Tritium as a Tracer of Leachate Contamination in Groundwater: A Brief Review of Tritium Anomalies Method
Abstract
1. Introduction
2. Background on Tritium
3. Tritium Activity Anomalies Method, a Summary
3.1. Background Concentration
3.2. Tritium as Leachate Tracer
- It was present in the leachate of many MSWs at high concentration;
- Being part of the water molecule, it is not subject to chemical reactions;
- “………………”, it is not absorbed by the soil;
- “………………”, it travels together with groundwater, since it is itself water.
- Some limitations were (and actually are!) instead connected to the following points:
- Few laboratories in the world own the proper facilities and human resources to perform such analysis;
- The cost is quite high;
- When low level tritium analysis is needed, samples are to be concentrated by electrolytical enrichment, which is a long and complex procedure.
3.3. Tritium Activity Anomalies: Overview of the TAAM Method
4. Literature Survey on the Application of Tritium in Landfill Pollution Studies
- The identification of the potential absence of pollution, when chemical studies in groundwater lead to uncertain or confusing results.
- The validation of chemical results in the case of landfill pollution.
- The assessment of contamination even before and better than chemistry.
- In these examples, tritium content in the leachate varies from a few tens to some thousands of TU.
| Reference | Location | Sampling Period | Leachate Tritium Content (TU) | |
|---|---|---|---|---|
| Avg | Min–Max | |||
| [52] | Western Australia | 1989–1992 | - | 2119–5339 |
| [52] | Western Australia | 2008–2009 | - | 1135–4297 |
| [52] | Western Australia | 2007–2009 | - | 237–2144 |
| [53] | - | - | - | 160–2800 |
| [54] | Piemonte, Italy | May-2009 | 415.2 | - |
| [66] | Emilia Romagna, Italy | Apr-1997 | 1665 | - |
| [66] | Emilia Romagna, Italy | Aug-1997 | 1080 | - |
| [66] | Emilia Romagna, Italy | Nov-1997 | 1700 | - |
| [66] | Emilia Romagna, Italy | Apr-1996 | 400 | - |
| [66] | Veneto, Italy | Mar-1998 | 983 | - |
| [66] | Veneto, Italy | Mar-1998 | 1545 | - |
| [66] | Veneto, Italy | Mar-1998 | 1129 | - |
| [66] | Veneto, Italy | May-2003 | 217 | - |
| [66] | Veneto, Italy | Apr-2004 | 119.4 | - |
| [66] | Veneto, Italy | Sep-2003 | 224.2 | - |
| [66] | Veneto, Italy | Nov-2008 | 428.9 | - |
| [66] | Veneto, Italy | - | 69.3 | - |
| [77] | Toscana, Italy | Aug-1989 | 980 | - |
| [77] | Toscana, Italy | Nov-1989 | 1003 | - |
| [77] | Emilia Romagna, Italy | Sep-1996 | 1100 | - |
| [77] | Emilia Romagna, Italy | Nov-1997 | 1581 | - |
| [78] | New York, US | 2006 | - | 393–60,152 |
| [79] | United Kingdom | - | *–39,270 | |
| [80] | Northeastern Scotland | 1996 | 2483 | - |
| [80] | Northeastern Scotland | 1999 | 1152 | - |
| [80] | Glasgow, Scotland | 1998 | 2135 | - |
| [80] | Glasgow, Scotland | 1995 | 11703 | - |
| [80] | Lowlands, Scotland | 1996 | 5330 | - |
| [80] | Lowlands, Scotland | 1999 | 602 | - |
| [68] | Veneto, Italy | Apr-2005 | 529 | - |
| [68] | Veneto, Italy | Jun-2005 | 2241 | - |
| [68] | Veneto, Italy | Sep-2005 | 1330 | - |
| [68] | Veneto, Italy | Apr-2005 | 629 | - |
| [68] | Veneto, Italy | Jun-2005 | 3416 | - |
| [70] | Veneto, Italy | Sep-2005 | 1330 | - |
| [70] | Veneto, Italy | Mar-2003 | 354 | - |
| [70] | Veneto, Italy | Jul-2003 | 225 | - |
| [70] | Veneto, Italy | Apr-2004 | 127 | - |
| [71] | Emilia Romagna, Italy | Jun-2006 | 658 | - |
| [71] | Emilia Romagna, Italy | Apr-1998 | 480 | - |
| [71] | Emilia Romagna, Italy | Jul-1997 | 304 | - |
| [71] | Emilia Romagna, Italy | May-1997 | 178 | - |
| [71] | Emilia Romagna, Italy | Dec-1997 | 280 | - |
| [72] | Veneto, Italy | Apr-1997 | 1587 | - |
| [74] | Marche, Italy | Mar-2002 | 15 | - |
| [74] | Marche, Italy | May-2003 | 13.2 | - |
| [74] | Marche, Italy | Dec-2001 | 291.2 | - |
| [74] | Marche, Italy | Nov-2001 | 53 | - |
| [74] | Marche, Italy | Jul-2002 | 29.9 | - |
| [74] | Marche, Italy | Nov-1993 | 520 | - |
| [73] | Emilia Romagna, Italy | Sep-1995 | 140 | - |
| [73] | Emilia Romagna, Italy | Sep-1995 | 960 | - |
| [73] | Emilia Romagna, Italy | Jul-1997 | 180 | - |
| [81] | Marche, Italy | May-2000 | 500 | - |
| [69] | Marche, Italy | Mar-1994 | 910 | - |
| [69] | Marche, Italy | Aug-1994 | 450 | - |
| [69] | Emilia Romagna, Italy | Apr-1996 | 83 | - |
| [69] | Emilia Romagna, Italy | Apr-1996 | 134 | - |
| [69] | Emilia Romagna, Italy | Apr-1996 | 82 | - |
| [69] | Emilia Romagna, Italy | Apr-1996 | 53 | - |
| [69] | Emilia Romagna, Italy | Sep-1996 | 270 | - |
| [69] | Emilia Romagna, Italy | Sep-1996 | 167 | - |
| [69] | Emilia Romagna, Italy | Apr-1996 | 1080 | - |
| [82] | Marche, Italy | Dec-1993 | 290 | - |
| [82] | Marche, Italy | Oct-1993 | 664 | - |
| [83] | Puglia, Italy | Nov-2014 | 235 | - |
| [83] | Puglia, Italy | Mar-2015 | 182 | - |
| [83] | Puglia, Italy | Jun-2015 | 225 | - |
| [84] | Sicily, Italy | 2019 | - | 48–374 |
| [85] | Abruzzo, Italy | Jun-2014 | 34.5 | - |
| [87] | Illinois, US | Pre 1992 | - | 227–338 |
| [88] | Austria | - | 2000 | |
| [89] | Austria | - | 3000 | |
| [90] | California, US | 1991 | 2421 | - |
| [91] | Scotland | 1995–1999 | - | 212–11,703 |
| [92] | California, US | 2003 | - | *–95,322 |
| [92] | Pennsylvania, US | 2004 | - | *–29,317 |
| [92] | Pennsylvania, US | 2005 | - | *–57,068 |
| [93] | Brazil | May-2002 | - | 111–141 |
| [94] | South Africa | 1995 | - | *–100,000 |
| [95] | South Africa | 1997 | 76000 | - |
| Leachate Tritium Content (TU) | δ18O | Cl− (mg/L) | NO3− (mg/L) | SO42− (mg/L) | EC (uS/cm) | pH | T (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | Location | Samp. Period | Avg | Min–Max | Min–Max | Avg | Min–Max | Avg | Min–Max | Avg | Min–Max | Avg | Min–Max | Avg | Min–Max | Avg | Min–Max |
| [56] | Illinois, US | - | - | 225–8001 | −8.8–−6.2 | - | - | - | - | - | - | - | - | - | - | - | - |
| [96] | Philippines | 2006–2008 | - | 750–820 | - | - | 8–204 | - | - | - | 10–34 | - | 3070–20,000 | - | - | - | - |
| [97] | Korea | 2002–2003 | - | 17–1196 | - | - | - | - | - | - | - | - | 5888–16,500 | - | 7.10–8.05 | - | - |
| [85] | Abruzzo, Italy | 2014 | 34.5 | - | - | 416 | - | - | - | 148.3 | - | - | 3267 | 7.1 | 22 | - | |
| [98] | Indonesia | Mar-2013 | - | 51–493 | −5.93–0.67 | - | - | - | - | - | - | - | 10.88–>20 | - | 7.61–80.4 | - | 30.5–35.2 |
| [99] | Greece | - | 38 | - | −7.16–−1.42 | 22404 | - | - | < 0.5 | 2.2 | - | 67100 | - | 7.5 | - | 28 | - |
| [95] | South Africa | Jul-2010 | 787.1 | - | - | 908 | - | 1.42 | - | 82.12 | - | 358.3 | - | 7.6 | - | - | - |
| [95] | South Africa | Jan-2012 | 781 | - | - | 1427 | - | 0.06 | - | 48.1 | - | 944 | - | 8.1 | - | - | - |
| [97] | Korea | 1994–2016 | - | 17–1196 | - | - | 132–3070 | - | 2–2600 | - | 2–90 | - | - | - | 7.36–8.29 | - | - |
| [100] | Central Europe | 1995–2002 | 540 | 10–150,000 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| [86] | Puglia, Italy | 2015 | - | 55–923 | - | - | 1230–14,600 | - | <0.5–53.1 | - | 1–156 | - | 15,500–35,400 | - | 7.5–7.9 | - | 19.9–42.5 |
4.1. Case History 1: Landfill Not Polluting the Environment
4.2. Case History 2: Landfill Polluting the Environment
4.3. Case History 3: Assessment of Landfill Leakage before Chemical Concentration Changes
5. Conclusions
- The current level of tritium in the leachate could not be high enough to ensure a good determination in groundwater and surface water, and this can also be dependent on the improvement in processes of waste storage;
- The analyses are expensive, there is the need of specific equipment (not used for other laboratory determination), the laboratory process is difficult and time-consuming, especially when using electrolytic enrichment;
- Often (and this is probably the greatest problem), there is a lack of knowledge about the potentiality of such a method in solving problems related to landfill contamination.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kendall, C.; Caldwell, E.A. Fundamentals of Isotope Geochemistry. In Isotope Tracers in Catchment Hydrology; Elsevier: Singapore, 1998; pp. 51–86. [Google Scholar]
- Philp, R.P. The Emergence of Stable Isotopes in Environmental and Forensic Geochemistry Studies: A Review. Environ. Chem. Lett. 2007, 5, 57–66. [Google Scholar] [CrossRef]
- IAEA, Environmental Isotopes in The Hydrological Cycle-Principles and Applications. Water Resour. Programme 2000, 1, 1–117.
- IAEA Isotopes in Environmental Studies. In Proceedings of the Aquatic Forum, Monaco, Austria, 25–29 October 2004.
- IAEA Use of Environmental Isotopes in Assessing Water Resources in Snow, Glacier, and Permafrost Dominated Areas under Changing Climatic Conditions. Available online: https://www.iaea.org/projects/crp/f32006 (accessed on 27 April 2015).
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 2013; ISBN 0-429-06957-X. [Google Scholar]
- Kendall, C.; Doctor, D.H. Stable Isotope Applications in Hydrologic Studies. Treatise Geochem. 2003, 5, 605. [Google Scholar]
- Kumar, S. Environmental Isotopes in Groundwater Applications. In Groundwater Development and Management; Springer: Berlin/Heidelberg, Germany, 2019; pp. 77–146. [Google Scholar]
- Barbieri, M. Isotopes in Hydrology and Hydrogeology; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2019; Volume 11, p. 291. [Google Scholar]
- Nisi, B.; Raco, B.; Dotsika, E. Groundwater Contamination Studies by Environmental Isotopes: A Review. Threat. Qual. Groundw. Resour. 2014, 40, 115–150. [Google Scholar]
- Sankoh, A.A.; Derkyi, N.S.A.; Frazer-williams, R.A.D.; Laar, C.; Kamara, I. A Review on the Application of Isotopic Techniques to Trace Groundwater Pollution Sources within Developing Countries. Water 2021, 14, 35. [Google Scholar] [CrossRef]
- Philp, R.P. Application of Stable Isotopes and Radioisotopes in Environmental Forensics. In Introduction to Environmental Forensics; Elsevier: Singapore, 2015; pp. 395–455. [Google Scholar]
- Rozanski, K.; Gonfiantini, R. Isotopes in Climatological Studies. IAEA Bull. 1990, 32, 9–15. [Google Scholar]
- Wilson, A.T. Isotope Evidence for Past Climatic and Environmental Change. J. Interdiscip. Hist. 1980, 10, 795–812. [Google Scholar] [CrossRef]
- Wright, J.D. Global Climate Change in Marine Stable Isotope Records. In Quaternary Geochronology: Methods and Applications; American Geophysical Union: Washington, DC, USA, 2000; pp. 671–682. [Google Scholar]
- Darling, W.G. The Isotope Hydrology of Quaternary Climate Change. J. Hum. Evol. 2011, 60, 417–427. [Google Scholar] [CrossRef]
- Dogramaci, S.S.; Herczeg, A.L. Strontium and Carbon Isotope Constraints on Carbonate-Solution Interactions and Inter-Aquifer Mixing in Groundwaters of the Semi-Arid Murray Basin, Australia. J. Hydrol. 2002, 262, 50–67. [Google Scholar] [CrossRef]
- Tan, S.P.V., VII; Mendoza, N.D.S.; Racadio, C.D.T.; Puthenpurekal, M.; Resurreccion, A.C.; Matsuzaki, H. Iodine-129 for Determining the Origin of Salinity in Groundwater in Pampanga, Philippines. J. Environ. Radioact. 2020, 218, 106239. [Google Scholar] [CrossRef]
- Brown, E.T.; Edmond, J.M.; Raisbeck, G.M.; Bourlès, D.L.; Yiou, F.; Measures, C.I. Beryllium Isotope Geochemistry in Tropical River Basins. Geochim. Cosmochim. Acta 1992, 56, 1607–1624. [Google Scholar] [CrossRef]
- Solomon, D.K.; Cook, P.G. 3 H and 3 He. In Environmental Tracers in Subsurface Hydrology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 397–424. [Google Scholar]
- Tadros, C.V.; Hughes, C.E.; Crawford, J.; Hollins, S.E.; Chisari, R. Tritium in Australian Precipitation: A 50 Year Record. J. Hydrol. 2014, 513, 262–273. [Google Scholar] [CrossRef]
- Jasechko, S. Global Isotope Hydrogeology―Review. Rev. Geophys. 2019, 57, 835–965. [Google Scholar] [CrossRef]
- Fronzi, D.; Mirabella, F.; Cardellini, C.; Caliro, S.; Palpacelli, S.; Cambi, C.; Valigi, D.; Tazioli, A. The Role of Faults in Groundwater Circulation before and after Seismic Events: Insights from Tracers, Water Isotopes and Geochemistry. Water 2021, 13, 1499. [Google Scholar] [CrossRef]
- Benítez, S.V.B. Understanding of (Bio) Geochemical Processes Which Control Chromium Release, Speciation and Isotopic Fractionation in Ultramafic Environments Impacted by Mining Activitites, Université Paris-Est; Università Degli Studi: Cassino, Italie, 2018. [Google Scholar]
- Fernex, F.; Richou, M.; Benamou, C.; Benaim, J. Evaluation of the Quality of Bottom Sediments from the North-Western Mediterranean Sea by Bio-Geochemical Criteria. Sci. Total Environ. 1992, 111, 25–46. [Google Scholar] [CrossRef]
- Caterina, D.; Orozco, A.F.; Nguyen, F. Long-Term ERT Monitoring of Biogeochemical Changes of an Aged Hydrocarbon Contamination. J. Contam. Hydrol. 2017, 201, 19–29. [Google Scholar] [CrossRef]
- Benner, S.G.; Smart, E.W.; Moore, J.N. Metal Behavior during Surface-Groundwater Interaction, Silver Bow Creek, Montana. Environ. Sci. Technol. 1995, 29, 1789–1795. [Google Scholar] [CrossRef]
- Hohl, S.V.; Jiang, S.-Y.; Viehmann, S.; Wei, W.; Liu, Q.; Wei, H.-Z.; Galer, S.J. Trace Metal and Cd Isotope Systematics of the Basal Datangpo Formation, Yangtze Platform (South China) Indicate Restrained (Bio) Geochemical Metal Cycling in Cryogenian Seawater. Geosciences 2020, 10, 36. [Google Scholar] [CrossRef]
- Bertrand, G.; Siergieiev, D.; Ala-Aho, P.; Rossi, P.M. Environmental Tracers and Indicators Bringing Together Groundwater, Surface Water and Groundwater-Dependent Ecosystems: Importance of Scale in Choosing Relevant Tools. Environ. Earth Sci. 2014, 72, 813–827. [Google Scholar] [CrossRef]
- Hintze, S.; Glauser, G.; Hunkeler, D. Influence of Surface Water–Groundwater Interactions on the Spatial Distribution of Pesticide Metabolites in Groundwater. Sci. Total Environ. 2020, 733, 139109. [Google Scholar] [CrossRef]
- Kalbus, E.; Reinstorf, F.; Schirmer, M. Measuring Methods for Groundwater–Surface Water Interactions: A Review. Hydrol. Earth Syst. Sci. 2006, 10, 873–887. [Google Scholar] [CrossRef]
- Kendall, C.; McDonnell, J.J. Isotope Tracers in Catchment Hydrology; Elsevier: Singapore, 2012; ISBN 0-08-092915-X. [Google Scholar]
- Ehleringer, J.R.; Rundel, P.W. Stable Isotopes:History, Units, and Instrumentation. In Stable Isotopes in Ecological Research; Rundel, P.W., Ehleringer, J.R., Nagy, K.A., Eds.; Ecological Studies; Springer: New York, NY, USA, 1989; Volume 68, pp. 1–15. ISBN 978-1-4612-8127-6. [Google Scholar]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing Anthropogenic Inputs of Nitrogen to Ecosystems. Stable Isot. Ecol. Environ. Sci. 2007, 2, 375–449. [Google Scholar]
- Nikolov, J.; Todorovic, N.; Jankovic, M.; Vostinar, M.; Bikit, I.; Veskovic, M. Different Methods for Tritium Determination in Surface Water by LSC. Appl. Radiat. Isot. 2013, 71, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Michel, R. Tritium in the Hydrologic Cycle. In Isotopes in the Water Cycle; Springer: Berlin/Heidelberg, Germany, 2005; pp. 53–66. [Google Scholar]
- IAEA Global Network of Isotopes in Rivers (GNIR). Available online: https://www.iaea.org/services/networks/gnir (accessed on 1 January 1998).
- IAEA WISER Portal. Available online: http://www-naweb.iaea.org/napc/ih/IHS_resources_isohis.html (accessed on 1 January 1998).
- Mammoliti, E.; Fronzi, D.; Mancini, A.; Valigi, D.; Tazioli, A. WaterbalANce, a WebApp for Thornthwaite–Mather Water Balance Computation: Comparison of Applications in Two European Watersheds. Hydrology 2021, 8, 34. [Google Scholar] [CrossRef]
- Tazioli, A.; Cervi, F.; Doveri, M.; Mussi, M.; Deiana, M.; Ronchetti, F. Estimating the Isotopic Altitude Gradient for Hydrogeological Studies in Mountainous Areas: Are the Low-Yield Springs Suitable? Insights from the Northern Apennines of Italy. Water 2019, 11, 1764. [Google Scholar] [CrossRef]
- Allen, S.T.; Keim, R.F.; Barnard, H.R.; McDonnell, J.J.; Renée Brooks, J. The Role of Stable Isotopes in Understanding Rainfall Interception Processes: A Review. Wiley Interdiscip. Rev. Water 2017, 4, e1187. [Google Scholar] [CrossRef] [PubMed]
- Barbecot, F.; Guillon, S.; Pili, E.; Larocque, M.; Gibert-Brunet, E.; Hélie, J.-F.; Noret, A.; Plain, C.; Schneider, V.; Mattei, A. Using Water Stable Isotopes in the Unsaturated Zone to Quantify Recharge in Two Contrasted Infiltration Regimes. Vadose Zone J. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Wassenaar, L.I.; Hendry, M.J.; Chostner, V.L.; Lis, G.P. High Resolution Pore Water Δ2H and Δ18O Measurements by H2O (Liquid)—H2O (Vapor) Equilibration Laser Spectroscopy. Environ. Sci. Technol. 2008, 42, 9262–9267. [Google Scholar] [CrossRef]
- Hendry, M.J.; Wassenaar, L.I. Inferring Heterogeneity in Aquitards Using High-resolution ΔD and Δ18O Profiles. Groundwater 2009, 47, 639–645. [Google Scholar] [CrossRef]
- Stumpp, C.; Hendry, M.J. Spatial and Temporal Dynamics of Water Flow and Solute Transport in a Heterogeneous Glacial till: The Application of High-Resolution Profiles of Δ18O and Δ2H in Pore Waters. J. Hydrol. 2012, 438, 203–214. [Google Scholar] [CrossRef]
- Mussi, M.; Nanni, T.; Tazioli, A.; Vivalda, P.M. The Mt Conero Limestone Ridge: The Contribution of Stable Isotopes to the Identification of the Recharge Area of Aquifers. Ital. J. Geosci. 2017, 136, 186–197. [Google Scholar] [CrossRef]
- Tazioli, A.; Tazioli, G.S. Landfill Contamination Problems: A General Perspective and Engineering Geology Aspects. G. Geol. Appl. 2005, 1, 203–211. [Google Scholar]
- Ramaroson, V.; Rakotomalala, C.U.; Rajaobelison, J.; Fareze, L.P.; Razafitsalama, F.A.; Rasolofonirina, M. Tritium as Tracer of Groundwater Pollution Extension: Case Study of Andralanitra Landfill Site, Antananarivo–Madagascar. Appl. Water Sci. 2018, 8, 57. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P.; Kennedy, C. Peak Waste: When Is It Likely to Occur? J. Ind. Ecol. 2015, 19, 117–128. [Google Scholar] [CrossRef]
- Mobbs, S.; Barraclough, I.; Napier, I. A Review of the Use and Disposal of Gaseous Tritium Light Devices. Bibliographic Information available from INIS. Available from British Library Document Supply Centre- DSC:GPE/3984. Available online: http://inis.iaea.org/search/search.aspx?orig_q=RN:31046469 (accessed on 1 January 1998).
- United Nations Scientific Committee on the Effects of Atomic Radiation. Report of the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 1977: Report to the General Assembly, with Scientific Annexes; United Nations: New York, NY, USA, 1977; pp. 1–259. [Google Scholar]
- Hughes, C.E.; Cendón, D.I.; Harrison, J.J.; Hankin, S.I.; Johansen, M.P.; Payne, T.E.; Vine, M.; Collins, R.N.; Hoffmann, E.L.; Loosz, T. Movement of a Tritium Plume in Shallow Groundwater at a Legacy Low-Level Radioactive Waste Disposal Site in Eastern Australia. J. Environ. Radioact. 2011, 102, 943–952. [Google Scholar] [CrossRef]
- Coleman, D.D.; Liu, C.L.; Hackley, K.C.; Benson, L.J. Identification of Landfill Methane Using Carbon and Hydrogen Isotope Analysis. In Proceedings of the International Madison Waste Conference Municipal and Industrial Waste; Department of Engineering Professional Development, Madison, WI, USA, 22–23 September 1993; p. 303. [Google Scholar]
- Losana, M.C.; Garbarino, G.; Gastaldo, S.; Marga, M.; Magnoni, M. Misure di Trizio nel Percolato di Discarica in Piemonte. In Proceedings of the AIRP—Atti del XXXV Congresso Nazionale di Radioprotezione, Venezia, Italy, 17–19 October 2012. [Google Scholar]
- Balouet, J.; Oudijk, G.; Petrisor, I.; Robert, D. Morrison Emerging Forensic Techniques. In Introduction to Environmental Forensics; Elsevier Academic Press: Cambridge, MA, USA, 2007; pp. 671–732. [Google Scholar]
- Hackley, K.C.; Liu, C.-L.; Coleman, D.D. Environmental Isotope Characteristics of Landfill Leachates and Gases. Groundwater 1996, 34, 827–836. [Google Scholar] [CrossRef]
- Global Network of Isotopes in Precipitation (GNIP). Available online: https://www.iaea.org/services/networks/gnip (accessed on 1 January 1998).
- Zhang, J.; Satake, H. The Chemical Characteristics of Submarine Groundwater Seepage in Toyama Bay, Central Japan. Land Mar. Hydrogeol. 2003, 45–60. [Google Scholar]
- Butler, M.J. Ground Water Pollution at Sanitary Landfill Sites: Geohydrological, Environmental Isotope and Hydrochemical Studies; University of the Witwatersrand: Johannesburg, South Africa, 1998. [Google Scholar]
- Fekri, A.; Wahbi, M.; Benbouziane, A.; Hammoumi, O.; Marah, H. The Tritium as an Indicator for Landfill Leachate Pollution (Case of Mediouna Landfill, Morocco). Geochim. Cosmochim. Acta Suppl. 2009, 73, A363. [Google Scholar]
- Preziosi, E.; Frollini, E.; Zoppini, A.; Ghergo, S.; Melita, M.; Parrone, D.; Rossi, D.; Amalfitano, S. Disentangling Natural and Anthropogenic Impacts on Groundwater by Hydrogeochemical, Isotopic and Microbiological Data: Hints from a Municipal Solid Waste Landfill. Waste Manag. 2019, 84, 245–255. [Google Scholar] [CrossRef]
- Morgenstern, U.; Stewart, M.K.; Stenger, R. Dating of Streamwater Using Tritium in a Post Nuclear Bomb Pulse World: Continuous Variation of Mean Transit Time with Streamflow. Hydrol. Earth Syst. Sci. 2010, 14, 2289–2301. [Google Scholar] [CrossRef]
- Lindsey, B.D.; Jurgens, B.C.; Belitz, K. Tritium as an Indicator of Modern, Mixed, and Premodern Groundwater Age; US Geological Survey: Reston, VA, USA, 2019. [Google Scholar]
- Onugba, A.; Aboh, H.O. The Tritium Content of Precipitation and Groundwater at Yola, Nigeria. Sci. World J. 2009, 4, 23–28. [Google Scholar] [CrossRef][Green Version]
- Brkić, Ž.; Kuhta, M.; Hunjak, T.; Larva, O. Regional Isotopic Signatures of Groundwater in Croatia. Water 2020, 12, 1983. [Google Scholar] [CrossRef]
- Tazioli, A.; Boschi, G.; Carlini, A. Monitoraggio Dell’inquinamento Da Discariche: Metodi Isotopici per Individuare La Presenza Di Contaminazione Delle Acque Sotterranee. G. Geol. Appl. 2002, 2, 130–136. [Google Scholar]
- Telloli, C.; Rizzo, A.; Salvi, S.; Pozzobon, A.; Marrocchino, E.; Vaccaro, C. Characterization of Groundwater Recharge through Tritium Measurements. Adv. Geosci. 2022, 57, 21–36. [Google Scholar] [CrossRef]
- Tazioli, A. Report Analysis on Isotopes and Tritium; Technical Report for SESA SpA; SESA SpA: Varese, Italy, 2006. [Google Scholar]
- Tazioli, G.S. Isotope and Tracer Techniques Applied to Studies of Sanitary Landfills and to an Industry for Galvanic Treatment. In Proceedings of the Consultants Meeting on Isotope Techniques in Groundwater Pollution Studies., Vienna, Austria, 6–9 December 1993; Volume 6. [Google Scholar]
- Tazioli, A. Report on Isotopes and Chemical Analysis of Leachate and Groundwater; Technical Report for APS SpA; SESA SpA: Varese, Italy, 2003. [Google Scholar]
- University of Ancona Collecchio Landfill: Sampling of Groundwater, Surface Water and Leachates for Isotopic Analysis; Technical Report for AMNU SpA; SESA SpA: Varese, Italy, 1997.
- University of Ancona Measures of Tritium on Samples from Water and Leachates; Analysis Report for Bastian Beton SpA; SESA SpA: Varese, Italy, 1998.
- University of Ancona Ravadese-2 Landfill: Sampling of Groundwater, Surface Water and Leachates for Isotopic Analysis; Technical Report for AMNU SpA; SESA SpA: Varese, Italy, 1996.
- University of Ancona Sanitary Landfills in Ancona Province, Tritium and Isotopes Levels; Analysis Report for Ancona Province; SESA SpA: Varese, Italy, 1994.
- Cidzikienė, V.; Jakimavičiūtė-Maselienė, V.; Girgždienė, R.; Mažeika, J.; Petrošius, R. Assessment of Tritium Activity in Groundwater at the Nuclear Objects Sites in Lithuania. Int. J. Nucl. Energy 2014, 2014, 1–6. [Google Scholar] [CrossRef][Green Version]
- Kashiwaya, K.; Muto, Y.; Kubo, T.; Ikawa, R.; Nakaya, S.; Koike, K.; Marui, A. Spatial Variations of Tritium Concentrations in Groundwater Collected in the Southern Coastal Region of Fukushima, Japan, after the Nuclear Accident. Sci. Rep. 2017, 7, 12578. [Google Scholar] [CrossRef]
- Calestani, G.; Masserano, M.; Pellegrini, M.; Tazioli, G.S.; Vannucchi, M. On the Methods for Monitoring the Interference of Sanitary Landfills Activity Processes with the Underground Environment. The Example of Two Monitored Landfills near the City of Parma, Italy. In Proceedings of the Waste Management and Landfill Symp, Cagliari, Italy, 4–8 October 1999; Volume 4, pp. 245–252. [Google Scholar]
- Mutch, R.D., Jr.; Mahony, J.D. A Study of Tritium in Municipal Solid Waste Leachate and Gas. Fusion Sci. Technol. 2008, 54, 305–310. [Google Scholar] [CrossRef]
- Robinson, H.D.; Gronow, J.R. Tritium Levels in Leachates and Condensates from Domestic Wastes in Landfill Sites. Water Environ. J. 1996, 10, 391–398. [Google Scholar] [CrossRef]
- Hicks, T.W.; Wilmot, R.D.; Bennett, D.G. Tritium in Scottish Landfill Sites; Galston Sciences Limited: Oakham, UK, 2000; p. 29. [Google Scholar]
- University of Ancona Sanitary Landfill of Maiolati Spontini; Analysis Report for Sogenus SpA; SESA SpA: Varese, Italy, 2001.
- Tomassoni, D.; Tazioli, G.S. Assessment Report on Tritium; Technical Report for Public Prosecutor’s Office of Ancona: Ancona, Italy, 1964. [Google Scholar]
- Cossu, R.; Zuffianò, L.E.; Limoni, P.P.; De Giorgio, G.; Pizzardini, P.; Miano, T.; Mondelli, D.; Garavaglia, R.; Carella, C.; Polemio, M. How Can the Role of Leachate on Nitrate Concentration and Groundwater Quality Be Clarified? An Approach for Landfills in Operation (Southern Italy). Waste Manag. 2018, 77, 156–165. [Google Scholar] [CrossRef]
- Rizzo, P.; Cappadonia, C.; Rotigliano, E.; Iacumin, P.; Sanangelantoni, A.M.; Zerbini, G.; Celico, F. Hydrogeological Behaviour and Geochemical Features of Waters in Evaporite-Bearing Low-Permeability Successions: A Case Study in Southern Sicily, Italy. Appl. Sci. 2020, 10, 8177. [Google Scholar] [CrossRef]
- Nigro, A.; Sappa, G.; Barbieri, M. Application of Boron and Tritium Isotopes for Tracing Landfill Contamination in Groundwater. J. Geochem. Explor. 2017, 172, 101–108. [Google Scholar] [CrossRef]
- Raco, B.; Battaglini, R. Tritium as a Tool to Assess Leachate Contamination: An Example from Conversano Landfill (Southern Italy). J. Geochem. Explor. 2022, 235, 106939. [Google Scholar] [CrossRef]
- Liu, C.L.; Hackley, K.C.; Baker, J. Application of Environmental Isotopes to Characterize Landfill Gases and Leachate. In Proceedings of the Geological Society of America, Abstracts with Programs, Cincinnati, OH, USA, 26–29 October 1992; Volume 24, p. A35. [Google Scholar]
- Rank, D.; Papesch, W.; Rajner, V.; Riehl-Herwirsch, G. Environmental Isotopes Study at the Breitenau Experimental Landfill (Lower Austria). Tracer Hydrol. 1992, 173, 176. [Google Scholar]
- Rank, D.; Papesch, W.; Rajner, V. Environmental Isotopes Study at a Research Landfill (Breitenau, Lower Austria). In Proceedings of the Isotopes in Water Resources Management, Breitenau, Austria, 1 March 1996, V. 1. Symposium on isotopes in water resources management, Vienna, Austria, 20–24 March 1995. [Google Scholar]
- Kerfoot, H.B.; Baker, J.A.; Burt, D.M. The Use of Isotopes to Identify Landfill Gas Effects on Groundwater. J. Environ. Monit. 2003, 5, 896. [Google Scholar] [CrossRef]
- Egboka, B.C.E.; Cherry, J.A.; Farvolden, R.N. Estimation of the Percentage of Annual Groundwater Recharge with Bomb Tritium Using a Cumulative Mass Balance Method. Pure Appl. Geophys. Pageoph 1982, 120, 330–347. [Google Scholar] [CrossRef]
- State Water Resources Control Board (SWRCB). Waste Discharge Requirments (Wdrs) For Storm Water Discharges From Small Municipal Separate Storm Sewer Systems (General Permit) 2003, 1–87. Available online: https://www.waterboards.ca.gov/board_decisions/adopted_orders/water_quality/2003/wqo/wqo2003_0005dwq.pdf (accessed on 23 April 2022).
- Bandeira, J.V.; Mingote, R.M.; Baptista, M.B.; Oliveira, D.M.; Lima, F.P. The Use of Tritium Content as an Indicator of the Groundwater Contamination by Sanitary Landfills Leachates in the Region of Belo Horizonte City, Brazil. Water Sci. Technol. 2008, 57, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, B.T. A Unique Approach to Evaluate the Utility of Landfill Monitoring Boreholes. In Geotechnics for Developing Africa: Proceedings of the 12th Regional Conference for Africa on Soil Mechanics and Geotechnical Engineering, Durban, South Africa, 25–27 October 1999; CRC Press: Boca Raton, FL, USA, 1999; Volume 12, p. 43. [Google Scholar]
- Levin, M.; Verhagen, B. Application of Isotope Techniques to Trace Location of Leakage from Dams and Reservoirs. In Technical Report to the Water Research Commission by School of Geosciences; University of the Witwatersrand: Johannesburg, South Africa, 2013; pp. 9–23. [Google Scholar]
- Castañeda, S.S.; Sucgang, R.J.; Almoneda, R.V.; Mendoza, N.D.S.; David, C.P.C. Environmental Isotopes and Major Ions for Tracing Leachate Contamination from a Municipal Landfill in Metro Manila, Philippines. J. Environ. Radioact. 2012, 110, 30–37. [Google Scholar] [CrossRef]
- Park, S.D.; Kim, J.G.; Kim, W.H.; Kim, H.S. Distribution of tritium in the leachates and methane gas condensates from municipal waste landfills in Korea. Water Environ. J. 2005, 19, 91–99. [Google Scholar] [CrossRef]
- Pujiindiyati, E.R.; Sidauruk, P. Study of Leachate Contamination in Bantar Gebang Landfill to Its Shallow Groundwater Using Natural Isotope Tracers of 18O, 2H and 3H. At. Indones. 2015, 41, 31. [Google Scholar] [CrossRef]
- Raco, B.; Dotsika, E.; Battaglini, R.; Bulleri, E.; Doveri, M.; Papakostantinou, K. A Quick and Reliable Method to Detect and Quantify Contamination from MSW Landfills: A Case Study. Water. Air. Soil Pollut. 2013, 224, 1380. [Google Scholar] [CrossRef]
- Fuganti, A.; Eichinger, L.; Morteani, G.; Preinfalk, C. L’utilizzo Degli Isotopi Trizio, Ossigeno-18, Deuterio e Carbonio-13 per La Valutazione Dei Rapporti Tra Discariche Di Rifiuti Ed Acque Sotterranee. Geol. Tec. E Ambient. 2003, 2, 5–10. [Google Scholar]
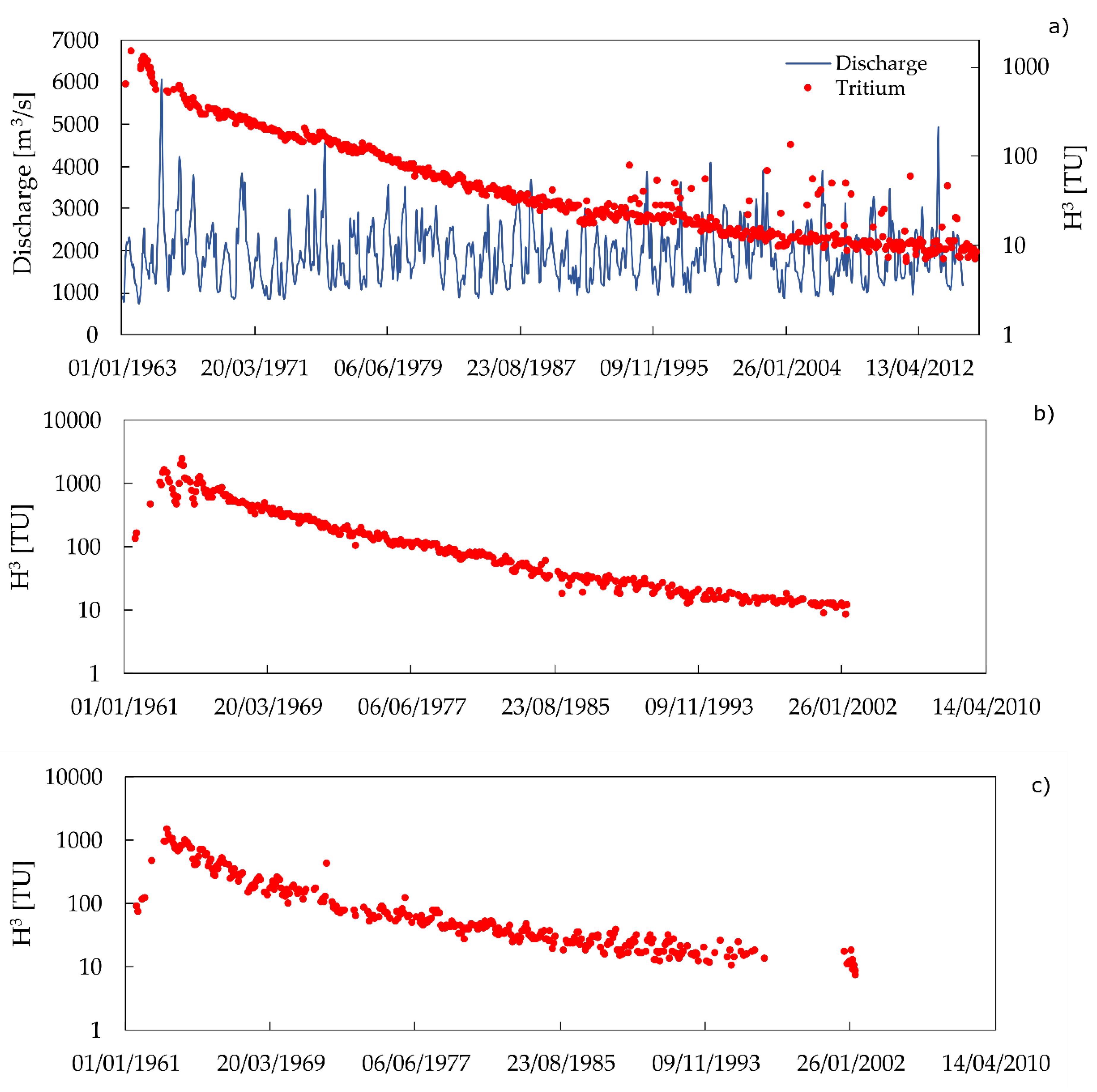
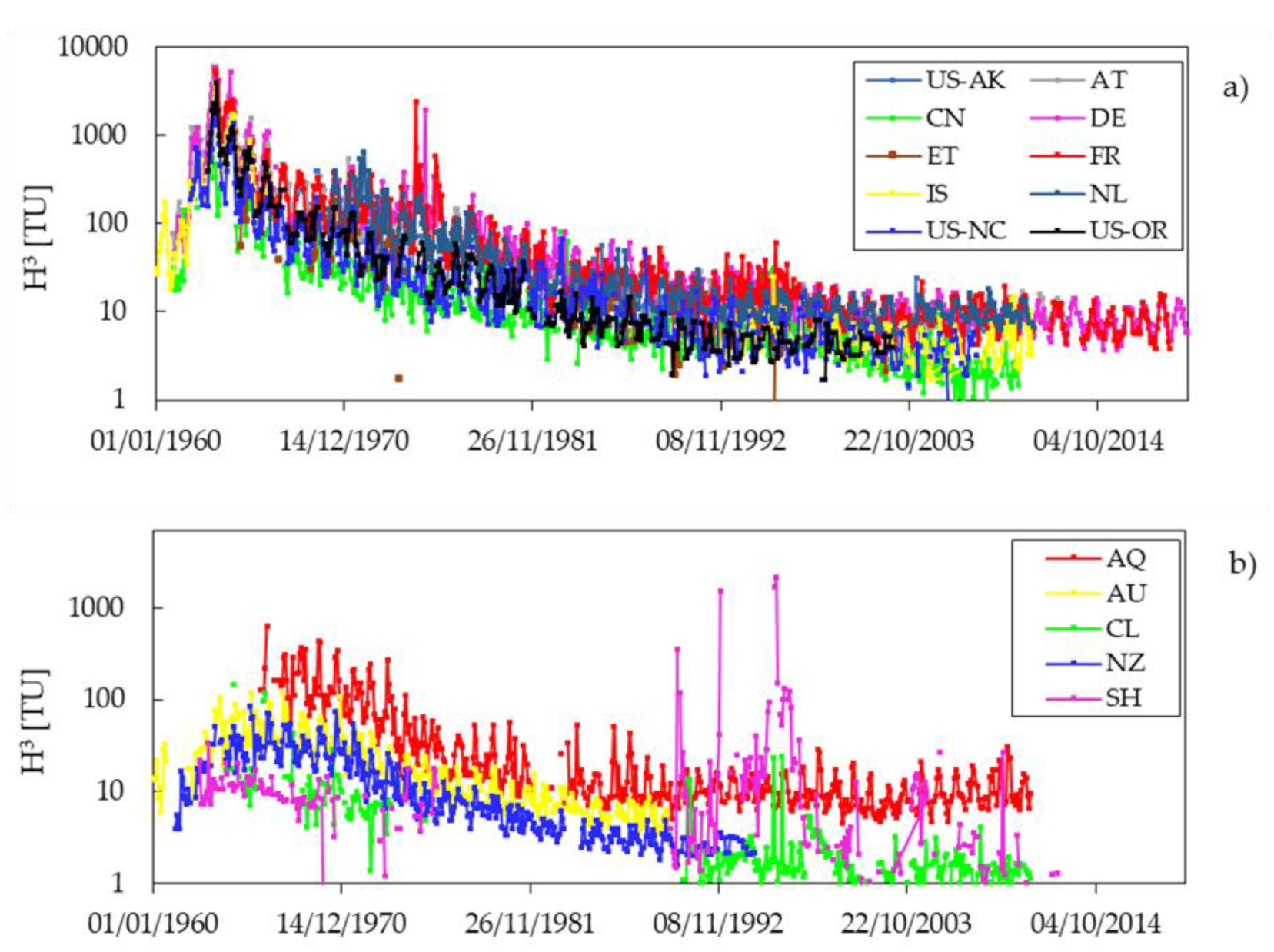



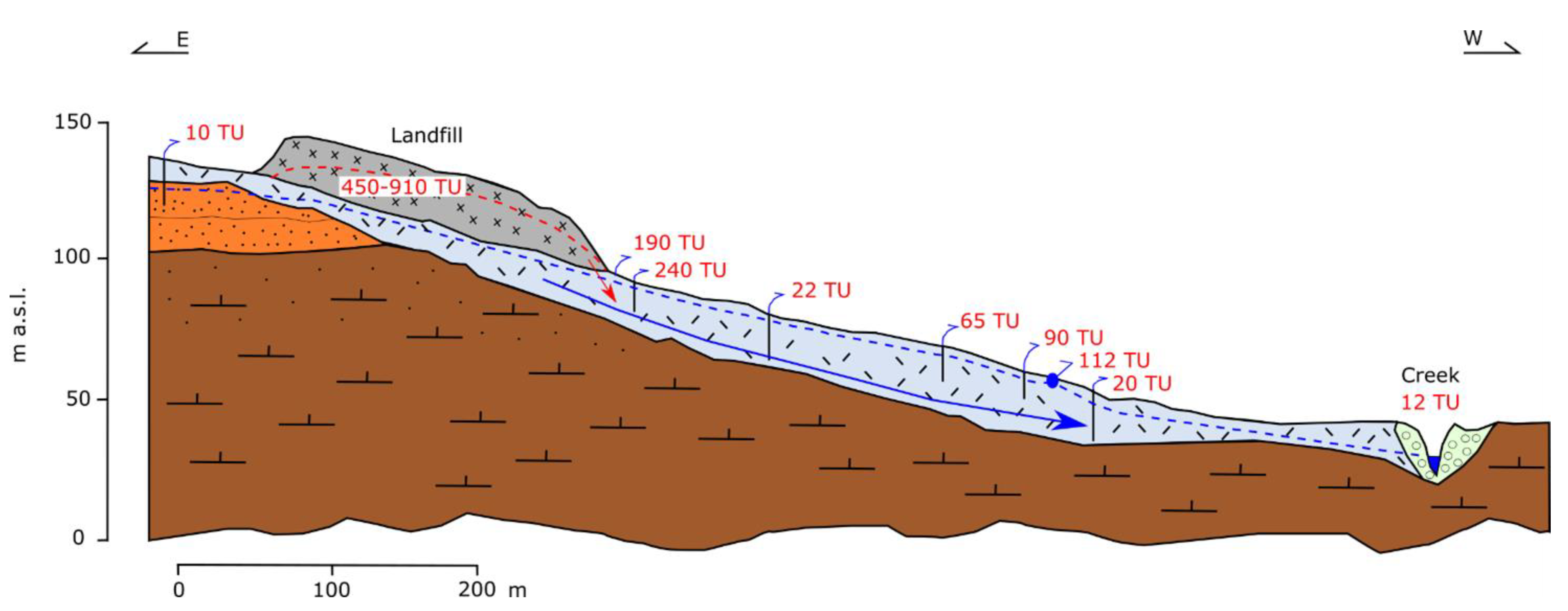
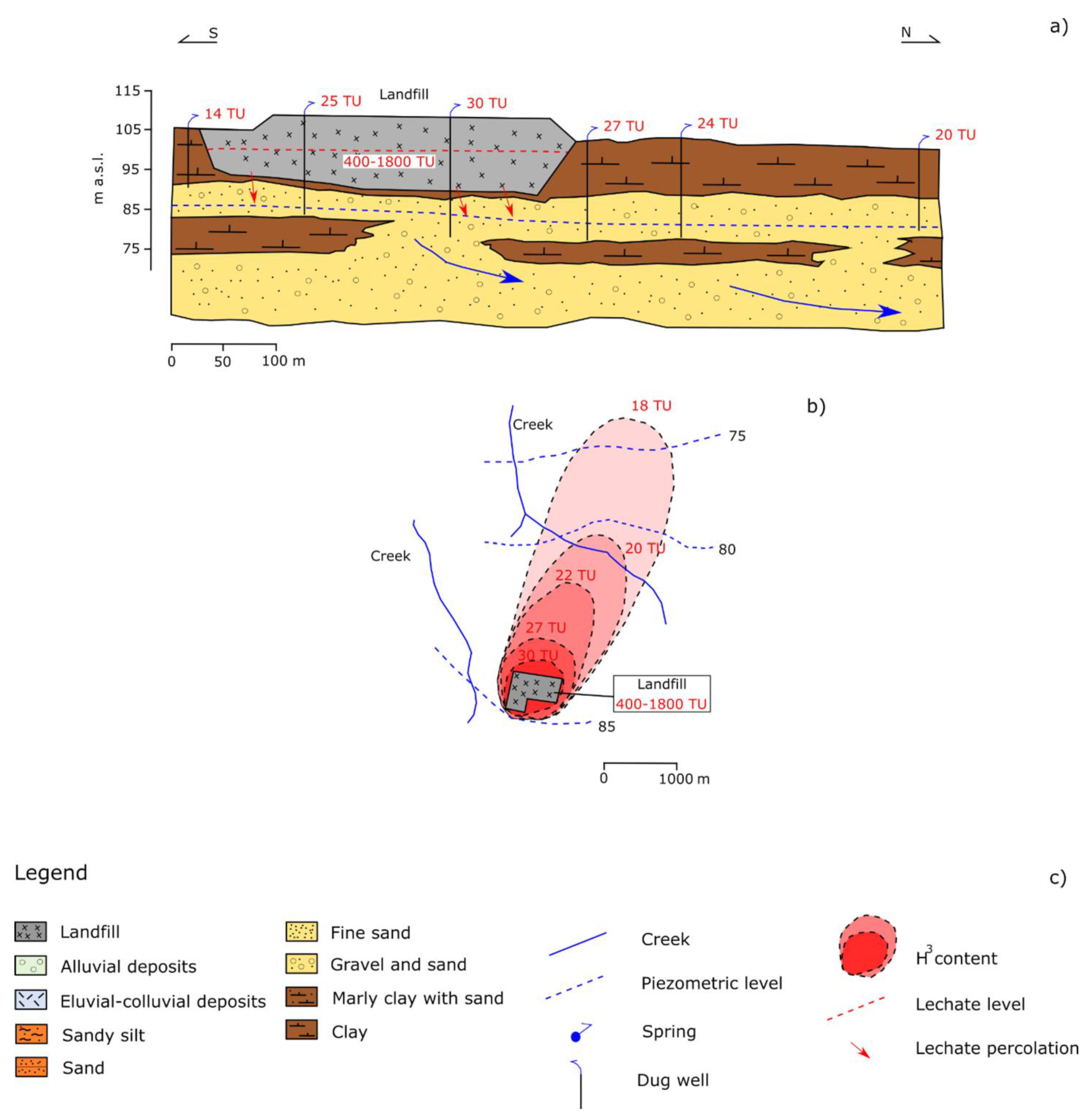
| Time Period | |||||||
|---|---|---|---|---|---|---|---|
| Country | Pre 1963 | 1963–1964 | 1965–1979 | 1980–1990 | 1991–2000 | 2001–2010 | Post 2010 |
| AQ | - | - | 90.97 | - | 9.82 | 10.05 | - |
| AT | 475.14 | 2374.21 | 259.98 | 25.95 | 15.07 | 11.12 | 10.37 |
| AU | 18.75 | 49.34 | 29.93 | 6.63 | - | - | - |
| CL | - | 26.78 | 11.57 | 1.1 | 2.40 | 1.43 | - |
| CN | 88.75 | 166.3 | 32.45 | 7.65 | 5.56 | 2.28 | - |
| DE | 425.97 | 2702.41 | 321.02 | 37.14 | 17.78 | 9.94 | 8.69 |
| ET | - | - | 100.09 | 11.42 | 8.18 | - | - |
| FR | - | 2373.65 | 171.232 | 26.00 | 15.12 | 9.38 | 7.71 |
| IS | 156.94 | 986.77 | 285.04 | - | 5.50 | 4.10 | - |
| NL | - | - | 113.43 | 20.80 | 11.85 | 10.02 | - |
| NZ | 9.90 | 28.17 | 20.05 | 3.68 | 2.54 | - | - |
| SH | 13.83 | 20.07 | 7.87 | 87.66 | 53.58 | 3.79 | - |
| US-NC | 259.38 | 642.62 | 53.45 | 13.54 | 5.75 | 3.58 | - |
| US-OR | - | 828.86 | 80.78 | 8.51 | 4.33 | 4.23 | |
| US-AK | - | - | 77.58 | 7.93 | 7.59 | 1.44 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tazioli, A.; Fronzi, D.; Mammoliti, E. Tritium as a Tracer of Leachate Contamination in Groundwater: A Brief Review of Tritium Anomalies Method. Hydrology 2022, 9, 75. https://doi.org/10.3390/hydrology9050075
Tazioli A, Fronzi D, Mammoliti E. Tritium as a Tracer of Leachate Contamination in Groundwater: A Brief Review of Tritium Anomalies Method. Hydrology. 2022; 9(5):75. https://doi.org/10.3390/hydrology9050075
Chicago/Turabian StyleTazioli, Alberto, Davide Fronzi, and Elisa Mammoliti. 2022. "Tritium as a Tracer of Leachate Contamination in Groundwater: A Brief Review of Tritium Anomalies Method" Hydrology 9, no. 5: 75. https://doi.org/10.3390/hydrology9050075
APA StyleTazioli, A., Fronzi, D., & Mammoliti, E. (2022). Tritium as a Tracer of Leachate Contamination in Groundwater: A Brief Review of Tritium Anomalies Method. Hydrology, 9(5), 75. https://doi.org/10.3390/hydrology9050075








