Concise Review: Bioengineering of Limbal Stem Cell Niche
Abstract
1. Introduction
2. Limbal Niche (LN)
2.1. Stem Cell Niche
2.2. LN Microstructure and Components
2.2.1. ECM of LN
2.2.2. Genes and Proteins Implicated in LN Regulation
3. LESCs’ Functions
3.1. Epithelial Maintenance
3.2. Epithelial Wound Healing
4. LSCD
5. Limbal Stem Cell Transplantation
5.1. Tissue Transplantation
5.2. LESC Culture and Expansion
6. LN Restoration
6.1. Bio-Scaffolds
6.1.1. Amniotic Membrane
6.1.2. Fabrication of Bio-Active ECMs
6.1.3. Others
6.2. Revitalization of Limbal Niche via Biological Factors
6.2.1. Blood-Derived Factors
6.2.2. Bio-Active Soluble Factors/Cocktails
6.3. Cell-Based Strategies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar]
- Tavakkoli, F.; Eleiwa, T.K.; Elhusseiny, A.M.; Damala, M.; Rai, A.K.; Cheraqpour, K.; Ansari, M.H.; Doroudian, M.H.; Keshel, S.; Soleimani, M. Corneal stem cells niche and homeostasis impacts in regenerative medicine; concise review. Eur. J. Ophthalmol. 2023, 11206721221150065. [Google Scholar] [CrossRef]
- Walther, V.; Graham, T.A. Location, location, location! The reality of life for an intestinal stem cell in the crypt. J. Pathol. 2014, 234, 1–4. [Google Scholar] [CrossRef]
- Nasser, W.; Amitai-Lange, A.; Soteriou, D.; Hanna, R.; Tiosano, B.; Fuchs, Y.; Shalom-Feuerstein, R. Corneal-committed cells restore the stem cell pool and tissue boundary following injury. Cell Rep. 2018, 22, 323–331. [Google Scholar] [CrossRef]
- Nubile, M.; Curcio, C.; Dua, H.S.; Calienno, R.; Lanzini, M.; Iezzi, M.; Mastropasqua, R.; Agnifili, L.; Mastropasqua, L. Pathological changes of the anatomical structure and markers of the limbal stem cell niche due to inflammation. Mol. Vis. 2013, 19, 516. [Google Scholar]
- Shortt, A.J.; Secker, G.A.; Munro, P.M.; Khaw, P.T.; Tuft, S.J.; Daniels, J.T. Characterization of the limbal epithelial stem cell niche: Novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells 2007, 25, 1402–1409. [Google Scholar] [CrossRef]
- Townsend, W.M. The limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1991, 89, 721. [Google Scholar]
- Dua, H.S.; Shanmuganathan, V.; Powell-Richards, A.; Tighe, P.; Joseph, A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005, 89, 529–532. [Google Scholar] [CrossRef]
- Grieve, K.; Ghoubay, D.; Georgeon, C.; Thouvenin, O.; Bouheraoua, N.; Paques, M.; Borderie, V. Three-dimensional structure of the mammalian limbal stem cell niche. Exp. Eye Res. 2015, 140, 75–84. [Google Scholar] [CrossRef]
- Notara, M.; Schrader, S.; Daniels, J.T. The porcine limbal epithelial stem cell niche as a new model for the study of transplanted tissue-engineered human limbal epithelial cells. Tissue Eng. Part A 2011, 17, 741–750. [Google Scholar] [CrossRef]
- Vantrappen, L.; Geboes, K.; Missotten, L.; Maudgal, P.; Desmet, V. Lymphocytes and Langerhans cells in the normal human cornea. Investig. Ophthalmol. Vis. Sci. 1985, 26, 220–225. [Google Scholar]
- Polisetti, N.; Gießl, A.; Zenkel, M.; Heger, L.; Dudziak, D.; Naschberger, E.; Stich, L.; Steinkasserer, A.; Kruse, F.E.; Schlötzer-Schrehardt, U. Melanocytes as emerging key players in niche regulation of limbal epithelial stem cells. Ocul. Surf. 2021, 22, 172–189. [Google Scholar] [CrossRef]
- Dziasko, M.A.; Tuft, S.J.; Daniels, J.T. Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments. Exp. Eye Res. 2015, 138, 70–79. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Haq, Z.; Kang, K.; Jabbehdari, S.; l Rosenblatt, M.; Djalilian, A.R. Strategies for reconstructing the limbal stem cell niche. Ocul. Surf. 2019, 17, 230–240. [Google Scholar] [CrossRef]
- Mathews, S.; Chidambaram, J.D.; Lanjewar, S.; Mascarenhas, J.; Prajna, N.V.; Muthukkaruppan, V.; Chidambaranathan, G.P. In vivo confocal microscopic analysis of normal human anterior limbal stroma. Cornea 2015, 34, 464. [Google Scholar] [CrossRef]
- Yamamoto, N.; Hirano, K.; Kojima, H.; Sumitomo, M.; Yamashita, H.; Ayaki, M.; Taniguchi, K.; Tanikawa, A.; Horiguchi, M. Cultured human corneal epithelial stem/progenitor cells derived from the corneal limbus. In Vitro Cell. Dev. Biol.-Anim. 2010, 46, 774–780. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Burgeson, R.E.; Butkowski, R.J.; Michael, A.F.; Sun, T.-T.; Kenney, M.C. Human corneal basement membrane heterogeneity: Topographical differences in the expression of type IV collagen and laminin isoforms. Lab. Investig. J. Tech. Methods Pathol. 1995, 72, 461–473. [Google Scholar]
- Schlötzer-Schrehardt, U.; Dietrich, T.; Saito, K.; Sorokin, L.; Sasaki, T.; Paulsson, M.; Kruse, F. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp. Eye Res. 2007, 85, 845–860. [Google Scholar] [CrossRef]
- Yeung, A.M.-H.; Schlötzer-Schrehardt, U.; Kulkarni, B.; Tint, N.L.; Hopkinson, A.; Dua, H.S. Limbal epithelial crypt: A model for corneal epithelial maintenance and novel limbal regional variations. Arch. Ophthalmol. 2008, 126, 665–669. [Google Scholar] [CrossRef]
- Sun, M.; Puri, S.; Mutoji, K.N.; Coulson-Thomas, Y.M.; Hascall, V.C.; Jackson, D.G.; Gesteira, T.F.; Coulson-Thomas, V.J. Hyaluronan derived from the limbus is a key regulator of corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1050–1062. [Google Scholar] [CrossRef]
- Gesteira, T.F.; Sun, M.; Coulson-Thomas, Y.M.; Yamaguchi, Y.; Yeh, L.-K.; Hascall, V.; Coulson-Thomas, V.J. Hyaluronan rich microenvironment in the limbal stem cell niche regulates limbal stem cell differentiation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4407–4421. [Google Scholar] [CrossRef]
- Yazdani, M.; Shahdadfar, A.; Jackson, C.J.; Utheim, T.P. Hyaluronan-based hydrogel scaffolds for limbal stem cell transplantation: A review. Cells 2019, 8, 245. [Google Scholar] [CrossRef]
- Seyed-Safi, A.G.; Daniels, J.T. The limbus: Structure and function. Exp. Eye Res. 2020, 197, 108074. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Ouyang, H.; Xue, Y.; Lin, Y.; Zhang, X.; Xi, L.; Patel, S.; Cai, H.; Luo, J.; Zhang, M.; Zhang, M. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature 2014, 511, 358–361. [Google Scholar] [CrossRef]
- Li, M.; Huang, H.; Li, L.; He, C.; Zhu, L.; Guo, H.; Wang, L.; Liu, J.; Wu, S.; Liu, J. Core transcription regulatory circuitry orchestrates corneal epithelial homeostasis. Nat. Commun. 2021, 12, 420. [Google Scholar] [CrossRef]
- Mei, H.; Nakatsu, M.N.; Baclagon, E.R.; Deng, S.X. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem Cells 2014, 32, 938–945. [Google Scholar] [CrossRef]
- González, S.; Halabi, M.; Ju, D.; Tsai, M.; Deng, S.X. Role of Jagged1-mediated Notch signaling activation in the differentiation and stratification of the human limbal epithelium. Cells 2020, 9, 1945. [Google Scholar] [CrossRef]
- Masood, F.; Chang, J.-H.; Akbar, A.; Song, A.; Hu, W.-Y.; Azar, D.T.; Rosenblatt, M.I. Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions. Cells 2022, 11, 3247. [Google Scholar] [CrossRef]
- Kaplan, N.; Wang, J.; Wray, B.; Patel, P.; Yang, W.; Peng, H.; Lavker, R.M. Single-cell RNA transcriptome helps define the limbal/corneal epithelial stem/early transit amplifying cells and how autophagy affects this population. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3570–3583. [Google Scholar] [CrossRef]
- Li, D.-Q.; Kim, S.; Li, J.-M.; Gao, Q.; Choi, J.; Bian, F.; Hu, J.; Zhang, Y.; Li, J.; Lu, R. Single-cell transcriptomics identifies limbal stem cell population and cell types mapping its differentiation trajectory in limbal basal epithelium of human cornea. Ocul. Surf. 2021, 20, 20–32. [Google Scholar] [CrossRef]
- Menzel-Severing, J.; Zenkel, M.; Polisetti, N.; Sock, E.; Wegner, M.; Kruse, F.E.; Schlötzer-Schrehardt, U. Transcription factor profiling identifies Sox9 as regulator of proliferation and differentiation in corneal epithelial stem/progenitor cells. Sci. Rep. 2018, 8, 10268. [Google Scholar] [CrossRef]
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.; Perez, V.L. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014, 511, 353–357. [Google Scholar] [CrossRef]
- Thoft, R.A. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 1983, 24, 1442–1443. [Google Scholar]
- Ahmad, S.; Kolli, S.; Lako, M.; Figueiredo, F.; Daniels, J.T. Stem cell therapies for ocular surface disease. Drug Discov. Today 2010, 15, 306–313. [Google Scholar] [CrossRef]
- Seyed-Safi, A.G.; Daniels, J.T. A validated porcine corneal organ culture model to study the limbal response to corneal epithelial injury. Exp. Eye Res. 2020, 197, 108063. [Google Scholar] [CrossRef]
- Cotsarelis, G.; Cheng, S.-Z.; Dong, G.; Sun, T.-T.; Lavker, R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef]
- Amitai-Lange, A.; Altshuler, A.; Bubley, J.; Dbayat, N.; Tiosano, B.; Shalom-Feuerstein, R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells 2015, 33, 230–239. [Google Scholar] [CrossRef]
- Park, M.; Richardson, A.; Pandzic, E.; Lobo, E.P.; Whan, R.; Watson, S.L.; Lyons, J.G.; Wakefield, D.; Di Girolamo, N. Visualizing the contribution of keratin-14+ limbal epithelial precursors in corneal wound healing. Stem Cell Rep. 2019, 12, 14–28. [Google Scholar] [CrossRef]
- Elhusseiny, A.M.; Soleimani, M.; Eleiwa, T.K.; ElSheikh, R.H.; Frank, C.R.; Naderan, M.; Yazdanpanah, G.; Rosenblatt, M.I.; Djalilian, A.R. Current and Emerging Therapies for Limbal Stem Cell Deficiency. Stem Cells Transl. Med. 2022, 11, 259–268. [Google Scholar] [CrossRef]
- Bonnet, C.; Roberts, J.S.; Deng, S.X. Limbal stem cell diseases. Exp. Eye Res. 2021, 205, 108437. [Google Scholar] [CrossRef]
- Tabatabaei, S.A.; Soleimani, M.; Mirshahi, R.; Zandian, M.; Ghasemi, H.; Hashemian, M.N.; Ghomi, Z. Selective localized tenonplasty for corneal burns based on the findings of ocular surface fluorescein angiography. Cornea 2017, 36, 1014–1017. [Google Scholar] [CrossRef]
- Barbaro, V.; Ferrari, S.; Fasolo, A.; Pedrotti, E.; Marchini, G.; Sbabo, A.; Nettis, N.; Ponzin, D.; Di Iorio, E. Evaluation of ocular surface disorders: A new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br. J. Ophthalmol. 2010, 94, 926–932. [Google Scholar] [CrossRef]
- Garcia, I.; Etxebarria, J.; Boto-de-Los-Bueis, A.; Díaz-Valle, D.; Rivas, L.; Martínez-Soroa, I.; Saenz, N.; López, C.; Del-Hierro-Zarzuelo, A.; Méndez, R. Comparative study of limbal stem cell deficiency diagnosis methods: Detection of MUC5AC mRNA and goblet cells in corneal epithelium. Ophthalmology 2012, 119, 923–929. [Google Scholar] [CrossRef]
- Moore, J.E.; McMullen, T.C.; Campbell, I.L.; Rohan, R.; Kaji, Y.; Afshari, N.A.; Usui, T.; Archer, D.B.; Adamis, A.P. The inflammatory milieu associated with conjunctivalized cornea and its alteration with IL-1 RA gene therapy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2905–2915. [Google Scholar]
- Le, Q.; Chauhan, T.; Yung, M.; Tseng, C.-H.; Deng, S.X. Outcomes of limbal stem cell transplant: A meta-analysis. JAMA Ophthalmol. 2020, 138, 660–670. [Google Scholar] [CrossRef]
- Fernandez-Buenaga, R.; Aiello, F.; Zaher, S.S.; Grixti, A.; Ahmad, S. Twenty years of limbal epithelial therapy: An update on managing limbal stem cell deficiency. BMJ Open Ophthalmol. 2018, 3, e000164. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef]
- Keivyon, K.R.; Tseng, S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Nikpoor, N.; Donthineni, P.R.; Singh, V.; Chodosh, J.; Basu, S. Autologous limbal stem cell transplantation: A systematic review of clinical outcomes with different surgical techniques. Br. J. Ophthalmol. 2020, 104, 247–253. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Sureka, S.P.; Shanbhag, S.S.; Kethiri, A.R.; Singh, V.; Sangwan, V.S. Simple limbal epithelial transplantation: Long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology 2016, 123, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Holland, E.J. Keratolimbal allograft. Curr. Opin. Ophthalmol. 2017, 28, 377–381. [Google Scholar] [CrossRef]
- Daya, S.M.; Ilari, F.L. Living related conjunctival limbal allograft for the treatment of stem cell deficiency. Ophthalmology 2001, 108, 126–133. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Saeed, H.N.; Paschalis, E.I.; Chodosh, J. Keratolimbal allograft for limbal stem cell deficiency after severe corneal chemical injury: A systematic review. Br. J. Ophthalmol. 2018, 102, 1114–1121. [Google Scholar] [CrossRef]
- Solomon, A.; Ellies, P.; Anderson, D.F.; Touhami, A.; Grueterich, M.; Espana, E.M.; Ti, S.-E.; Goto, E.; Feuer, W.J.; Tseng, S.C. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology 2002, 109, 1159–1166. [Google Scholar] [CrossRef]
- Espana, E.; Di Pascuale, M.; Grueterich, M.; Solomon, A.; Tseng, S. Keratolimbal allograft in corneal reconstruction. Eye 2004, 18, 406–417. [Google Scholar] [CrossRef]
- Tsubota, K.; Shimmura, S.; Shinozaki, N.; Holland, E.J.; Shimazaki, J. Clinical application of living-related conjunctival-limbal allograft. Am. J. Ophthalmol. 2002, 133, 134–135. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Eslani, M.; Jamali, H.; Karimian, F.; Tailor, U.A.; Djalilian, A.R. Postoperative complications of conjunctival limbal autograft surgery. Cornea 2012, 31, 893–899. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A.; Kethiri, A.R.; Sangwan, V.S. Current perspectives of limbal-derived stem cells and its application in ocular surface regeneration and limbal stem cell transplantation. Stem Cells Transl. Med. 2021, 10, 1121–1128. [Google Scholar] [CrossRef]
- Daya, S.M. Conjunctival–limbal autograft. Curr. Opin. Ophthalmol. 2017, 28, 370–376. [Google Scholar] [CrossRef]
- Yin, J.; Jurkunas, U. Limbal stem cell transplantation and complications. In Seminars in Ophthalmology; Taylor & Francis: Oxford, UK, 2018; pp. 134–141. [Google Scholar]
- Basu, S.; Ali, H.; Sangwan, V.S. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am. J. Ophthalmol. 2012, 153, 643–650.e642. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Vazirani, J.; Ali, M.H.; Sharma, N.; Gupta, N.; Mittal, V.; Atallah, M.; Amescua, G.; Chowdhury, T.; Abdala-Figuerola, A.; Ramirez-Miranda, A. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: Multicentre results. Br. J. Ophthalmol. 2016, 100, 1416–1420. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Cabral, J.V.; Jackson, C.J.; Utheim, T.P.; Jirsova, K. Ex vivo cultivated oral mucosal epithelial cell transplantation for limbal stem cell deficiency: A review. Stem Cell Res. Ther. 2020, 11, 301. [Google Scholar] [CrossRef]
- Han, E.S.; Wee, W.R.; Lee, J.H.; Kim, M.K. Long-term outcome and prognostic factor analysis for keratolimbal allografts. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1697–1704. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Eslani, M.; Djalillian, A.R. Complications of keratolimbal allograft surgery. Cornea 2013, 32, 561–566. [Google Scholar] [CrossRef]
- Liang, L.; Sheha, H.; Li, J.; Tseng, S. Limbal stem cell transplantation: New progresses and challenges. Eye 2009, 23, 1946–1953. [Google Scholar] [CrossRef]
- Chen, J.; Tseng, S. Corneal epithelial wound healing in partial limbal deficiency. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1301–1314. [Google Scholar]
- Chen, J.; Tseng, S. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2219–2233. [Google Scholar]
- Moldovan, S.; Borderie, V.; Baudrimont, M.; Laroche, L. Treatment of unilateral limbal stem cell deficiency syndrome by limbal autograft. J. Fr. D’ophtalmologie 1999, 22, 302–309. [Google Scholar]
- Rao, S.K.; Rajagopal, R.; Sitalakshmi, G.; Padmanabhan, P. Limbal autografting: Comparison of results in the acute and chronic phases of ocular surface burns. Cornea 1999, 18, 164–171. [Google Scholar] [CrossRef]
- Atallah, M.R.; Palioura, S.; Perez, V.L.; Amescua, G. Limbal stem cell transplantation: Current perspectives. Clin. Ophthalmol. (Auckl. NZ) 2016, 10, 593. [Google Scholar]
- Ghareeb, A.E.; Lako, M.; Figueiredo, F.C. Recent advances in stem cell therapy for limbal stem cell deficiency: A narrative review. Ophthalmol. Ther. 2020, 9, 809–831. [Google Scholar] [CrossRef]
- Shortt, A.J.; Secker, G.A.; Rajan, M.S.; Meligonis, G.; Dart, J.K.; Tuft, S.J.; Daniels, J.T. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology 2008, 115, 1989–1997. [Google Scholar] [CrossRef]
- Behaegel, J.; Zakaria, N.; Tassignon, M.-J.; Leysen, I.; Bock, F.; Koppen, C.; Dhubhghaill, S.N. Short-and long-term results of xenogeneic-free cultivated autologous and allogeneic limbal epithelial stem cell transplantations. Cornea 2019, 38, 1543. [Google Scholar] [CrossRef]
- Borderie, V.M.; Ghoubay, D.; Georgeon, C.; Borderie, M.; Sousa, C.; Legendre, A.; Rouard, H. Long-term results of cultured limbal stem cell versus limbal tissue transplantation in stage III limbal deficiency. Stem Cells Transl. Med. 2019, 8, 1230–1241. [Google Scholar] [CrossRef]
- Pedrotti, E.; Passilongo, M.; Fasolo, A.; Nubile, M.; Parisi, G.; Mastropasqua, R.; Ficial, S.; Bertolin, M.; Di Iorio, E.; Ponzin, D. In vivo confocal microscopy 1 year after autologous cultured limbal stem cell grafts. Ophthalmology 2015, 122, 1660–1668. [Google Scholar] [CrossRef]
- Mishan, M.A.; Yaseri, M.; Baradaran-Rafii, A.; Kanavi, M.R. Systematic review and meta-analysis investigating autograft versus allograft cultivated limbal epithelial transplantation in limbal stem cell deficiency. Int. Ophthalmol. 2019, 39, 2685–2696. [Google Scholar] [CrossRef]
- Kolli, S.; Ahmad, S.; Mudhar, H.S.; Meeny, A.; Lako, M.; Figueiredo, F.C. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells 2014, 32, 2135–2146. [Google Scholar] [CrossRef]
- Ilmarinen, T.; Laine, J.; Juuti-Uusitalo, K.; Numminen, J.; Seppänen-Suuronen, R.; Uusitalo, H.; Skottman, H. Towards a defined, serum-and feeder-free culture of stratified human oral mucosal epithelium for ocular surface reconstruction. Acta Ophthalmol. 2013, 91, 744–750. [Google Scholar]
- Ricardo, J.R.S.; Cristovam, P.C.; Pedro Filho, A.; Farias, C.C.; de Araujo, A.L.; Loureiro, R.R.; Covre, J.L.; de Barros, J.N.; Barreiro, T.P.; dos Santos, M.S. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea 2013, 32, 221–228. [Google Scholar] [CrossRef]
- Inoue, K.; Aoi, N.; Sato, T.; Yamauchi, Y.; Suga, H.; Eto, H.; Kato, H.; Araki, J.; Yoshimura, K. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab. Investig. 2009, 89, 844–856. [Google Scholar] [CrossRef]
- Blazejewska, E.A.; Schlötzer-Schrehardt, U.; Zenkel, M.; Bachmann, B.; Chankiewitz, E.; Jacobi, C.; Kruse, F.E. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells 2009, 27, 642–652. [Google Scholar]
- Hayashi, R.; Ishikawa, Y.; Sasamoto, Y.; Katori, R.; Nomura, N.; Ichikawa, T.; Araki, S.; Soma, T.; Kawasaki, S.; Sekiguchi, K. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016, 531, 376–380. [Google Scholar]
- Hongisto, H.; Vattulainen, M.; Ilmarinen, T.; Mikhailova, A.; Skottman, H. Efficient and scalable directed differentiation of clinically compatible corneal limbal epithelial stem cells from human pluripotent stem cells. J. Vis. Exp. 2018, 140, e58279. [Google Scholar] [CrossRef]
- Japan Team Proves iPS-Based Cornea Transplant Safe in World-1st Trial. Available online: https://english.kyodonews.net/news/2022/04/c8af6b7913b2-japan-team-proves-ips-based-cornea-transplant-safe-in-world-1st-trial.html (accessed on 13 December 2022).
- Gomes, J.Á.P.; Monteiro, B.G.; Melo, G.B.; Smith, R.L.; da Silva, M.C.P.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar]
- Monteiro, B.; Serafim, R.; Melo, G.; Silva, M.; Lizier, N.; Maranduba, C.; Smith, R.; Kerkis, A.; Cerruti, H.; Gomes, J. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009, 42, 587–594. [Google Scholar]
- Reza, H.M.; Ng, B.-Y.; Gimeno, F.L.; Phan, T.T.; Ang, L.P.-K. Umbilical cord lining stem cells as a novel and promising source for ocular surface regeneration. Stem Cell Rev. Rep. 2011, 7, 935–947. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Hardarson, T.; Ellerström, C.; Nordberg, M.; Caisander, G.; Rao, M.; Hyllner, J.; Stenevi, U. Transplantation of human embryonic stem cells onto a partially wounded human cornea in vitro. Acta Ophthalmol. 2013, 91, 127–130. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, K.; Sun, Y.; Gao, X.; Li, Y.; Chen, Z.; Wu, X. Reconstruction of functional ocular surface by acellular porcine cornea matrix scaffold and limbal stem cells derived from human embryonic stem cells. Tissue Eng. Part A 2013, 19, 2412–2425. [Google Scholar] [CrossRef]
- da Mata Martins, T.M.; da Silva Cunha, P.; Rodrigues, M.A.; de Carvalho, J.L.; de Souza, J.E.; de Carvalho Oliveira, J.A.; Gomes, D.A.; de Goes, A.M. Epithelial basement membrane of human decellularized cornea as a suitable substrate for differentiation of embryonic stem cells into corneal epithelial-like cells. Mater. Sci. Eng. C 2020, 116, 111215. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Stewart, R.; Yung, S.; Kolli, S.; Armstrong, L.; Stojkovic, M.; Figueiredo, F.; Lako, M. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells 2007, 25, 1145–1155. [Google Scholar] [CrossRef]
- Zhang, C.; Du, L.; Pang, K.; Wu, X. Differentiation of human embryonic stem cells into corneal epithelial progenitor cells under defined conditions. PLoS ONE 2017, 12, e0183303. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-G.; Alizadeh, H.; Kinoshita, K.; McCulley, J.P. Experimental transplantation of cultured human limbal and amniotic epithelial cells onto the corneal surface. Cornea 1999, 18, 570–579. [Google Scholar] [CrossRef]
- Fatimah, S.S.; Ng, S.L.; Chua, K.H.; Hayati, A.R.; Tan, A.E.; Tan, G.C. Value of human amniotic epithelial cells in tissue engineering for cornea. Hum. Cell 2010, 23, 141–151. [Google Scholar] [CrossRef]
- Hernáez-Moya, R.; González, S.; Urkaregi, A.; Pijoan, J.I.; Deng, S.X.; Andollo, N. Expansion of Human Limbal Epithelial Stem/Progenitor Cells Using Different Human Sera: A Multivariate Statistical Analysis. Int. J. Mol. Sci. 2020, 21, 6132. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.; Ahmad, S.; Lako, M.; Figueiredo, F. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells 2010, 28, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Brejchova, K.; Trosan, P.; Studeny, P.; Skalicka, P.; Utheim, T.P.; Bednar, J.; Jirsova, K. Characterization and comparison of human limbal explant cultures grown under defined and xeno-free conditions. Exp. Eye Res. 2018, 176, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Bobba, S.; Chow, S.; Watson, S.; Di Girolamo, N. Clinical outcomes of xeno-free expansion and transplantation of autologous ocular surface epithelial stem cells via contact lens delivery: A prospective case series. Stem Cell Res. Ther. 2015, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Deng, S.X. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Exp. Eye Res. 2013, 116, 169–176. [Google Scholar] [CrossRef]
- Mei, H.; González, S.; Nakatsu, M.N.; Baclagon, E.R.; Chen, F.V.; Deng, S.X. Human adipose-derived stem cells support the growth of limbal stem/progenitor cells. PLoS ONE 2017, 12, e0186238. [Google Scholar] [CrossRef]
- Nakatsu, M.N.; González, S.; Mei, H.; Deng, S.X. Human limbal mesenchymal cells support the growth of human corneal epithelial stem/progenitor cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6953–6959. [Google Scholar] [CrossRef]
- González, S.; Mei, H.; Nakatsu, M.N.; Baclagon, E.R.; Deng, S.X. A 3D culture system enhances the ability of human bone marrow stromal cells to support the growth of limbal stem/progenitor cells. Stem Cell Res. 2016, 16, 358–364. [Google Scholar] [CrossRef]
- Djalilian, A.R.; Mahesh, S.P.; Koch, C.A.; Nussenblatt, R.B.; Shen, D.; Zhuang, Z.; Holland, E.J.; Chan, C.-C. Survival of donor epithelial cells after limbal stem cell transplantation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 803–807. [Google Scholar] [CrossRef]
- Niknejad, H.; Yazdanpanah, G.; Ahmadiani, A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell Tissue Res. 2016, 363, 599–608. [Google Scholar] [CrossRef]
- Ma, D.H.-K.; Chen, H.-C.; Ma, K.S.-K.; Lai, J.-Y.; Yang, U.; Yeh, L.-K.; Hsueh, Y.-J.; Chu, W.-K.; Lai, C.-H.; Chen, J.-K. Preservation of human limbal epithelial progenitor cells on carbodiimide cross-linked amniotic membrane via integrin-linked kinase-mediated Wnt activation. Acta Biomater. 2016, 31, 144–155. [Google Scholar] [CrossRef]
- Tsai, R.J.-F.; Tsai, R.Y.-N. From stem cell niche environments to engineering of corneal epithelium tissue. Jpn. J. Ophthalmol. 2014, 58, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, X.-Y.; Ruan, Y.-X.; Wang, L.; Jiang, M.-M.; Wu, J.; Chen, J. Construction of corneal epithelium with human amniotic epithelial cells and repair of limbal deficiency in rabbit models. Hum. Cell 2015, 28, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Konomi, K.; Satake, Y.; Shimmura, S.; Tsubota, K.; Shimazaki, J. Long-term results of amniotic membrane transplantation for partial limbal deficiency. Cornea 2013, 32, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Levis, H.J.; Daniels, J.T. Recreating the human limbal epithelial stem cell niche with bioengineered limbal crypts. Curr. Eye Res. 2016, 41, 1153–1160. [Google Scholar] [CrossRef]
- Shafiq, M.A.; Gemeinhart, R.A.; Yue, B.Y.; Djalilian, A.R. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng. Part C Methods 2012, 18, 340–348. [Google Scholar] [CrossRef]
- Amin, S.; Jalilian, E.; Katz, E.; Frank, C.; Yazdanpanah, G.; Guaiquil, V.H.; Rosenblatt, M.I.; Djalilian, A.R. The Limbal Niche and Regenerative Strategies. Vision 2021, 5, 43. [Google Scholar] [CrossRef]
- Shi, Y.; Bikkuzin, T.; Song, Z.; Jin, X.; Jin, H.; Li, X.; Zhang, H. Comprehensive evaluation of decellularized porcine corneal after clinical transplantation. Xenotransplantation 2017, 24, e12338. [Google Scholar] [CrossRef]
- Shi, W.; Xie, L. Focus on the clinical application of the first artificial bioengineered cornea in China. [Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2016, 52, 161–163. [Google Scholar]
- Ahearne, M.; Lynch, A.P. Early observation of extracellular matrix-derived hydrogels for corneal stroma regeneration. Tissue Eng. Part C Methods 2015, 21, 1059–1069. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, Q.K.; Feng, B.; Yan, C.X.; Zhu, M.Y.; Chen, J.Z.; Fu, W.; Fu, Y. Characterization of a hydrogel derived from decellularized corneal extracellular matrix. J. Biomater. Tissue Eng. 2015, 5, 951–960. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Jiang, Y.; Rabiee, B.; Omidi, M.; Rosenblatt, M.I.; Shokuhfar, T.; Pan, Y.; Naba, A.; Djalilian, A.R. Fabrication, Rheological, and Compositional Characterization of Thermoresponsive Hydrogel from Cornea. Tissue Eng. Part C Methods 2021, 27, 307–321. [Google Scholar] [CrossRef]
- Duan, X.; Sheardown, H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: Mechanical properties and corneal epithelial cell interactions. Biomaterials 2006, 27, 4608–4617. [Google Scholar] [CrossRef] [PubMed]
- Myung, D.; Farooqui, N.; Zheng, L.L.; Koh, W.; Gupta, S.; Bakri, A.; Noolandi, J.; Cochran, J.R.; Frank, C.W.; Ta, C.N. Bioactive interpenetrating polymer network hydrogels that support corneal epithelial wound healing. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 70–81. [Google Scholar] [CrossRef]
- Lee, H.J.; Fernandes-Cunha, G.M.; Na, K.S.; Hull, S.M.; Myung, D. Bio-orthogonally crosslinked, in situ forming corneal stromal tissue substitute. Adv. Healthc. Mater. 2018, 7, 1800560. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Lawrence, B.D.; Gao, X.R.; Luo, Y.; Zhou, Q.; Liu, A.; Guaiquil, V.H.; Rosenblatt, M.I. Micro-and nanoscale topographies on silk regulate gene expression of human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6388–6398. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef]
- Dehghani, S.; Rasoulianboroujeni, M.; Ghasemi, H.; Keshel, S.H.; Nozarian, Z.; Hashemian, M.N.; Zarei-Ghanavati, M.; Latifi, G.; Ghaffari, R.; Cui, Z. 3D-Printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: An in vitro & in vivo study. Biomaterials 2018, 174, 95–112. [Google Scholar]
- Nguyen, K.N.; Bobba, S.; Richardson, A.; Park, M.; Watson, S.L.; Wakefield, D.; Di Girolamo, N. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater. 2018, 65, 21–35. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolym. Orig. Res. Biomol. 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Ma, A.; Zhao, B.; Bentley, A.J.; Brahma, A.; MacNeil, S.; Martin, F.L.; Rimmer, S.; Fullwood, N.J. Corneal epithelialisation on surface-modified hydrogel implants. J. Mater. Sci. Mater. Med. 2011, 22, 663–670. [Google Scholar] [CrossRef]
- Myung, D.; Koh, W.; Bakri, A.; Zhang, F.; Marshall, A.; Ko, J.; Noolandi, J.; Carrasco, M.; Cochran, J.R.; Frank, C.W. Design and fabrication of an artificial cornea based on a photolithographically patterned hydrogel construct. Biomed. Microdevices 2007, 9, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Radosevich, M.; Goubran, H.; Burnouf, T. Fibrin sealant: Scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997, 72, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Atrah, H. Fibrin Glue; British Medical Journal Publishing Group: London, UK, 1994; Volume 308, pp. 933–934. [Google Scholar]
- Azari, A.A.; Rapuano, C.J. Autologous serum eye drops for the treatment of ocular surface disease. Eye Contact Lens 2015, 41, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Shin, Y.-T.; Kim, H.K. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn. J. Ophthalmol. 2012, 56, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Baradaran-Rafii, A.; Asl, N.S.; Ebrahimi, M.; Jabbehdari, S.; Bamdad, S.; Roshandel, D.; Eslani, M.; Momeni, M. The role of amniotic membrane extract eye drop (AMEED) in in vivo cultivation of limbal stem cells. Ocul. Surf. 2018, 16, 146–153. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Han, B.; Zhu, Y.-T.; Mahabole, M.; Huang, J.; Beebe, D.C.; Tseng, S.C. HC-HA/PTX3 purified from amniotic membrane promotes BMP signaling in limbal niche cells to maintain quiescence of limbal epithelial progenitor/stem cells. Stem Cells 2015, 33, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chen, S.-L.; Wu, J.-Y.; Ho, M.-Y.; Chen, L.-J.; Hsieh, J.-W.; Cheng, H.-C.; Tsao, Y.-P. PEDF promotes self-renewal of limbal stem cell and accelerates corneal epithelial wound healing. Stem Cells 2013, 31, 1775–1784. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Na, K.-S.; Putra, I.; Lee, H.J.; Hull, S.; Cheng, Y.-C.; Blanco, I.J.; Eslani, M.; Djalilian, A.R.; Myung, D. Corneal wound healing effects of mesenchymal stem cell secretome delivered within a viscoelastic gel carrier. Stem Cells Transl. Med. 2019, 8, 478–489. [Google Scholar] [CrossRef]
- Tirassa, P.; Rosso, P.; Iannitelli, A. Ocular nerve growth factor (NGF) and NGF eye drop application as paradigms to investigate NGF neuroprotective and reparative actions. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2018; pp. 19–38. [Google Scholar]
- Samaeekia, R.; Eslani, M.; Putra, I.; Rabiee, B.; Shen, X.; Park, Y.J.; Hematti, P.; Djalilian, A.R. Role of Human Corneal Mesenchymal Stromal Cell-derived Exosomes in Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3454. [Google Scholar]
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Toledano Furman, N.E.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S., Jr.; Olson, S.D. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells 2018, 36, 79–90. [Google Scholar] [CrossRef]
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Eslani, M.; Putra, I.; Shen, X.; Hamouie, J.; Afsharkhamseh, N.; Besharat, S.; Rosenblatt, M.I.; Dana, R.; Hematti, P.; Djalilian, A.R. Corneal mesenchymal stromal cells are directly antiangiogenic via PEDF and sFLT-1. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5507–5517. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic transplants of bone marrow. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Neofytou, E.; Deuse, T.; Beygui, R.E.; Schrepfer, S. Mesenchymal stromal cell therapy: Different sources exhibit different immunobiological properties. Transplantation 2015, 99, 1113–1118. [Google Scholar] [CrossRef]
- McCorry, M.C.; Puetzer, J.L.; Bonassar, L.J. Characterization of mesenchymal stem cells and fibrochondrocytes in three-dimensional co-culture: Analysis of cell shape, matrix production, and mechanical performance. Stem Cell Res. Ther. 2016, 7, 39. [Google Scholar] [CrossRef]
- Amirjamshidi, H.; Milani, B.; Sagha, H.; Movahedan, A.; Shafiq, M.; Lavker, R.; Yue, B.; Djalilian, A. Limbal fibroblast conditioned media: A non-invasive treatment for limbal stem cell deficiency. Mol. Vis. 2011, 17, 658. [Google Scholar]
- Ye, J.; Yao, K.; Kim, J. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: Engraftment and involvement in wound healing. Eye 2006, 20, 482–490. [Google Scholar] [CrossRef]
- Cejkova, J.; Trosan, P.; Cejka, C.; Lencova, A.; Zajicova, A.; Javorkova, E.; Kubinova, S.; Sykova, E.; Holan, V. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp. Eye Res. 2013, 116, 312–323. [Google Scholar] [CrossRef]
- Lee, M.J.; Ko, A.Y.; Ko, J.H.; Lee, H.J.; Kim, M.K.; Wee, W.R.; Khwarg, S.I.; Oh, J.Y. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol. Ther. 2015, 23, 139–146. [Google Scholar] [CrossRef]
- Holan, V.; Trosan, P.; Cejka, C.; Javorkova, E.; Zajicova, A.; Hermankova, B.; Chudickova, M.; Cejkova, J. A comparative study of the therapeutic potential of mesenchymal stem cells and limbal epithelial stem cells for ocular surface reconstruction. Stem Cells Transl. Med. 2015, 4, 1052–1063. [Google Scholar] [CrossRef]
- Espandar, L.; Caldwell, D.; Watson, R.; Blanco-Mezquita, T.; Zhang, S.; Bunnell, B. Application of adipose-derived stem cells on scleral contact lens carrier in an animal model of severe acute alkaline burn. Eye Contact Lens 2014, 40, 243. [Google Scholar] [CrossRef] [PubMed]
- Bu, P.; Vin, A.P.; Sethupathi, P.; Ambrecht, L.A.; Zhai, Y.; Nikolic, N.; Qiao, L.; Bouchard, C.S. Effects of activated omental cells on rat limbal corneal alkali injury. Exp. Eye Res. 2014, 121, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Acar, U.; Pinarli, F.A.; Acar, D.E.; Beyazyildiz, E.; Sobaci, G.; Ozgermen, B.B.; Sonmez, A.A.; Delibasi, T. Effect of allogeneic limbal mesenchymal stem cell therapy in corneal healing: Role of administration route. Ophthalmic Res. 2015, 53, 82–89. [Google Scholar] [CrossRef]
- Zeng, W.; Li, Y.; Zeng, G.; Yang, B.; Zhu, Y. Transplantation with cultured stem cells derived from the human amniotic membrane for corneal alkali burns: An experimental study. Ann. Clin. Lab. Sci. 2014, 44, 73–81. [Google Scholar] [PubMed]
- Lan, Y.; Kodati, S.; Lee, H.S.; Omoto, M.; Jin, Y.; Chauhan, S.K. Kinetics and function of mesenchymal stem cells in corneal injury. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhu, M.; Madigan, M.C.; You, J.; King, N.J.; Billson, F.A.; McClellan, K.; Sutton, G.; Petsoglou, C. Immunomodulatory effects of bone marrow-derived mesenchymal stem cells on pro-inflammatory cytokine-stimulated human corneal epithelial cells. PLoS ONE 2014, 9, e101841. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Wu, Y.; Cui, X.; Liu, X.; Yu, M.; Yang, C.; Li, X. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS ONE 2015, 10, e0119725. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, Y.; Xiao, Z.; Yang, W.; Zhang, C.; Song, E.; Du, Y.; Li, L. Reconstruction of chemically burned rat corneal surface by bone marrow–derived human mesenchymal stem cells. Stem Cells 2006, 24, 315–321. [Google Scholar] [CrossRef]
- Cejka, Č.; Cejkova, J.; Trosan, P.; Zajicova, A.; Sykova, E.; Holan, V. Transfer of mesenchymal stem cells and cyclosporine A on alkali-injured rabbit cornea using nanofiber scaffolds strongly reduces corneal neovascularization and scar formation. Histol. Histopathol. 2016, 31, 969–980. [Google Scholar]
- Eslani, M.; Putra, I.; Hamouie, J.; Shen, X.; Afsharkhamseh, N.; Hematti, P.; Djalilian, A.R. The Effect of Corneal-Derived Versus Bone Marrow-Derived Mesenchymal Stromal Cell Secretome on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4421. [Google Scholar]
- Polisetti, N.; Agarwal, P.; Khan, I.; Kondaiah, P.; Sangwan, V.S.; Vemuganti, G.K. Gene expression profile of epithelial cells and mesenchymal cells derived from limbal explant culture. Mol. Vis. 2010, 16, 1227. [Google Scholar] [PubMed]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2019, 206, 18–40. [Google Scholar] [CrossRef]
- Galindo, S.; de la Mata, A.; López-Paniagua, M.; Herreras, J.M.; Pérez, I.; Calonge, M.; Nieto-Miguel, T. Subconjunctival injection of mesenchymal stem cells for corneal failure due to limbal stem cell deficiency: State of the art. Stem Cell Res. Ther. 2021, 12, 60. [Google Scholar] [CrossRef]
- Mittal, S.K.; Omoto, M.; Amouzegar, A.; Sahu, A.; Rezazadeh, A.; Katikireddy, K.R.; Shah, D.I.; Sahu, S.K.; Chauhan, S.K. Restoration of corneal transparency by mesenchymal stem cells. Stem Cell Rep. 2016, 7, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Safety of Locally Delivered Allogeneic Mesenchymal Stromal Cells. Available online: https://clinicaltrials.gov/ct2/show/NCT04626583 (accessed on 13 December 2022).
- Margolis, M.; Jung, R.; Tu, G.; An, S.; Dana, R.; Jeng, B.H.; Basu, S.; Rosenblatt, M.; Hematti, P.; Mahmud, N. Phase I Study of the Safety of Locally Delivered Allogeneic Mesenchymal Stem Cells for Promoting Corneal Repair: Early Results. Investig. Ophthalmol. Vis. Sci. 2022, 63, 91-A0189. [Google Scholar]
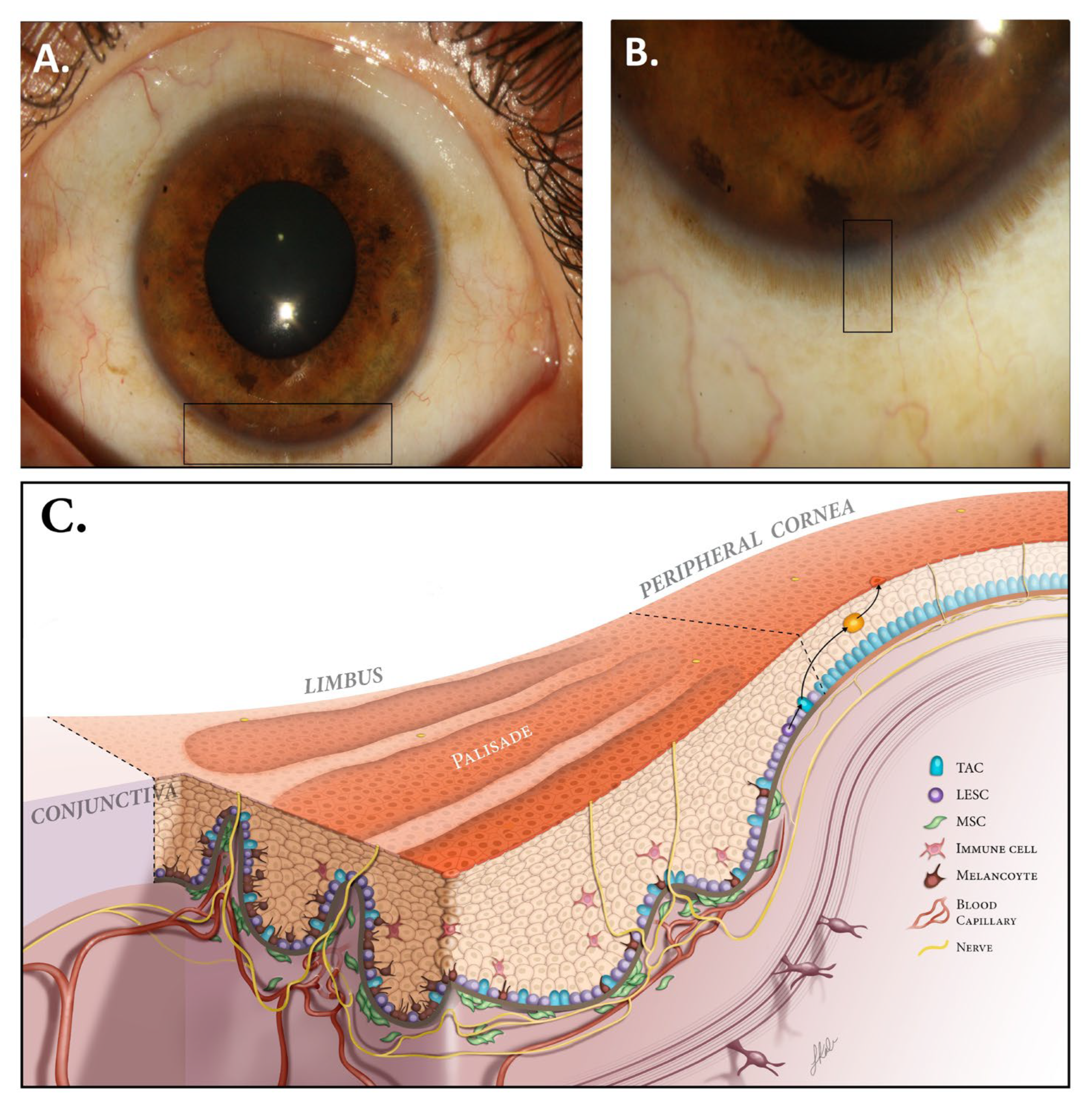
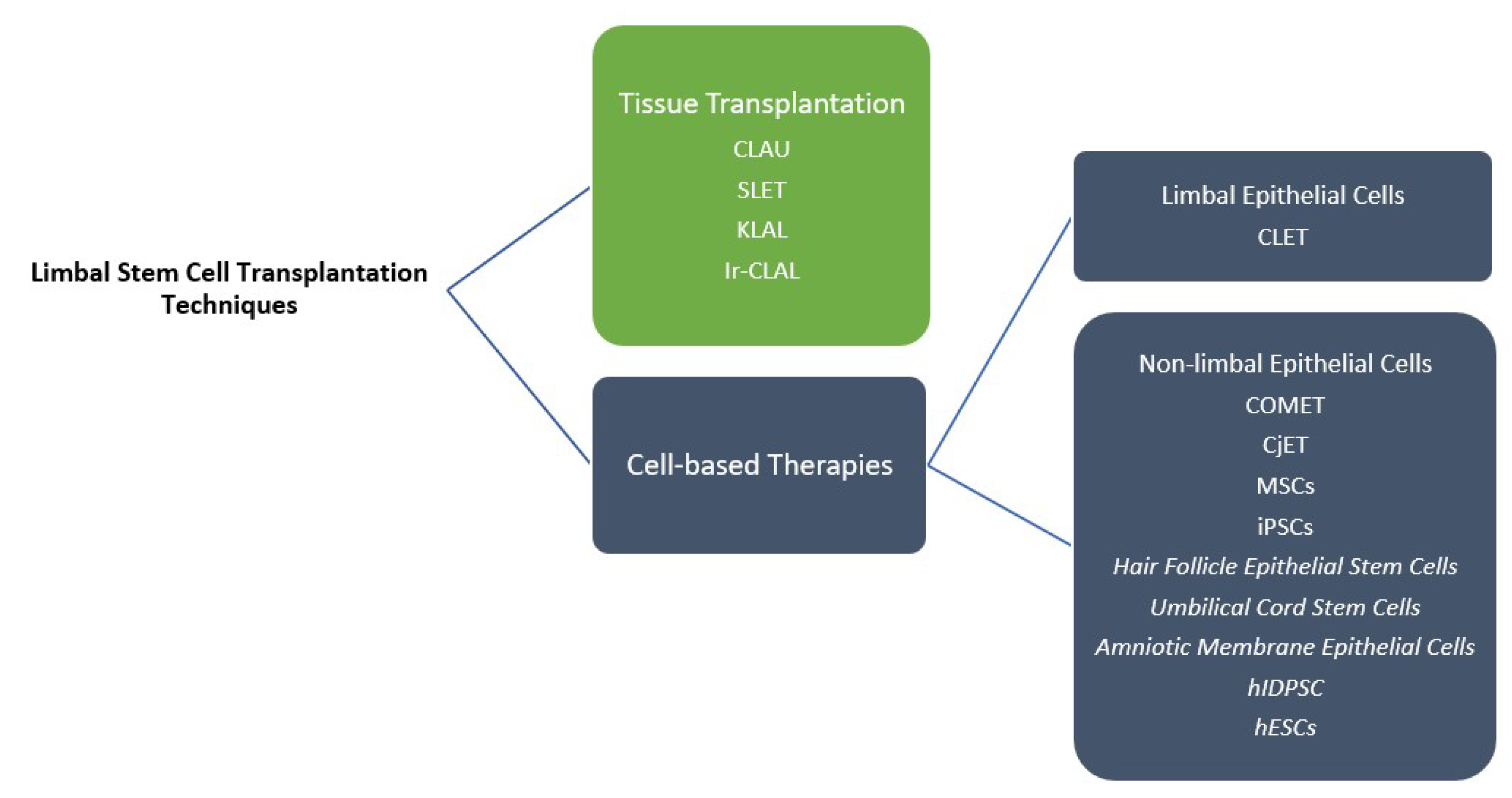
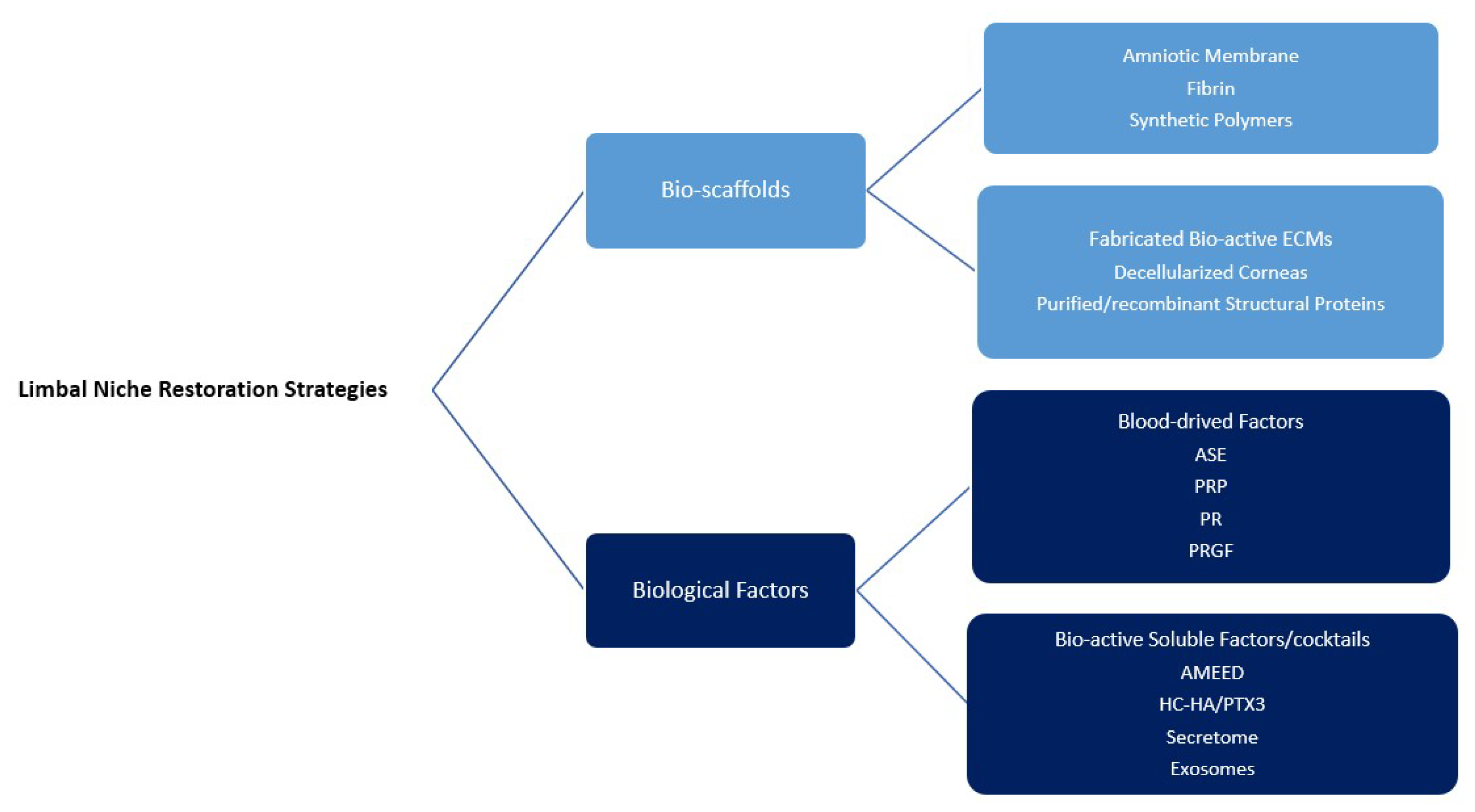
| Technique | Reference | Advantages | Disadvantages | Complications | |
|---|---|---|---|---|---|
| CLAU | [60,61,62] | -Acceptable outcomes -Application of conjunctival patch in ocular surface reconstruction | Risk of iatrogenic LSCD | -Delayed epithelial healing -PED -Corneal perforation -Progressive conjunctival ingrowth | |
| CLET | [63,64,65] | -Acceptable outcomes -Requirement of small donor tissue | -Expense -Technical difficulties -Risk of prion disease transmission via animal product usage during culture | -Postoperative hemorrhage under the graft -Infection -PED -Corneal perforation | |
| SLET | [51,66] | -Acceptable outcomes -Requirement of small donor tissue | -Risk of donor tissue loss | -Focal recurrence of LSCD -Progressive conjunctivalization and symblepharon -Keratitis -PED | |
| COMET | [67,68] | Applicable in bilateral cases | -Peripheral corneal neovascularization -Suboptimal visual outcomes | -PED -Corneal perforation -Glaucoma -Infection | |
| Limbal allografts | lr-CLAL | [54,63] | -Applicable in bilateral cases -Utilizes a large conjunctival patch, which can be used in ocular surface reconstruction | -Requirement of immunosuppression regimen -Delayed epithelialization -Limited long-term success | -Rejection -Glaucoma -PED -Corneal melting and perforation -Graft-related issues -Infection -Posterior segment complications such as retinal detachment, vitreous hemorrhage, and cystoid macular edema |
| KLAL | [63,69,70] | -Applicable in bilateral cases -Providing a larger number of LESCs compared to lr-CLAL | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleimani, M.; Cheraqpour, K.; Koganti, R.; Baharnoori, S.M.; Djalilian, A.R. Concise Review: Bioengineering of Limbal Stem Cell Niche. Bioengineering 2023, 10, 111. https://doi.org/10.3390/bioengineering10010111
Soleimani M, Cheraqpour K, Koganti R, Baharnoori SM, Djalilian AR. Concise Review: Bioengineering of Limbal Stem Cell Niche. Bioengineering. 2023; 10(1):111. https://doi.org/10.3390/bioengineering10010111
Chicago/Turabian StyleSoleimani, Mohammad, Kasra Cheraqpour, Raghuram Koganti, Seyed Mahbod Baharnoori, and Ali R. Djalilian. 2023. "Concise Review: Bioengineering of Limbal Stem Cell Niche" Bioengineering 10, no. 1: 111. https://doi.org/10.3390/bioengineering10010111
APA StyleSoleimani, M., Cheraqpour, K., Koganti, R., Baharnoori, S. M., & Djalilian, A. R. (2023). Concise Review: Bioengineering of Limbal Stem Cell Niche. Bioengineering, 10(1), 111. https://doi.org/10.3390/bioengineering10010111






