Abstract
The development, optimization, and analysis of downstream processes are challenged by a high number of potentially critical process parameters that need to be investigated using lab-scale experiments. These process parameters are spread across multiple unit operations and potentially show interactions across unit operations. In this contribution, we present a novel strategy for bioprocess development that considers the risk of parameter interactions across unit operations for efficient experimental design. A novel risk assessment tool (interaction matrix) is introduced to the Quality by Design (QbD) workflow. Using this tool, the risk of interaction across unit operations is rated. Subsequently, a design of experiments (DoE) across unit operations is conducted that has the power to reveal multivariate interdependencies. The power of the presented strategy is demonstrated for protein isolation steps of an inclusion body process, focusing on the quality attribute inclusion body purity. The concentration of Triton X-100 in the course of inclusion body (IB) purification was shown to interact with the g-number of the subsequent centrifugation step. The presented strategy targets a holistic view on the process and allows handling of a high number of experimental parameters across unit operations using minimal experimental effort. It is generically applicable for process development along QbD principles.
1. Introduction
1.1. Process Development along QbD Principles
Significant changes of biopharmaceutical manufacturing have taken place in the past decades. Recombinant protein titers have improved from a mg scale to more than 10 g per liter. This emerged as a major challenge for downstream processing because its capacity could not keep up with the larger titers [1]. The need for innovation demands industry to adapt its production processes to deal with these increasing product titers. Traditional biopharmaceutical production processes that are mainly based on empirical process knowledge are facing the problem of laborious post approval changes when adapting the process or implementing new technologies to raise their efficiency. Furthermore the inability to predict effects of scale-up on final product quality is inherent in empirical process development. This often leads to higher costs and difficulties in implementing manufacturing changes [2]. A reorganization of the regulatory approval and an initiative towards a more science-based process understanding was launched by the Food and Drug Administration (FDA) to address these issues [3]. The aim is to lead the industry to a state of deeper process understanding resulting in higher flexibility and freedom to operate within a so-called design space. The concept of design space is part of the “Quality by Design” (QbD) paradigm and is well described in literature [2,4,5,6,7,8,9]. To accompany manufacturers towards a more science-based process development several guidelines have been published [4,10,11,12]. In short, the QbD concept involves to first define a Quality Target Product Profile (QTPP) for product performance and to identify its Critical Quality Attributes (CQAs). On the basis of this information, experimental design and analysis assist in understanding the impact of Critical Process Parameters (CPP) on CQAs and as a consequence to identify and control the sources of variability.
1.2. Parameter Interactions
Downstream processes (DSP) in biopharmaceutical processes include harvesting, isolation, and purification of biosynthetic products. Different unit operations are implemented depending on (i) the product location (extracellular or intracellular, if intracellular: periplasm or cytoplasm); (ii) the nature of the product (size, charge, and solubility); (iii) the value of the product (various forms of expensive chromatography steps for high value pharmaceutical products); (iv) final conformation (need for refolding) or other constraints. The chosen process sequence forms an integrated process, whereby a change in one unit operation can possibly show and procreate an effect in subsequent unit operations. Thus, interaction effects of parameters across unit operations are possible and need to be considered during process development. Interactions occur when the effect of one parameter on the response depends on the setting of a second parameter. This means that the combined effects of two parameters from different unit operations cannot be predicted from the separate effects and therefore cannot be predicted by investigating unit operations separately. Current state of the art DSP development starts with process characterization employing risk assessments to identify the most critical parameters, which are then analyzed within single unit operations (SUO), as exemplified in several contributions [13,14,15,16]. However, the regulatory authorities underline that interactions of parameters need to be considered within the QbD approach [11], which in our understanding also includes interactions across unit operations (AUO). So far, few contributions consider interactions across unit operations for process optimization purposes [17]. Especially the application of partition designs for the assessment of parameters across unit operations to build predictive models across entire processes is noteworthy [18]. However, to our knowledge, there is no easy and comprehensive strategy published so far which is applicable to be applied within the QbD context.
1.3. Design of Experiments
Design of experiments (DoE) emerged as the primary tool for process development along QbD principles [19,20,21]. DoE is a formal mathematical method for systematically planning and conducting scientific experiments. The principle involved is the change of experimental variables together in order to determine their effect of a given response. DoE presents a substantial step forward compared to the previously used one-factor-at-a-time (OFAT) method of designing experiments in terms of the lower number of experimental runs needed to precisely estimate effects and interactions of parameters and the reduced risk of missing optimal settings of parameters [22,23]. The goal of experimental design is to extract the maximum information from as few experiments as possible. The information content is expressed as design resolution and assigns the risk that certain interaction effects may be confounded with other effects, which is well described in literature [24,25]. An example of the relationship between the number of parameters to be investigated and the number of experimental runs for screening designs and optimization design is shown in Figure 1. This data set was generated using the experimental design software MODDE (Umetrics, Umeå, Sweden), which automatically suggests a number of experimental runs depending on the number of factors entered and the selected experimental design (screening or optimization). Optimization designs are able to resolve quadratic interaction effects, but the number of runs increases exponentially with the number of investigated parameters when the experimenter targets to find process optima. Screening designs with lower resolution (III/IV) have a much lower number of experiments, but a high risk for confounding interaction effects. Increasing the resolution of screening designs leads inevitably to the increase of experimental runs.
As the number of experimental observations increases significantly with the level of targeted information content, process developers have to decide carefully which design to choose and even more importantly: which parameters to investigate in more detail. To save time and money in process development it is mandatory to keep the number of experiments low. To achieve this, risk assessments are the major task to identify criticality by risk ranking in order to reduce the number of parameters for experimental design studies based on prior knowledge.
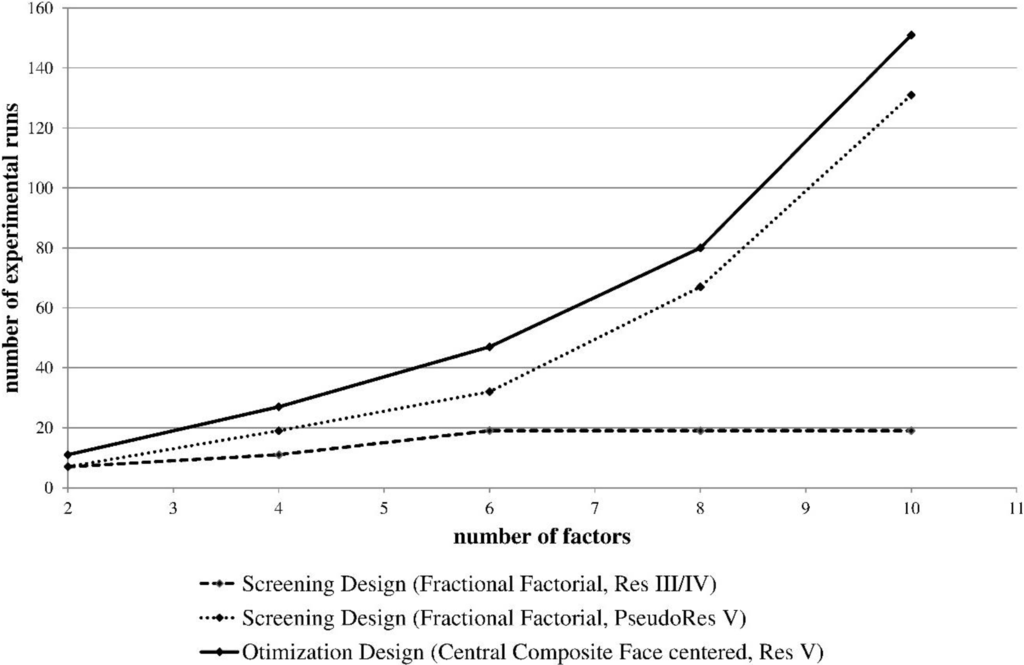
Figure 1.
Correlation between the number of experimental runs and the number of investigated parameters. For optimization designs the number of runs increases exponentially while for screening designs the number of runs remains low for low resolution and increases significantly with the degree of resolution.
1.4. Risk Assessments
For risk evaluation the identification of QTPP and CQAs, a definition process design space is realized in the first steps of the process development as described in the ICH guidance Q9 [10] and reviewed in several publications [8,9,26]. Once the QTPP and CQAs have been identified, the criticality of process parameters has to be evaluated. Commonly applied techniques for the rating of severity, occurrence, and detectability of CPPs are cause and effect diagrams (e.g., Ishikawa diagram) and Failure Mode and Effect Analysis (FMEAs) [2,27,28]. In traditional evaluations, risk assessments are carried out for single unit operations separately, and consequently, the experimental studies are performed accordingly to single unit operation separately [29].
In process development screening designs are often employed, with the advantage that the number of experiments is low and the disadvantage that significant main effects are often confounded with two factor interactions. In enhanced approaches these studies are followed by the evaluation of significant parameters in an optimization designs for single unit operations [30], nonetheless, there is still the risk of missing a critical parameter that acts across unit operations. At the end, all CQAs have to meet the acceptance criteria, and quality assurance has to be demonstrated according to the ICH definition of design space [4], so that the “multidimensional combination and interactions of input variables and process parameters have been demonstrated to provide assurance of quality”. The need for an adapted risk assessment for scientific process development is encouraged by ICH Q11_step 5 “A design space that spans multiple unit operations can provide more operational flexibility” [11]. In our opinion one step towards this request is a rearrangement of the QbD workflow realized by the introduction of an early criticality analysis of parameters potentially interacting across unit operations.
1.5. Goal
With this contribution we aim to propose a strategy for integrated process DoE design, which allows building experimental designs across unit operations and allows the investigation of possible interacting parameters. The power of this strategy is exemplified by the product isolation steps of a recombinant inclusion body process. The investigation is focused on the CQA inclusion body purity (IB purity), which is known to have a critical impact on further DSP steps [31]. The impact of this area on overall process variability is underestimated, and it is normally operated rather as a black box approach, because product related quality attributes are difficult to measure effectively in a timely manner. Investigation of parameters from this perspective might significantly reduce the risk of missing important parameter interactions and thereby it may also eliminate repeated design cycles. The overall goal is the development of a strategy that is capable of building a knowledge space across multiple process steps, thereby giving the opportunity to define a design space and control space in the course of process development. For this purpose we present a novel risk-based strategy that allows the user to (i) deal with a high number of process parameters that are possibly critical, (ii) achieve a holistic view of the process, (iii) efficiently design statistical experimental plans across unit operations (iv) reveal interdependencies across unit operations.
2. Experimental Section
2.1. Strain and Media
E. coli strain C41 (F-ompT hsdSB (rB- mB-) gal dcm (DE3); Lucigene, Middleton, WI, USA) harboring an isopropyl-beta-D-thiogalactopyranoside (IPTG) inducible pET expression system was used for the production of the recombinant growth factor. A Techfors-S bioreactor (Infors, Bottmingen, Switzerland) with 10 L working volume was used. A defined minimal medium according to DeLisa et al. [32] was used. Kanamycin was added to the batch media at a final concentration of 0.05 g/L. Production of recombinant protein was induced by addition of IPTG at a final concentration of 1 mM. D-glucose was used as carbon source in the batch medium with a concentration of 20 g/L. The fed-batch medium contained D-glucose at a concentration of 400 g/L. Dissolved oxygen levels (DO2) were kept above 40% saturation. The pH was kept constant at 7.2 by adding 12.5% NH4OH, which also served as nitrogen source.
2.2. Harvest and Inclusion Body (IB) Processing
The cell broth was harvested by centrifugation (4300 g, 20 min, 4 °C) and the pellets were washed (50 mM Tris, 100 mM NaCl). After centrifugation (4,300 RCF, 20 min, 4 °C), the pellets were resuspended (50 mM Tris, 5 mM DTT, 1 mM EDTA, pH 8) and homogenized (10 g/L dry cell weight). Homogenization (Avestin EmulsiFlex©, Ottawa, Canada) was performed in a continuous mode at 1500 bar and alternating for two, four, and six passages (according to the experimental run number; see Table 1).

Table 1.
Experimental design worksheet. Three levels from each investigated factor were varied according to the experimental setup in a randomized run order.
| Exp No | Exp Name | Run Order | Triton X-100 concentration | Passages | Time centrifugation | g-number centrifugation |
|---|---|---|---|---|---|---|
| 1 | N1 | 2 | 0 | 2 | 10 | 3000 |
| 2 | N2 | 15 | 1 | 2 | 10 | 3000 |
| 3 | N3 | 1 | 0 | 6 | 10 | 3000 |
| 4 | N4 | 23 | 1 | 6 | 10 | 3000 |
| 5 | N5 | 6 | 0 | 2 | 30 | 3000 |
| 6 | N6 | 11 | 1 | 2 | 30 | 3000 |
| 7 | N7 | 8 | 0 | 6 | 30 | 3000 |
| 8 | N8 | 19 | 1 | 6 | 30 | 3000 |
| 9 | N9 | 9 | 0 | 2 | 10 | 13,000 |
| 10 | N10 | 25 | 1 | 2 | 10 | 13,000 |
| 11 | N11 | 20 | 0 | 6 | 10 | 13,000 |
| 12 | N12 | 12 | 1 | 6 | 10 | 13,000 |
| 13 | N13 | 24 | 0 | 2 | 30 | 13,000 |
| 14 | N14 | 14 | 1 | 2 | 30 | 13,000 |
| 15 | N15 | 27 | 0 | 6 | 30 | 13,000 |
| 16 | N16 | 22 | 1 | 6 | 30 | 13,000 |
| 17 | N17 | 3 | 0 | 4 | 20 | 8000 |
| 18 | N18 | 5 | 1 | 4 | 20 | 8000 |
| 19 | N19 | 7 | 0.5 | 2 | 20 | 8000 |
| 20 | N20 | 21 | 0.5 | 6 | 20 | 8000 |
| 21 | N21 | 10 | 0.5 | 4 | 10 | 8000 |
| 22 | N22 | 26 | 0.5 | 4 | 30 | 8000 |
| 23 | N23 | 18 | 0.5 | 4 | 20 | 3000 |
| 24 | N24 | 17 | 0.5 | 4 | 20 | 13,000 |
| 25 | N25 | 13 | 0.5 | 4 | 20 | 8000 |
| 26 | N26 | 16 | 0.5 | 4 | 20 | 8000 |
| 27 | N27 | 4 | 0.5 | 4 | 20 | 8000 |
Homogenized samples were centrifuged (20 min, 4 °C) at 3000, 8000 or 13,000 g (according to the experimental run number; see Table 1) and washed with Triton X-100 buffer (50 mM Tris, 1 mM EDTA, 0, 0.5 or 1% w/v Triton X-100 according to the experimental run number; see Table 1), Another washing step with deionized water was performed (3000, 8000, 13,000 g, 20 min, RT) to remove residual Triton X-100. The resulting inclusion body pellets were solubilized (GuHCl 6 M, 10 mM Tris, 50 mM 2ME) for three hours at room temperature on a shaking platform. After centrifugation (13,000 g, 20 min, RT) the supernatant was precipitated using 10% trichloroacetic acid (TCA) by adding one volume of TCA to one volume of the protein sample. The pellets were then resuspended in Laemmli sample buffer containing 5% 2ME for reduction and prepared for SDS PAGE (10 min heating at 95 °C, then 5 min centrifugation at 13,000 g) and loaded onto a Gel (8–16%; GE Healtcare, Chalfont St. Gilles, UK). The gels were run at 160 V, 60 min and subsequently stained overnight in a sensitive Coomassie solution (0.02% (w/v) Coomassie Brilliant Blue G 250, 5% (w/v) Aluminium Sulfate-(14-18)-Hydrate, 10% (v/v) Ethanol, 2% (v/v) Ortho phosphoric Acid, distilled water). Gel analysis was done using the software Image Lab (Bio-Rad, Hercules, CA, USA). Assessing the purity of a sample is one application that uses quantification of all components of a sample relative to each other in one lane. Results are expressed as percent of all bands identified (band %) and represents the purity of the recombinant growth factor in %.
2.3. Experimental Design
Setup and data evaluation of the experimental design were done using the software MODDE Version 9.0 (Umetrics, Umeå, Sweden).
3. Results and Discussion
3.1. Strategy Overview
The proposed strategy for science and risk-based bioprocess development is displayed in Figure 2. The individual steps will be described in more detail hereafter. At first, classical risk assessment tools such as Ishikawa diagrams and failure mode and effect analysis (FMEA) are used for an initial ranking of all process parameters according to severity, occurrence, and detectability. The subsequent step within the classical QbD workflow is the conduction of screening and optimization experimental designs for process investigation and optimization. To capture potential interaction effects, multivariate studies are to be preferred; otherwise optimization and design space establishment can be impaired. However, the high amount of possible critical parameters to be optimized or investigated excludes the conduction of optimization experimental designs for all parameters that are possibly critical. Hence, a classification of parameters has to be made: Parameters that are to be optimized/investigated using univariate approaches and parameters that are to be optimized using multivariate approaches. Based on that classification we introduced a new step in the QbD workflow: a second risk assessment step (RA 2) that evaluates not only the criticality of parameters but also the possibility of interaction (pi) between process parameters across unit operations. Figure 2 gives an overview of such a process characterization cycle displaying the consecutive steps: (i) identification of parameter criticality; (ii) identification of potential interaction across unit operations; (iii) risk based DoE design across unit operations; (iv) transformation of data into knowledge.
According to the prior defined QTPP, the capture of potential CPPs for corresponding CQAs is performed using fishbone diagrams, which, additionally to criticality, already indicate the potential risk of interactions across unit operations. The novel combination of the potential interacting parameters from different process steps in the form of an interaction matrix is the key step to entire process understanding and decision-making for grouping CPPs in experimental designs. This allows to efficiently design experimental plans across unit operations targeting enhanced process knowledge.
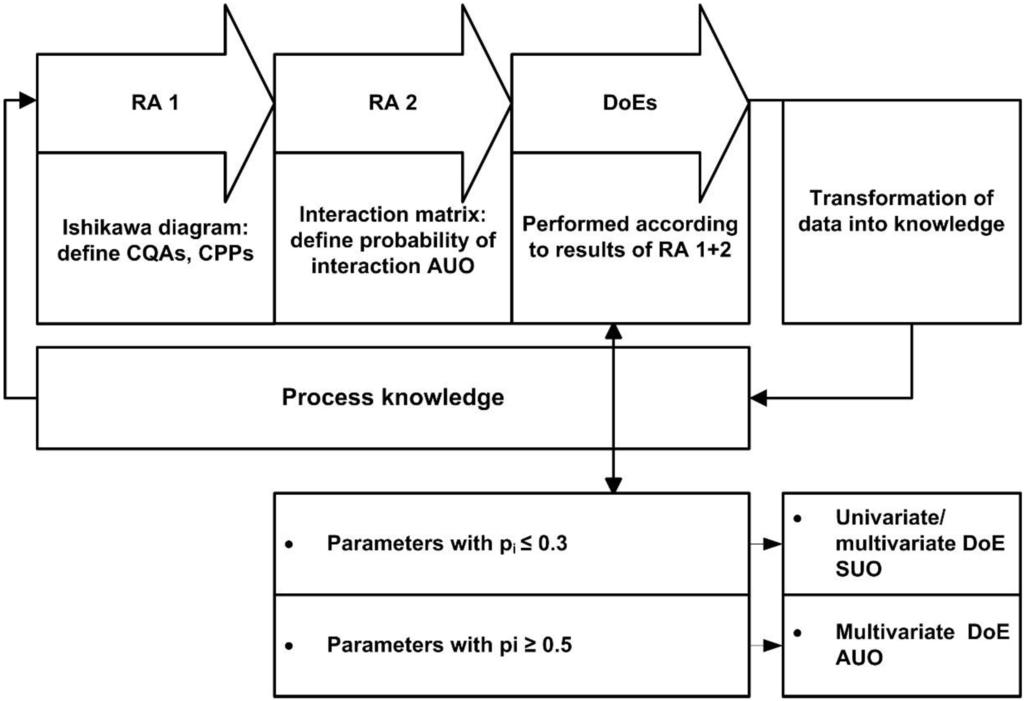
Figure 2.
Novel Quality by Design (QbD) workflow comprising the interaction matrix in risk assessment 2 (RA 2) as key step for the integrated design of experiments (DoE) design across unit operations. Risk analysis starts with a double-stage assessment of criticality. RA 1 defines Critical Quality Attributes (CQAs), Critical Process Parameters (CPPs) and gives a first evaluation of possible Across Unit Operations (AUO)-interactions. RA 2 rates the potential interacting parameters by assigning a probability of interaction (pi) value. Experimental designs across unit operations are planned based on the pi values for single unit operations (SUO) or across unit operations (AUO). Extraction of information from data and transformation to knowledge leads the way towards integrated process knowledge.
3.2. Risk Assessment 1—Identification of Parameters for Process Characterization
An initial risk analysis for the downstream process development of a recombinant human Growth Factor (rhGF) was performed by a multidisciplinary team from the fields of process technology, microbiology, chemical engineering, and protein chemistry. After definition of QTPP and CQAs the first step on the way to the establishment of a design space was an initial risk analysis 1 (RA 1) to identify parameters for process characterization using Ishikawa diagrams. Definition of parameters, which could have a critical impact on CQAs, was done for each unit operation, hereafter exemplified for the CQA IB purity. Furthermore each unit operation was evaluated with special regard to parameters potentially interacting across unit operations (AUO) and accordingly marked in the Ishikawa diagrams. For further investigations of the process units including potential critical AUO interacting parameters, they are combined in one Ishikawa diagram, shown in Figure 3. The potential criticality of parameters and their probability of interaction (pi) across unit operations were assessed and are indicated by bold letters in the diagram. This first evaluation of potential cross-interacting parameters forms the basis for an integrated risk analysis accomplished by the Risk Assessment 2 in the following section. This is demonstrated for the unit operations of the isolation steps homogenization, Triton X-100 washing, and centrifugation. These unit operations form an “interaction block” (Figure 4) that is further rated in risk assessment 2.
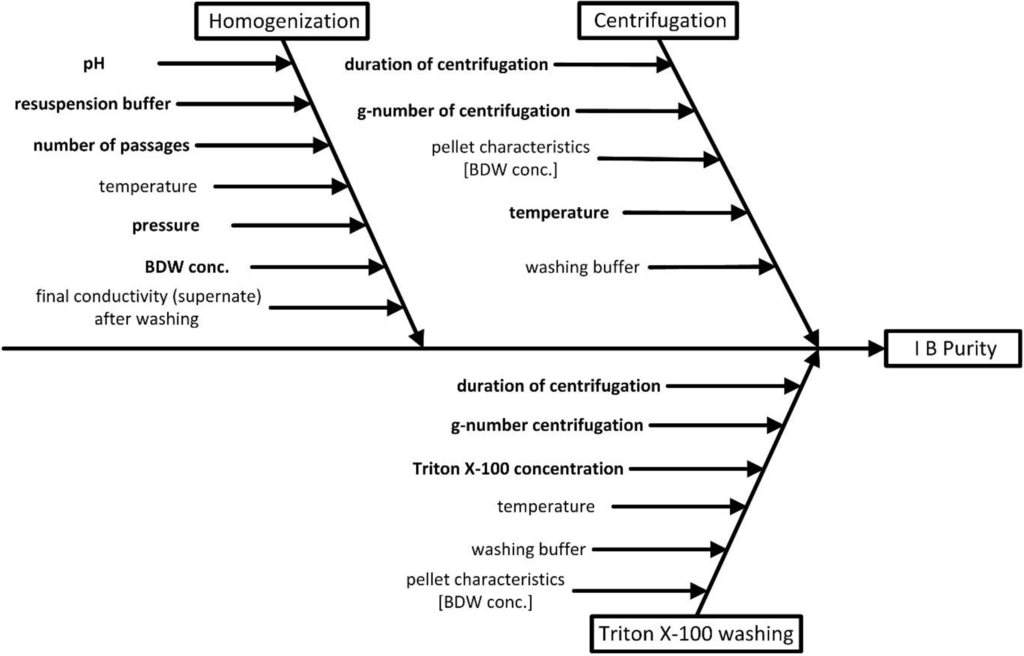
Figure 3.
Ishikawa diagram for visualization of critical process parameters. All parameters named by the expert team are listed for each unit operation. With respect to criticality and potential interactions across unit operations, the corresponding unit operations were combined for further risk evaluation.
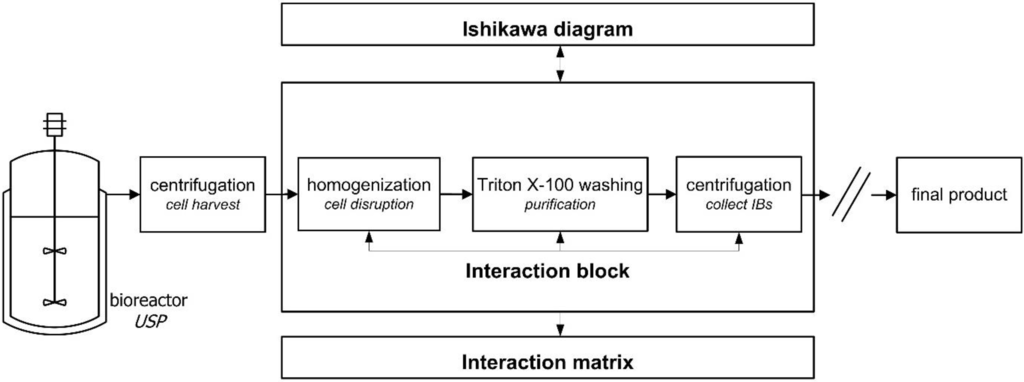
Figure 4.
Interaction block comprising potentially interacting unit operations. All parameters from these unit operations potentially interacting across unit operations enter the interaction matrix.
3.3. Risk Assessment 2—Interaction Matrix across Unit Operations (AUO)
This section provides an example of how to proceed with potential CPPs across unit operations. For this, a novel step was introduced in the QbD workflow: the interaction matrix. All parameters, the result of which had a potential critical from RA1 were included in further evaluations. Those parameters were used to create the interaction matrix to evaluate and rate the probability of interaction (pi) with each other across unit operations. The pi represents the importance of parameters for AUO interactions, and parameters with a high pi are considered to be high risk. The respective pi values depict a subjective user evaluation of the risk of interactions. This constitutes an additional risk assessment step similar to FMEA approaches.
Rating of criticality with respect to pi was done for all possible combinations using the rating values: 0.1 = minimal pi, 0.3 = low pi, 0.5 = medium pi, 0.7 = high pi, 1 = proven pi. The threshold value for the decision to perform a multivariate experimental design across unit operations was defined as 0.5. All parameters giving a pi equal or higher than 0.5 are included in the experimental plans across unit operations. The selection of the threshold value is a trade-off between the number of experiments and the risk of missing significant parameters. Ideally, the threshold value is kept constant and consequently all parameters with medium and high pi values are investigated across unit operations. If a high number of potentially interacting parameters is found, the user might be forced to adjust the threshold value if the number of experiments is not manageable. In Table 2, a clear assignment of risk can be provided by this matrix to select parameters for DoE design. Parameters giving a pi lower than 0.5 undergo a conventional experimental plan (not shown in this contribution.

Table 2.
Interaction matrix. Parameters are rated according to their potential of interaction (pi) across unit operations. This is done for every parameter combination and all parameters giving a probability of interaction equal or higher than 0.5 are evaluated via multivariate DoE analysis across unit operations in the next step.
| probability of interaction | washing buffer pH | washing buffer Triton X-100 concentration | washing buffer temperature | washing buffer DTT -concentration | number of passages homogenizer | temperature homogenizer | pressure homogenizer | BDW concentration homogenizer | duration centrifugation | g-number centrifugation | temperature centrifugation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| washing buffer pH | 0.1 | 0.3 | 0.1 | 0.1 | 0.3 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 |
| washing buffer Triton X-100 concentration | 0.3 | 0.1 | 0.1 | 0.1 | 0.5 | 0.3 | 0.3 | 0.3 | 0.7 | 0.7 | 0.1 |
| washing buffer temperature | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| washing buffer DTT - concentration | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| number of passages homogenizer | 0.3 | 0.5 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.5 | 0.5 | 0.1 |
| temperature homogenizer | 0.1 | 0.3 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| pressure homogenizer | 0.3 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 | 0.1 |
| BDW concentration homogenizer | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 | 0.1 |
| duration centrifugation | 0.1 | 0.7 | 0.1 | 0.1 | 0.5 | 0.1 | 0.3 | 0.3 | 0.1 | 0.7 | 0.1 |
| g-number centrifugation | 0.1 | 0.7 | 0.1 | 0.1 | 0.5 | 0.1 | 0.3 | 0.3 | 0.7 | 0.1 | 0.3 |
| temperature centrifugation | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 |
The parameters Triton X-100 concentration, duration of centrifugation, g-number of centrifugation, and number of passages during homogenization resulted in pi values equal or higher than 0.5 for the CQA IB purity and were included in DoE AUO.
3.4. Power of Approach is Demonstrated for Early Downstream Protein Isolation Steps for rhGF
This section demonstrates the applicability of the new strategy on the multivariate investigation of interactions across unit operations by the example of the isolation steps of an rhGF for the CQA IB purity.
3.4.1. DoE Design
Based on the risk assessments of the previous sections four parameters were included in the multivariate study across unit operations. The CPPs Triton X-100 concentration in washing step, duration of centrifugation, g-number of centrifugation, and passages during homogenization was investigated for the response IB purity. With the purpose of determining interactions across unit operations and optimal operating conditions we selected a central composite face centered design (CCF) with three levels and resolution V. Within this design no main effects or two-factor interactions are aliased with any other main effects or two-factor interactions, which facilitates optimization and design space establishment across unit operations. Evaluation of IB purity was done by densitometric analysis of the ratio of impurity amount per target protein amount using SDS-Gels.
The design consists of 27 randomized IB protein isolation runs including three center points. Two runs were excluded due to sample precipitation in the SDS Gel pocket. The design region comprising the remaining 25 runs is shown in Figure 5.
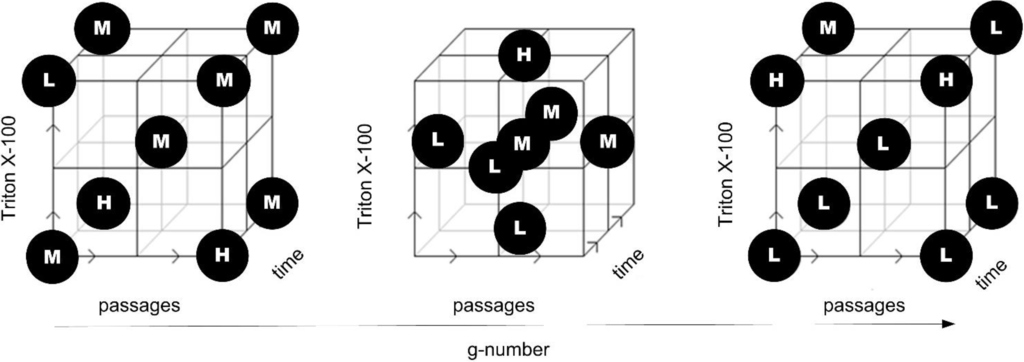
Figure 5.
Central composite face centered design for DoE across unit operations. Parameter passages from unit operation homogenization, Triton X-100 concentration from washing step, and time plus g-number of the unit operation centrifugation are combined in this design space exploration; H = high, M = middle and L = low level of response.
3.4.2. Multivariate DoE Study across Unit Operations
The rationale behind the criticality rating in this multivariate study was discussed as follows. Triton X-100 is known to increase IB purity by solubilizing membrane proteins, thereby solubilizing membrane fractions that otherwise would accumulate in the IB/cell debris fraction during centrifugation. The degree of cell disruption and the size of cell fragments vary depending on the number of passages during homogenization. Homogenization of bacterial cells at low numbers of passages leads to a higher viscosity of the cell suspension while increasing the number of passages decreases the viscosity. This effect is mainly associated with released DNA that is sheared during repeated homogenization passages. Both the concentration of Triton X-100 and the viscosity of the cell suspension after homogenization might interact with the centrifugation parameters, as the centrifugation time is proportional to the viscosity, inversely proportional to the density difference and to the square of both, the particle diameter and angular velocity/RCF. Centrifugation time is also influenced by the particle characteristics diameter and the density, both described by Stokes’ law.
The multivariate study revealed that the following parameters impacted process performance and product quality.
- The Triton X-100 concentration during the washing step showed a main effect on CQA IB purity
- A significant interaction effect between the process parameters Triton X-100 concentration and g-number of centrifugation was observed
Experimental design and data evaluation were done using the experimental design software MODDE (Umetrics, Umeå, Sweden). The response IB purity was fitted using multilinear regression (MLR). The software automatically tests for collinearities using singular value decomposition, which can be read out as condition number. A condition number is defined as the square root of the ratio of the largest and the smallest eigenvalue of the scatter matrix. Serious collinearity problems occur at condition numbers >8. The condition number of this experimental setup was 1.355, which means no collinearities were detected. The significance of factors is estimated on the basis of a t-test; the factor selection was done using a manual backward selection resulting in the statistically significant factors/terms at the 5% level: Triton X-100 concentration (p = 0.0048), Triton X-100 concentration*g-number (p = 0.0020) and g-number (p = 0.0962, this non-significant factor is not removed due to the hierarchical structure of the model). The confidence intervals on the model coefficients are generated on the basis of the t-test and are shown by the bars on the significant model coefficients (Figure 6a).
Evaluation of the CCF model revealed a quadratic interaction term across process steps of Triton X-100 concentration in the washing step and the g number of the centrifugation step. Although the predictive power of the model is low (Q2 = 0.348, R2 = 0.564) due to high replicate errors, the model is valid (probability based on the fact that the lack of fit was not significant at the 5% level p = 0.301). The predicted responses based on the developed model showed a maximum purity within the investigated range at 1% Triton X-100 and 13,000 g. The IB purity is shown in percentage along the isobars in Figure 6b and was assessed as described in IB purification (Section 2.2 Harvest and IB Processing). These findings regarding IB purity can be explained by the fact that by increasing the g-numbers at constant 0% Triton X-100 concentration, smaller cell fractions pelletize and hence reduce IB purity. The lowest IB purity within the investigated range can therefore be found at the highest g-numbers for 0% Triton X-100 concentration. The study demonstrated an inverse effect for IB purity at high g-numbers when the concentration of Triton X-100 was raised to 1%. The capability of Triton X-100 to dissolve membrane proteins and increase purity rises to a maximum at 1% within the investigated range. One percent was chosen as a constraint as this value is often reported in the literature for inclusion body purification and is therefore assumed not to attack the IB surface [33]. In traditional approaches IB are often reported to be collected at low speed centrifugation between 5000 and 8000 g and to be separated from cell debris based on their higher density [34]. The study revealed that centrifugation at higher g-numbers increased purity in the presence of 1% Triton X-100. This investigation across unit operation demonstrates the power to reveal interaction effects of parameters from different unit operations.
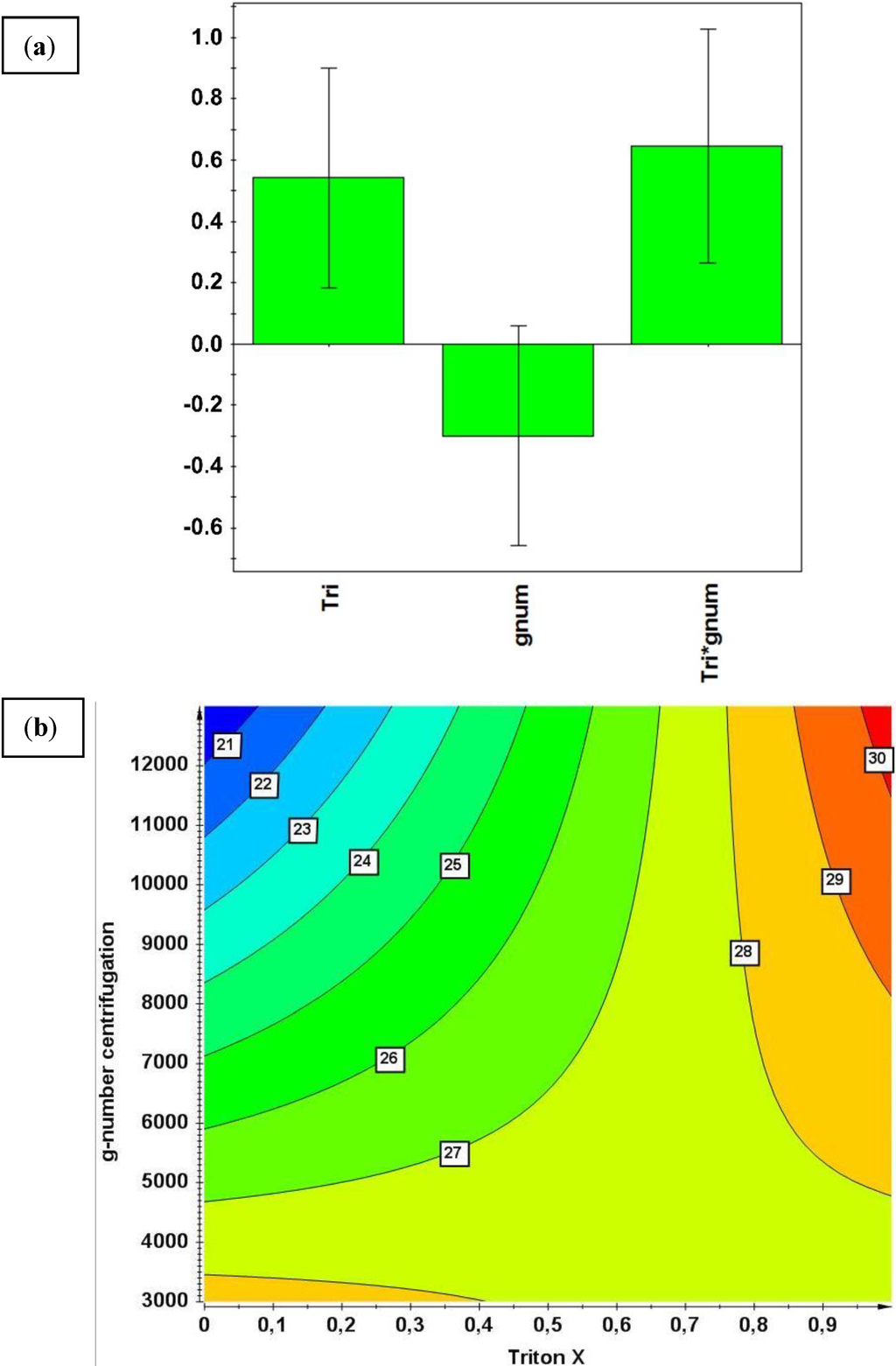
Figure 6.
(a) Normalized coefficient plot for the significant factors Triton X-100 concentration (Tri) and g-number of centrifugation (gnum). (b) Prediction plot for IB purity. IB purity is shown as a function of Triton X-100 concentration in the washing step and g-number of the centrifugation step.
Investigation of one factor at a time starting with the g-number would result in a local IB purity maximum at about 3000–4500 g. Increasing then Triton X-100 concentration at this fixed g-number level would show no significant effect on IB purity. The risk of missing potential important parameter interactions in OFAT approaches can be compared with the risk in traditional approaches where parameters from different unit operations are covered in an isolated way. The perceived outcomes of different risk assessment approaches are compared in Figure 7. Isolated examination of parameters that act across unit operations hold the risk of finding local maxima (or minima) instead of real maxima (or minima) (Approaches 1 and 2). For pharmaceutical processes, the regulatory authorities urge to consider a more integrated view, which was put into practice within the A-Mab case study [30]. The A-Mab case study constitutes the state of the art reference document for process development along QbD principles. While the A-Mab approach considers interactions within unit operations, it provides no strategy about how to deal with the risk of interactions across unit operations. The novel risk based approach presented within this contribution (Approach 3) holds the potential of uncovering hidden interdependencies as shown for the early protein isolation steps in this study while being fully in-line with the regulatory concepts of QbD.
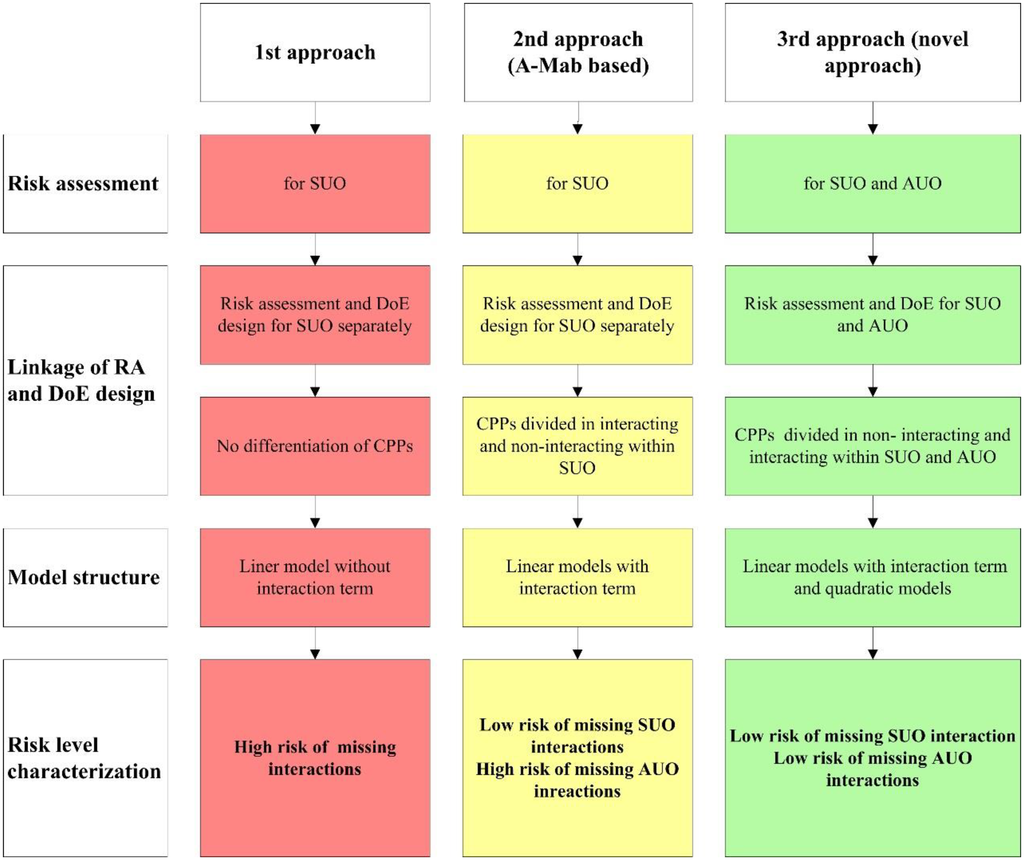
Figure 7.
Comparison of different risk assessment approaches. The first approach displays the traditional way of linkage between risk assessment (RA) and DoE design, the second approach was a development towards a more integrated view (A-Mab study [30]). The perceived reduction of risk from Approach 1 to our novel Approach 3 results from minimizing the risk of missing interactions within SUO and AUO.
4. Conclusions
Within process development of integrated downstream processes and factor-to-factor interactions across unit operations need to be taken into account. Otherwise, global optima cannot be identified, and the risk of factor interactions cannot be considered, as required by the regulatory authorities’ Quality by Design initiative.
For the first time we report a strategy that allows the user to deal with the risk of process parameter interactions across unit operations based on a novel risk assessment tool (interaction matrix).
A comparison of traditional, enhanced, and novel AUO risk assessment approaches (Figure 7) represents the perceived risk reduction up to the novel approach, ranging from high risk when considering only isolated parameters in single unit operations to significantly reduced risk when considering the whole process. The presented approach used traditional CCF designs with factors spreading across multiple operations. The use of partition designs, which were reported to be applicable for this purpose [18], instead of CCF designs would be possible.
Although the number of experiments is a sensitive issue in terms of time and cost investment, a step forward to a more science based process development is essential for future drug development. The roadmap for downstream process development along QbD principles presented here targets at facilitating the development of flexible production processes that are adaptable as a response to increasing market demands.
Acknowledgements
Funding Institutions within the framework of the Austrian K1—Program were the Austrian Research Promotion Agency (FFG), Structural Programs Styrian provincial government (Land Steiermark)—Department for Science and Research, Styrian Business Promotion Agency (SFG), BIRD-C GmbH & CoKG, Kritzendorf and Morphoplant GmbH, Bochum. Thanks to Dominik Sauer who contributed to the experimental section.
Author Contributions
TL managed the first risk evaluations/FMEA, AM, PS and CH co-developed the new risk strategy concept, AM drafted the manuscript and conducted the study. All authors approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gottschalk, U. The future of downstream processing-2013. BioPharm. Int. 2013, 24, S39–S45. [Google Scholar]
- Rathore, A.S.; Winkle, H. Quality by design for biopharmaceuticals. Nat. Biotechnol. 2009, 27, 26–34. [Google Scholar] [CrossRef] [PubMed]
- PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine, Office of Regulatory Affairs: Rockville, MD, USA, 2004.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Pharmaceutical Development Q8(R2). 2009. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf (accessed on 31 July 2014).
- Rathore, A.S. Implementation of quality by design (QbD) for biopharmaceutical products. PDA J. Pharm. Sci. Technol. 2010, 64, 495–496. [Google Scholar] [PubMed]
- Rathore, A.S. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009, 27, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Devine, R. PDA workshop on “Quality by Design for Biopharmaceuticals: Concepts and Implementation”, May 21–22, 2007, Bethesda, Maryland. PDA J. Pharm. Sci. Technol. 2008, 62, 380–390. [Google Scholar] [PubMed]
- Mandenius, C.F.; Graumann, K.; Schultz, T.W.; Premstaller, A.; Olsson, I.M.; Petiot, E.; Clemens, C.; Welin, M. Quality-by-design for biotechnology-related pharmaceuticals. Biotechnol. J. 2009, 4, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Branning, R.; Cecchini, D. Quality: Design space for biotech products. Biopharm. Int. 2007, 20. Available online: http://www.biopharminternational.com/biopharm/Article/Quality-Design-Space-for-Biotech-Products/ArticleStandard/Article/detail/415832 (accessed on 31 July 2014).
- ICH Harmonised Tripartite Guideline. Quality Risk Management Q9. 2005. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf (accessed on 31 July 2014).
- ICH Harmonised Tripartite Guideline. Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) Q11. 2012. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q11/Q11_Step_4.pdf (accessed on 31 July 2014).
- ICH Harmonised Tripartite Guideline. Pharmaceutical Quality System Q10. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q10/Step4/Q10_Guideline.pdf (accessed on 31 July 2014).
- Bade, P.D.; Kotu, S.P.; Rathore, A.S. Optimization of a refolding step for a therapeutic fusion protein in the quality by design (QbD) paradigm. J. Separ. Sci. 2012, 35, 3160–3169. [Google Scholar] [CrossRef]
- Bhambure, R.; Rathore, A.S. Chromatography process development in the quality by design paradigm I: Establishing a high-throughput process development platform as a tool for estimating “characterization space” for an ion exchange chromatography step. Biotechnol. Progr. 2013, 29, 403–414. [Google Scholar] [CrossRef]
- Rathore, A.S.; Yu, M.; Yeboah, S.; Sharma, A. Case study and application of process analytical technology (PAT) towards bioprocessing: use of on-line high-performance liquid chromatography (HPLC) for making real-time pooling decisions for process chromatography. Biotechnol. Bioeng. 2008, 100, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Rao Dasari, V.K.; Are, D.; Rao Joginapally, V.; Mangamoori, L.N.; Rao Adibhatla, K.S.B. Optimization of the downstream process for high recovery of rhG-CSF from inclusion bodies expressed in Escherichia coli. Process Biochem. 2008, 43, 566–575. [Google Scholar] [CrossRef]
- Gao, W.J.J.; Lin, H.J.; Leung, K.T.; Liao, B.Q. Influence of elevated pH shocks on the performance of a submerged anaerobic membrane bioreactor. Process Biochem. 2010, 45, 1279–1287. [Google Scholar] [CrossRef]
- Pieracci, J.; Perry, L.; Conley, L. Using partition designs to enhance purification process understanding. Biotechnol. Bioeng. 2010, 107, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Harms, J.; Wang, X.Y.; Kim, T.; Yang, X.M.; Rathore, A.S. Defining process design space for biotech products: Case study of Pichia pastoris fermentation. Biotechnol. Progr. 2008, 24, 655–662. [Google Scholar] [CrossRef]
- Looby, M.; Ibarra, N.; Pierce, J.J.; Buckley, K.; O’Donovan, E.; Heenan, M.; Moran, E.; Farid, S.S.; Baganz, F. Application of quality by design principles to the development and technology transfer of a major process improvement for the manufacture of a recombinant protein. Biotechnol. Progr. 2011, 27, 1718–1729. [Google Scholar] [CrossRef]
- Montgomery, D.C. Experimental design for product and process design and development. J. Royal Statistical Soc. Ser. D (The Statistician) 1999, 48, 159–177. [Google Scholar] [CrossRef]
- Czitrom, V. One-Factor-at-a-Time versus designed experiments. Am. Statistician 1999, 53, 126–131. [Google Scholar]
- Wahid, Z.; Nadir, N. Improvement of one factor at a time through design of experiments. WASJ 2013, 21, 56–61. [Google Scholar]
- NIST/SEMATECH e-Handbook of Statistical Methods. Available online: http://itl.nist.gov/div898/handbook/index.htm (accessed on 31 July 2014).
- Telford, J.K. A brief introduction to design of experiments. Johns Hopkins Apl. Technical. Digest. 2007, 27, 224–232. [Google Scholar]
- Garcia, T.; Cook, G.; Nosal, R. PQLI key topics—Criticality, design space, and control strategy. J. Pharm. Innov. 2008, 3, 60–68. [Google Scholar] [CrossRef]
- Guebitz, B.; Schnedl, H.; Khinast, J.G. A risk management ontology for Quality-by-Design based on a new development approach according GAMP 5.0. Expert Syst. Appl. 2012, 39, 7291–7301. [Google Scholar] [CrossRef]
- Schmidt, R.; Riedel, G.J.; Kangas, K. Risk Assessment Using Design Review Based on Failure Mode. In Proceedings of 2011 Annual Reliability and Maintainability Symposium (RAMS), Lake Buena Vista, FL, USA, 24–27 January 2011; pp. 1–6.
- Dizon-Maspat, J.; Bourret, J.; D’Agostini, A.; Li, F. Single pass tangential flow filtration to debottleneck downstream processing for therapeutic antibody production. Biotechnol. Bioeng. 2012, 109, 962–970. [Google Scholar] [CrossRef] [PubMed]
- CMC Biotech Working Group. A-Mab: A case Study in Bioprocess Development. Available online: http://www.casss.org/?page=286 (accessed on 31 July 2014).
- Maachupalli-Reddy, J.; Kelley, B.D.; Clark, E.D.B. Effect of inclusion body contaminants on the oxidative renaturation of hen egg white lysozyme. Biotechnol. Progr. 1997, 13, 144–150. [Google Scholar] [CrossRef]
- DeLisa, M.P.; Li, J.; Rao, G.; Weigand, W.A.; Bentley, W.E. Monitoring GFP-operon fusion protein expression during high cell density cultivation of Escherichia coli using an on-line optical sensor. Biotechnol. Bioeng. 1999, 65, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Margreiter, G.; Messner, P.; Caldwell, K.D.; Bayer, K. Size characterization of inclusion bodies by sedimentation field-flow fractionation. J. Biotechnol. 2008, 138, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Batas, B.; Schiraldi, C.; Chaudhuri, J.B. Inclusion body purification and protein refolding using microfiltration and size exclusion chromatography. J. Biotechnol. 1999, 68, 149–158. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).