Advancement in the Cuffless and Noninvasive Measurement of Blood Pressure: A Review of the Literature and Open Challenges
Abstract
1. Introduction
1.1. Current Literature Survey
1.2. Survey Goal
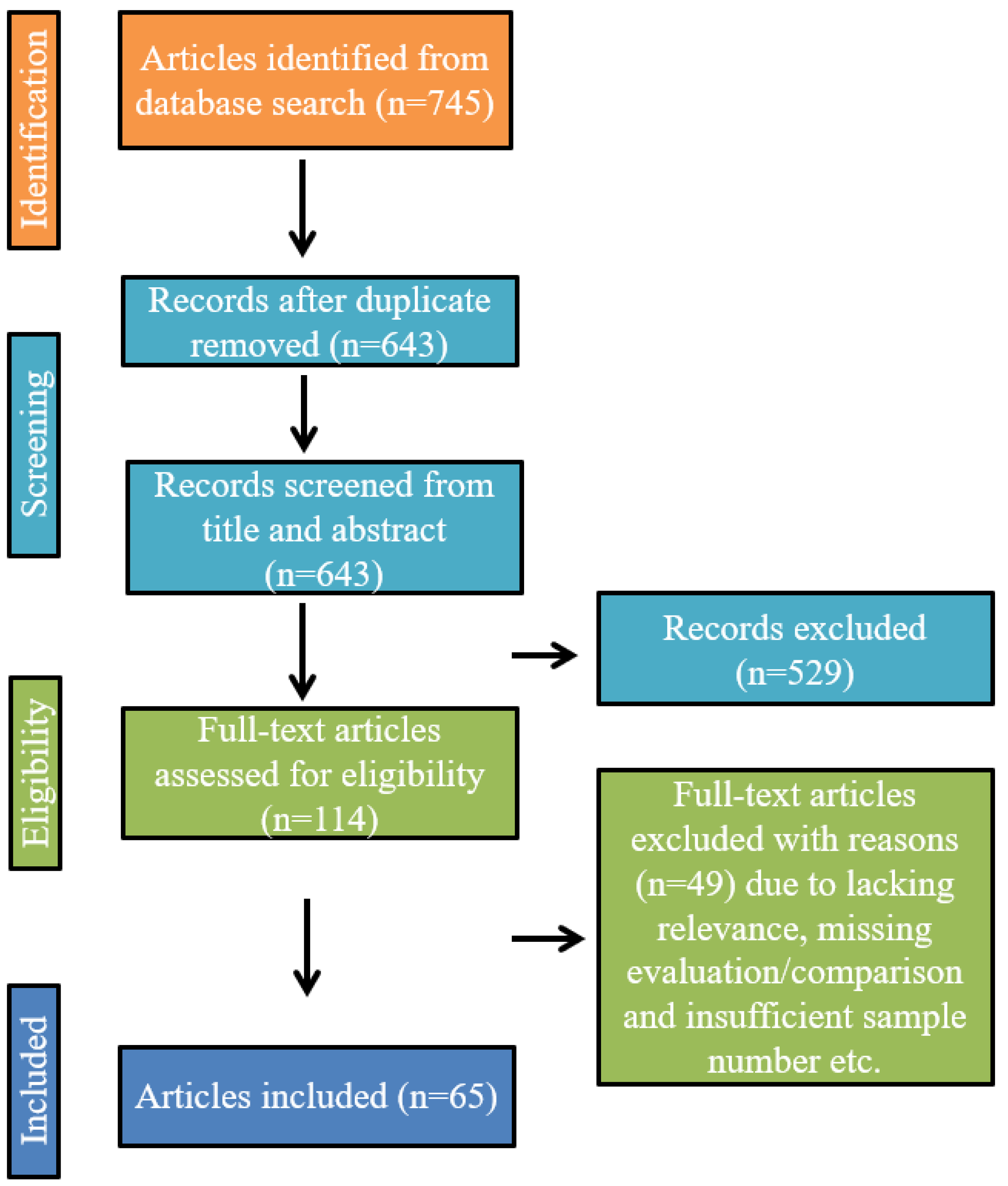
2. Framework for the Search and Selection Process
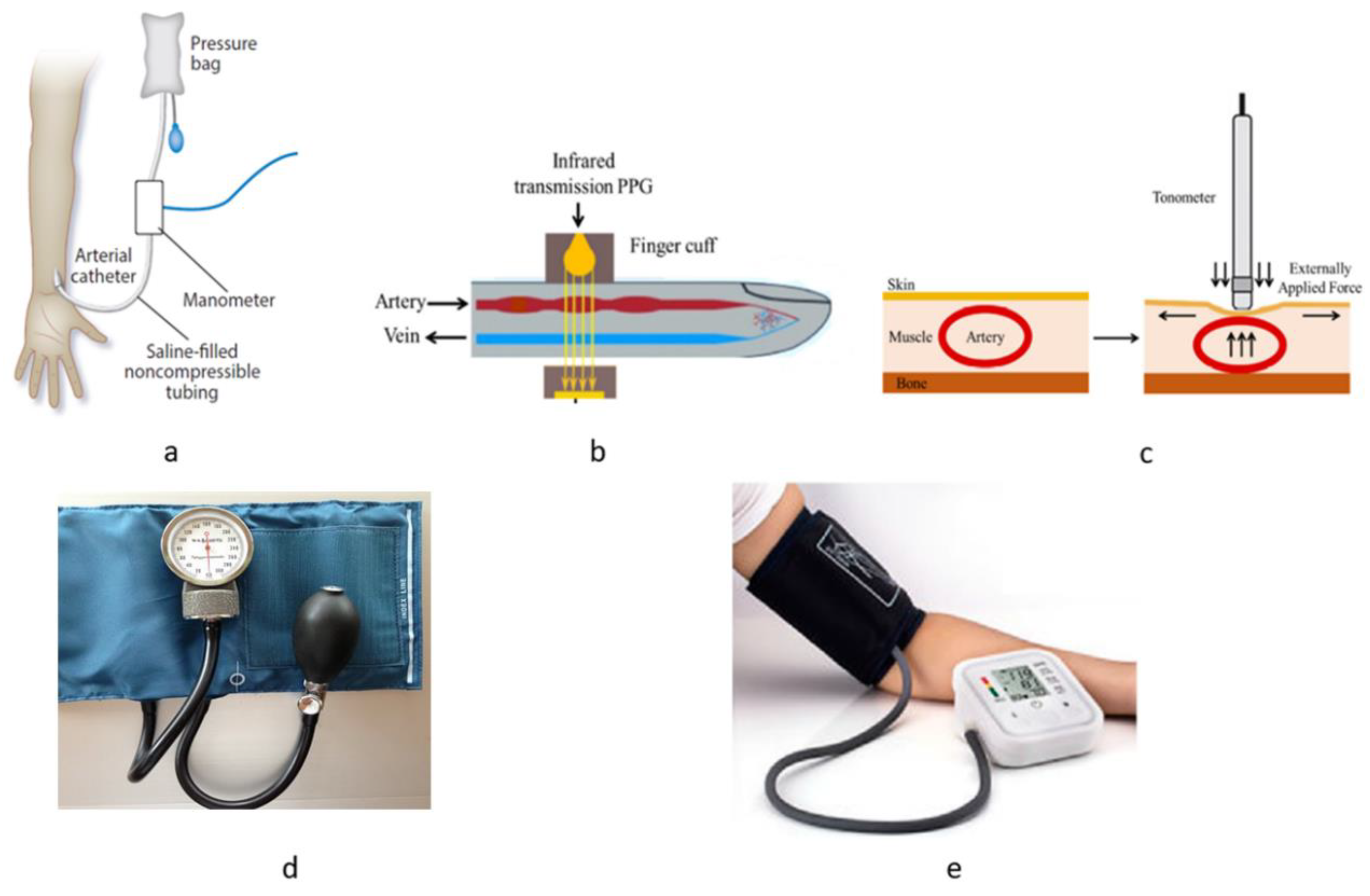
3. Traditional Techniques for BP Measurement
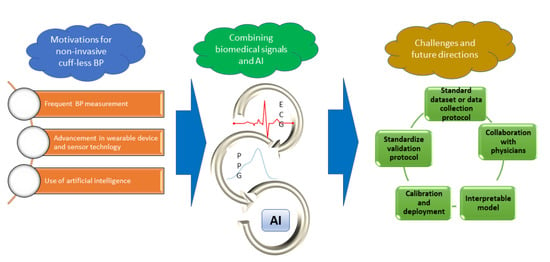
4. Motivation for Cuffless Measurements of BP
4.1. Limitations with Traditional Noninvasive Methods for BP Measurement
4.2. Necessity for Validation Protocol for Existing and Future BP Measurement Device
4.3. Improvement in Technology Helping Research with Cuffless BP Measurement
4.4. Existing Wearable Device for Hypertension Management
4.5. Rationale behind the Necessity to Continue Research on Cuffless BP Measurement
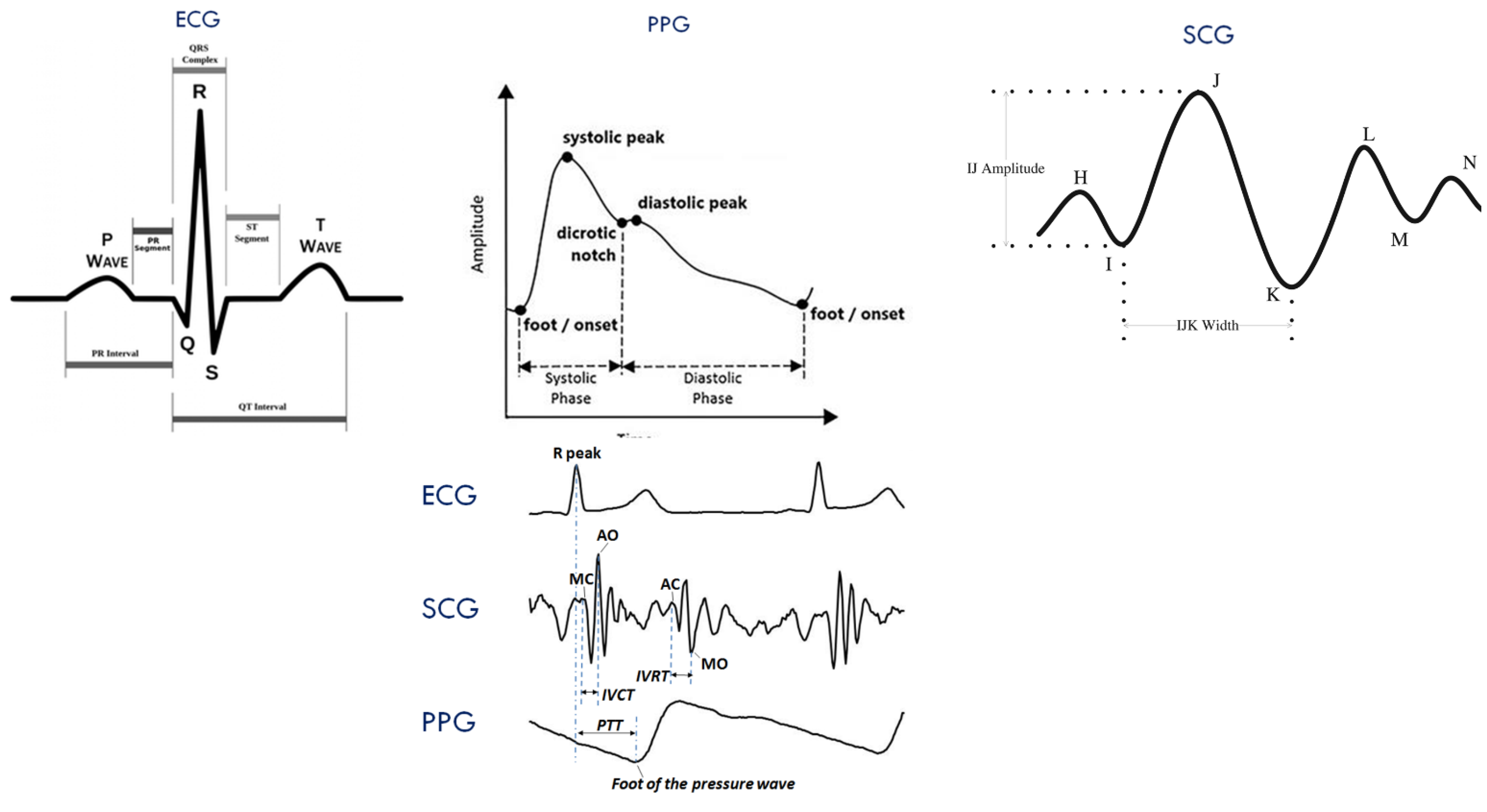
5. Features from Biomedical Signals for BP Measurement
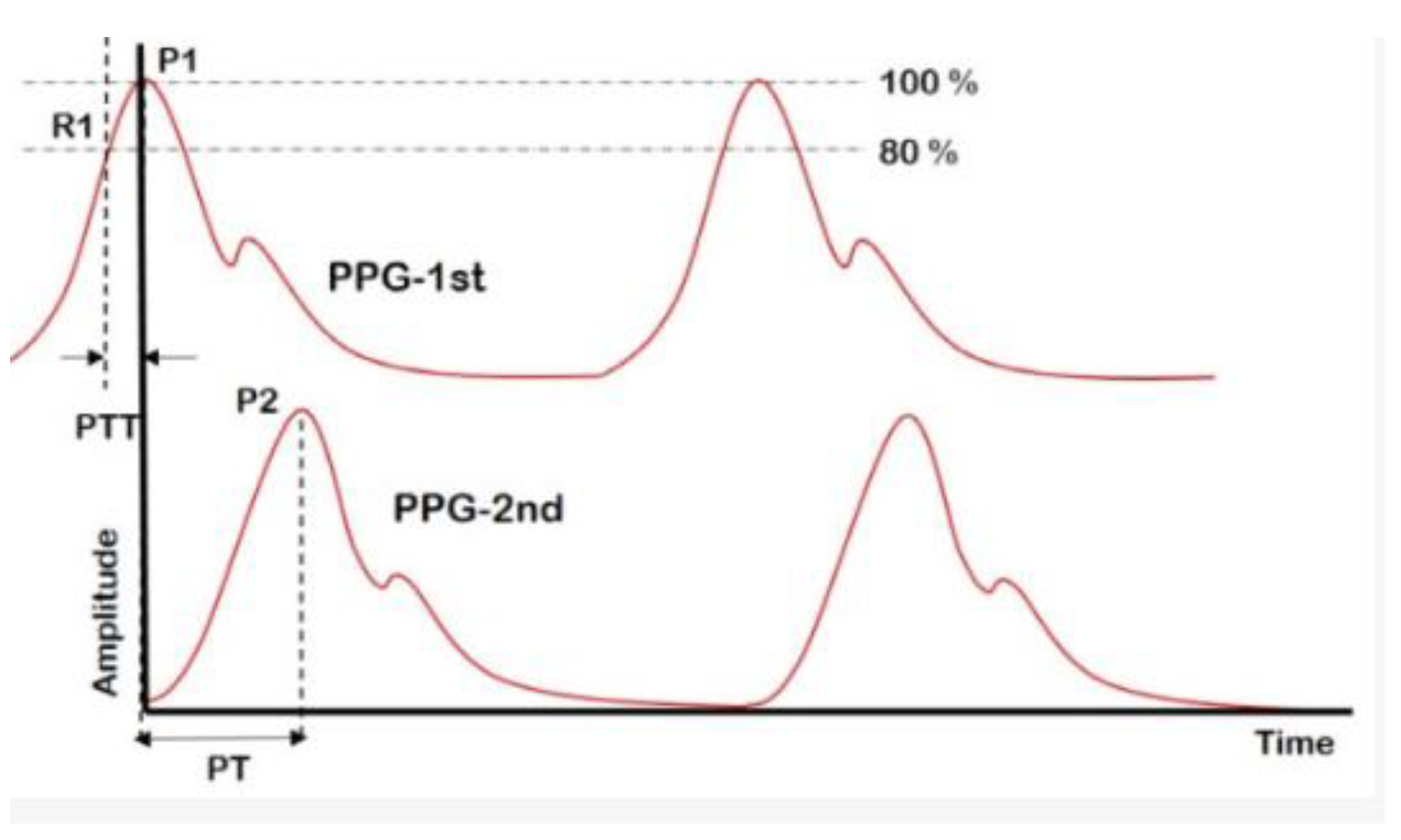
5.1. Pulse Transit Time and Pulse Arrival Time
5.2. Pulse Wave Velocity
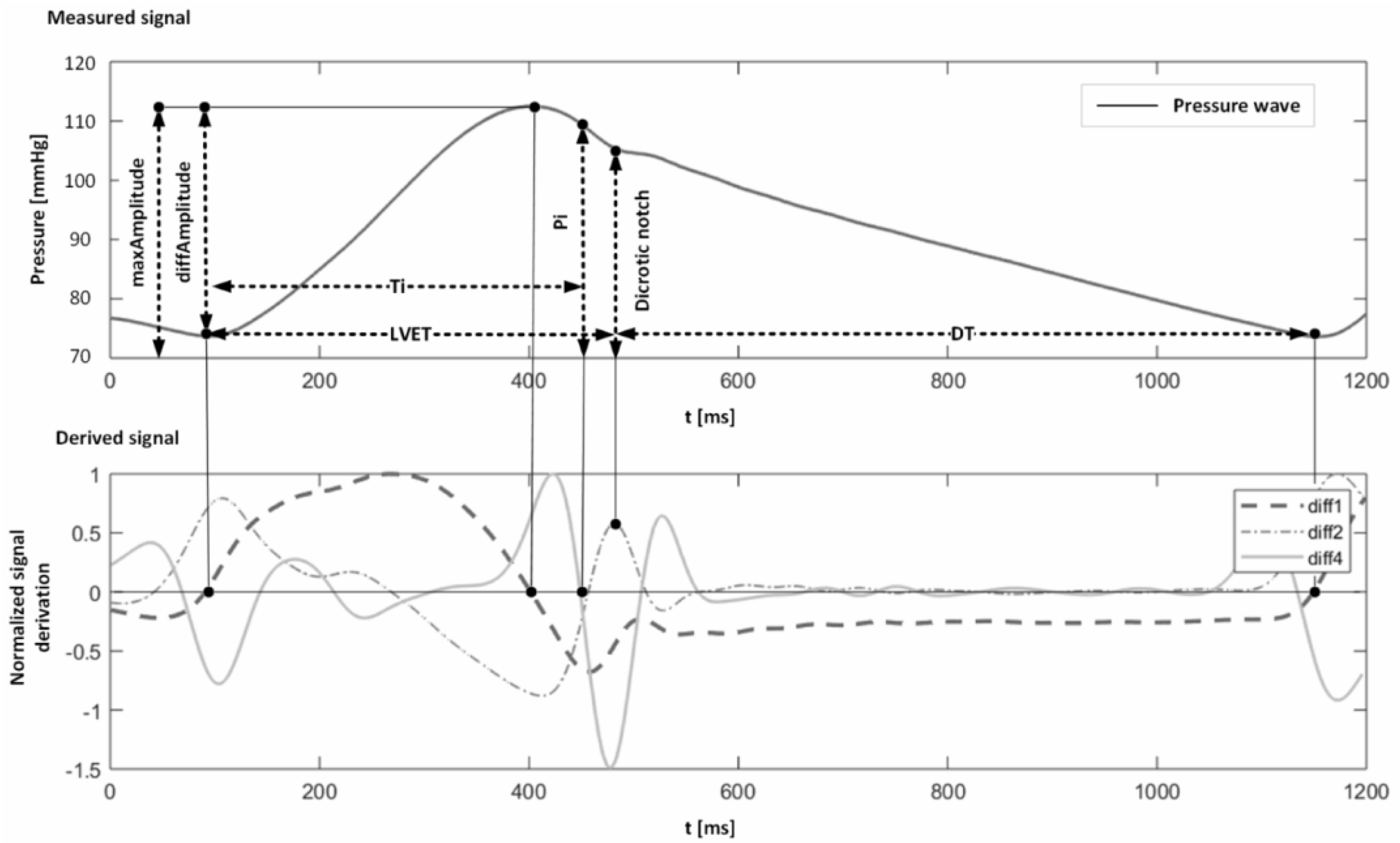
5.3. Pulse Wave Analysis
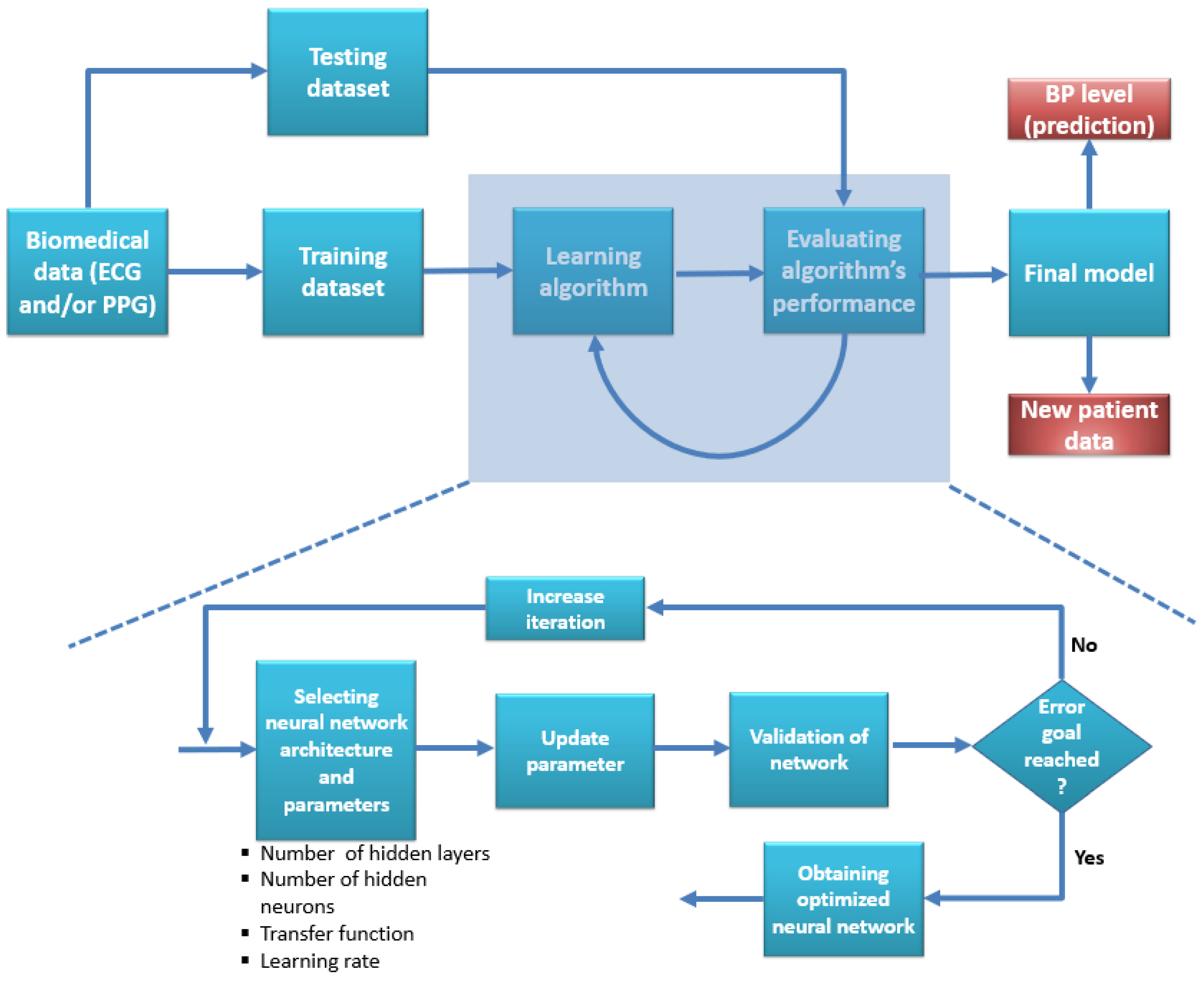
6. Machine Learning and Deep Learning in Cuffless Noninvasive Blood Pressure Measurement
6.1. Conventional Machine Learning Algorithms
- The model must reproduce the claimed result using other datasets or real-time data.
- The rationale behind the performance should accompany the use of algorithms.
- The number of features and optimization algorithms needs to be explainable.
- AAMI and BHS standards need to be passed for both SBP and DBP.
- Since all the features are from biomedical signals due to physiological activities, calibration is mandatory. The purpose, frequency, and procedure of calibration need to be addressed.
- The database (either online or real patient data) needs to be well distributed regarding demographic data.
- There has to be a generalized validation protocol in place so that the performance of all the techniques can be compared without any bias.
6.2. Deep Learning Algorithms
- Although deep learning models provided better accuracy compared to shallow machine learning algorithms, the number of studies that matched the AAMI and BHS standards is deficient.
- Since the approaches are mainly data-driven, the performance using the author’s choice of the dataset for their model should match using another random dataset. Nearly all the studies used the same dataset for test and validation.
- Since deep learning algorithms require large samples to be adequately trained, there is a high chance that all the experiments using microscopic subjects may result in overfitting the technique.
- There is no general validation approach available to compare performance among different studies.
- No clinically acceptable method was produced or implemented to have a gold standard using deep learning techniques.
7. Dataset Used for BP Measurement Model
8. Challenges and Future Recommendations
8.1. Data Collection and Accuracy of Control Data
8.2. Validation Protocol
8.3. Calibration
8.4. The Model or Technique Needs to Be Interpretable
8.5. Model Deployment
8.6. Necessity of Collaboration with a Health Professional
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ringrose, J.; Padwal, R. Wearable Technology to Detect Stress-Induced Blood Pressure Changes: The Next Chapter in Ambulatory Blood Pressure Monitoring? Am. J. Hypertens. 2021, 34, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.; Li, C.; Stuchlik, P.; Bu, X.; Kelly, T.; Mills, K.; He, H.; Chen, J.; Whelton, P.; He, J. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: A systematic review and network meta-analysis. JAMA Cardiol. 2017, 2, 775–781. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Siu, A.; US Preventive Services Task Force*. Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2015, 163, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Shimbo, D.; Carey, R.; Charleston, J.; Gaillard, T.; Misra, S.; Myers, M.; Ogedegbe, G.; Schwartz, J.; Townsend, R. Measurement of blood pressure in humans: A scientific statement from the American Heart Association. Hypertension 2019, 73, e35–e66. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Verhaeverbeke, I.; Mets, T. Drug-induced orthostatic hypotension in the elderly. Drug Saf. 1997, 17, 105–118. [Google Scholar] [CrossRef]
- Mamun; Khan, M.M.R.; Alouani, A. Myocardial Infarction Detection Using Multi Biomedical Sensors. In Proceedings of the 10th International Conference on Bioinformatics and Computational Biology (BICOB-2018), Las Vegas, NV, USA, 19–21 March 2018. [Google Scholar]
- Tewelde, S.; Liu, S.; Winters, M. Cardiogenic Shock. Cardiol. Clin. 2018, 36, 53–61. [Google Scholar] [CrossRef]
- Man, P.; Cheung, K.; Sangsiri, N.; Shek, W.; Wong, K.; Chin, J.; Chan, T.; So, R. Blood Pressure Measurement: From Cuff-Based to Contactless Monitoring. Healthcare 2022, 10, 2113. [Google Scholar] [CrossRef]
- Salkic, S.; Batic-Mujanovic, O.; Ljuca, F.; Brkic, S. Clinical presentation of hypertensive crises in emergency medical services. Mater. Sociomed 2014, 26, 12–16. [Google Scholar] [CrossRef]
- Giles, T.; Materson, B. Treating stage 2 hypertension. J. Clin. Hypertens. 2005, 7, 464–470. [Google Scholar] [CrossRef]
- Oparil, S.; Zaman, M.; Calhoun, D. Pathogenesis of hypertension. Ann. Intern. Med. 2003, 139, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Jamal, S. Essential Hypertension. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tsyrlin, V.; Pliss, M.; Kuzmenko, N. The history of blood pressure measurement: From Hales to our days. Arter. Gipertenz 2016, 22, 144–152. [Google Scholar] [CrossRef]
- Lewis, O. Stephen Hales and the measurement of blood pressure. J. Hum. Hypertens 1994, 8, 865–871. [Google Scholar] [PubMed]
- Quinney, D. Daniel Bernoulli and the Making of the Fluid Equation; Keele University: Keele, UK, 1997; Available online: http://pass.maths.org.uk/issue1/bern (accessed on 20 December 2022).
- Peterson, L.; Dripps, R.; Risman, G. A method for recording the arterial pressure pulse and blood pressure in man. Am. Heart J. 1949, 37, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Athaya, T.; Choi, S. A Review of Noninvasive Methodologies to Estimate the Blood Pressure Waveform. Sensors 2022, 22, 3953. [Google Scholar] [CrossRef] [PubMed]
- Seldinger, S.I. Catheter replacement of the needle in percutaneous arteriography: A new technique. Acta Radiol. 2008, 49, 47–52. [Google Scholar] [CrossRef]
- E.C.PEIRCE. Percutaneous femoral artery catheterization in man with special reference to aortography. Surg. Gynecol. Obstet. 1951, 93, 56–74. [Google Scholar]
- McGhee, B.; Bridges, E. Monitoring arterial blood pressure: What you may not know. Crit. Care Nurse 2002, 22, 60–79. [Google Scholar] [CrossRef]
- Lam, S.; Liu, H.; Jian, Z.; Settels, J.; Bohringer, C. Intraoperative Invasive Blood Pressure Monitoring and the Potential Pitfalls of Invasively Measured Systolic Blood Pressure. Cureus 2021, 13, e17610. [Google Scholar] [CrossRef]
- Hill, B.; Rakocz, N.; Rudas, Á.; Chiang, J.; Wang, S.; Hofer, I.; Cannesson, M.; Halperin, E. Imputation of the continuous arterial line blood pressure waveform from non-invasive measurements using deep learning. Sci. Rep. 2021, 11, 1–12. [Google Scholar]
- Haque, C.; Kwon, T.; Kim, K. Cuffless Blood Pressure Estimation Based on Monte Carlo Simulation Using Photoplethysmography Signals. Sensors 2022, 22, 1175. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, J.; Chen, X.; Wang, Y.; Zhao, Z. Continuous blood pressure estimation based on multiple parameters from eletrocardiogram and photoplethysmogram by Back-propagation neural network. Comput. Ind. 2017, 89, 50–59. [Google Scholar] [CrossRef]
- Miao, F.; Wen, B.; Hu, Z.; Fortino, G.; Wang, X.; Liu, Z.; Tang, M.; Li, Y. Continuous blood pressure measurement from one-channel electrocardiogram signal using deep-learning techniques. Artif. Intell. Med. 2020, 108, 101919. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, A.; Huang, A.; Zubajlo, R.; Anthony, B. Real-time blood pressure estimation from force-measured ultrasound. IEEE Trans. Biomed. Eng. 2018, 65, 2405–2416. [Google Scholar] [CrossRef]
- Zakrzewski, A.; Anthony, B. Noninvasive blood pressure estimation using ultrasound and simple finite element models. IEEE Trans. Biomed. Eng. 2017, 65, 2011–2022. [Google Scholar] [CrossRef]
- Mamun, K.; Rahman, M.M.; Alouani, A. Automatic Detection of Heart Diseases Using Biomedical Signals: A Literature Review of Current Status and Limitations. In Proceedings of the Future of Information and Communication Conference, San Francisco, CA, USA, 3–4 March 2022. [Google Scholar]
- Landry, C.; Peterson, S.; Arami, A. Estimation of the Blood Pressure Waveform Using Electrocardiography. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar]
- Sadrawi, M.; Lin, Y.; Lin, C.; Mathunjwa, B.; Fan, S.; Abbod, M.; Shieh, J. Genetic deep convolutional autoencoder applied for generative continuous arterial blood pressure via photoplethysmography. Sensors 2020, 20, 3829. [Google Scholar] [CrossRef]
- Qin, K.; Huang, W.; Zhang, T. Deep generative model with domain adversarial training for predicting arterial blood pressure waveform from photoplethysmogram signal. Biomed. Signal Process. Control. 2021, 70, 102972. [Google Scholar] [CrossRef]
- Athaya, T.; Choi, S. An estimation method of continuous non-invasive arterial blood pressure waveform using photoplethysmography: A U-Net architecture-based approach. Sensors 2021, 21, 1867. [Google Scholar] [CrossRef]
- Ibtehaz, N.; Rahman, M. Ppg2abp: Translating photoplethysmogram (ppg) signals to arterial blood pressure (abp) waveforms using fully convolutional neural networks. arXiv 2020, arXiv:2005.01669. [Google Scholar]
- Mukkamala, R.; Hahn, J.; Inan, O.; Mestha, L.; Kim, C.; Töreyin, H.; Kyal, S. Toward ubiquitous blood pressure monitoring via pulse transit time: Theory and practice. IEEE Trans. Biomed. Eng. 2015, 62, 1879–1901. [Google Scholar] [CrossRef]
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.; Abbott, D.; Lim, K.; Ward, R. The use of photoplethysmography for assessing hypertension. NPJ Digit. Med. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Ogedegbe, G.; Pickering, T. Principles and techniques of blood pressure measurement. Cardiol. Clin. 2010, 28, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, N.; Yang, G.; Pettigrew, R.; Lo, B.; Miao, F.; Li, Y.; Liu, J.; Zhang, Y. Continuous blood pressure measurement from invasive to unobtrusive: Celebration of 200th birth anniversary of Carl Ludwig. IEEE J. Biomed. Health Inform. 2016, 20, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Pandit, J.; Lores, E.; Batlle, D. Cuffless Blood Pressure Monitoring: Promises and Challenges. Clin. J. Am. Soc. Nephrol. 2020, 15, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Xu, S.; Tran, S.; Garg, S.; Springer, M.; Karunanithi, M.; Mohawesh, R. A survey: From shallow to deep machine learning approaches for blood pressure estimation using biosensors. Expert Syst. Appl. 2022, 197, 116788. [Google Scholar] [CrossRef]
- Hong, S.; Zhou, Y.; Shang, J.; Xiao, C.; Sun, J. Opportunities and challenges of deep learning methods for electrocardiogram data: A systematic review. Comput. Biol. Med. 2020, 122, 103801. [Google Scholar] [CrossRef] [PubMed]
- Mukkamala, R.; Stergiou, G.; Avolio, A. Cuffless blood pressure measurement. Annu. Rev. Biomed. Eng. 2022, 24, 203–230. [Google Scholar] [CrossRef]
- Sharma, M.; Rajput, J.; Tan, R.; Acharya, U. Automated Detection of Hypertension Using Physiological Signals: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5838. [Google Scholar] [CrossRef]
- Mukkamala, R.; Yavarimanesh, M.; Natarajan, K.; Hahn, J.; Kyriakoulis, K.; Avolio, A.; Stergiou, G. Evaluation of the accuracy of cuffless blood pressure measurement devices: Challenges and proposals. Hypertension 2021, 78, 1161–1167. [Google Scholar] [CrossRef]
- Koshimizu, H.; Kojima, R.; Okuno, Y. Future possibilities for artificial intelligence in the practical management of hypertension. Hypertens. Res. 2020, 43, 1327–1337. [Google Scholar] [CrossRef]
- Kario, K. Management of hypertension in the digital era: Small wearable monitoring devices for remote blood pressure monitoring. Hypertension 2020, 76, 640–650. [Google Scholar] [CrossRef]
- Pilz, N.; Patzak, A.; Bothe, T. Continuous cuffless and non-invasive measurement of arterial blood pressure—Concepts and future perspectives. Blood Press. 2022, 31, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.; Mukkamala, R.; Avolio, A.; Kyriakoulis, K.; Mieke, S.; Murray, A.; Parati, G.; Schutte, A.; Sharman, J.; Asmar, R.; et al. Cuffless blood pressure measuring devices: Review and statement by the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. J. Hypertens 2022, 40, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, W.; Asayama, K.; Zhang, Z.; Thijs, L.; Li, Y.; O’Brien, E.; Staessen, J. Ambulatory blood pressure monitoring to diagnose and manage hypertension. Hypertension 2021, 77, 254–264. [Google Scholar] [CrossRef]
- Panula, T.; Sirkia, J.; Wong, D.; Kaisti, M. Advances in non-invasive blood pressure measurement techniques. IEEE Rev. Biomed. Eng. 2022. [Google Scholar] [CrossRef]
- Marino, P. The ICU Book; Lippincott williams & wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Wesseling, K.; Jansen, J.; Settels, J.; Schreuder, J. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J. Appl. Physiol. 1985, 74, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.; Palatini, P.; Asmar, R.; Ioannidis, J.; Kollias, A.; Lacy, P.; McManus, R.; Myers, M.; Parati, G.; Shennan, A. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J. Hypertens. 2019, 37, 459–466. [Google Scholar]
- Peter, L.; Noury, N.; Cerny, M. A review of methods for non-invasive and continuous blood pressure monitoring: Pulse transit time method is promising? Irbm 2014, 35, 271–282. [Google Scholar] [CrossRef]
- Booth, J. A Short History of Blood Pressure Measurement. Proc. R. Soc. Med. 1977, 70, 793–799. [Google Scholar] [CrossRef]
- Shimek, J.; Emmanuel, J.; Orris, P.; Chartier, Y.; World Health Organization. Replacement of Mercury Thermometers and Sphygmomanometers in Healthcare: Technical Guidance; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Geddes, L. Handbook of Blood Pressure Measurement; Humana Press: Totowa, NJ, USA, 2013. [Google Scholar]
- Liu, J.; Cheng, H.; Chen, C.; Sung, S.; Moslehpour, M.; Hahn, J.; Mukkamala, R. Patient-specific oscillometric blood pressure measurement. IEEE Trans. Biomed. Eng. 2015, 63, 1220–1228. [Google Scholar] [CrossRef]
- Forouzanfar, M.; Dajani, H.; Groza, V.; Bolic, M.; Rajan, S.; Batkin, I. Oscillometric blood pressure estimation: Past, present, and future. IEEE Rev. Biomed. Eng. 2015, 8, 44–63. [Google Scholar] [CrossRef]
- Babbs, C. Oscillometric measurement of systolic and diastolic blood pressures validated in a physiologic mathematical model. Biomed. Eng. Online 2012, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, A.; Yavarimanesh, M.; Hahn, J.; Sung, S.; Chen, C.; Cheng, H.; Mukkamala, R. Formulas to explain popular oscillometric blood pressure estimation algorithms. Front. Physiol. 2019, 10, 1415. [Google Scholar] [CrossRef]
- Liu, J.; Hahn, J.; Mukkamala, R. Error mechanisms of the oscillometric fixed-ratio blood pressure measurement method. Ann. Biomed. Eng. 2013, 41, 587–597. [Google Scholar] [CrossRef]
- Wesseling, K.; Settels, J.; Wit, B. The Measurement of Continuous Finger Arterial Pressure Noninvasively in Stationary Subjects. In Biological and Psychological Factors in Cardiovascular Disease; Springer: Berlin/Heidelberg, Germany, 1986; pp. 355–375. [Google Scholar]
- Fortin, J.; Marte, W.; Grüllenberger, R.; Hacker, A.; Habenbacher, W.; Heller, A.; Wagner, C.; Wach, P.; Skrabal, F. Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput. Biol. Med. 2006, 36, 941–957. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.; Kazanskiy, N.; Khonina, S. Revolution in Flexible Wearable Electronics for Temperature and Pressure Monitoring—A Review. Electronics 2022, 11, 716. [Google Scholar] [CrossRef]
- Nelson, M.; Stepanek, J.; Cevette, M.; Covalciuc, M.; Hurst, R.; Tajik, A. Noninvasive measurement of central vascular pressures with arterial tonometry: Clinical revival of the pulse pressure waveform? Mayo Clin. Proc. 2010, 85, 460–472. [Google Scholar] [CrossRef]
- Chen, C.; Nevo, E.; Fetics, B.; Pak, P.; Yin, F.; Maughan, W.; Kass, D. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure: Validation of generalized transfer function. Circulation 1997, 95, 1827–1836. [Google Scholar] [CrossRef]
- Matthys, K.; Verdonck, P. Development and modelling of arterial applanation tonometry: A review. Technol. Health Care 2002, 10, 65–76. [Google Scholar] [CrossRef]
- Salvi, P.; Grillo, A.; Parati, G. Noninvasive estimation of central blood pressure and analysis of pulse waves by applanation tonometry. Hypertens. Res. 2015, 38, 646–648. [Google Scholar] [CrossRef]
- Drzewiecki, G.; Melbin, J.; Noordergraaf, A. Arterial tonometry: Review and analysis. J. Biomech. 1983, 16, 141–152. [Google Scholar] [CrossRef]
- Picone, D.; Schultz, M.; Otahal, P.; Aakhus, S.; Al-Jumaily, A.; Black, J.; Bos, W.; Chambers, J.; Chen, C.; Cheng, H. Accuracy of cuff-measured blood pressure: Systematic reviews and meta-analyses. J. Am. Coll. Cardiol. 2017, 70, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.; Alpert, B.; Mieke, S.; Wang, J.; O’Brien, E. Validation protocols for blood pressure measuring devices in the 21st century. J. Clin. Hypertens. 2018, 20, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.; Alpert, B.; Mieke, S.; Asmar, R.; Atkins, N.; Eckert, S.; Frick, G.; Friedman, B.; Graßl, T.; Ichikawa, T. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension 2018, 71, 368–374. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; O’Rourke, M.; Nichols, W. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Giorgini, P.; Weder, A.; Jackson, E.; Brook, R. A review of blood pressure measurement protocols among hypertension trials: Implications for “evidence-based” clinical practice. J. Am. Soc. Hypertens. 2014, 8, 670–676. [Google Scholar] [CrossRef]
- Attaei, M.; Khatib, R.; McKee, M.; Lear, S.; Dagenais, G.; Igumbor, E.; AlHabib, K.; Kaur, M.; Kruger, L.; Teo, K. Availability and affordability of blood pressure-lowering medicines and the effect on blood pressure control in high-income, middle-income, and low-income countries: An analysis of the PURE study data. Lancet Public Health 2 2017, 2, e411–e419. [Google Scholar] [CrossRef]
- Pickering, T.; Shimbo, D.; Haas, D. Ambulatory blood-pressure monitoring. N. Engl. J. Med. 2006, 354, 2368–2374. [Google Scholar] [CrossRef]
- Rosner, B.; Polk, B. Predictive values of routine blood pressure measurements in screening for hypertension. Am. J. Epidemiol. 1983, 117, 429–442. [Google Scholar] [CrossRef]
- Sessler, D. Beyond ‘failure to rescue’: The time has come for continuous ward monitoring. Br. J. Anaesth. 2019, 122, 304–306. [Google Scholar] [CrossRef]
- Maheshwari, K.; Nathanson, B.; Munson, S.; Khangulov, V.; Stevens, M.; Badani, H.; Khanna, A.; Sessler, D. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Med. 2018, 44, 857–867. [Google Scholar] [CrossRef]
- Zenati, M.; Billiar, T.; Townsend, R.; Peitzman, A.; Harbrecht, B. A brief episode of hypotension increases mortality in critically ill trauma patients. J. Trauma Acute Care Surg. 2002, 53, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Taira, D.; Sentell, T.; Albright, C.; Lansidell, D.; Nakagawa, K.; Seto, T.; Stevens, J. Insights in Public Health: Ambulatory Blood Pressure Monitoring: Underuse in Clinical Practice in Hawai’i. Hawaii. J. Med. Public. Health 2017, 76, 314–317. [Google Scholar] [PubMed]
- Pickering, T.; Hall, J.; Appel, L.; Falkner, B.; Graves, J.; Hill, M.; Jones, D.; Kurtz, T.; Sheps, S.; Roccella, E.; et al. Recommendations for blood pressure measurement in humans: An AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J. Clin. Hypertens. 2005, 7, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Appel, L.; Sheps, S.; Roccella, E.; Lenfant, C. Measuring blood pressure accurately: New and persistent challenges. JAMA 2003, 289, 1027–1030. [Google Scholar] [CrossRef]
- Campbell, N.; Padwal, R.; Picone, D.; Su, H.; Sharman, J. The impact of small to moderate inaccuracies in assessing blood pressure on hypertension prevalence and control rates. J. Clin. Hypertens. 2020, 22, 939–942. [Google Scholar] [CrossRef]
- Campbell, N.; McKay, D. Accurate blood pressure measurement: Why does it matter? CMAJ 1999, 161, 277–278. [Google Scholar]
- Joffres, M.; Campbell, N.; Manns, B.; Tu, K. Estimate of the benefits of a population-based reduction in dietary sodium additives on hypertension and its related health care costs in Canada. Can. J. Cardiol. 2007, 23, 437–443. [Google Scholar] [CrossRef]
- Frieden, T.; Varghese, C.; Kishore, S.; Campbell, N.; Moran, A.; Padwal, R.; Jaffe, M. Scaling up effective treatment of hypertension—A pathfinder for universal health coverage. J. Clin. Hypertens. 2019, 21, 1442–1449. [Google Scholar] [CrossRef]
- Parati, G.; Goncalves, A.; Soergel, D.; Bruno, R.; Caiani, E.; Gerdts, E.; Mahfoud, F.; Mantovani, L.; McManus, R.; Santalucia, P. New perspectives for hypertension management: Progress in methodological and technological developments. Eur. J. Prev. Cardiol. 2022. [Google Scholar] [CrossRef]
- Padwal, R.; Campbell, N.; Weber, M.; Lackland, D.; Shimbo, D.; Zhang, X.; Schutte, A.; Rakotz, M.; Wozniak, G.; Townsend, R. The Accuracy in Measurement of Blood Pressure (AIM-BP) collaborative: Background and rationale. J. Clin. Hypertens. 2019, 21, 1780–1783. [Google Scholar] [CrossRef]
- Campbell, N.; Berbari, A.; Cloutier, L.; Gelfer, M.; Kenerson, J.; Khalsa, T.; Lackland, D.; Lemogoum, D.; Mangat, B.; Mohan, S.; et al. Policy statement of the world hypertension league on noninvasive blood pressure measurement devices and blood pressure measurement in the clinical or community setting. J. Clin. Hypertens. 2014, 16, 320–322. [Google Scholar] [CrossRef] [PubMed]
- John, O.; Campbell, N.; Brady, T.; Farrell, M.; Varghese, C.; Berumen, A.V.; Gaitan, V.R.; Laura, A.; Toffelmire, N.; Ameel, M.; et al. The 2020 “WHO technical specifications for automated non-invasive blood pressure measuring devices with cuff”. Hypertension 2021, 77, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Picone, D.; Deshpande, R.; Schultz, M.; Fonseca, R.; Campbell, N.; Delles, C.; Olsen, M.H.; Schutte, A.; Stergiou, G.; Padwal, R. Nonvalidated home blood pressure devices dominate the online marketplace in Australia: Major implications for cardiovascular risk management. Hypertension 2020, 75, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.; Khalsa, T.; Ordunez, P.; Morales, Y.R.; Zhang, X.; Parati, G.; Padwal, R.; Tsuyuki, R.; Cloutier, L.; Sharman, J. Brief online certification course for measuring blood pressure with an automated blood pressure device. A free new resource to support World Hypertension Day Oct 17. J. Clin. Hypertens. 2020, 22, 1754–1756. [Google Scholar]
- Zheng, Y.; Ding, X.; Poon, C.; Lo, B.; Zhang, H.; Zhou, X.; Yang, G.; Zhao, N.; Zhang, Y. Unobtrusive sensing and wearable devices for health informatics. IEEE Trans. Biomed. Eng. 2014, 61, 1538–1554. [Google Scholar] [CrossRef]
- Luz, E.J.d.S.; Schwartz, W.; Cámara-Chávez, G.; Menotti, D. ECG-based heartbeat classification for arrhythmia detection: A survey. Comput. Methods Programs Biomed. 2016, 127, 144–164. [Google Scholar] [CrossRef]
- Estrada, G.M.; Mendoza, L.; Molina, V. Relationship of blood pressure with the electrical signal of the heart using signal processing. Tecciencia 2014, 9, 9–14. [Google Scholar] [CrossRef]
- Sharma, M.; Dhiman, H.; Acharya, U. Automatic identification of insomnia using optimal antisymmetric biorthogonal wavelet filter bank with ECG signals. Comput. Biol. Med. 2021, 131, 104246. [Google Scholar] [CrossRef]
- Sharma, M.; Agarwal, S.; Acharya, U. Application of an optimal class of antisymmetric wavelet filter banks for obstructive sleep apnea diagnosis using ECG signals. Comput. Biol. Med. 2018, 100, 100–113. [Google Scholar] [CrossRef]
- Sharma, M.; Tan, R.S.; Acharya, U. A novel automated diagnostic system for classification of myocardial infarction ECG signals using an optimal biorthogonal filter bank. Comput. Biol. Med. 2018, 102, 341–356. [Google Scholar] [CrossRef]
- Reisner, A.; Shaltis, P.; McCombie, D.; Asada, H.; Warner, D.; Warner, M. Utility of the photoplethysmogram in circulatory monitoring. J. Am. Soc. Anesthesiol. 2008, 108, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable photoplethysmographic sensors—Past and present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Hypertension assessment using photoplethysmography: A risk stratification approach. J. Clin. Med. 2018, 8, 12. [Google Scholar] [CrossRef]
- Inan, O.; Migeotte, P.; Park, K.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G. Ballistocardiography and seismocardiography: A review of recent advances. IEEE J. Biomed. Health Inform. 2014, 19, 1414–1427. [Google Scholar] [CrossRef]
- Kim, C.; Ober, S.; McMurtry, M.; Finegan, B.; Inan, O.; Mukkamala, R.; Hahn, J. Ballistocardiogram: Mechanism and potential for unobtrusive cardiovascular health monitoring. Sci. Rep. 2016, 6, 31297. [Google Scholar] [CrossRef] [PubMed]
- Gill, R. Measurement of blood flow by ultrasound: Accuracy and sources of error. Ultrasound Med. Biol. 1985, 11, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Bera, T. Bioelectrical impedance methods for noninvasive health monitoring: A review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R. Fundamentals of impedance cardiography. IEEE Eng. Med. Biol. Mag. 1989, 8, 35–38. [Google Scholar] [CrossRef]
- Verkruysse, W.; Svaasand, L.; Nelson, J. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef]
- Jonathan, E.; Leahy, M. Investigating a smartphone imaging unit for photoplethysmography. Physiol. Meas. 2010, 31, N79. [Google Scholar] [CrossRef]
- Landreani, F.; Caiani, E. Smartphone accelerometers for the detection of heart rate. Expert Rev. Med. Devices 2017, 14, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, G.; Durand, F.; Guttag, J. Detecting Pulse from Head Motions in Video. In Proceedings of Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Portland, OR, USA, 23–28 June 2013; pp. 3430–3437. [Google Scholar]
- Chung, H.; Rwei, A.; Hourlier-Fargette, A.; Xu, S.; Lee, K.; Dunne, E.; Xie, Z.; Liu, C.; Carlini, A.; Kim, D. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 2020, 26, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Tee, B.; Mei, J.; Appleton, A.; Kim, D.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Ha, T.; Tran, J.; Liu, S.; Jang, H.; Jeong, H.; Mitbander, R.; Huh, H.; Qiu, Y.; Duong, J.; Wang, R. A chest-laminated ultrathin and stretchable E-Tattoo for the measurement of electrocardiogram, seismocardiogram, and cardiac time intervals. Adv. Sci. 2019, 6, 1900290. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Rizzo, G.; Işilay, Z.; Lombardi, P. SeisMote: A multi-sensor wireless platform for cardiovascular monitoring in laboratory, daily life, and telemedicine. Sensors 2020, 20, 680. [Google Scholar] [CrossRef]
- Yano, Y.; Hoshide, S.; Inokuchi, T.; Kanemaru, Y.; Shimada, K.; Kario, K. Association between morning blood pressure surge and cardiovascular remodeling in treated elderly hypertensive subjects. Am. J. Hypertens. 2009, 22, 1177–1182. [Google Scholar] [CrossRef]
- Shimizu, M.; Ishikawa, J.; Yano, Y.; Hoshide, S.; Shimada, K.; Kario, K. The relationship between the morning blood pressure surge and low-grade inflammation on silent cerebral infarct and clinical stroke events. Atherosclerosis 2011, 219, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Metoki, H.; Ohkubo, T.; Kikuya, M.; Asayama, K.; Obara, T.; Hashimoto, J.; Totsune, K.; Hoshi, H.; Satoh, H.; Imai, Y. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: The Ohasama study. Hypertension 2006, 47, 149–154. [Google Scholar] [CrossRef]
- Kuwajima, I.; Mitani, K.; Miyao, M.; Suzuki, Y.; Kuramoto, K.; Ozawa, T. Cardiac implications of the morning surge in blood pressure in elderly hypertensive patients: Relation to arising time. Am. J. Hypertens. 1995, 8, 29–33. [Google Scholar] [CrossRef]
- Kario, K.; Pickering, T.; Umeda, Y.; Hoshide, S.; Hoshide, Y.; Morinari, M.; Murata, M.; Kuroda, T.; Schwartz, J.; Shimada, K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: A prospective study. Circulation 2003, 107, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, R.; Kario, K.; Hoshide, S.; Umeda, Y.; Hoshide, Y.; Shimada, K. Morning blood pressure hyper-reactivity is an independent predictor for hypertensive cardiac hypertrophy in a community-dwelling population. Am. J. Hypertens. 2005, 18, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Y.; Zhang, J.; Wang, Y.; Ling, H.; Chen, K.; Gao, P.; Zhu, D. Association between ambulatory systolic blood pressure during the day and asymptomatic intracranial arterial stenosis. Hypertension 2014, 63, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Acciaroli, G.; Facchinetti, A.; Pillonetto, G.; Sparacino, G. Non-Invasive Continuous-Time Blood Pressure Estimation from a Single Channel PPG Signal Using Regularized ARX Models. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 3630–3633. [Google Scholar]
- Kario, K.; Shimada, K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: Antihypertensive strategy for nocturnal blood pressure. Clin. Exp. Hypertens. 2004, 26, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Matsuo, T.; Kobayashi, H.; Imiya, M.; Matsuo, M.; Shimada, K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients: Advanced silent cerebrovascular damage in extreme dippers. Hypertension 1996, 27, 130–135. [Google Scholar] [CrossRef]
- Hoshide, S.; Kario, K.; Hoshide, Y.; Umeda, Y.; Hashimoto, T.; Kunii, O.; Ojima, T.; Shimada, K. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am. J. Hypertens. 2003, 16, 434–438. [Google Scholar] [CrossRef]
- Burke, L.; Ma, J.; Azar, K.; Bennett, G.; Peterson, E.; Zheng, Y.; Riley, W.; Stephens, J.; Shah, S.; Suffoletto, B. Current science on consumer use of mobile health for cardiovascular disease prevention: A scientific statement from the American Heart Association. Circulation 2015, 132, 1157–1213. [Google Scholar] [CrossRef]
- Kuwabara, M.; Harada, K.; Hishiki, Y.; Kario, K. Validation of a wrist-type home nocturnal blood pressure monitor in the sitting and supine position according to the ANSI/AAMI/ISO81060-2: 2013 guidelines: Omron HEM-9600T. J. Clin. Hypertens. 2019, 21, 463–469. [Google Scholar] [CrossRef]
- Kuwabara, M.; Harada, K.; Hishiki, Y.; Kario, K. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2: 2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J. Clin. Hypertens. 2019, 21, 853–858. [Google Scholar] [CrossRef]
- Kollias, A.; Ntineri, A.; Kyriakoulis, K.; Stambolliu, E.; Lagou, S.; Boubouchairopoulou, N.; Stergiou, G. Validation of the professional device for blood pressure measurement Microlife WatchBP Office in adults and children according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization standard. Blood Press. Monit. 2018, 23, 112–114. [Google Scholar]
- Kario, K.; Shimbo, D.; Tomitani, N.; Kanegae, H.; Schwartz, J.; Williams, B. The first study comparing a wearable watch-type blood pressure monitor with a conventional ambulatory blood pressure monitor on in-office and out-of-office settings. J. Clin. Hypertens. 2020, 22, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Cartledge, S.; Karmakar, C.; Rawstorn, J.; Fraser, S.; Chow, C.; Maddison, R. Validation and acceptability of a cuffless wrist-worn wearable blood pressure monitoring device among users and health care professionals: Mixed methods study. JMIR mHealth uHealth 2019, 7, e14706. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Bando, Y.; Kawachi, T.; Yamakita, H.; Futatsuyama, K.; Honda, Y.; Yasui, H.; Nishimura, K.; Kamihara, T.; Okumura, T. Development and validation of a novel cuff-less blood pressure monitoring device. Basic Transl. Sci. 2017, 2, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Lazazzera, R.; Belhaj, Y.; Carrault, G. A new wearable device for blood pressure estimation using photoplethysmogram. Sensors 2019, 19, 2557. [Google Scholar] [CrossRef]
- Chan, G.; Cooper, R.; Hosanee, M.; Welykholowa, K.; Kyriacou, P.; Zheng, D.; Allen, J.; Abbott, D.; Lovell, N.; Fletcher, R. Multi-site photoplethysmography technology for blood pressure assessment: Challenges and recommendations. J. Clin. Med. 2019, 8, 1827. [Google Scholar] [CrossRef] [PubMed]
- Bilo, G.; Zorzi, C.; Munera, J.O.; Torlasco, C.; Giuli, V.; Parati, G. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision. Blood Press. Monit. 2015, 20, 291–294. [Google Scholar] [CrossRef]
- O’Brien, E.; Pickering, T.; Asmar, R.; Myers, M.; Parati, G.; Staessen, J.; Mengden, T.; Imai, Y.; Waeber, B.; Palatini, P. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press. Monit. 2002, 7, 3–17. [Google Scholar] [CrossRef]
- Ibrahim, B.; Jafari, R. Continuous Blood Pressure Monitoring Using Wrist-Worn Bio-Impedance Sensors with Wet Electrodes. In Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar]
- Qiu, C.; Wu, T.; Redouté, J.; Yuce, M. A Wireless Wearable Sensor Patch for the Real-Time Estimation of Continuous Beat-to-Beat Blood Pressure. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6842–6845. [Google Scholar]
- Hosanee, M.; Chan, G.; Welykholowa, K.; Cooper, R.; Kyriacou, P.; Zheng, D.; Allen, J.; Abbott, D.; Menon, C.; Lovell, N. Cuffless single-site photoplethysmography for blood pressure monitoring. J. Clin. Med. 2020, 9, 723. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Seo, J.; Ihm, S.; Kim, K.; Cho, E.; Kim, H.; Shin, J.; Park, S.; Sohn, I. Smartphone/smartwatch-based cuffless blood pressure measurement: A position paper from the Korean Society of Hypertension. Clin. Hypertens. 2021, 27, 1–8. [Google Scholar] [CrossRef]
- Arakawa, T. Recent research and developing trends of wearable sensors for detecting blood pressure. Sensors 2018, 18, 2772. [Google Scholar] [CrossRef]
- Boubouchairopoulou, N.; Kollias, A.; Chiu, B.; Chen, B.; Lagou, S.; Anestis, P.; Stergiou, G. A novel cuffless device for self-measurement of blood pressure: Concept, performance and clinical validation. J. Hum. Hypertens. 2017, 31, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Mamun; Khan, M.M.R. Significance of Features from Biomedical Signals in Heart Health Monitoring. BioMed 2022, 2, 391–408. [Google Scholar] [CrossRef]
- Vybornova, A.; Polychronopoulou, E.; Wurzner-Ghajarzadeh, A.; Fallet, S.; Sola, J.; Wuerzner, G. Blood pressure from the optical Aktiia Bracelet: A 1-month validation study using an extended ISO81060-2 protocol adapted for a cuffless wrist device. Blood Press. Monit. 2021, 26, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Kenney, M.; Seals, D. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension 1993, 22, 653–664. [Google Scholar] [CrossRef]
- Mukkamala, R.; Hahn, J. Initialization of Pulse Transit Time-Based Blood Pressure Monitors. In The Handbook of Cuffless Blood Pressure Monitoring; Springer: Berlin/Heidelberg, Germany, 2019; pp. 163–190. [Google Scholar]
- Miao, F.; Liu, Z.; Liu, J.; Wen, B.; He, Q.; Li, Y. Multi-sensor fusion approach for cuff-less blood pressure measurement. IEEE J. Biomed. Health Inform. 2019, 24, 79–91. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, B.; Li, Y.; Tang, M.; Miao, F. Continuous blood pressure estimation from electrocardiogram and photoplethysmogram during arrhythmias. Front. Physiol. 2020, 11, 575407. [Google Scholar] [CrossRef]
- Xing, X.; Ma, Z.; Zhang, M.; Zhou, Y.; Dong, W.; Song, M. An unobtrusive and calibration-free blood pressure estimation method using photoplethysmography and biometrics. Sci. Rep. 2019, 9, 8611 . [Google Scholar] [CrossRef]
- Poon, C.; Zhang, Y. Cuff-Less and Noninvasive Measurements of Arterial Blood Pressure by Pulse Transit Time. In Proceedings of the IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 5877–5880. [Google Scholar]
- Schoettker, P.; Degott, J.; Hofmann, G.; Proença, M.; Bonnier, G.; Lemkaddem, A.; Lemay, M.; Schorer, R.; Christen, U.; Knebel, J. Blood pressure measurements with the OptiBP smartphone app validated against reference auscultatory measurements. Sci. Rep. 2020, 10, 17827. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, J.; Ruiz-Sanmartín, A.; Ribas, V.; Caballero, J.; García-Roche, A.; Riera, J.; Nuvials, X.; de Nadal, M.; de Sola-Morales, O.; Serra, J. Innovative continuous non-invasive cuffless blood pressure monitoring based on photoplethysmography technology. Intensive Care Med. 2013, 39, 1618–1625. [Google Scholar] [CrossRef]
- Luo, H.; Yang, D.; Barszczyk, A.; Vempala, N.; Wei, J.; Wu, S.; Zheng, P.; Fu, G.; Lee, K.; Feng, Z. Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ. Cardiovasc. Imaging 2019, 12, e008857. [Google Scholar] [CrossRef]
- Nachman, D.; Gepner, Y.; Goldstein, N.; Kabakov, E.; Ishay, A.; Littman, R.; Azmon, Y.; Jaffe, E.; Eisenkraft, A. Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Sci. Rep. 2020, 10, 16116. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [PubMed]
- Harma, M.; Barbosa, K.; Ho, V.; Griggs, D.; Ghirmai, T.; Krishnan, S.; Hsiai, T.; Chiao, J.; Cao, H. Cuff-less and continuous blood pressure monitoring: A methodological review. Technologies 2017, 5, 21. [Google Scholar]
- Gao, M.; Olivier, N.; Mukkamala, R. Comparison of noninvasive pulse transit time estimates as markers of blood pressure using invasive pulse transit time measurements as a reference. Physiol. Rep. 2016, 4, e12768. [Google Scholar] [CrossRef]
- Allen, J.; Murray, A. Age-related changes in peripheral pulse timing characteristics at the ears, fingers and toes. J. Hum. Hypertens. 2002, 16, 711–717. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, C.; Tao, G.; Bi, M. Continuous and noninvasive measurement of systolic and diastolic blood pressure by one mathematical model with the same model parameters and two separate pulse wave velocities. Ann. Biomed. Eng. 2012, 40, 871–882. [Google Scholar] [CrossRef]
- Mamun, K.; Rahman, M.M.; Alouani, A. Using Photoplethysmography & ECG towards a Non-Invasive Cuff Less Blood Pressure Measurement Technique. In Proceedings of the 2019 IEEE Canadian Conference of Electrical and Computer Engineering (CCECE), Edmonton, AB, Canada, 5–8 May 2019; pp. 1–4. [Google Scholar]
- Nitzan, M.; Khanokh, B.; Slovik, Y. The difference in pulse transit time to the toe and finger measured by photoplethysmography. Physiol. Meas. 2001, 23, 85. [Google Scholar] [CrossRef]
- Liang, Y.; Abbott, D.; Howard, N.; Lim, K.; Ward, R.; Elgendi, M. How effective is pulse arrival time for evaluating blood pressure? Challenges and recommendations from a study using the MIMIC database. J. Clin. Med. 2019, 8, 337. [Google Scholar] [CrossRef]
- Kim, C.; Carek, A.; Inan, O.; Mukkamala, R.; Hahn, J. Ballistocardiogram-based approach to cuffless blood pressure monitoring: Proof of concept and potential challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2384–2391. [Google Scholar] [CrossRef]
- Lee, J.; Yang, S.; Lee, S.; Kim, H. Analysis of pulse arrival time as an indicator of blood pressure in a large surgical biosignal database: Recommendations for developing ubiquitous blood pressure monitoring methods. J. Clin. Med. 2019, 8, 1773. [Google Scholar]
- Sharwood-Smith, G.; Bruce, J. Drummond. Assessment of pulse transit time to indicate cardiovascular changes during obstetric spinal anaesthesia. Br. J. Anaesth. 2006, 96, 100–105. [Google Scholar] [CrossRef]
- Yoon, Y.; Kang, J.; Kwon, Y.; Park, S.; Noh, S.; Kim, Y.; Park, J.; Hwang, S. Cuff-less blood pressure estimation using pulse waveform analysis and pulse arrival time. IEEE J. Biomed. Health Inform. 2017, 22, 1068–1074. [Google Scholar] [CrossRef]
- Noordergraaf, A. Circulatory System Dynamics; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Zhang, G.; Gao, M.; Xu, D.; Olivier, N.; Mukkamala, R. Pulse arrival time is not an adequate surrogate for pulse transit time as a marker of blood pressure. J. Appl. Physiol. 2011, 111, 1681–1686. [Google Scholar] [CrossRef]
- Chen, W.; Kobayashi, T.; Ichikawa, S.; Takeuchi, Y.; Togawa, T. Continuous estimation of systolic blood pressure using the pulse arrival time and intermittent calibration. Med. Biol. Eng. Comput. 2000, 38, 569–574. [Google Scholar] [CrossRef]
- Forouzanfar, M.; Ahmad, S.; Batkin, I.; Dajani, H.; Groza, V.; Bolic, M. Model-based mean arterial pressure estimation using simultaneous electrocardiogram and oscillometric blood pressure measurements. IEEE Trans. Instrum. Meas. 2015, 64, 2443–2452. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, Y. A Correlation Study on the Variabilities in Pulse Transit Time, Blood Pressure, and Heart Rate Recorded Simultaneously from Healthy Subjects. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 996–999. [Google Scholar]
- Sigrist, R.; Liau, J.; Kaffas, A.; Chammas, M.; Willmann, J. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Chen, Z.; Deng, G.; Wang, H.; Mavromoustakis, C.; Song, H.; Ming, Z. Design of a continuous blood pressure measurement system based on pulse wave and ECG signals. IEEE J. Transl. Eng. Health Med. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Byfield, R.; Miller, M.; Miles, J.; Guidoboni, G.; Lin, J. Towards Robust Blood Pressure Estimation From Pulse Wave Velocity Measured by Photoplethysmography Sensors. IEEE Sens. J. 2021, 22, 2475–2483. [Google Scholar] [CrossRef]
- Kao, Y.; Chao, P.; Wey, C. Design and validation of a new PPG module to acquire high-quality physiological signals for high-accuracy biomedical sensing. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Kachuee, M.; Kiani, M.; Mohammadzade, H.; Shabany, M. Cuffless blood pressure estimation algorithms for continuous health-care monitoring. IEEE Trans. Biomed. Eng. 2016, 64, 859–869. [Google Scholar] [CrossRef]
- Kachuee, M.; Kiani, M.; Mohammadzade, H.; Shabany, M. Cuff-Less High-Accuracy Calibration-Free Blood Pressure Estimation Using Pulse Transit Time. In Proceedings of the 2015 IEEE International Symposium on Circuits and Systems (ISCAS), Lisbon, Portugal, 24–27 May 2015; pp. 1006–1009. [Google Scholar]
- Goldberger, A.; Amaral, L.; Glass, L.; Hausdorff, J.; Ivanov, P.; Mark, R.; Mietus, J.; Moody, G.; Peng, C.; Stanley, H. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Oguri, K. Cuffless Blood Pressure Estimation by Error-Correcting Output Coding Method Based on an Aggregation of AdaBoost with a Photoplethysmograph Sensor. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 6765–6768. [Google Scholar]
- Teng, X.; Zhang, Y. Continuous and noninvasive estimation of arterial blood pressure using a photoplethysmographic approach. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No. 03CH37439), Cancun, Mexico, 17–21 September 2003; 4, pp. 3153–3156. [Google Scholar]
- Peter, L.; Kracik, J.; Cerny, M.; Noury, N.; Polzer, S. Mathematical model based on the shape of pulse waves measured at a single spot for the non-invasive prediction of blood pressure. Processes 2020, 8, 442. [Google Scholar] [CrossRef]
- Monte-Moreno, E. Non-invasive estimate of blood glucose and blood pressure from a photoplethysmograph by means of machine learning techniques. Artif. Intell. Med. 2011, 53, 127–138. [Google Scholar] [CrossRef]
- Kavsaoğlu, A.; Polat, K.; Hariharan, M. Non-invasive prediction of hemoglobin level using machine learning techniques with the PPG signal’s characteristics features. Appl. Soft Comput. 2015, 37, 983–991. [Google Scholar] [CrossRef]
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, Z.; Rabbani, K. Frequency domain analysis to identify neurological disorders from evoked EMG responses. J. Biol. Phys. 2007, 33, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Z. A SVM Method for Continuous Blood Pressure Estimation from a PPG Signal. In Proceedings of the 9th International Conference on Machine Learning and Computing, New York, NY, USA, 24–26 February 2017; pp. 128–132. [Google Scholar]
- Xing, X.; Sun, M. Optical blood pressure estimation with photoplethysmography and FFT-based neural networks. Biomed. Opt. Express 2016, 7, 3007–3020. [Google Scholar] [CrossRef]
- Cattivelli, F.; Garudadri, H. Noninvasive Cuffless Estimation of Blood Pressure from Pulse Arrival Time and Heart Rate with Adaptive Calibration. In Proceedings of the 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 114–119. [Google Scholar]
- Chowdhury, M.; Khandakar, A.; Alzoubi, K.; Mansoor, S.; Tahir, A.M.; Reaz, M.; Al-Emadi, N. Real-time smart-digital stethoscope system for heart diseases monitoring. Sensors 2019, 19, 2781. [Google Scholar] [CrossRef]
- Chowdhury, M.; Alzoubi, K.; Khandakar, A.; Khallifa, R.; Abouhasera, R.; Koubaa, S.; Ahmed, R.; Hasan, A. Wearable real-time heart attack detection and warning system to reduce road accidents. Sensors 2019, 19, 2780. [Google Scholar] [CrossRef]
- Yi, C.; Jian, C.; Wenqiang, J. Continuous Blood Pressure Measurement Based on Photoplethysmography. In Proceedings of the 14th IEEE International Conference on Electronic Measurement & Instruments (ICEMI), Changsha, China, 1–3 November 2019; pp. 1656–1663. [Google Scholar]
- Miao, F.; Fu, N.; Zhang, Y.; Ding, X.; Hong, X.; He, Q.; Li, Y. A novel continuous blood pressure estimation approach based on data mining techniques. IEEE J. Biomed. Health Inform. 2017, 21, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.; Wu, C.; Lu, S.; Tsao, Y. A Linear Regression Model with Dynamic Pulse Transit Time Features for Noninvasive Blood Pressure Prediction. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, 17–19 October 2016; pp. 604–607. [Google Scholar]
- Ghosh, S.; Banerjee, A.; Ray, N.; Wood, P.; Boulanger, P.; Padwal, R. Continuous Blood Pressure Prediction from Pulse Transit Time Using ECG and PPG Signals. In Proceedings of the 2016 IEEE Healthcare Innovation Point-Of-Care Technologies Conference (HI-POCT), Cancun, Mexico, 9–11 November 2016; pp. 188–191. [Google Scholar]
- Datta, S.; Banerjee, R.; Choudhury, A.; Sinha, A.; Pal, A. Blood Pressure Estimation from Photoplethysmogram Using Latent Parameters. In Proceedings of the 2016 IEEE International Conference on Communications (ICC), Kuala Lumpur, Malaysia, 22–27 May 2016; pp. 1–7. [Google Scholar]
- Choudhury, A.; Banerjee, R.; Sinha, A.; Kundu, S. Estimating Blood Pressure Using Windkessel Model on Photoplethysmogram. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4567–4570. [Google Scholar]
- Wong, M.; Poon, C.; Zhang, Y. An evaluation of the cuffless blood pressure estimation based on pulse transit time technique: A half year study on normotensive subjects. Cardiovasc. Eng. 2009, 9, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Hassan; Ali, M.K.B.; Mashor, M.; Nasir, N.; Mohamed, S. Measuring Blood Pressure Using a Photoplethysmography Approach. In 4th Kuala Lumpur International Conference on Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2008; pp. 591–594. [Google Scholar]
- Gao, S.; Wittek, P.; Zhao, L.; Jiang, W. Data-Driven Estimation of Blood Pressure Using Photoplethysmographic Signals. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 766–769. [Google Scholar]
- Liu, M.; Po, L.; Fu, H. Cuffless blood pressure estimation based on photoplethysmography signal and its second derivative. Int. J. Comput. Theory Eng. 2017, 9, 202. [Google Scholar] [CrossRef]
- Khalid, S.; Zhang, J.; Chen, F.; Zheng, D. Blood pressure estimation using photoplethysmography only: Comparison between different machine learning approaches. J. Healthc. Eng. 2018, 2018, 1548647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ren, H.; Huang, G.; Cheng, Y.; Hu, C. Predicting blood pressure from physiological index data using the SVR algorithm. BMC Bioinform. 2019, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dey, J.; Gaurav, A.; Tiwari, V. InstaBP: Cuff-Less Blood Pressure Monitoring on Smartphone Using Single PPG Sensor. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5002–5005. [Google Scholar]
- Fujita, D.; Suzuki, A.; Ryu, K. PPG-based systolic blood pressure estimation method using PLS and level-crossing feature. Appl. Sci. 2019, 9, 304. [Google Scholar] [CrossRef]
- Mousavi, S.; Firouzmand, M.; Charmi, M.; Hemmati, M.; Moghadam, M.; Ghorbani, Y. Blood pressure estimation from appropriate and inappropriate PPG signals using A whole-based method. Biomed. Signal Process. Control. 2019, 47, 196–206. [Google Scholar] [CrossRef]
- Mamun, K.; Rahman, M.M.; Alouani, A. Cuffless Blood Pressure Measurement Using Linear and Nonlinear Optimized Feature Selection. Diagnostics 2022, 12, 408. [Google Scholar] [CrossRef]
- Landry, C.; Peterson, S.; Arami, A. Nonlinear dynamic modeling of blood pressure waveform: Towards an accurate cuffless monitoring system. IEEE Sens. J. 2020, 20, 5368–5378. [Google Scholar] [CrossRef]
- Mehrabadi, M.; Aqajari, S.; Zargari, A.; Dutt, N.; Rahmani, A. Novel Blood Pressure Waveform Reconstruction from Photoplethysmography using Cycle Generative Adversarial Networks. arXiv 2022, arXiv:2201.09976. [Google Scholar]
- Lamonaca, F.; Barbe, K.; Kurylyak, Y.; Grimaldi, D.; Van Moer, W.; Furfaro, A.; Spagnuolo, V. Application of the Artificial Neural Network for Blood Pressure Evaluation with Smartphones. In Proceedings of the 2013 IEEE 7th International Conference on Intelligent Data Acquisition and Advanced Computing Systems (IDAACS), Berlin, Germany, 12–14 September 2013; pp. 408–412. [Google Scholar]
- Su, P.; Ding, X.; Zhang, Y.; Liu, J.; Miao, F.; Zhao, N. Long-Term Blood Pressure Prediction with Deep Recurrent Neural Networks. In Proceedings of the 2018 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Las Vegas, NV, USA, 4 March 2018; pp. 323–328. [Google Scholar]
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Photoplethysmography and deep learning: Enhancing hypertension risk stratification. Biosensors 2018, 8, 101. [Google Scholar] [PubMed]
- Panwar, M.; Gautam, A.; Biswas, D.; Acharyya, A. PP-Net: A deep learning framework for PPG-based blood pressure and heart rate estimation. IEEE Sensors J. 2020, 20, 10000–10011. [Google Scholar] [CrossRef]
- Tazarv, A.; Levorato, M. A Deep Learning Approach to Predict Blood Pressure from PPG Signals. In Proceedings of the 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 31 October–4 November 2021; pp. 5658–5662. [Google Scholar]
- Jeong, D.; Lim, K. Combined deep CNN–LSTM network-based multitasking learning architecture for noninvasive continuous blood pressure estimation using difference in ECG-PPG features. Sci. Rep. 2021, 11, 13539. [Google Scholar] [CrossRef]
- Schrumpf, F.; Frenzel, P.; Aust, C.; Osterhoff, G.; Fuchs, M. Assessment of non-invasive blood pressure prediction from ppg and rppg signals using deep learning. Sensors 2021, 21, 6022. [Google Scholar] [CrossRef] [PubMed]
- Harfiya, L.; Chang, C.; Li, Y. Continuous blood pressure estimation using exclusively photopletysmography by LSTM-based signal-to-signal translation. Sensors 2021, 21, 2952. [Google Scholar] [CrossRef]
- Huang, B.; Chen, W.; Lin, C.; Juang, C.; Wang, J. MLP-BP: A novel framework for cuffless blood pressure measurement with PPG and ECG signals based on MLP-Mixer neural networks. Biomed. Signal Process. Control 2022, 73, 103404. [Google Scholar] [CrossRef]
- Wu, B.; Chiu, L.; Wu, Y.; Lai, C.; Chu, P. Contactless Blood Pressure Measurement via Remote Photoplethysmography with Synthetic Data Generation Using Generative Adversarial Network. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, LA, USA, 19–20 June 2022; pp. 2130–2138. [Google Scholar]
- Mamun, K.; Rahman, M.M.; Alouani, A. FA-1D-CNN Implementation to Improve Diagnosis of Heart Disease Risk Level. In Proceedings of the 6th World Congress on Electrical Engineering and Computer Systems and Science, Prague, Czech Republic, 13–15 August 2020; pp. 122.1–122.9. [Google Scholar]
- Wu, D.; Xu, L.; Zhang, R.; Zhang, H.; Ren, L.; Zhang, Y. Continuous cuff-less blood pressure estimation based on combined information using deep learning approach. J. Med. Imaging Health Inform. 2018, 8, 1290–1299. [Google Scholar]
- Vardhan, K.; Vedanth, S.; Poojah, G.; Abhishek, K.; Kumar, M.; Vijayaraghavan, V. BP-Net: Efficient Deep Learning for Continuous Arterial Blood Pressure Estimation using Photoplethysmogram. In Proceedings of the 2021 20th IEEE International Conference on Machine Learning and Applications (ICMLA), Pasadena, CA, USA, 13–16 December 2021; pp. 1495–1500. [Google Scholar]
- Liu, D.; Gorges, M.; Jenkins, S. University of Queensland vital signs dataset: Development of an accessible repository of anesthesia patient monitoring data for research. Anesth. Analg. 2012, 114, 584–589. [Google Scholar] [CrossRef]
- Johnson, A.; Pollard, T.; Shen, L.; Lehman, L.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Celi, L.A.; Mark, R. MIMIC-III, a freely accessible critical care database. Sci. Data 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Saeed, M.; Villarroel, M.; Reisner, A.; Clifford, G.; Lehman, L.; Moody, G.; Heldt, T.; Kyaw, T.; Moody, B.; Mark, R. Multiparameter Intelligent Monitoring in Intensive Care II: A public-access intensive care unit database. Crit. Care Med. 2011, 39, 952–960. [Google Scholar] [CrossRef]
- Schlesinger, O.; Vigderhouse, N.; Eytan, D.; Moshe, Y. Blood Pressure Estimation from PPG Signals Using Convolutional Neural Networks and Siamese Network. In Proceedings of the ICASSP 2020–2020 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Barcelona, Spain, 4–8 May 2020; pp. 1135–1139. [Google Scholar]
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Hypertension assessment via ECG and PPG signals: An evaluation using MIMIC database. Diagnostics 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Melillo, P.; Izzo, R.; Orrico, A.; Scala, P.; Attanasio, M.; Mirra, M.; De Luca, N.; Pecchia, L. Automatic prediction of cardiovascular and cerebrovascular events using heart rate variability analysis. PloS ONE 2015, 10, e0118504. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Gajbhiye, P.; Tripathy, R.; Acharya, U. A two-stage Deep CNN Architecture for the Classification of Low-risk and High-risk Hypertension Classes using Multi-lead ECG Signals. Inform. Med. Unlocked 2020, 21, 100479. [Google Scholar] [CrossRef]
- Ding, X.; Yan, B.; Zhang, Y.; Liu, J.; Zhao, N.; Tsang, H. Pulse transit time based continuous cuffless blood pressure estimation: A new extension and a comprehensive evaluation. Sci. Rep. 2017, 7, 11554. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.; Jafari, R.; Chung, W. Noninvasive cuffless blood pressure estimation using pulse transit time and impedance plethysmography. IEEE Trans. Biomed. Eng. 2018, 66, 967–976. [Google Scholar] [CrossRef]
- Seals, S.; Colantonio, L.; Tingle, J.; Shimbo, D.; Correa, A.; Griswold, M.; Muntner, P. Calibration of blood pressure measurements in the Jackson Heart Study. Blood Press. Monit. 2019, 24, 130–136. [Google Scholar] [CrossRef]



| Dataset | Data | Signal | Description | Reference Research |
|---|---|---|---|---|
| MIMIC II [229] | 26,870 | PPG | It contains different physiological signals, including blood pressure, PPG, and ECG. There is confusion about whether the data were synchronized, so using MIMIC II for any data such as PAT is not recommended. | [230] |
| MIMIC III [228] | 40,000 | ECG, PPG | It is a collection of physiological data from a different hospital; most research with clinical data for the BP measurement model has used this dataset. | [231] |
| University of Queensland’s vital signs dataset [227] | 23,617 | PPG | This dataset includes blood pressure measurement along with PPG signal. The case number is limited compared to MIMIC II and III. | [218] |
| SHAREE (Smart Health for Assessing the Risk of Events via ECG) [232] | 24 h electrocardiographic (ECG) Holter recordings of 139 hypertensive patients | ECG | Anti-hypertensive treatment was given to 139 patients, and after one month, the ECG Holter was used to record data. Each recording was of 24 h, containing three ECG leads sampling at 128 Hz. | [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan Mamun, M.M.R.; Sherif, A. Advancement in the Cuffless and Noninvasive Measurement of Blood Pressure: A Review of the Literature and Open Challenges. Bioengineering 2023, 10, 27. https://doi.org/10.3390/bioengineering10010027
Khan Mamun MMR, Sherif A. Advancement in the Cuffless and Noninvasive Measurement of Blood Pressure: A Review of the Literature and Open Challenges. Bioengineering. 2023; 10(1):27. https://doi.org/10.3390/bioengineering10010027
Chicago/Turabian StyleKhan Mamun, Mohammad Mahbubur Rahman, and Ahmed Sherif. 2023. "Advancement in the Cuffless and Noninvasive Measurement of Blood Pressure: A Review of the Literature and Open Challenges" Bioengineering 10, no. 1: 27. https://doi.org/10.3390/bioengineering10010027
APA StyleKhan Mamun, M. M. R., & Sherif, A. (2023). Advancement in the Cuffless and Noninvasive Measurement of Blood Pressure: A Review of the Literature and Open Challenges. Bioengineering, 10(1), 27. https://doi.org/10.3390/bioengineering10010027