State of the Art and Future of Stem Cell Therapy in Ischemic Stroke: Why Don’t We Focus on Their Administration?
Abstract
1. Introduction
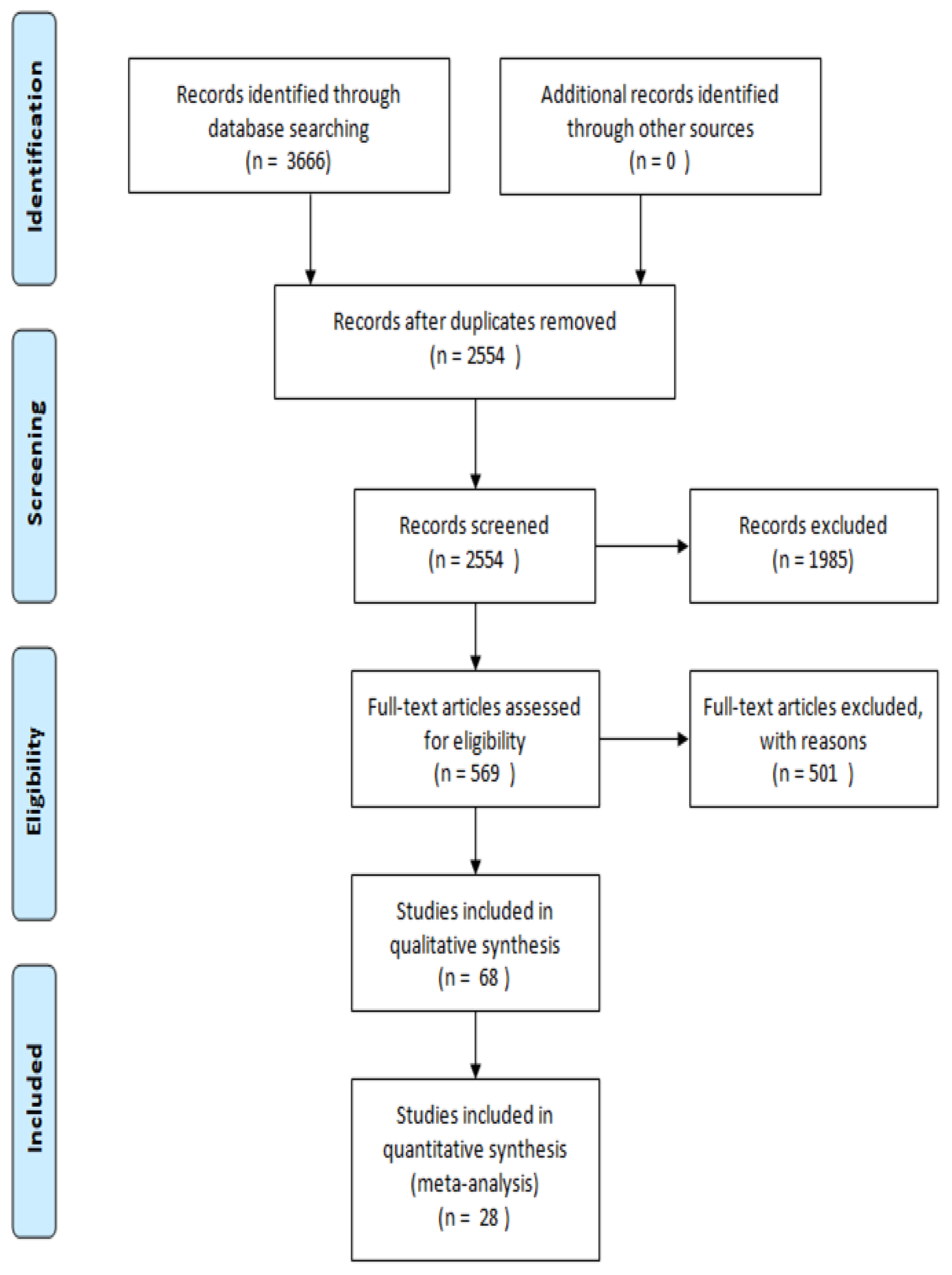
2. Methodology
3. Stroke: Description, Outcomes and Possible Therapies
4. Stem Cells: Definition, Types and Characteristics
5. In Vitro Preconditioning: Enhancing Capacities for Future Applications
6. Routes of Administration for Stem Cell Therapy
6.1. In Vivo Tests with Classical Routes of Administration: Optimizing Existing Techniques
6.1.1. Intracerebral Administration
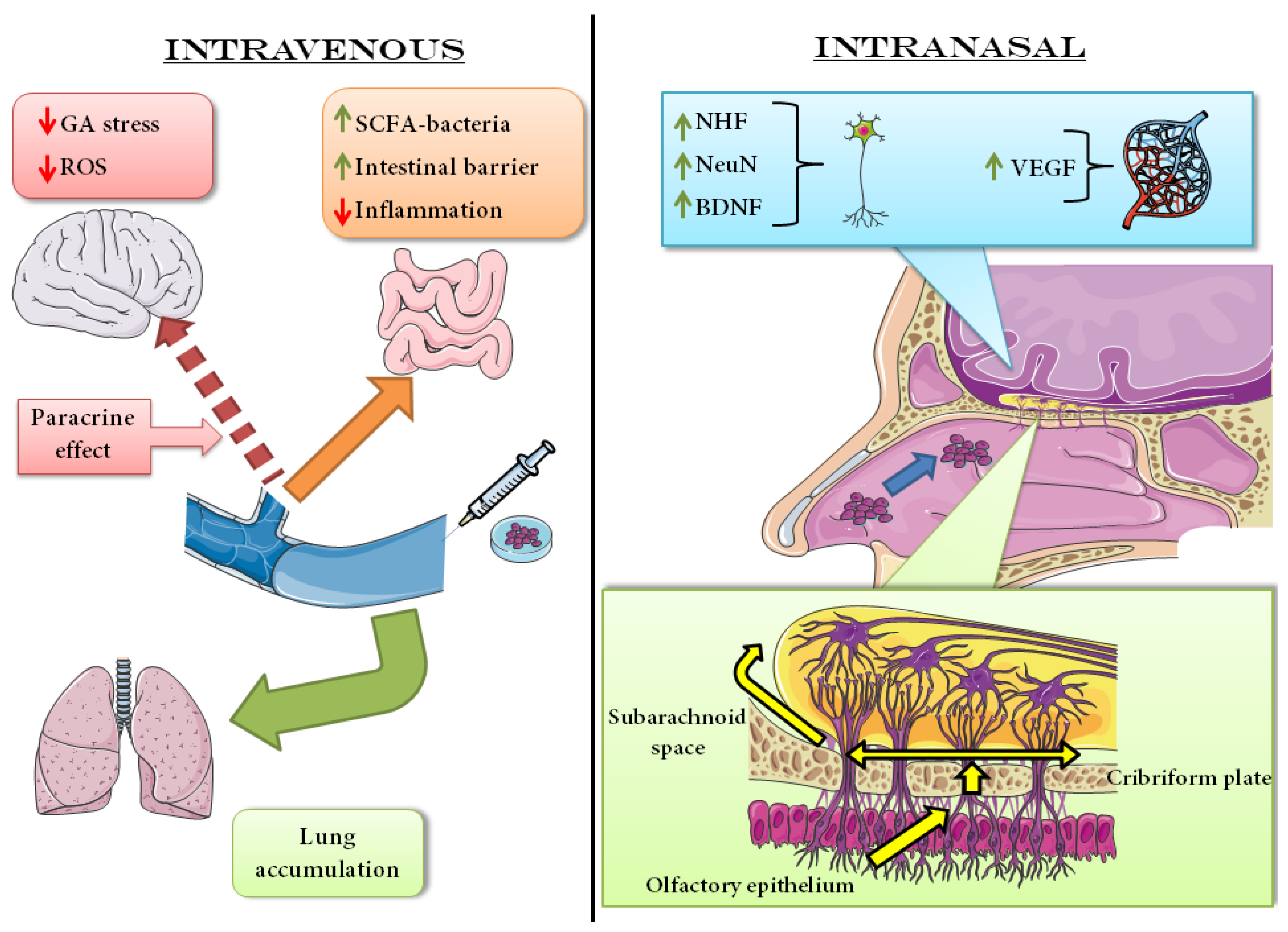
6.1.2. Intravenous Administration
6.2. In Vivo Tests with Non-Classical Routes of Administration: Intrathecal, Intraperitoneal and Intranasal Administration
6.2.1. Intrathecal Administration
6.2.2. Intraperitoneal Administration
6.2.3. Intranasal Administration
7. Clinical Evidence
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Association of Neurological Surgeon. Cerebrovascular Disease. Available online: https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Cerebrovascular-Disease (accessed on 26 October 2022).
- Ramalho-Santos, M.; Willenbring, H. On the Origin of the Term “Stem Cell”. Cell Stem Cell 2007, 1, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.M. Current state of stem cell-based therapies: An overview. Stem Cell Investig. 2020, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung and Blood Institute. What Is a Stroke? Available online: https://www.nhlbi.nih.gov/health/stroke (accessed on 26 October 2022).
- Phipps, M.S.; Cronin, C.A. Management of acute ischemic stroke. BMJ 2020, 368, l6983. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic Cascades in Ischemic Stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Denes, A.; Vidyasagar, R.; Feng, J.; Narvainen, J.; McColl, B.W.; Kauppinen, R.A.; Allan, S.M. Proliferating resident microglia after focal cerebral ischaemia in mice. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007, 27, 1941–1953. [Google Scholar] [CrossRef]
- Banati, R.B.; Gehrmann, J.; Schubert, P.; Kreutzberg, G.W. Cytotoxicity of microglia. Glia 1993, 7, 111–118. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Mendelow, A.D.; Hanley, D.F. Intracerebral haemorrhage. Lancet 2009, 373, 1632–1644. [Google Scholar] [CrossRef]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. What Are Some Common Outcomes of Stroke & Some Common Treatments for These Outcomes? Available online: https://www.nichd.nih.gov/health/topics/stroke/conditioninfo/treatment# (accessed on 26 October 2022).
- Roth, J.M. Recombinant tissue plasminogen activator for the treatment of acute ischemic stroke. Proc. (Bayl. University. Med. Cent.) 2011, 24, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Matei, N.; Camara, J.; Zhang, J.H. The Next Step in the Treatment of Stroke. Front. Neurol. 2021, 11, 582605. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.T.; Ben Assayag, E.; Shabashov-Stone, D.; Liraz-Zaltsman, S.; Mazzitelli, J.; Arenas, M.; Abduljawad, N.; Kliper, E.; Korczyn, A.D.; Thareja, N.S.; et al. CCR5 Is a Therapeutic Target for Recovery after Stroke and Traumatic Brain Injury. Cell 2019, 176, 1143–1157.e13. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Xue, T.; Chen, S.; Wu, X.; Yan, Z.; Wang, Z.; Wu, D.; Chen, Z.; Wang, Z. Is Fluoxetine Good for Subacute Stroke? A Meta-Analysis Evidenced From Randomized Controlled Trials. Front. Neurol. 2021, 12, 633781. [Google Scholar] [CrossRef]
- Richards, L.G.; Cramer, S.C. Advances in Stroke: Therapies Targeting Stroke Recovery. Stroke 2021, 52, 348–350. [Google Scholar] [CrossRef]
- Bliss, T.M.; Andres, R.H.; Steinberg, G.K. Optimizing the success of cell transplantation therapy for stroke. Neurobiol. Dis. 2010, 37, 275–283. [Google Scholar] [CrossRef]
- Singh, M.; Pandey, P.K.; Bhasin, A.; Padma, M.V.; Mohanty, S. Application of Stem Cells in Stroke: A Multifactorial Approach. Front. Neurosci. 2020, 14, 473. [Google Scholar] [CrossRef]
- Tan, N.; Xin, W.; Huang, M.; Mao, Y. Mesenchymal stem cell therapy for ischemic stroke: Novel insight into the crosstalk with immune cells. Front Neurol 2022, 13, 1048113. [Google Scholar] [CrossRef]
- Sobhani, A.; Khanlarkhani, N.; Baazm, M.; Mohammadzadeh, F.; Najafi, A.; Mehdinejadiani, S.; Sargolzaei Aval, F. Multipotent Stem Cell and Current Application. Acta Med. Iran. 2017, 55, 6–23. [Google Scholar]
- Fortier, L.A. Stem Cells: Classifications, Controversies, and Clinical Applications. Vet. Surg. 2005, 34, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G. Embryo-derived stem cells: Of mice and men. Annu. Rev. Cell Dev. Biol. 2001, 17, 435–462. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef]
- Huelsken, J. Tissue-specific stem cells: Friend or foe? Cell Res. 2009, 19, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Alatyyat, S.M.; Alasmari, H.M.; Aleid, O.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Umbilical cord stem cells: Background, processing and applications. Tissue Cell 2020, 65, 101351. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008, 26, 101–106. [Google Scholar] [CrossRef]
- Zayed, M.A.; Sultan, S.; Alsaab, H.O.; Yousof, S.M.; Alrefaei, G.I.; Alsubhi, N.H.; Alkarim, S.; Al Ghamdi, K.S.; Bagabir, S.A.; Jana, A.; et al. Stem-Cell-Based Therapy: The Celestial Weapon against Neurological Disorders. Cells 2022, 11, 3476. [Google Scholar] [CrossRef]
- Monsour, M.; Gorsky, A.; Nguyen, H.; Castelli, V.; Lee, J.Y.; Borlongan, C.V. Enhancing oxidative phosphorylation over glycolysis for energy production in cultured mesenchymal stem cells. Neuroreport 2022, 33, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Gorsky, A.; Monsour, M.; Nguyen, H.; Castelli, V.; Lee, J.Y.; Borlongan, C.V. Metabolic Switching of Cultured Mesenchymal Stem Cells Creates Super Mitochondria in Rescuing Ischemic Neurons. Neuromol. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, S.; Xu, W.; Hong, Y.; Dou, R.; Shen, H.; Liu, X.; Wu, T.; He, J.C. Interleukin-17A promotes the differentiation of bone marrow mesenchymal stem cells into neuronal cells. Tissue Cell 2021, 69, 101482. [Google Scholar] [CrossRef]
- Zhuo, Y.; Wang, L.; Ge, L.; Li, X.; Duan, D.; Teng, X.; Jiang, M.; Liu, K.; Yuan, T.; Wu, P.; et al. Hypoxic Culture Promotes Dopaminergic-Neuronal Differentiation of Nasal Olfactory Mucosa Mesenchymal Stem Cells via Upregulation of Hypoxia-Inducible Factor-1α. Cell Transplant. 2017, 26, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, S.; Beppu, M.; Takagi, T.; Nakano-Doi, A.; Nakagomi, N.; Matsuyama, T.; Nakagomi, T.; Yoshimura, S. Ischemia-Induced Multipotent Stem Cells Isolated from Stroke Patients Exhibit Higher Neurogenic Differentiation Potential than Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2020, 29, 994–1006. [Google Scholar] [CrossRef]
- Chiang, T.; Messing, R.O.; Chou, W.H. Mouse model of middle cerebral artery occlusion. J. Vis. Exp. JoVE 2011, 48, e2761. [Google Scholar] [CrossRef] [PubMed]
- Kazemi Arababadi, M.; Rahmani, M.R.; Asadi, F.; Shabanizadeh, A.; Yousefi-Ahmadipour, A. Involvement of T-bet and GATA3 transcription factors in Mesenchymal stem cells and royal jelly combination treatment in brain stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2022, 31, 106678. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.; Komai, S.; Eliava, M.; Seeburg, P.H.; Osten, P. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 2006, 1, 3166–3173. [Google Scholar] [CrossRef]
- Rodríguez-Frutos, B.; Otero-Ortega, L.; Gutiérrez-Fernández, M.; Fuentes, B.; Ramos-Cejudo, J.; Díez-Tejedor, E. Stem Cell Therapy and Administration Routes After Stroke. Transl. Stroke Res. 2016, 7, 378–387. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, K.R.; Jang, H.; Lee, N.K.; Jung, Y.H.; Kim, J.P.; Lee, J.I.; Chang, J.W.; Park, S.; Kim, S.T.; et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase I clinical trial. Alzheimer’s Res. Ther. 2021, 13, 154. [Google Scholar] [CrossRef]
- Muir, K.W.; Bulters, D.; Willmot, M.; Sprigg, N.; Dixit, A.; Ward, N.; Tyrrell, P.; Majid, A.; Dunn, L.; Bath, P.; et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J. Neurol. Neurosurg. Psychiatry 2020, 91, 396. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, X.; Yao, C.; Bingwa, L.A.; Wang, H.; Lin, Z.; Jin, K.; Zhuge, Q.; Yang, S. Transplantation of Roxadustat-preconditioned bone marrow stromal cells improves neurological function recovery through enhancing grafted cell survival in ischemic stroke rats. CNS Neurosci. Ther. 2022, 28, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, Y.; Zhang, Q.; Li, J.; Lin, B.; Zhao, J.; Zhang, F.; Li, Y.; Yang, X.; Lu, L.; et al. Upregulating HIF-1α to Boost the Survival of Neural Stem Cells via Functional Peptides-Complexed MRI-Visible Nanomedicine for Stroke Therapy. Adv. Healthc. Mater. 2022, e2201630. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Wan, J.; Li, X.; Liu, R.; Xu, C.; An, Y.; Chen, J. Preconditioning with Trehalose Protects the Bone Marrow-Derived Mesenchymal Stem Cells Under Oxidative Stress and Enhances the Stem Cell-Based Therapy for Cerebral Ischemic Stroke. Cell. Reprogramming 2022, 24, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Patkar, S.; Uwanogho, D.; Modo, M.; Tate, R.J.; Plevin, R.; Carswell, H.V.O. Targeting 17β-estradiol biosynthesis in neural stem cells improves stroke outcome. Front. Cell. Neurosci. 2022, 16, 917181. [Google Scholar] [CrossRef]
- McCrary, M.R.; Jiang, M.Q.; Jesson, K.; Gu, X.; Logun, M.T.; Wu, A.; Gonsalves, N.; Karumbaiah, L.; Yu, S.P.; Wei, L. Glycosaminoglycan scaffolding and neural progenitor cell transplantation promotes regenerative immunomodulation in the mouse ischemic brain. Exp. Neurol. 2022, 357, 114177. [Google Scholar] [CrossRef]
- Deng, M.; Liu, J.; He, J.; Lan, Z.; Cheng, S.; Hu, Z.; Xiao, H. Bone marrow-derived mesenchymal stem cells overexpressed with miR-182-5p protects against brain injury in a mouse model of cerebral ischemia. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2022, 31, 106748. [Google Scholar] [CrossRef]
- Gong, P.; Tian, Q.; He, Y.; He, P.; Wang, J.; Guo, Y.; Ye, Q.; Li, M. Dental pulp stem cell transplantation facilitates neuronal neuroprotection following cerebral ischemic stroke. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 152, 113234. [Google Scholar] [CrossRef]
- Barbash, I.M.; Chouraqui, P.; Baron, J.; Feinberg, M.S.; Etzion, S.; Tessone, A.; Miller, L.; Guetta, E.; Zipori, D.; Kedes, L.H.; et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation 2003, 108, 863–868. [Google Scholar] [CrossRef]
- Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells 2008, 1, 1–7. [Google Scholar] [CrossRef]
- Tögel, F.; Yang, Y.; Zhang, P.; Hu, Z.; Westenfelder, C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008, 295, F315–F321. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.; Janowski, M.; Walczak, P. Intra-Arterial Delivery of Cell Therapies for Stroke. Stroke 2018, 49, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Álvarez-Grech, J.; Vallejo-Cremades, M.T.; Expósito-Alcaide, M.; Merino, J.; Roda, J.M.; Díez-Tejedor, E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience 2011, 175, 394–405. [Google Scholar] [CrossRef]
- Chia, Y.C.; Anjum, C.E.; Yee, H.R.; Kenisi, Y.; Chan, M.K.S.; Wong, M.B.F.; Pan, S.Y. Stem Cell Therapy for Neurodegenerative Diseases: How Do Stem Cells Bypass the Blood-Brain Barrier and Home to the Brain? Stem Cells Int. 2020, 2020, 8889061. [Google Scholar] [CrossRef]
- Zhuo, Y.; Chen, W.; Li, W.; Huang, Y.; Duan, D.; Ge, L.; He, J.; Liu, J.; Hu, Z.; Lu, M. Ischemic-hypoxic preconditioning enhances the mitochondrial function recovery of transplanted olfactory mucosa mesenchymal stem cells via miR-181a signaling in ischemic stroke. Aging 2021, 13, 11234–11256. [Google Scholar] [CrossRef]
- Zhao, L.N.; Ma, S.W.; Xiao, J.; Yang, L.J.; Xu, S.X.; Zhao, L. Bone marrow mesenchymal stem cell therapy regulates gut microbiota to improve post-stroke neurological function recovery in rats. World J. Stem Cells 2021, 13, 1905–1917. [Google Scholar] [CrossRef]
- Li, T.; Sun, Q.; Feng, L.; Yan, D.; Wang, B.; Li, M.; Xiong, X.; Ma, D.; Gao, Y. Uncovering the characteristics of the gut microbiota in patients with acute ischemic stroke and phlegm-heat syndrome. PLoS ONE 2022, 17, e0276598. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, J.; Huang, Y.; Tang, X.; Xiao, H.; Liu, Z.; Jiang, Z.; Zeng, L.; Hu, Z.; Lu, M. OM-MSCs Alleviate the Golgi Apparatus Stress Response following Cerebral Ischemia/Reperfusion Injury via the PEDF-PI3K/Akt/mTOR Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 4805040. [Google Scholar] [CrossRef]
- Intracerebroventricular, Intracerebral and Intrathecal. In Encyclopedia of Pain; Schmidt, R.F., Willis, W.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; p. 1017. [Google Scholar] [CrossRef]
- Ran, Y.; Dong, Y.; Li, Y.; Xie, J.; Zeng, S.; Liang, C.; Dai, W.; Tang, W.; Wu, Y.; Yu, S. Mesenchymal stem cell aggregation mediated by integrin α4/VCAM-1 after intrathecal transplantation in MCAO rats. Stem Cell Res. Ther. 2022, 13, 507. [Google Scholar] [CrossRef]
- Kaiser, E.E.; Waters, E.S.; Yang, X.; Fagan, M.M.; Scheulin, K.M.; Sneed, S.E.; Cheek, S.R.; Jeon, J.H.; Shin, S.K.; Kinder, H.A.; et al. Tanshinone IIA-Loaded Nanoparticle and Neural Stem Cell Therapy Enhances Recovery in a Pig Ischemic Stroke Model. Stem Cells Transl. Med. 2022, 11, 1061–1071. [Google Scholar] [CrossRef]
- Yousefi, F.; Ebtekar, M.; Soleimani, M.; Soudi, S.; Hashemi, S.M. Comparison of in vivo immunomodulatory effects of intravenous and intraperitoneal administration of adipose-tissue mesenchymal stem cells in experimental autoimmune encephalomyelitis (EAE). Int. Immunopharmacol. 2013, 17, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, M.; Vali, R.; Yaghoobi, Z.; Sehati, F.; Jashn, V.; Kolur, S.M.; Akhondzadeh, F.; Ashabi, G. Adipose-derived mesenchymal stem cells reduced transient cerebral ischemia injury by modulation of inflammatory factors and AMPK signaling. Behav. Brain Res. 2022, 433, 114001. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, M.; Vital, S.; Stokes, K.Y.; Wang, Y.; Yun, J.W.; White, L.A.; Chernyshev, O.; Kelley, R.E.; Alexander, J.S. Human placenta mesenchymal stem cell protection in ischemic stroke is angiotensin converting enzyme-2 and masR receptor-dependent. Stem Cells (Dayt. Ohio) 2021, 39, 1335–1348. [Google Scholar] [CrossRef]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, M.; Taguchi, A.; Tsuda, H.; Sato, Y.; Yamahara, K.; Harada-Shiba, M.; Miyazato, M.; Ikeda, T.; Iida, H.; Tsuji, M. Intraperitoneal and intravenous deliveries are not comparable in terms of drug efficacy and cell distribution in neonatal mice with hypoxia-ischemia. Brain Dev. 2015, 37, 376–386. [Google Scholar] [CrossRef]
- Danielyan, L.; Schäfer, R.; von Ameln-Mayerhofer, A.; Buadze, M.; Geisler, J.; Klopfer, T.; Burkhardt, U.; Proksch, B.; Verleysdonk, S.; Ayturan, M.; et al. Intranasal delivery of cells to the brain. Eur. J. Cell Biol. 2009, 88, 315–324. [Google Scholar] [CrossRef]
- van Velthoven, C.T.J.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Nasal Administration of Stem Cells: A Promising Novel Route to Treat Neonatal Ischemic Brain Damage. Pediatr. Res. 2010, 68, 419–422. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Akbarpour, B.; Karimi-Haghighi, S.; Pandamooz, S.; Belém-Filho, I.J.A.; Masís-Calvo, M.; Salimi, H.; Lashanizadegan, R.; Pouramini, A.; Owjfard, M.; et al. Therapeutic potential of hair follicle-derived stem cell intranasal transplantation in a rat model of ischemic stroke. BMC Neurosci. 2022, 23, 47. [Google Scholar] [CrossRef]
- Lu, S.; Li, K.; Yang, Y.; Wang, Q.; Yu, Y.; Wang, Z.; Luan, Z. Optimization of an Intranasal Route for the Delivery of Human Neural Stem Cells to Treat a Neonatal Hypoxic-Ischemic Brain Injury Rat Model. Neuropsychiatr. Dis. Treat. 2022, 18, 413–426. [Google Scholar] [CrossRef]
- Shen, H.; Gu, X.; Wei, Z.Z.; Wu, A.; Liu, X.; Wei, L. Combinatorial intranasal delivery of bone marrow mesenchymal stem cells and insulin-like growth factor-1 improves neurovascularization and functional outcomes following focal cerebral ischemia in mice. Exp. Neurol. 2021, 337, 113542. [Google Scholar] [CrossRef]
- Larpthaveesarp, A.; Pathipati, P.; Ostrin, S.; Rajah, A.; Ferriero, D.; Gonzalez, F.F. Enhanced Mesenchymal Stromal Cells or Erythropoietin Provide Long-Term Functional Benefit After Neonatal Stroke. Stroke 2021, 52, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.J.; Deveau, T.C.; Gu, X.; Kim, Y.S.; Xu, Y.; Yu, S.P.; Wei, L. Delayed and repeated intranasal delivery of bone marrow stromal cells increases regeneration and functional recovery after ischemic stroke in mice. BMC Neurosci. 2018, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- van Velthoven, C.T.; Dzietko, M.; Wendland, M.F.; Derugin, N.; Faustino, J.; Heijnen, C.J.; Ferriero, D.M.; Vexler, Z.S. Mesenchymal stem cells attenuate MRI-identifiable injury, protect white matter, and improve long-term functional outcomes after neonatal focal stroke in rats. J. Neurosci. Res. 2017, 95, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- González-Reimers, E.; Martín-González, C.; Romero-Acevedo, L.; Quintero-Platt, G.; Gonzalez-Arnay, E.; Santolaria-Fernández, F. Chapter 15—Effects of Alcohol on the Corpus Callosum. In Neuroscience of Alcohol; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 143–152. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Z.Z.; Gu, X.; Zhang, J.Y.; Zhang, Y.; Li, J.; Wei, L. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp. Neurol. 2015, 272, 78–87. [Google Scholar] [CrossRef]
- Savitz, S.I.; Misra, V.; Kasam, M.; Juneja, H.; Cox, C.S., Jr.; Alderman, S.; Aisiku, I.; Kar, S.; Gee, A.; Grotta, J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011, 70, 59–69. [Google Scholar] [CrossRef]
- Baak, L.M.; Wagenaar, N.; van der Aa, N.E.; Groenendaal, F.; Dudink, J.; Tataranno, M.L.; Mahamuud, U.; Verhage, C.H.; Eijsermans, R.; Smit, L.S.; et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): A first-in-human, open-label intervention study. Lancet. Neurol. 2022, 21, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Kim, E.H.; Cha, J.M.; Moon, G.J. Adult Stem Cell Therapy for Stroke: Challenges and Progress. J. Stroke 2016, 18, 256–266. [Google Scholar] [CrossRef]
| Cells Precondition | Cell Type | Effects | Mechanisms of Action | Ref. |
|---|---|---|---|---|
| IL-17A exposure | BM-MSCs | Neuronal differentiation | IKKα/β phosphorylation decrease Hes-1 increase Enolase increase Notch/Wnt/Nf-κB partway induction | [36] |
| Metabolic switch | UC-MSCs | OCR/ECAR increase | Oxidative phosphorylation as a primary source of energy | [34] |
| Metabolic switch | MSCs | “Super mitochondria” development | ROS reduction Energy production increase | [35] |
| Hypoxia | OM-MSCs | Increased dopamine release Promote dopaminergic development | HIF-1α upregulation Expression of Lmx1b, Pixt3, Nurr1, En1 and En2 | [37] |
| None | iSCs + BM-MSCs | iSCs can better differentiate into neurons | Not applicable | [38] |
| Route | Cells Precondition | Cell Type | Experimental Model | Effects | Mechanisms of Action | Ref. |
|---|---|---|---|---|---|---|
| i.c. | Hypoxia + FG-4592 | BM-MSC | pMCAO | Enhancement of autophagy Increased survivability | HIF-1α/BNIP3 signaling pathway activation | [45] |
| i.c. | Trehalose | BM-MSCs | In vitro + MCAO | Increased autophagy Neuroprotection New vascularization | AMPK pathway induction Oxidative stress protection Bcl-2/Bax ratio increased Activation mTOR pathway BDNF, VEGF and HGF secretion | [47] |
| i.c. | Dax1 KD | MHP36 | MCAO | Better and faster recover after model induction Reduction of the lesion site | 17β-estradiol increased production Synaptogenesis increase GAP-43 and MAP-2 expression | [48] |
| i.v. | Hypoxia | OM-MSCs | In vitro + MCAO | Downregulation of miR-181a | Neuronal mitochondria protective function enhancement ROS decrease Regulation of HSP70, GRP78, Bcl-2 and Mcl-1 | [58] |
| i.c. | CS-A encapsulation | NPCs | MCAO (3–6 ligations) | Increased angiogenesis Increased neurogenesis Score improvement in behavioral tests | MCP-1 increase in microglia PPARγ-expressing microglia recruitment IL-10 levels increase VEGFR2, TIE2 and FGF2 increase expression in microglia | [49] |
| i.c. | Nanoparticles (HIF-1α upregulation) | NSCs | PTI | Infarction volume reduction Efficient migration to the infarcted area | Casp8ap2 decrease Increased HIF-1α Increased CXCR4 | [46] |
| i.c. | miR-185-5p overexpression | BM-MSCs | MCAO | Better scores in behavioral tests Less reactive microglia | TLR4/Nf-κB signaling block IL-6, IL-1β and TNF-α reduction | [50] |
| Not specified | None | MSCs + Royal jelly | MCAO | Compensation of long-term inflammation | IFN-γ serum level decrease IL-1β brain level decrease Th1 and Th2 presence increase | [40] |
| i.v. | None | BM-MSCs | MCAO | Reduced intestinal damage Reduced inflammation Mitigate stroke effect on microbiota | SCFA-producing bacteria population increase Stimulation of tight-junction proteins Butyrate production | [59] |
| i.v. | None | OM-MSCs | MCAO | Reduction of GA stress Restoring of PI3K/Akt/mTOR pathway | PEDF secretion GOLPH3 level reduction | [61] |
| i.c. | None | DPSCs | tMCAO | Better outcome for neurological functions | Brain edema reduction Bcl-2/Bax ratio increase NeuN increased expression | [51] |
| Route | Cells Precondition | Cell Type | Experimental Model | Effects | Mechanisms of Action | Ref. |
|---|---|---|---|---|---|---|
| i.t. | 3D culture | MSCs | In vitro + MCAO | Better differentiation potential Increase secretion of beneficial factors Fewer aggregates | Decrease expression of VCAM and integrin α4 | [63] |
| i.t. | Nanoparticles (Tan IIA) | iNSC | MCAO | Reduction of tissue degradation Induction of neurogenesis Enhanced survivability | Iba1+ cells activation reduction Doublecortin+ neuroblasts increase in SVZ and lesion borders IL-4 and IL-13 increase PI3K/Akt/mTOR pathway enhancement | [64] |
| i.p. | None | AD-MSCs | CCAO | Reduced oxidative stress BBB protection Reduction of inflammation | IL-6 and TNF-α reduction Bax/Bcl-2 ratio decrease AMPK action on oxidative stress Klotho-α action on inflammation | [66] |
| i.p. | None | h-PMSCs | MCAO | Improved neurological functions | ACE-2 increased expression | [67] |
| i.p and i.v. | None | MSCs | wt | i.v. Primarily targets lungs i.p Primarily targets liver, spleen and kidneys | Not applicable | [69] |
| i.n. | None | HFSCs | MCAO | Reduction infarcted area Better scores in behavioral tests | NeuN e VEGF level restoration NT-3 level regulated | [72] |
| i.n. | Hypoxia | hNSCs | Neonatal HI | Brain engraftment Better migration | CXCR4 enhancement Apoptosis ratio decrease | [73] |
| i.n. | Erythropoietin | MSCs | MCAO | Ameliorate sensorimotor capacity and long-lasting cognitive improve | Increased brain volume Paracrine effects | [75] |
| i.n. | IGF-1 | BM-MSCs | In vitro + pMCAO (2-3 branches) + tCCAO | Prevention of apoptosis Better migration Increased neurogenesis Angiogenesis induction | PI3K/Akt pathway activation CXCR4 increased expression VEGF, Ang-1 and BDNF secretion | [74] |
| i.n. | Hypoxia | BM-MSCs | pMCAO (2-3 branches) + tCCAO | Increased neurogenesis Sensorimotor score improved Increase cerebral blood flow | NeuN/BrdU positive cells amount increased Glut-1/BrdU positive cells amount increased Angiogenesis increased | [76] |
| i.n. | None | MSCs | MCAO | Improvement in white matter condition Improved sensorimotor functionality Decreased lateralizing behavior | NHF increased in the lesioned area | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valeri, A.; Mazzon, E. State of the Art and Future of Stem Cell Therapy in Ischemic Stroke: Why Don’t We Focus on Their Administration? Bioengineering 2023, 10, 118. https://doi.org/10.3390/bioengineering10010118
Valeri A, Mazzon E. State of the Art and Future of Stem Cell Therapy in Ischemic Stroke: Why Don’t We Focus on Their Administration? Bioengineering. 2023; 10(1):118. https://doi.org/10.3390/bioengineering10010118
Chicago/Turabian StyleValeri, Andrea, and Emanuela Mazzon. 2023. "State of the Art and Future of Stem Cell Therapy in Ischemic Stroke: Why Don’t We Focus on Their Administration?" Bioengineering 10, no. 1: 118. https://doi.org/10.3390/bioengineering10010118
APA StyleValeri, A., & Mazzon, E. (2023). State of the Art and Future of Stem Cell Therapy in Ischemic Stroke: Why Don’t We Focus on Their Administration? Bioengineering, 10(1), 118. https://doi.org/10.3390/bioengineering10010118






