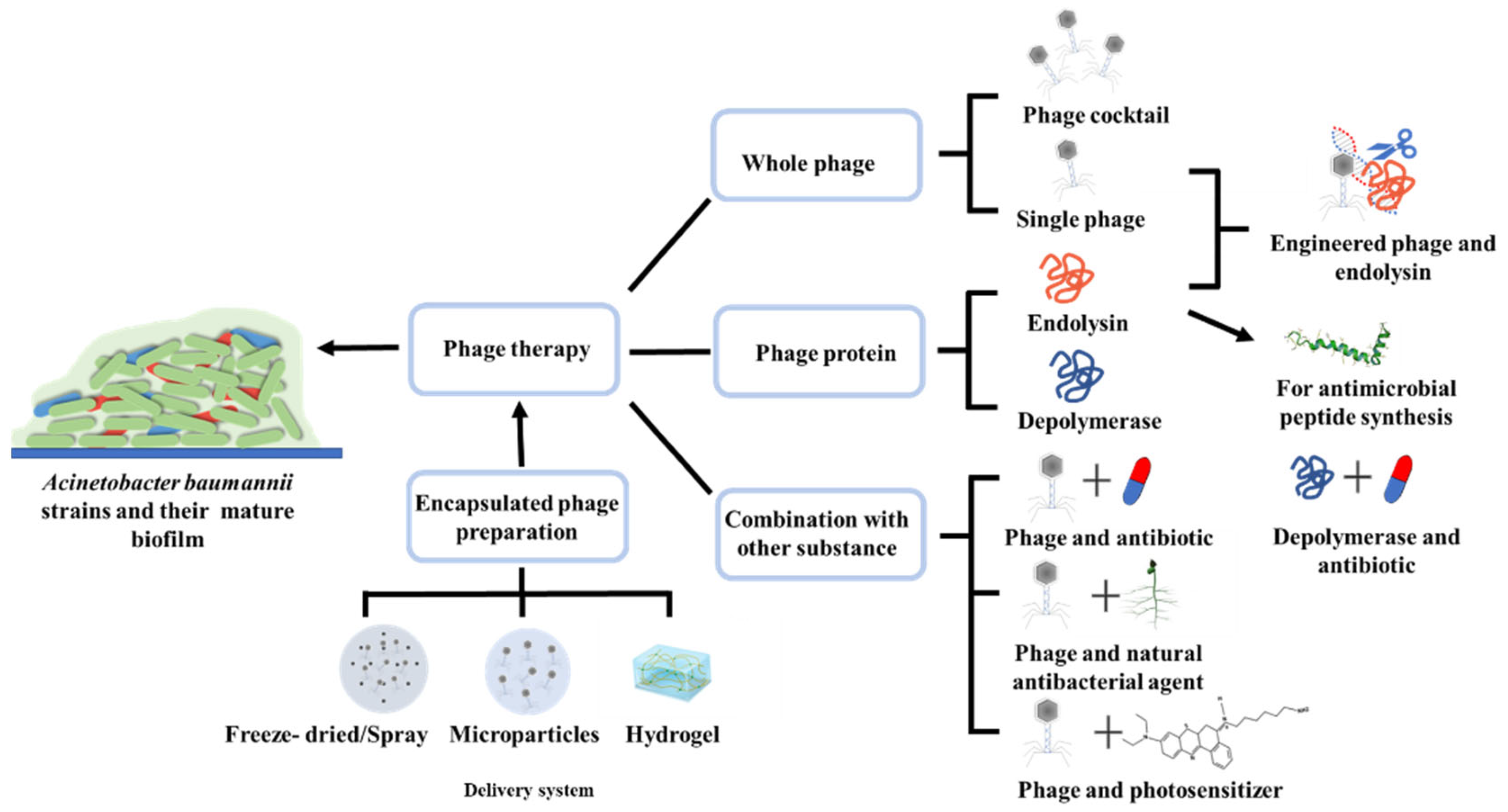
1. Introduction
2. Routine Phage-Based Therapy
2.1. Whole Phage and Phage Cocktail
2.2. Endolysins
2.3. Depolymerases
3. Combination of Phage Therapy and Other Substances
3.1. Phages in Combination with Antibiotics
3.2. Phages in Combination with Natural Antibacterial Agents
3.3. Phages in Combination with Photosensitizer
4. Engineered Phages and Endolysins
5. Delivery and Encapsulated Phage Preparation
6. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Kumar, A. Bacteriophage therapeutics to confront multidrug-resistant Acinetobacter baumannii—A global health menace. Environ. Microbiol. Rep. 2021, 14, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Khazaal, S.S.; Al-Saryi, N.; Ibrahim, S.A. Immunomodulation by Acinetobacter baumannii of endotracheal tube biofilm in ventilator-associated pneumonia. Meta Gene 2020, 24, 100672. [Google Scholar] [CrossRef]
- Rahman, M.; Ahmed, S. Prevalence of colistin resistance gene mcr-1 in clinical Isolates Acinetobacter Baumannii from India. Int. J. Infect. Dis. 2020, 101, 81. [Google Scholar] [CrossRef]
- Martins-Sorenson, N.; Snesrud, E.; Xavier, D.E.; Cacci, L.C.; Iavarone, A.T.; McGann, P.; Riley, L.W.; Moreira, B.M. A novel plasmid-encoded mcr-4.3 gene in a colistin-resistant Acinetobacter baumannii clinical strain. J. Antimicrob. Chemother. 2020, 75, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, F.L.G.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar]
- Tamma, P.D.; Suh, G.A. Phage Are All the Rage: Bacteriophage in Clinical Practice. J. Pediatr. Inf. Dis. Soc. 2021, 10, 749–753. [Google Scholar] [CrossRef]
- Wei, J.W.; Peng, N.; Liang, Y.X.; Li, K.K.; Li, Y.J. Phage Therapy: Consider the Past, Embrace the Future. Appl. Sci. 2020, 10, 7654. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Amirmozafari, N.; Shariati, A.; Hallajzadeh, M.; Mirkalantari, S.; Khoshbayan, A.; Jazi, F.M. How Phages Overcome the Challenges of Drug Resistant Bacteria in Clinical Infections. Infect. Drug Resist. 2020, 13, 45–61. [Google Scholar] [CrossRef]
- Lai, W.C.B.; Chen, X.; Ho, M.K.Y.; Xia, J.; Leung, S.S.Y. Bacteriophage-derived endolysins to target gram-negative bacteria. Int. J. Pharm. 2020, 589, 119833. [Google Scholar] [CrossRef]
- Liu, Y.; Leung, S.S.Y.; Guo, Y.; Zhao, L.; Jiang, N.; Mi, L.; Li, P.; Wang, C.; Qin, Y.; Mi, Z.; et al. The Capsule Depolymerase Dpo48 Rescues Galleria mellonella and Mice from Acinetobacter baumannii Systemic Infections. Front. Microbiol. 2019, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Drobiazko, A.Y.; Kasimova, A.A.; Evseev, P.V.; Shneider, M.M.; Klimuk, E.I.; Shashkov, A.S.; Dmitrenok, A.S.; Chizhov, A.O.; Slukin, P.V.; Skryabin, Y.P.; et al. Capsule-Targeting Depolymerases Derived from Acinetobacter baumannii Prophage Regions. Int. J. Mol. Sci. 2022, 23, 4971. [Google Scholar] [CrossRef] [PubMed]
- Shahed-Al-Mahmud, M.; Roy, R.; Sugiokto, F.G.; Islam, M.N.; Lin, M.D.; Lin, L.C.; Lin, N.T. Phage phi AB6-Borne Depolymerase Combats Acinetobacter baumannii Biofilm Formation and Infection. Antibiotics 2021, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Kisil, O.V.; Efimenko, T.A.; Gabrielyan, N.I.; Efremenkova, O.V. Development of Antimicrobial Therapy Methods to Overcome the Antibiotic Resistance of Acinetobacter baumannii. Acta Nat. 2020, 12, 34–45. [Google Scholar] [CrossRef]
- Baginska, N.; Cieslik, M.; Gorski, A.; Jonczyk-Matysiak, E. The Role of Antibiotic Resistant A. baumannii in the Pathogenesis of Urinary Tract Infection and the Potential of Its Treatment with the Use of Bacteriophage Therapy. Antibiotics 2021, 10, 281. [Google Scholar] [CrossRef]
- El Haddad, L.; Harb, C.P.; Gebara, M.A.; Stibich, M.A.; Chemaly, R.F. A Systematic and Critical Review of Bacteriophage Therapy against Multidrug-resistant ESKAPE Organisms in Humans. Clin. Infect. Dis. 2019, 69, 167–178. [Google Scholar] [CrossRef]
- Sisakhtpour, B.; Mirzaei, A.; Karbasizadeh, V.; Hosseini, N.; Shabani, M.; Moghim, S. The characteristic and potential therapeutic effect of isolated multidrug-resistant Acinetobacter baumannii lytic phage. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 1. [Google Scholar] [CrossRef]
- Torabi, L.R.; Doudi, M.; Naghavi, N.S.; Monajemi, R. Isolation, characterization, and effectiveness of bacteriophage P Phi-Bw-Ab against XDR Acinetobacter baumannii isolated from nosocomial burn wound infection. Iran. J. Basic Med. Sci. 2021, 24, 1254–1263. [Google Scholar] [CrossRef]
- Xu, J.Z.; Li, X.B.; Kang, G.B.; Bai, L.; Wang, P.; Huang, H. Isolation and Characterization of AbTJ, an Acinetobacter baumannii Phage, and Functional Identification of Its Receptor-Binding Modules. Viruses 2020, 12, 205. [Google Scholar] [CrossRef]
- Asif, M.; Alvi, I.A.; Tabassum, R.; Rehman, S.U. TAC1, an unclassified bacteriophage of the family Myoviridae infecting Acinetobacter baumannii with a large burst size and a short latent period. Arch. Virol. 2020, 165, 419–424. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Yong, D. Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Kuo, C.F.; Yeh, C.M.; Chen, J.R.; Cheng, M.F.; Hung, C.H. Efficacy of φkm18p phage therapy in a murine model of extensively drug-resistant Acinetobacter baumannii infection. Infect. Drug Resist. 2018, 11, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hernandez-Morales, A.; Clark, J.; Le, T.; Biswas, B.; Bishop-Lilly, K.A.; Henry, M.; Quinones, J.; Voegtly, L.J.; Cer, R.Z.; et al. Comparative genomics of Acinetobacter baumannii and therapeutic bacteriophages from a patient undergoing phage therapy. Nat. Commun. 2022, 13, 3776. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Y.; Wang, L.L.; Li, X.Y.; Tan, D.M.; Cong, C.; Xu, Y.P. Efficacy of a phage cocktail in controlling phage resistance development in multidrug resistant Acinetobacter baumannii. Virus Res. 2019, 272, 197734. [Google Scholar] [CrossRef]
- Regeimbal, J.M.; Jacobs, A.C.; Corey, B.W.; Henry, M.S.; Thompson, M.G.; Pavlicek, R.L.; Quinones, J.; Hannah, R.M.; Ghebremedhin, M.; Crane, N.J.; et al. Personalized Therapeutic Cocktail of Wild Environmental Phages Rescues Mice from Acinetobacter baumannii Wound Infections. Antimicrob. Agents Chemother. 2016, 60, 5806–5816. [Google Scholar] [CrossRef]
- Leshkasheli, L.; Kutateladze, M.; Balarjishvili, N.; Bolkvadze, D.; Save, J.; Oechslin, F.; Que, Y.A.; Resch, G. Efficacy of newly isolated and highly potent bacteriophages in a mouse model of extensively drug-resistant Acinetobacter baumannii bacteraemia. J. Glob. Antimicrob. Resist. 2019, 19, 255–261. [Google Scholar] [CrossRef]
- Wu, N.N.; Dai, J.; Guo, M.Q.; Li, J.H.; Zhou, X.; Li, F.; Gao, Y.; Qu, H.P.; Lu, H.Z.; Jin, J.; et al. Pre-optimized phage therapy on secondary Acinetobacter baumannii infection in four critical COVID-19 patients. Emerg. Microbes Infect. 2021, 10, 612–618. [Google Scholar] [CrossRef]
- Patel, S.R.; Pratap, C.B.; Nath, G. Evaluation of bacteriophage cocktail on septicaemia caused by colistin-resistant Acinetobacter baumannii in immunocompromised mice model. Indian J. Med. Res. 2021, 154, 141–149. [Google Scholar] [CrossRef]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts are Easier than the Whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Wang, L.; Li, G.; Cong, C.; Li, R.; Cui, H.; Murtaza, B.; Xu, Y. The endolysin of the Acinetobacter baumannii phage vB_AbaP_D2 shows broad antibacterial activity. Microb. Biotechnol. 2021, 14, 403–418. [Google Scholar] [CrossRef]
- Chu, J.J.K.; Poh, W.H.; Hasnuddin, N.T.B.; Hew, E.Y.; Dam, L.C.; Sahili, A.E.; Rice, S.A.; Goh, B.C. Novel Phage Lysin Abp013 against Acinetobacter baumannii. Antibiotics 2022, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Thiagarajan, V.; Walmagh, M.; Sillankorva, S.; Lavigne, R.; Neves-Petersen, M.T.; Kluskens, L.D.; Azeredo, J. A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS ONE 2014, 9, e108376. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Boas, D.V.; Mesnage, S.; Kluskens, L.D.; Lavigne, R.; Sillankorva, S.; Secundo, F.; Azeredo, J. Structural and Enzymatic Characterization of ABgp46, a Novel Phage Endolysin with Broad Anti-Gram-Negative Bacterial Activity. Front. Microbiol. 2016, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Walmagh, M.; Lavigne, R. Use of bacteriophage endolysin EL188 and outer membrane permeabilizers against Pseudomonas aeruginosa. J. Appl. Microbiol. 2011, 110, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; You, R.I.; Lai, M.J.; Lin, N.T.; Chen, L.K.; Chang, K.C. Highly potent antimicrobial modified peptides derived from the Acinetobacter baumannii phage endolysin LysAB2. Sci. Rep. 2017, 7, 11477. [Google Scholar] [CrossRef] [PubMed]
- Latka, A.; Maciejewska, B.; Majkowska-Skrobek, G.; Briers, Y.; Drulis-Kawa, Z. Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process. Appl. Microbiol. Biotechnol. 2017, 101, 3103–3119. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.Y.; Zhu, Y.; Huang, Y.; Gao, M.M.; Yuan, X.; Niu, W.K.; Liu, H.Y.; Fan, H.; Qin, Y.H.; et al. Identification of a Novel Acinetobacter baumannii Phage-Derived Depolymerase and Its Therapeutic Application in Mice. Front. Microbiol. 2020, 11, 1407. [Google Scholar] [CrossRef]
- Liu, Y.N.; Mi, Z.Q.; Mi, L.Y.; Huang, Y.; Li, P.Y.; Liu, H.Y.; Yuan, X.; Niu, W.K.; Jiang, N.; Bai, C.Q.; et al. Identification and characterization of capsule depolymerase Dpo48 from Acinetobacter baumannii phage IME200. PeerJ 2019, 7, e6173. [Google Scholar] [CrossRef]
- Lee, I.M.; Tu, I.F.; Yang, F.L.; Ko, T.P.; Liao, J.H.; Lin, N.T.; Wu, C.Y.; Ren, C.T.; Wang, A.H.J.; Chang, C.M.; et al. Structural basis for fragmenting the exopolysaccharide of Acinetobacter baumannii by bacteriophage Phi AB6 tailspike protein. Sci. Rep. 2017, 7, 42711. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Roszak, M.; Golec, P.; Śleboda-Taront, D.; Łubowska, N.; Górska, M.; Jursa-Kulesza, J.; Rakoczy, R.; Wojciuk, B.; Dołęgowska, B. Antibiotics Act with vB_AbaP_AGC01 Phage against Acinetobacter baumannii in Human Heat-Inactivated Plasma Blood and Galleria mellonella Models. Int. J. Mol. Sci. 2020, 21, 4390. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Wahida, A.; Latz, S.; Kruttgen, A.; Hafner, H.; Buhl, E.M.; Ritter, K.; Horz, H.P. Enhanced antibacterial effect of the novel T4-like bacteriophage KARL-1 in combination with antibiotics against multi-drug resistant Acinetobacter baumannii. Sci. Rep. 2018, 8, 14140. [Google Scholar] [CrossRef] [PubMed]
- Blasco, L.; Ambroa, A.; Lopez, M.; Fernandez-Garcia, L.; Bleriot, I.; Trastoy, R.; Ramos-Vivas, J.; Coenye, T.; Fernandez-Cuenca, F.; Vila, J.; et al. Combined Use of the Ab105-2φΔCI Lytic Mutant Phage and Different Antibiotics in Clinical Isolates of Multi-Resistant Acinetobacter baumannii. Microorganisms 2019, 7, 556. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.H.; Hsu, Y.H.; Wu, W.J.; Chang, K.C.; Yeh, C.S. Phage Digestion of a Bacterial Capsule Imparts Resistance to Two Antibiotic Agents. Microorganisms 2021, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, M.; Zhang, P.; Xu, M.; Yuan, W.; Bian, L.; Liu, Y.; Xia, J.; Leung, S.S.Y. Phage-Derived Depolymerase as an Antibiotic Adjuvant Against Multidrug-Resistant Acinetobacter baumannii. Front. Microbiol. 2022, 13, 845500. [Google Scholar] [CrossRef]
- Wang, X.; Loh, B.; Altamirano, F.G.; Yu, Y.; Hua, X.; Leptihn, S. Colistin-phage combinations decrease antibiotic resistance in A. baumannii via changes in envelope architecture. Emerg. Microbes Infect. 2021, 10, 2205–2219. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Kostoulias, X.; Subedi, D.; Korneev, D.; Peleg, A.Y.; Barr, J.J. Phage-antibiotic combination is a superior treatment against Acinetobacter baumannii in a preclinical study. eBiomedicine 2022, 80, 104045. [Google Scholar] [CrossRef]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-Derived Products as Antibacterial and Antifungal Agents in Human Health Care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef]
- Wintachai, P.; Voravuthikunchai, S.P. Characterization of Novel Lytic Myoviridae Phage Infecting Multidrug-Resistant Acinetobacter baumannii and Synergistic Antimicrobial Efficacy between Phage and Sacha Inchi Oil. Pharmaceuticals 2022, 15, 291. [Google Scholar] [CrossRef]
- Gonzalez-Aspajo, G.; Belkhelfa, H.; Haddioui-Hbabi, L.; Bourdy, G.; Deharo, E. Sacha Inchi Oil (Plukenetia volubilis L.), effect on adherence of Staphylococus aureus to human skin explant and keratinocytes in vitro. J. Ethnopharmacol. 2015, 171, 330–334. [Google Scholar] [CrossRef]
- Ran, B.; Yuan, Y.Y.; Xia, W.X.; Li, M.L.; Yao, Q.C.; Wang, Z.K.; Wang, L.L.; Li, X.Y.; Xu, Y.P.; Peng, X.J. A photo-sensitizable phage for multidrug-resistant Acinetobacter baumannii therapy and biofilm ablation. Chem. Sci. 2021, 12, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xia, J.; Tian, R.; Wang, J.; Fan, J.; Du, J.; Long, S.; Song, X.; Foley, J.W.; Peng, X. Near-Infrared Light-Initiated Molecular Superoxide Radical Generator: Rejuvenating Photodynamic Therapy against Hypoxic Tumors. J. Am. Chem. Soc. 2018, 140, 14851–14859. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shao, Y.; Kim, J.H.; Pu, Z.; Zhao, X.; Huang, H.; Xiong, T.; Kang, Y.; Li, G.; Shao, K.; et al. Unimolecular Photodynamic O(2)-Economizer To Overcome Hypoxia Resistance in Phototherapeutics. J. Am. Chem. Soc. 2020, 142, 5380–5388. [Google Scholar] [CrossRef] [PubMed]
- Al-Shayeb, B.; Sachdeva, R.; Chen, L.X.; Ward, F.; Munk, P.; Devoto, A.; Castelle, C.J.; Olm, M.R.; Bouma-Gregson, K.; Amano, Y.; et al. Clades of huge phages from across Earth’s ecosystems. Nature 2020, 578, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, I.A. Phage engineering and the evolutionary arms race. Curr. Opin. Biotechnol. 2021, 68, 23–29. [Google Scholar] [CrossRef]
- Łobocka, M.; Dąbrowska, K.; Górski, A. Engineered Bacteriophage Therapeutics: Rationale, Challenges and Future. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2021, 35, 255–280. [Google Scholar] [CrossRef]
- Barnard, A.M.L.; Fairhead, H.I.M. A commentary on the development of engineered phage as therapeutics. Drug Discov. Today 2021, 26, 2095–2098. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.P.; Chen, Y.; Hua, X.T.; Yu, Y.S.; Ji, Q.J. A Highly Efficient CRISPR-Cas9-Based Genome Engineering Platform in Acinetobacter baumannii to Understand the H2O2-Sensing Mechanism of OxyR. Cell Chem. Biol. 2019, 26, 1732–1742.e5. [Google Scholar] [CrossRef]
- He, Y.; Guo, Y. The experimental scheme in editing the phage for Acinetobacter baumannii by CRISPR-Cas9. Bull. Biol. 2020, 55, 50–53. [Google Scholar]
- Abdelkader, K.; Gutiérrez, D.; Tamés-Caunedo, H.; Ruas-Madiedo, P.; Safaan, A.; Khairalla, A.S.; Gaber, Y.; Dishisha, T.; Briers, Y. Engineering a Lysin with Intrinsic Antibacterial Activity (LysMK34) by Cecropin A Fusion Enhances Its Antibacterial Properties against Acinetobacter baumannii. Appl. Environ. Microbiol. 2022, 88, e0151521. [Google Scholar] [CrossRef]
- Pinto, A.M.; Silva, M.D.; Pastrana, L.M.; Banobre-Lopez, M.; Sillankorva, S. The clinical path to deliver encapsulated phages and lysins. FEMS Microbiol. Rev. 2021, 45, fuab019. [Google Scholar] [CrossRef] [PubMed]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Chadha, P.; Katare, O.P.; Chhibber, S. Liposome loaded phage cocktail: Enhanced therapeutic potential in resolving Klebsiella pneumoniae mediated burn wound infections. Burn. J. Int. Soc. Burn. Inj. 2017, 43, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Iszatt, J.J.; Larcombe, A.N.; Chan, H.K.; Stick, S.M.; Garratt, L.W.; Kicic, A. Phage Therapy for Multi-Drug Resistant Respiratory Tract Infections. Viruses 2021, 13, 1809. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Wallin, M.; Lin, Y.; Leung, S.S.Y.; Wang, H.; Morales, S.; Chan, H.K. Phage therapy for respiratory infections. Adv. Drug Deliv. Rev. 2018, 133, 76–86. [Google Scholar] [CrossRef]
- Rao, S.; Betancourt-Garcia, M.; Kare-Opaneye, Y.O.; Swierczewski, B.E.; Bennett, J.W.; Horne, B.; Fackler, J.; Suazo Hernandez, L.P.; Brownstein, M.J. Critically Ill Patient with Multidrug-Resistant Acinetobacter baumannii Respiratory Infection Successfully Treated with Intravenous and Nebulized Bacteriophage Therapy. Antimicrob. Agents Chemother. 2022, 66, e0082421. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Ilomuanya, M.O.; Enwuru, N.V.; Adenokun, E.; Fatunmbi, A.; Adeluola, A.; Igwilo, C.I. Chitosan-Based Microparticle Encapsulated Acinetobacter baumannii Phage Cocktail in Hydrogel Matrix for the Management of Multidrug Resistant Chronic Wound Infection. Turk. J. Pharm. Sci. 2022, 19, 187–195. [Google Scholar] [CrossRef]
- Campos, W.F.; Silva, E.C.; Oliveira, T.J.; Oliveira, J.M., Jr.; Tubino, M.; Pereira, C.; Vila, M.M.; Balcão, V.M. Transdermal permeation of bacteriophage particles by choline oleate: Potential for treatment of soft-tissue infections. Future Microbiol. 2020, 15, 881–896. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Wojciuk, B.; Roszak, M.; Lubowska, N.; Blazejczak, P.; Jursa-Kulesza, J.; Rakoczy, R.; Masiuk, H.; Dolegowska, B. Environmental Phage-Based Cocktail and Antibiotic Combination Effects on Acinetobacter baumannii Biofilm in a Human Urine Model. Microb. Drug Resist. 2021, 27, 25–35. [Google Scholar] [CrossRef]
- Caflisch, K.M.; Suh, G.A.; Patel, R. Biological challenges of phage therapy and proposed solutions: A literature review. Expert Rev. Anti-Infect. Ther. 2019, 17, 1011–1041. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.; Salabarria, A.C.; Edwards, R.A.; Roach, D.R. Standardized bacteriophage purification for personalized phage therapy. Nat. Protoc. 2020, 15, 2867–2890. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Guo, X.; Feng, T.; Li, L. Exploring the whole standard operating procedure for phage therapy in clinical practice. J. Transl. Med. 2019, 17, 373. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Su, J.; Fu, M.; Zhang, H.; Zeng, H. Recent Advances in Phage-Based Therapeutics for Multi-Drug Resistant Acinetobacter baumannii. Bioengineering 2023, 10, 35. https://doi.org/10.3390/bioengineering10010035
Tan Y, Su J, Fu M, Zhang H, Zeng H. Recent Advances in Phage-Based Therapeutics for Multi-Drug Resistant Acinetobacter baumannii. Bioengineering. 2023; 10(1):35. https://doi.org/10.3390/bioengineering10010035
Chicago/Turabian StyleTan, Yujing, Jianhui Su, Minghui Fu, Hongmei Zhang, and Haiyan Zeng. 2023. "Recent Advances in Phage-Based Therapeutics for Multi-Drug Resistant Acinetobacter baumannii" Bioengineering 10, no. 1: 35. https://doi.org/10.3390/bioengineering10010035
APA StyleTan, Y., Su, J., Fu, M., Zhang, H., & Zeng, H. (2023). Recent Advances in Phage-Based Therapeutics for Multi-Drug Resistant Acinetobacter baumannii. Bioengineering, 10(1), 35. https://doi.org/10.3390/bioengineering10010035





