REY Patterns and Their Natural Anomalies in Waters and Brines: The Correlation of Gd and Y Anomalies
Abstract
1. Introduction
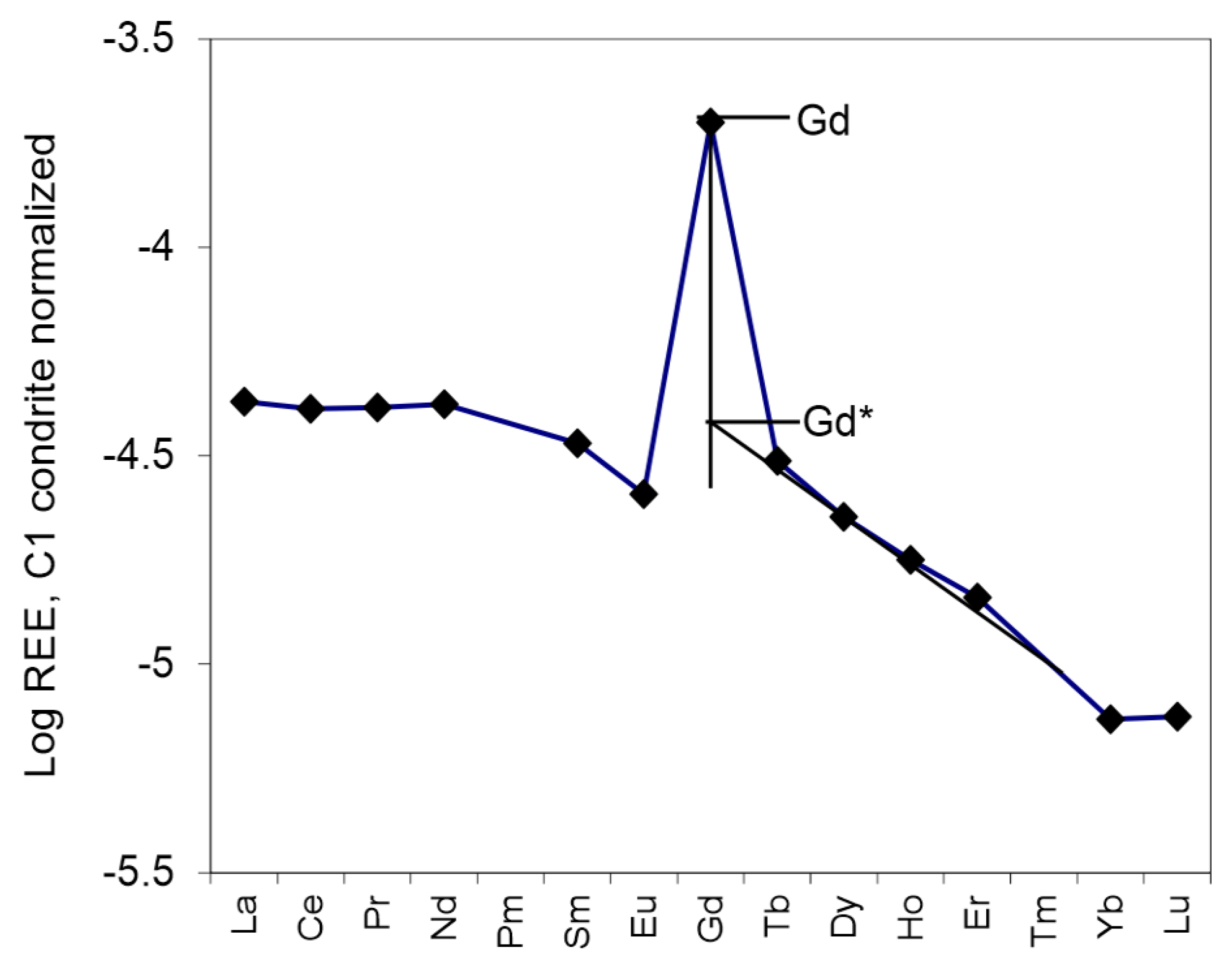
2. Quantification of Ce, Eu, Gd and Y Anomalies
3. Results
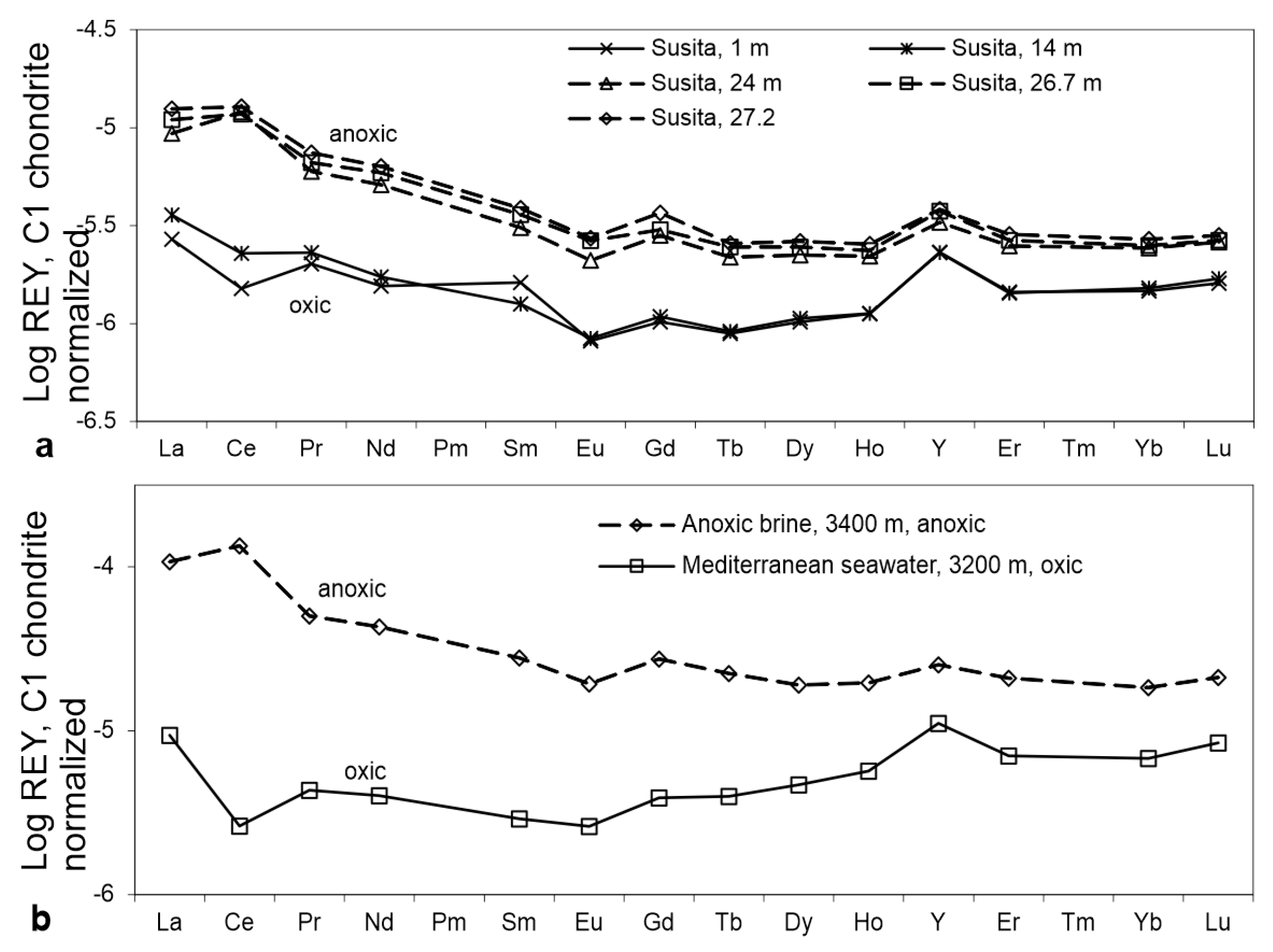
3.1. Seawater
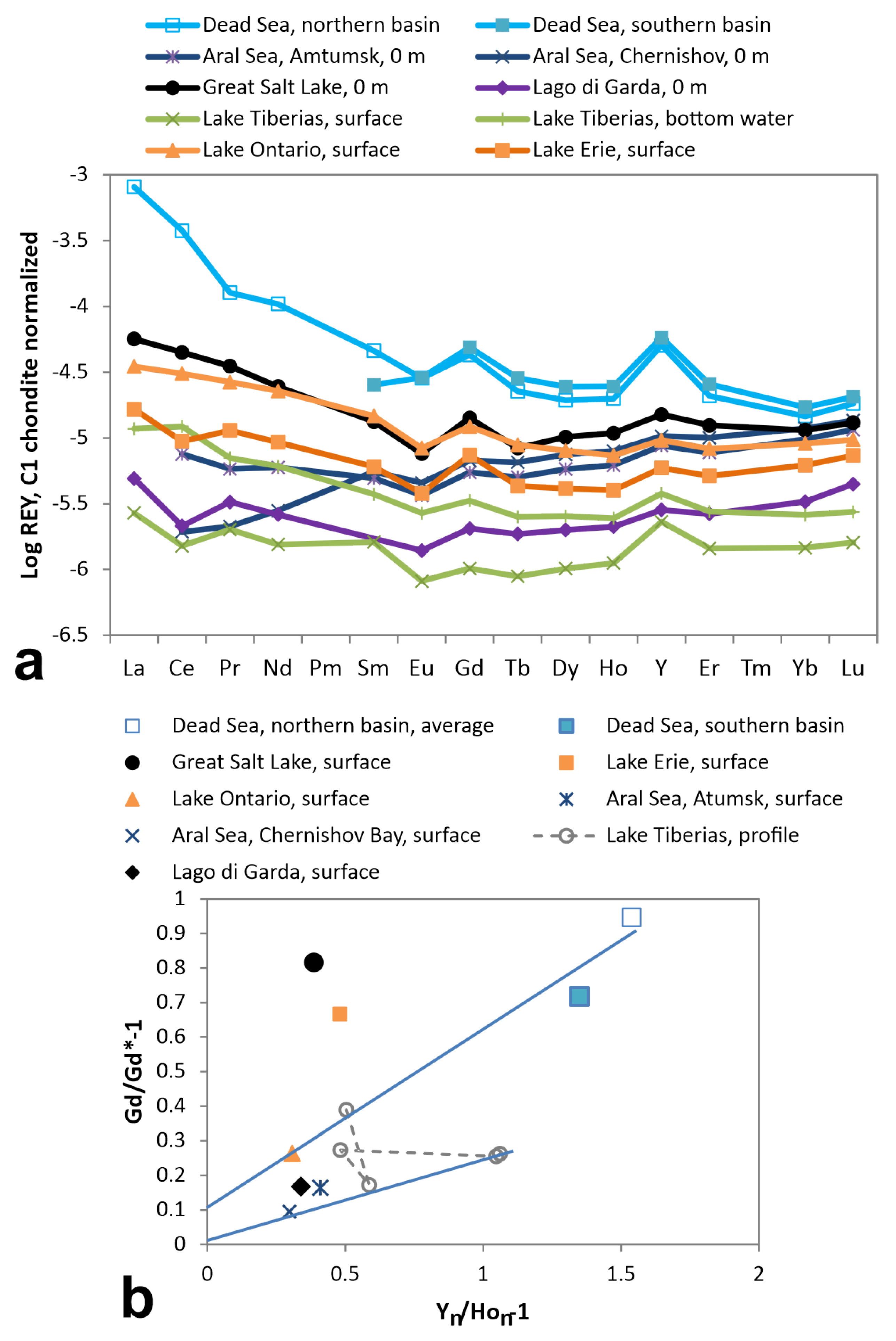
3.2. Lakes and Continental Seas
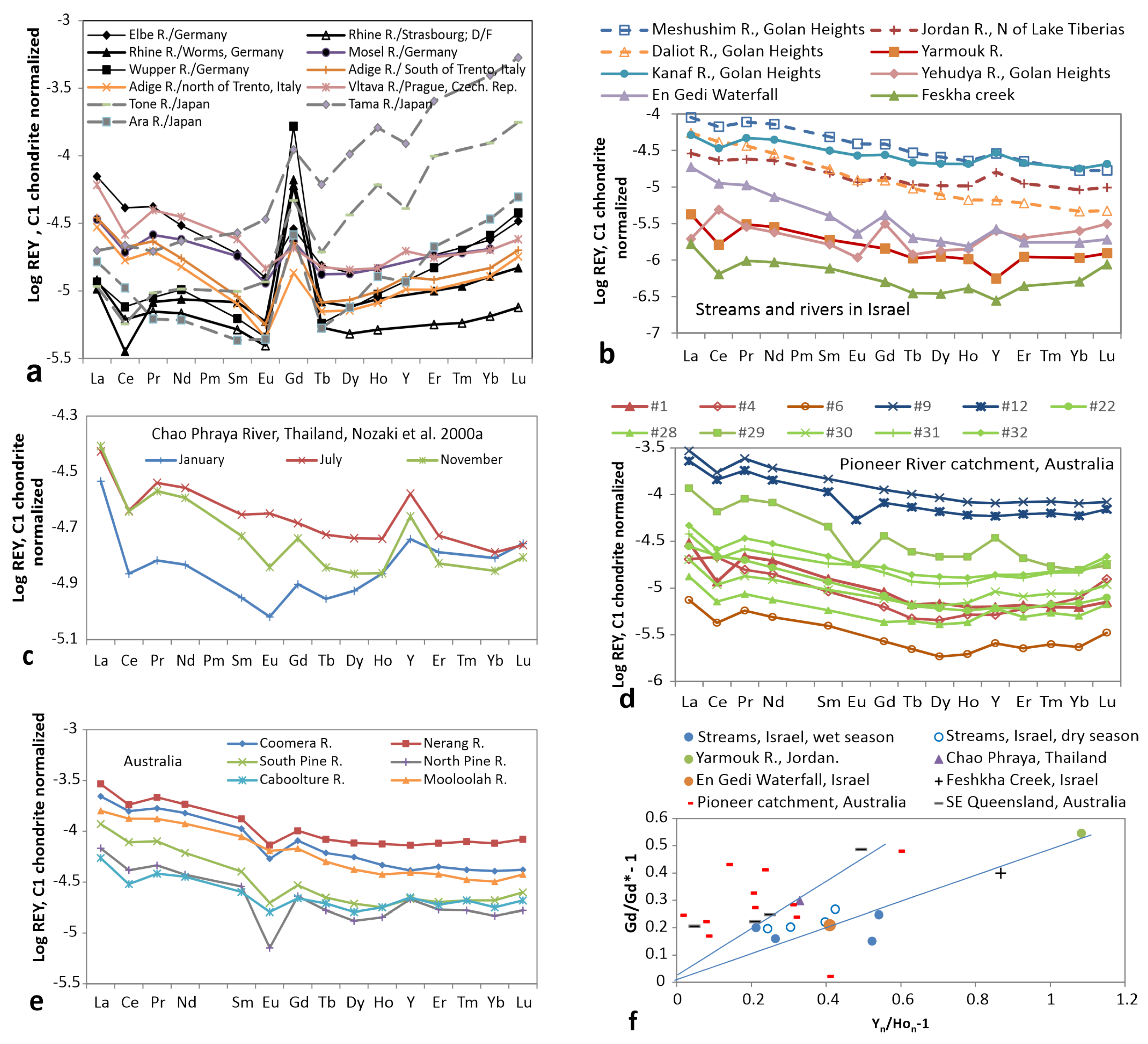
3.3. Rivers and Streams
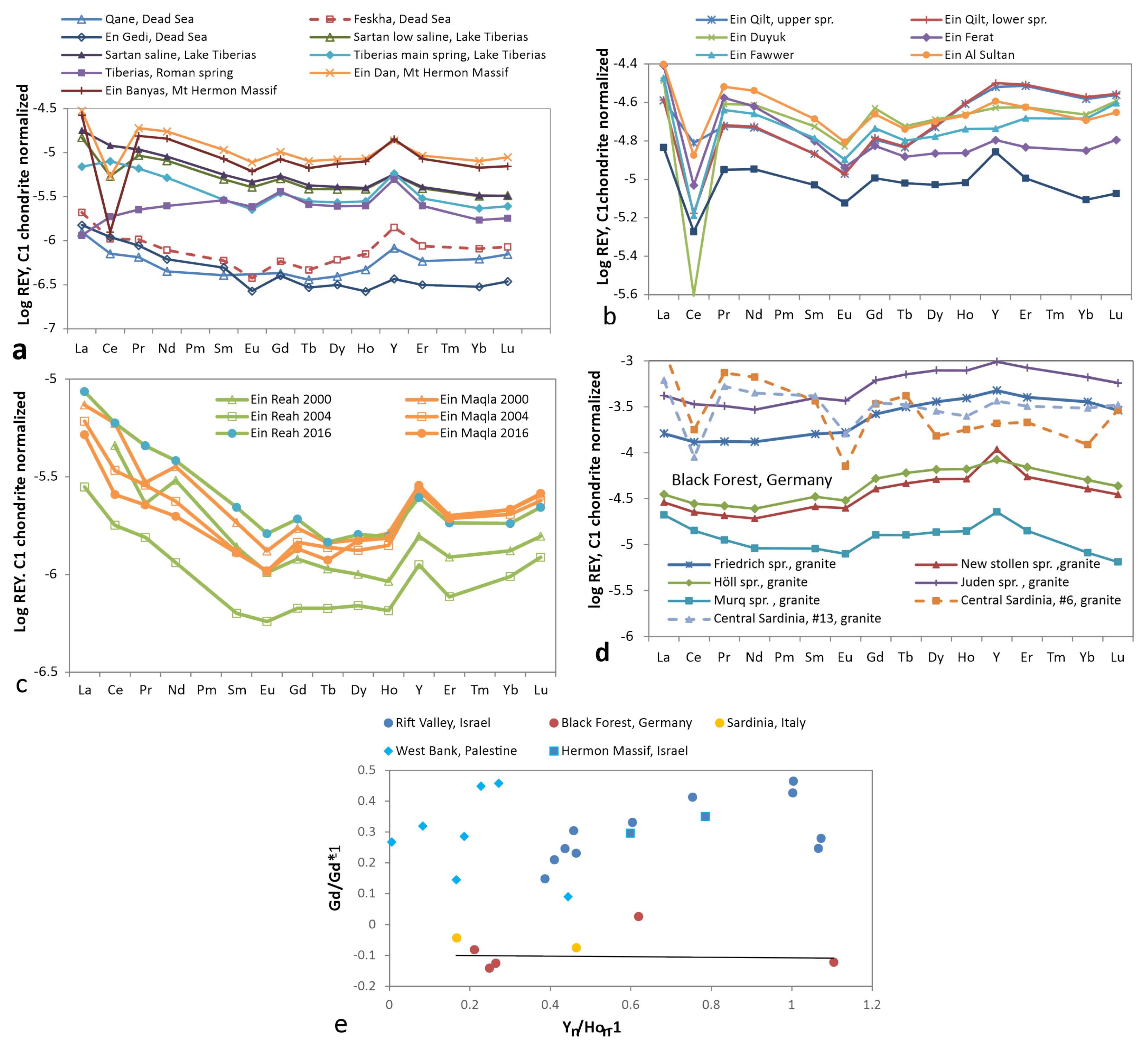
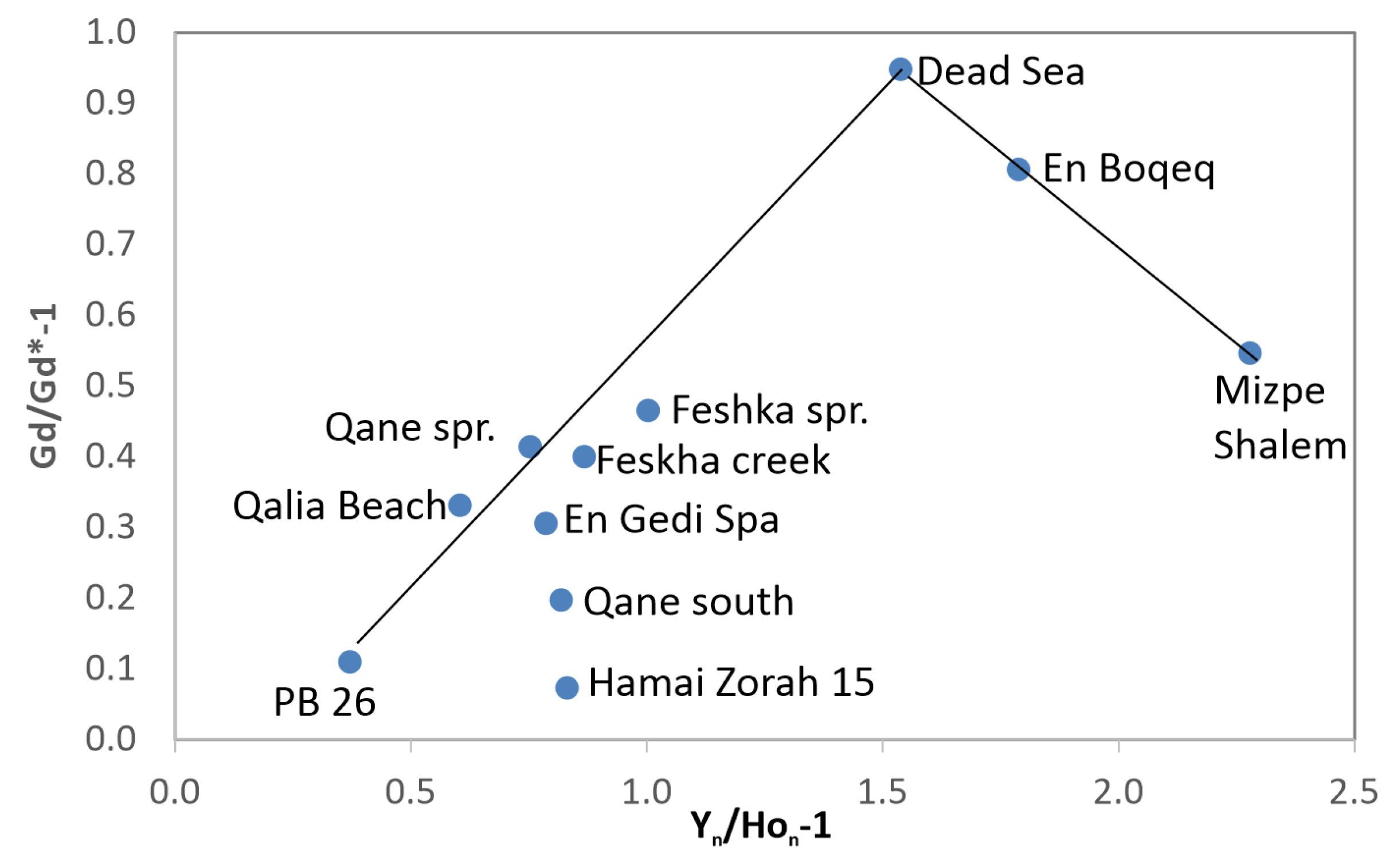
3.4. Spring Water
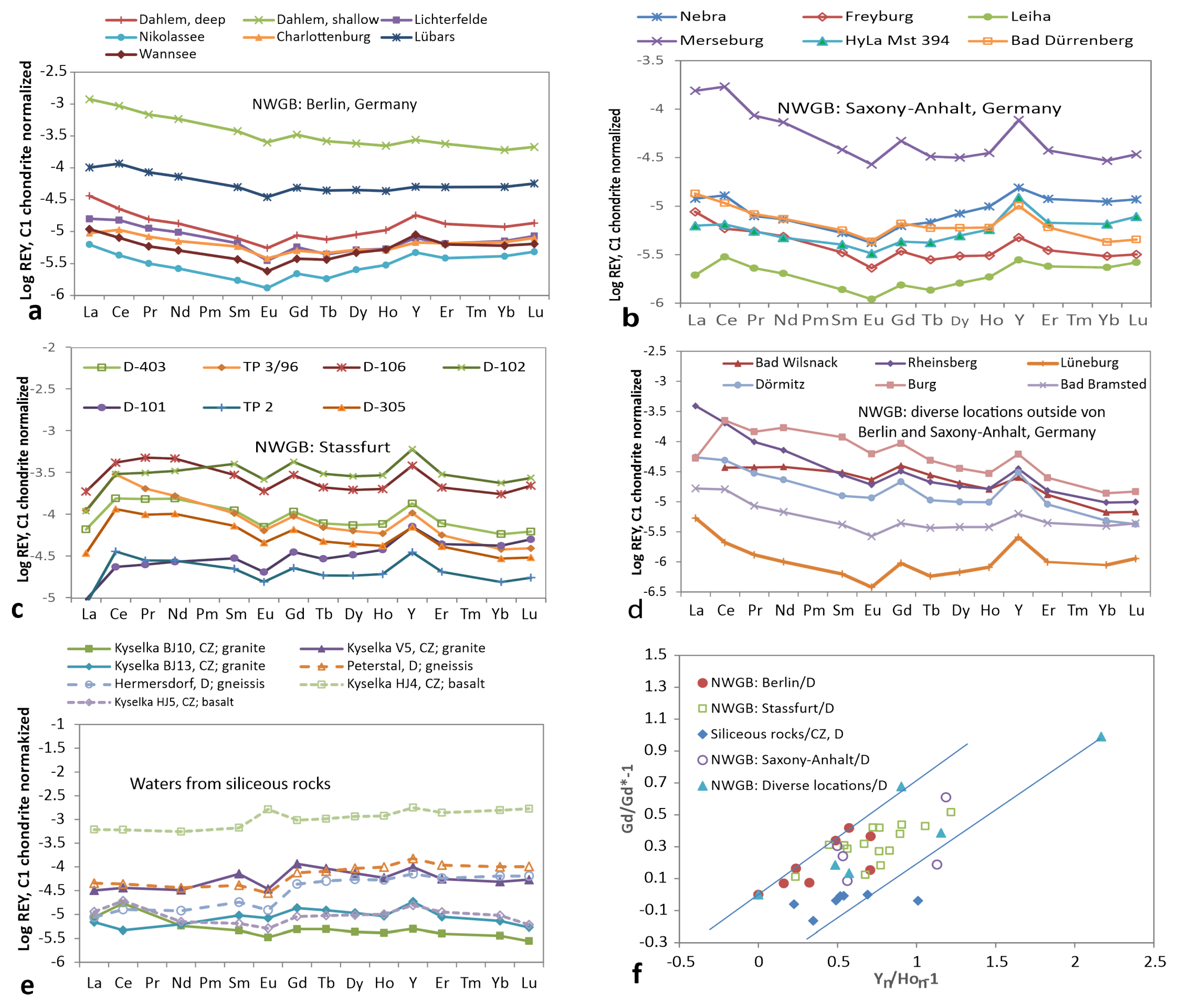
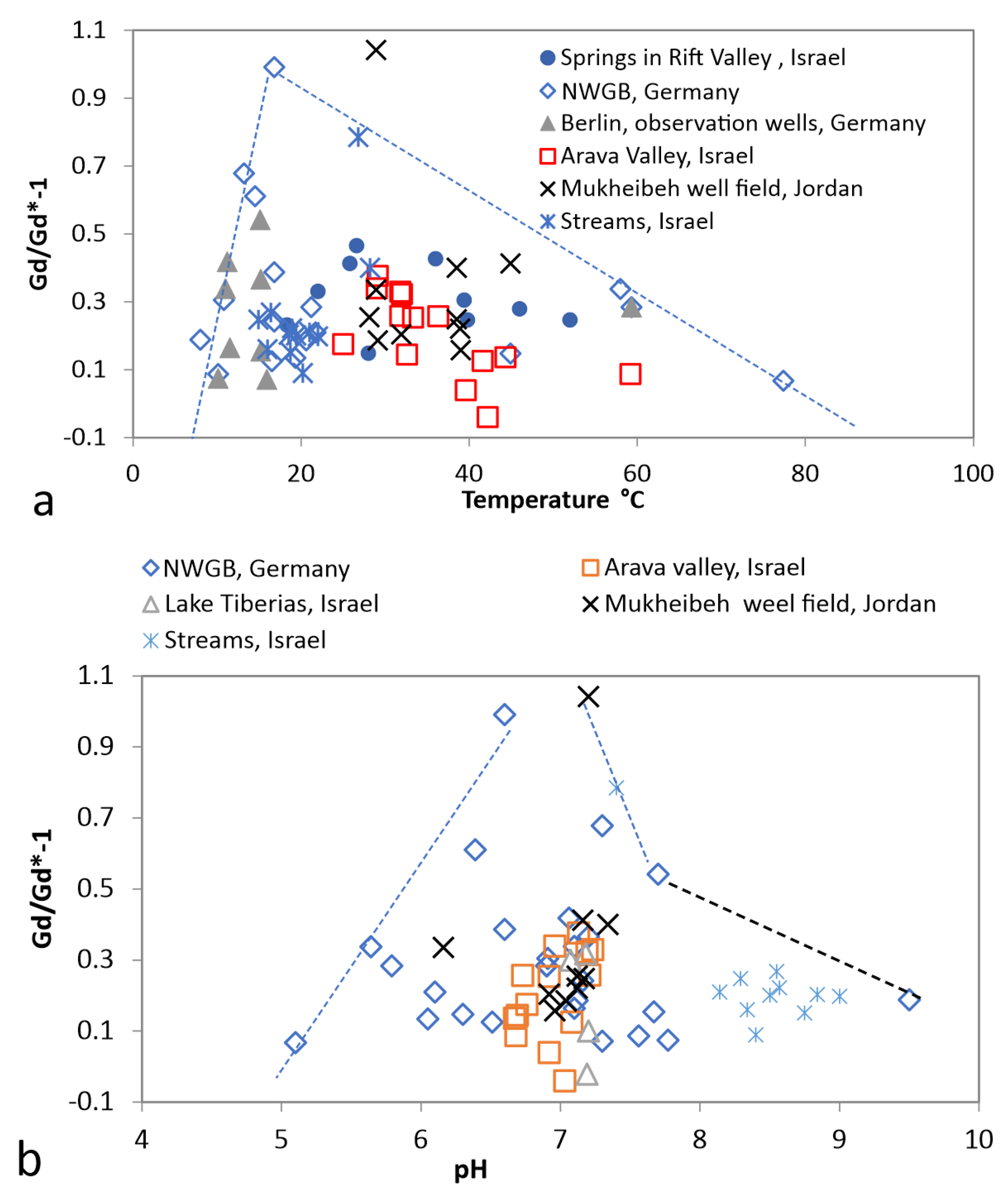
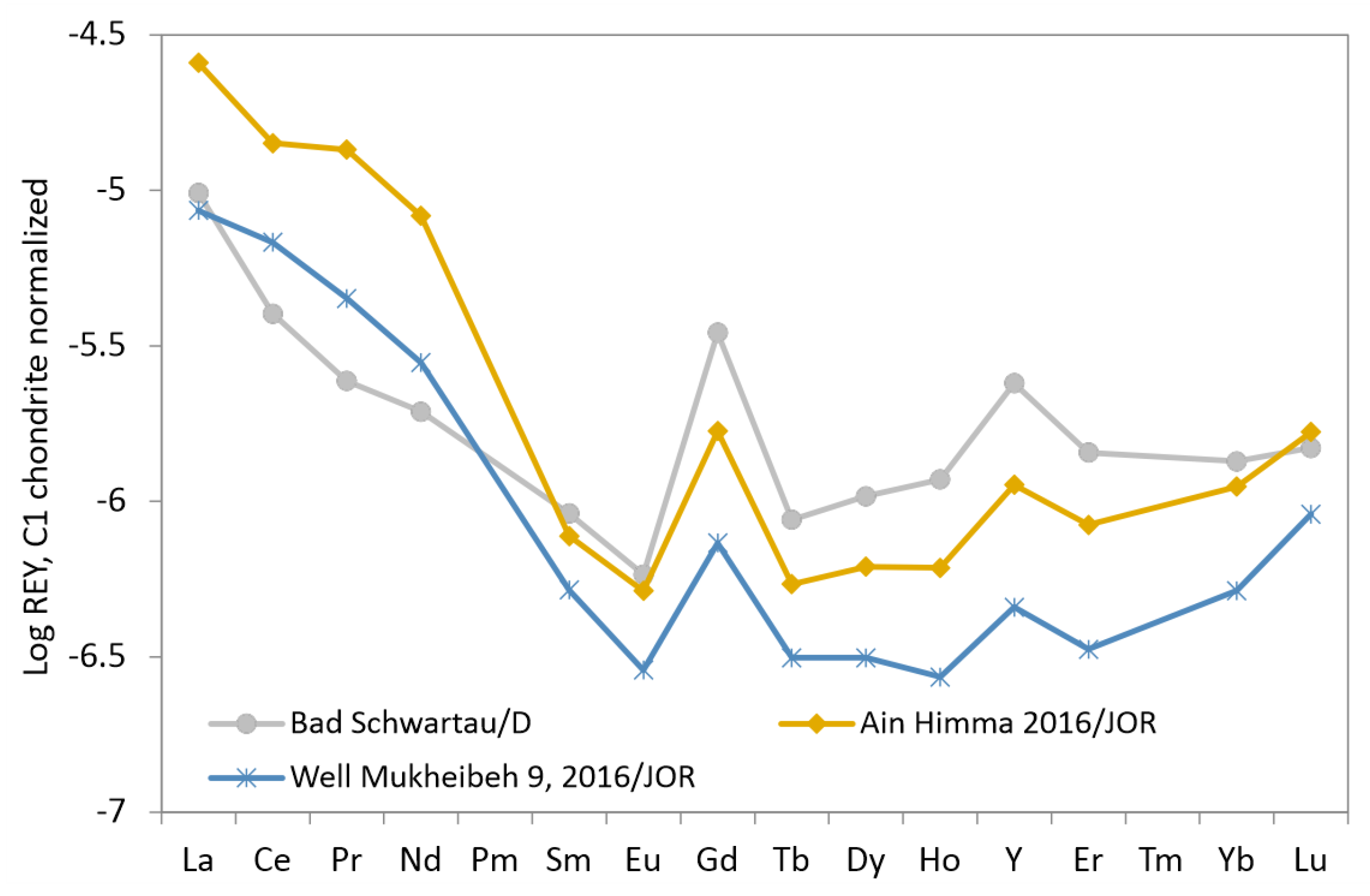
3.5. Groundwater from Wells with Temperature below 20 C
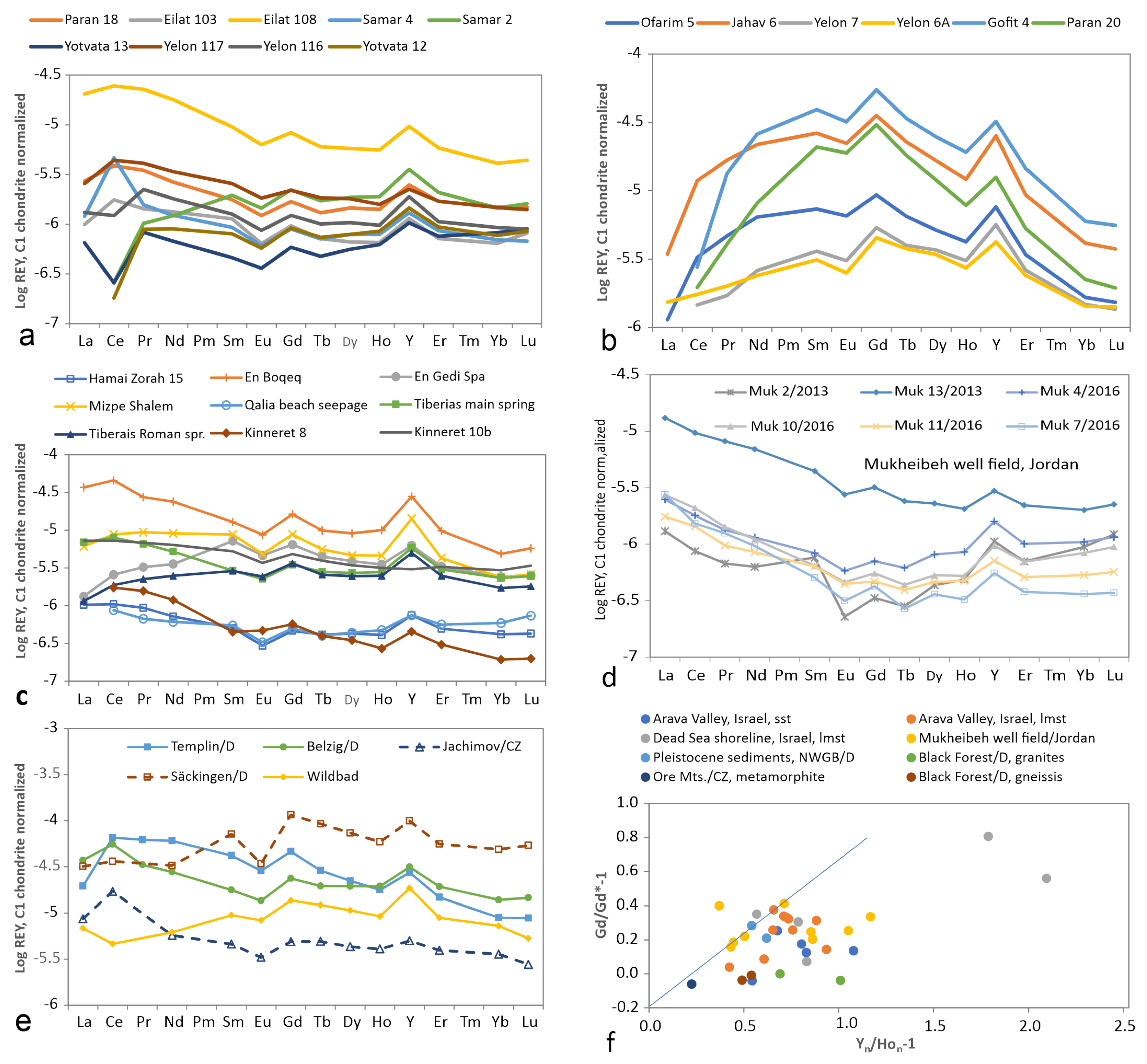
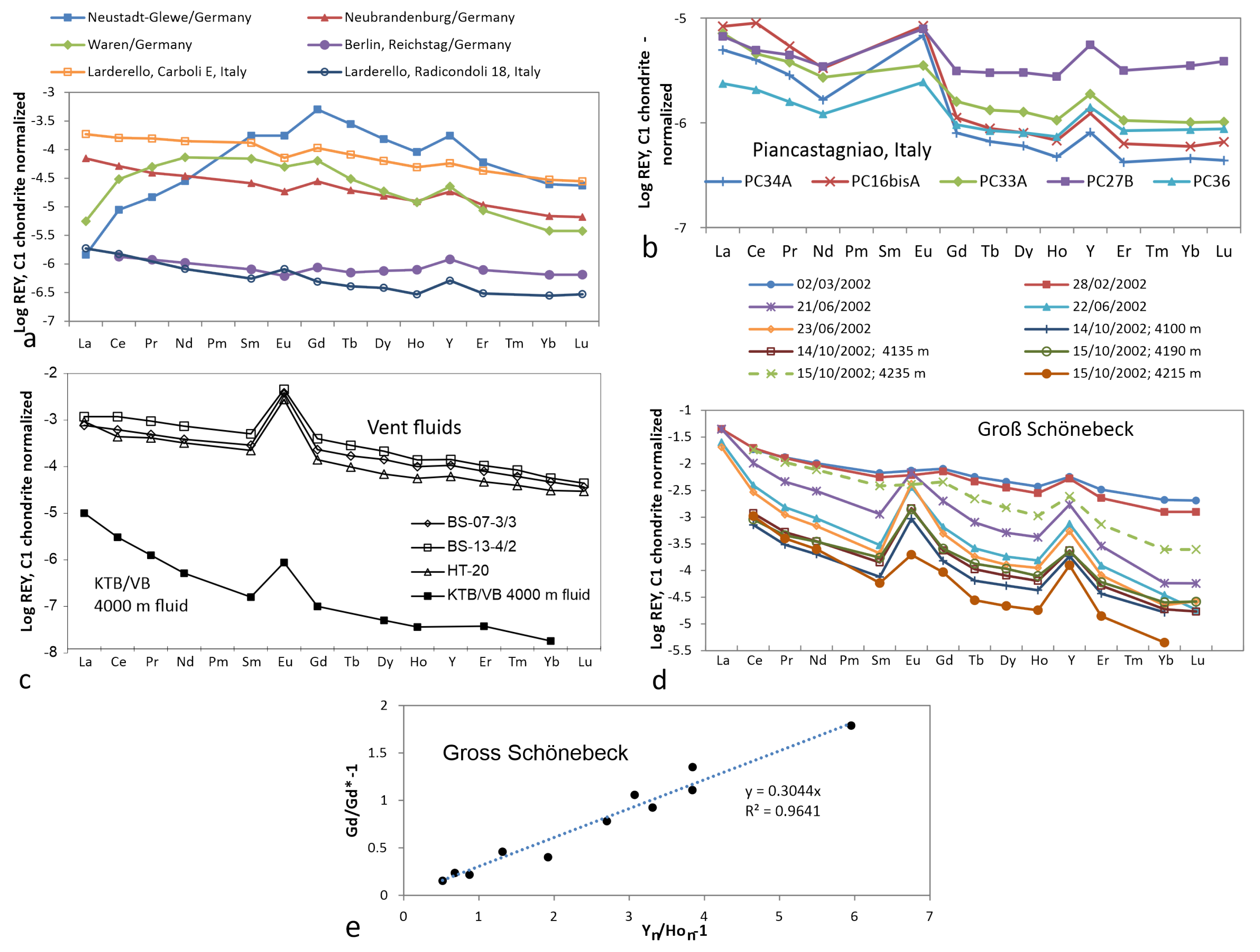
3.6. Thermal Water/Brine (20–50 C)
3.7. Geothermal Water/Brine
4. Discussion
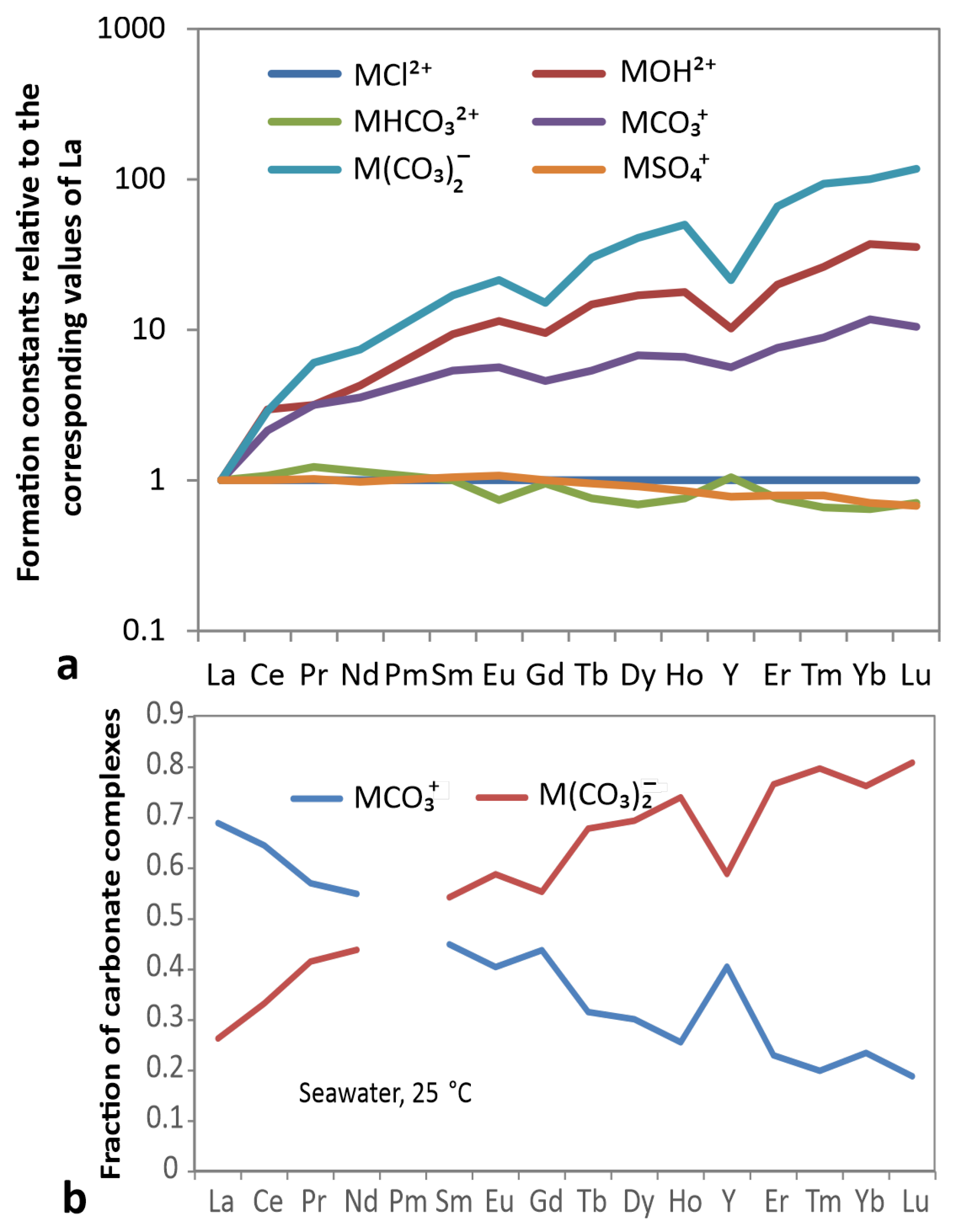
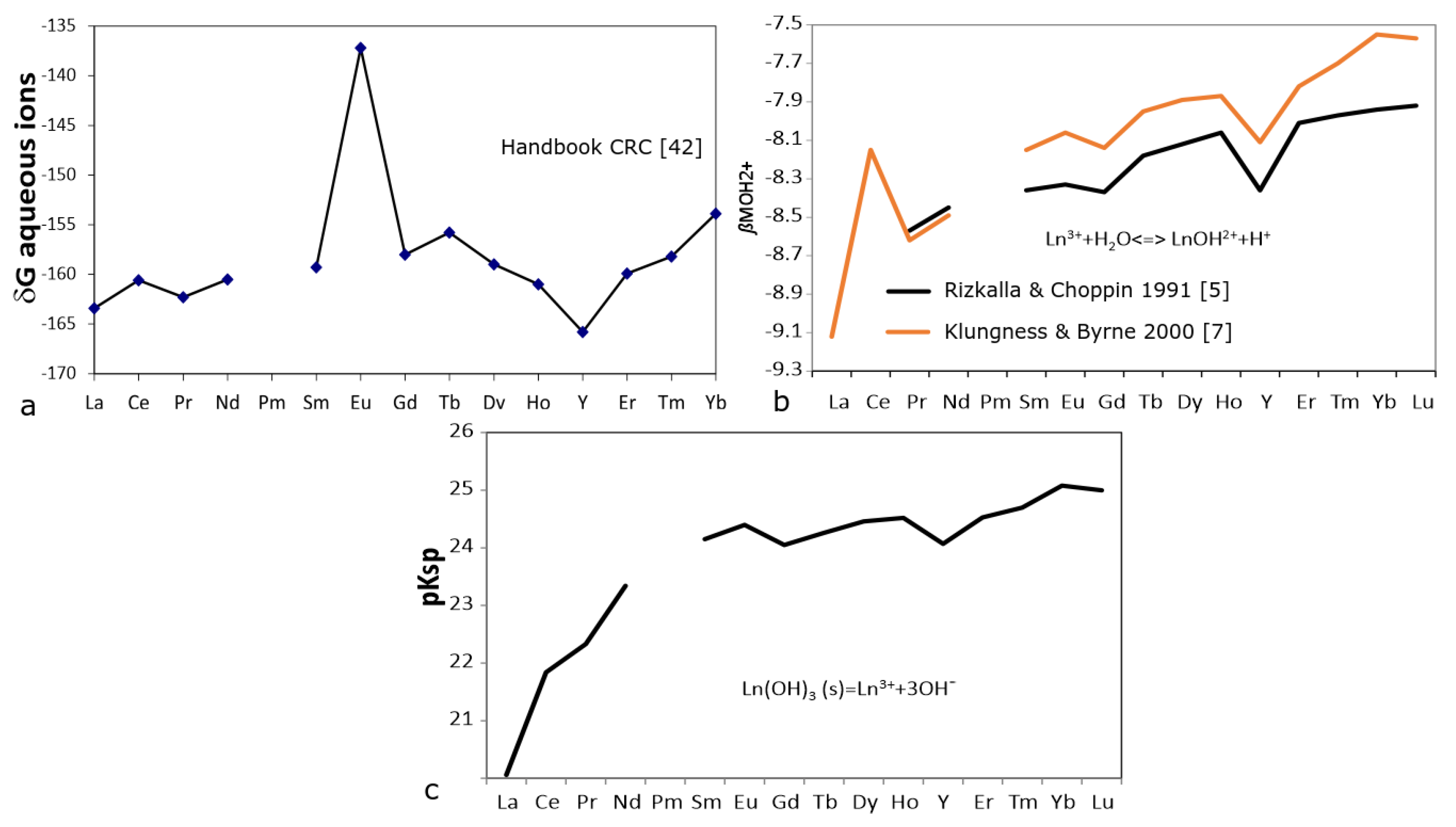
4.1. Water–Rock Interaction
4.2. Scavenging by Fe-Mn-Oxihydroxides
4.3. Gd–Y Anomaly Correlations
4.4. High Gd Anomalies in Aqueous Phases
5. Conclusions
- carbonato and probably phosphato complexes and scavenging by FeOOH colloids generate positive Gd and Y anomalies;
- Gd anomalies decrease with increasing Y anomalies when surface brines interact with carbonate rocks. In such processes, REE are lowered by adsorption and ion exchange but this process does not affect Y to the same degree;
- redox-cycling in stratified water bodies transport of REE by settling particles is more efficient than that of Y. For that reason, Y/Ho is lower in the anoxic than in the oxic zone; for Gd anomalies it is the opposite (e.g., Lake Tiberias and Eastern Mediterranean);
- under chemically reducing or acidic conditions Gd and Y anomalies are not generated;
- Gd and Y anomalies seem to be controlled by pH;
- excepting Eu anomalies, the anomalies of Ce, Gd and Y vanish with increasing temperatures by ion exchange with other minerals and are therefore mostly absent in geothermal waters;
- anthropogenic contamination of surface waters produces Gd anomalies uncorrelated with Y anomalies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf and lanthanide tetrad effect. Contrib. Miner. Pet. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Nozaki, Y.; Zhang, J.; Amakawa, H. The fractionation between Y and Ho in the marine environment. Earth Planet. Sci. Lett. 1997, 148, 329–340. [Google Scholar] [CrossRef]
- Peppard, D.F.; Mason, G.W.; Lewey, S. A tetrad effect in the liquid-liquid extraction ordering of lanthanide(III). J. Inorg. Nucl. Chem. 1969, 31, 2271–2272. [Google Scholar] [CrossRef]
- Spedding, F.H.; Pikal, M.J.; Ayers, B.O. Apparent molar volumes of some aqueous rare earth chloride and nitrate solutions at 25°. J. Phys. Chem. 1966, 70, 2440–2449. [Google Scholar] [CrossRef]
- Rizkalla, E.N.; Choppin, G.R. Hydration and hydrolysis of lanthanides. In Handbook of Physics and Chemistry of Rare Earths; Gschneider, K.A., Eyring, L., Eds.; North Holland Publ.: Amsterdam, The Netherlands; London, UK; New York, NY, USA; Tokyo, Japan, 1991; Volume 15, pp. 391–442. [Google Scholar]
- Diakonov, I.I.; Ragnarsdottir, K.V.; Tagirov, B.R. Standard thermodynamic properties and heat capacity equations of rare earth hydroxides: II. Ce(III)-, Pr-, Sm-, Eu(III)-, Gd-, Tb-, Dy-, Ho-, Tm-, Yb-, and Y-hydroxides. Comparison of thermodynamical and solubility data. Chem. Geol. 1998, 151, 327–347. [Google Scholar] [CrossRef]
- Klungnes, G.D.; Byrne, R.H. Comparative hydrolysis behavior of the rare earths and yttrium: The influence of temperature and ionic strength. Polyhedron 2000, 19, 99–107. [Google Scholar] [CrossRef]
- Liu, X.; Byrne, R.H. Comprehensive investigation of yttrium and rare earth element complexation by carbonate ions using ICP-mass spectrometry. J. Sol. Chem. 1998, 27, 803–815. [Google Scholar] [CrossRef]
- Kawabe, I.; Ohta, A.; Ishii, S.; Tokumura, M.; Miyaichi, K. REE partitioning between Fe-Mn oxihydroxide precipitates and weakly acid NaCl solutions: Convex tetrad effect and fractionation of Y and Sc from heavy lanthanides. Geochem. J. 1999, 33, 167–179. [Google Scholar] [CrossRef]
- Kawabe, I. Hydration change of aqueous lanthanide ions and tetrad effects in lanthanide(III)-carbonate complexation. Geochem. J. 1999, 33, 267–275. [Google Scholar] [CrossRef][Green Version]
- Luo, Y.-R.; Byrne, R.H. Carbonate complexation of yttrium and the rare earth elements in natural waters. Geochim. Cosmochim. Acta 2004, 68, 691–699. [Google Scholar] [CrossRef]
- Byrne, R.H.; Liu, X.; Schijf, J. The influence of phosphate coprecipitation on rare earth distribution in natural waters. Geochim. Cosmochim. Acta 1996, 60, 3341–3346. [Google Scholar] [CrossRef]
- Liu, X.; Byrne, R.H. Rare earth and yttrium phosphate solubilities in aqueous solution. Geochim. Cosmochim. Acta 1997, 6, 1625–1633. [Google Scholar] [CrossRef]
- Luo, Y.-R.; Byrne, R.H. Yttrium and rare earth element complexation by chloride ions at 25 °C. J. Sol. Chem. 2001, 30, 837–845. [Google Scholar] [CrossRef]
- Luo, Y.-R.; Byrne, R.H. The ionic strength dependence of rare earth and yttrium fluoride complexation. J. Sol. Chem. 2000, 29, 1089–1099. [Google Scholar] [CrossRef]
- Schijf, J.; Byrne, R.H. Determination of stability constants for the mono and difluoro complexes of Y and REE, using a cation-exchange resin and ICP-MS. Polyhedron 1999, 18, 2839–2844. [Google Scholar] [CrossRef]
- Schijf, J.; Byrne, R.H. Determination of SO4ß1 for yttrium and rare earth elements at I = 0.66 m and t = 25 °C-Implications for YREE solution speciation in sulphate rich waters. Geochim. Cosmochim. Acta 2004, 68, 2825–2837. [Google Scholar] [CrossRef]
- De Baar, H.J.W. The Marine Geochemistry of the Rare Earth Elements. Ph.D. Thesis, Woods Hole Oceanographic Institute/Massachusets Institute of Technology, Cambridge, MA, USA, 1983; 278p. [Google Scholar]
- De Baar, H.J.W.; Bacon, M.P.; Brewer, P.G. Rare earth elements in the Pacific and Atlantic Ocean. Geochim. Cosmochim. Acta 1985, 49, 1943–1959. [Google Scholar] [CrossRef]
- De Baar, H.J.W.; Brewer, P.; Bacon, M.P. Anomalies in rare earth distributions in seawater: Gd and Tb. Geochim. Cosmochim. Acta 1985, 49, 1961–1969. [Google Scholar] [CrossRef]
- Piepgras, D.J.; Jacobsen, S.B. The behavior of rare earth elements in seawater: Precise determination of variations in the North Pacific water column. Geochim. Cosmochim. Acta 1992, 56, 1851–1861. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman Iron Formations, Transvaal Supergroup, South Africa. Precamb. Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Bau, M.; Möller, P.; Dulski, P. Yttrium and lanthanides in eastern Mediterranean seawater and their fractionation during redox-cycling. Mar. Chem. 1997, 56, 123–131. [Google Scholar] [CrossRef]
- Nozaki, Y.; Alib, A.S.; Amakawa, H.; Gamo, T.; Hasumoto, H. Dissolved rare earth elements and hydrography in the Sulu Sea. Geochim. Cosmochim. Acta 1999, 63, 2171–2181. [Google Scholar] [CrossRef]
- Möller, P.; Morteani, G.; Dulski, P. Anomalous gadolinium, cerium, and yttrium contents in the Adige and Isarco river waters and in the water of their tributaries (Provinces Trento and Bolzano/Bozen, NE Italy). Acta Hydrochim. Hydrobiol. 2003, 31, 225–239. [Google Scholar] [CrossRef]
- Goldstein, S.; Jacobsen, S.B. REE in the Great Whale River estuary, northwest Quebec. Earth Planet. Sci. Lett. 1988, 88, 241–252. [Google Scholar] [CrossRef]
- Elderfield, H.; Upstill-Goddard, R.; Sholkovitz, E.R. The rare earth elements in rivers, estuaries, and coastal seas and their significance to the composition of ocean waters. Geochim. Cosmochim. Acta 1990, 57, 513–518. [Google Scholar] [CrossRef]
- Sholkovitz, E.R. Chemical evolution of rare earth elements: Fractionation between colloidal and solution phases of filtered river water. Earth Planet. Sci. Lett. 1992, 114, 77–84. [Google Scholar] [CrossRef]
- Terakado, Y.; Masuda, A. The coprecipitation of rare earth elements with calcite and aragonite. Chem. Geol. 1988, 69, 101–110. [Google Scholar] [CrossRef]
- Zhong, S.; Mucci, A. Partitioning of rare earth elements (REES) between calcite and seawater solutions at 25 °C and 1 atm, and high dissolved REE concentrations. Geochim. Cosmochim. Acta 1995, 59, 4443–4453. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawabe, I. REE abundance in ancient seawater inferred from marine limestones and experimental REE partition coefficients between calcite and aqueous solution. Geochem. J. 2006, 40, 425–435. [Google Scholar] [CrossRef]
- Toyama, K.; Terakado, Y. Estimation of the practical partition coefficients of rare earth elements between limestones and seawater: Discussion and application. Geochem. J. 2019, 43, 139–150. [Google Scholar] [CrossRef]
- Bau, M. Scavenging of dissolved yttrium and rare earths by precipitating iron oxihydroxed: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochim. Cosmochim. Acta 1999, 63, 67–77. [Google Scholar] [CrossRef]
- Kawabe, I.; Ohta, A.; Miura, N. Distribution coefficients of REE between Fe oxyhydroxide precipitates and NaCl solutions affected by REE-carbonate complexation. Geochem. J. 1999, 33, 181–197. [Google Scholar] [CrossRef]
- Quinn, K.A.; Byrne, R.H.; Schijf, J. Comparative scavenging of yttrium and the rare earth elements in seawater: Competitive influences of solution and surface chemistry. Aquat. Chem. 2004, 100, 59–80. [Google Scholar] [CrossRef]
- Chopin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of lanthanides on smectite and kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar]
- Irber, W. Laugungsexperimente an Peraluminischen Graniten als Sonde für Alterationsprozesse im Finalen Stadium der Granitkristallisation mit Anwendung auf das Rb-Sr-Isotopensystem. Ph.D. Thesis, Free University Berlin, Berlin, Germany, 1996; 319p. [Google Scholar]
- Hannigan, R.E.; Sholkovitz, E.R. The development of middle rare earth elements in freshwaters: Weathering of phosphate minerals. Chem. Geol. 2001, 175, 495–508. [Google Scholar] [CrossRef]
- Kagi, H.; Dohmoto, Y.; Takano, S.; Masuda, A. Tetrad effect in lanthanides partitioning between calcium sulfate crystal and its saturated solution. Chem. Geol. 1993, 107, 71–82. [Google Scholar] [CrossRef]
- Toulkeridis, T.; Podwojewski, P.; Clauer, N. Tracing of the source of gypsum in New Caledonian soils by REE contents and S-Sr isotopic compositions. Chem. Geol. 1998, 145, 61–71. [Google Scholar] [CrossRef]
- Playa, E.; Cendon, D.I.; Trave, A.; Chivas, A.R.; Garcia, A. Non-marine evaporites with both inherited marine and continental signatures: The Gulf of Carpentaria, Australia, at ~70 ka. Sediment. Geol. 2007, 201, 267–285. [Google Scholar] [CrossRef]
- Handbook of Chemistry and Physics; Selected Values of Chemical Thermodynamic Properties; CRC Press: Boca Raton, FL, USA, 1988; pp. D51–D93.
- Tanaka, K.; Takahashi, Y.; Shimizu, H. Local structure of Y and Ho in calcite and its relevance to Y fractionation from Ho in partitioning between calcite and aqueous solution. Chem. Geol. 2008, 248, 104–113. [Google Scholar] [CrossRef]
- De Carlo, E.H.; Wen, X.Y.; Irving, M. The influence of redox reactions on the uptake of dissolved Ce by suspended Fe and Mn oxide particles. Aquat. Geochem. 1997, 3, 357–389. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Europium redox equilibria in aqueous solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Bilal, B.A. Thermodynamic study of Eu3+/Eu2+ redox reaction in aqueous solution at elevated temperatures and pressures by means of cyclic voltammetry. Z. Naturforsch. 1991, 46, 1108–1116. [Google Scholar] [CrossRef]
- Möller, P.; Dulski, P.; Gerstenberger, H.; Morteani, G.; Fuganti, A. Rare earth elements, yttrium and H, O, C, Sr, Nd, and Pb isotope studies in mineral waters and corresponding rocks from NW -Bohemia, Czech Republic. Appl. Geochem. 1998, 13, 975–994. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet. Sci. Lett. 1996, 143, 245–256. [Google Scholar] [CrossRef]
- Fuganti, A.; Möller, P.; Morteani, G.; Dulski, P. Gadolinio ed altre terre rare usabili come traccianti per stabilire l’eta il movimento ed i rischi delle acque sotterranee: Esempio dell’area di Trento. Geol. Tec. Ambient. 1996, 4, 13–18. [Google Scholar]
- Knappe, A.; Sommer-von Jarmersted, C.; Pekdeger, A.; Bau, M.; Dulski, P. Gadolinium in aquatic systems as indicator of sewage water contamination. In Geochemistry of the Earth’s Surface; Armannsson, H., Ed.; Balkema: Rotterdam, The Netherlands, 1999; pp. 187–190. [Google Scholar]
- Knappe, A.; Möller, P.; Dulski, P.; Pekdeger, A. Positive gadolinium anomaly in surface water and ground water of the urban area Berlin Germany. Chem. Erde 2005, 65, 167–189. [Google Scholar] [CrossRef]
- Möller, P.; Paces, T.; Dulski, P.; Morteani, G. Anthropogenic Gd in surface water, drainage systems, and the water supply of the city of Prague, Czech Republic. Environ. Sci. Technol. 2002, 36, 2387–2394. [Google Scholar] [CrossRef]
- Siebert, C.; Möller, P.; Magri, F.; Shalev, E.; Rosenthal, E.; Al-Raggad, M.; Rödiger, T. Applying rare earth elements, uranium and 87Sr/86Sr to disentangle structurally forced confluence of regional groundwater resources; The case of the lower Yarmouk Gorge. Geofluids 2019, 2019, 6727681. [Google Scholar] [CrossRef]
- Möller, P.; De Lucia, M. Incorporation of rare earths and yttrium in calcite: A critical evaluation. Aquat. Geochem. 2020, 26, 89–117. [Google Scholar] [CrossRef]
- Anders, E.; Grevesse, N. Abundance of elements: Meteoric and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Jupiter, S.D.; Kamber, B.S. Aquatic geochemistry of rare earth elements and yttrium in the Pioneer River catchment, Australia. Mar. Freshw. Res. 2006, 57, 725–736. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Greig, A.; Collerson, K.D.; Kamber, B.S. Rare earth elements and Yttrium variability in South East Queensland waterways. Aquat. Geochem. 2006, 12, 39–72. [Google Scholar] [CrossRef]
- Wang, X.; Barrat, J.A.; Bayon, G.; Chauvaud, L.; Feng, D. Lanthanum anomalies as fingerprints of methanotrophy. Geochem. Perspect. Lett. 2020, 14, 26–30. [Google Scholar] [CrossRef]
- Möller, P.; Dulski, P.; De Lucia, M. Compilation of Data Used in the Manuscript “REY Patterns and Their Natural Anomalies in Waters and Brines: The Correlation of Gd- and Y-Anomalies”. 2021. Available online: https://zenodo.org/record/4899253#.YQyny0C-tPY (accessed on 4 August 2021). [CrossRef]
- Elderfield, H.; Greaves, M.J. The rare earth elements in seawater. Nature 1982, 296, 214–219. [Google Scholar] [CrossRef]
- Möller, P.; Dulski, P.; Bau, M. Rare-earth element adsorption in a seawater profile above the East Pacific Rise. Chem. Erde 1994, 54, 129–149. [Google Scholar]
- Zhang, J.; Nozaki, A. Rare earth elements and yttrium in seawater: ICP-MS determinations in the East Caroline, Coral Sea, and South Fidgi basins of the western South Pacific Ocean. Geochim. Cosmochim. Acta 1996, 60, 4631–4644. [Google Scholar] [CrossRef]
- Möller, P.; Stober, I.; Dulski, P. Seltenerdelement-, Yttrium Gehalte und Bleiisotope in Thermal- und Mineralwässern des Schwarzwaldes. Grundwasser 1997, 3, 118–132. [Google Scholar] [CrossRef]
- Nozaki, Y.; Lerche, D.; Alibo, D.S.; Sinidvongs, A. The estuarine geochemistry of rare earth elements and Indium in the Chao Phraya River, Thailand. Geochim. Cosmochim. Acta 2000, 64, 3983–3994. [Google Scholar] [CrossRef]
- Möller, P.; Rosenthal, E.; Dulski, P.; Geyer, S. Characterization of recharge areas by rare earth elements and stable isotopes of H2O. In The Water of the Jordan Valley; Hötzl, H., Möller, P., Rosenthal, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 123–147. [Google Scholar]
- Bau, M.; Knappe, A.; Dulski, P. Anthropogenic gadolinium as a micropollutant in river waters in Pennsylvania and in Lake Erie, northwestern United States. Chem. Erde/Geochem. 2006, 66, 143–152. [Google Scholar]
- Siebert, C. Saisonale Chemische Variationen des See Genezareth, Seiner Zuflüsse und Deren Ursachen. Ph.D. Thesis, Free University Berlin, Berlin, Germany, 2006; 222p. [Google Scholar]
- Nozaki, Y.; Lerche, D.; Alibo, D.S.; Tsutsumi, M. Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: Evidence for anthropogenic Gd and In. Geochim. Cosmochim. Acta 2000, 64, 3975–3982. [Google Scholar] [CrossRef]
- Verplanck, P.L.; Taylor, H.E.; Nordstrom, D.K.; Barber, L.B. Aqueous stability of Gadolinium in surface waters receiving sewage treatment plant effluent, Boulder Creek, Colorado. Environ. Sci. Technol. 2005, 39, 6923–6929. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Helmers, E. Hospital effluents as a source of gadolinium in the aquatic ebvironment. Environ. Sci. Technol. 2002, 34, 573–577. [Google Scholar] [CrossRef]
- Kulaksiz, S.; Bau, M. Contrasting behavior of anthropogenic gadolinium and natural rare earth elements in estuaries and the gadolinium input into the North Sea. Earth Planet. Sci. Lett. 2007, 260, 361–371. [Google Scholar] [CrossRef]
- Kulaksiz, S.; Bau, M. Rare earth elements in the Rhine River Germany: First case of anthropogenic lanthanum as a dissolved microcomponent in the hydrophere. Environ. Intern. 2011, 37, 973–979. [Google Scholar] [CrossRef]
- Banks, D.; Hall, G.; Reimann, C.; Siewers, U. Distribution of rare earth elements in crystalline bedrock groundwaters: Oslo and Bergen regions, Norway. Appl. Geochem. 1999, 14, 27–39. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Zhou, X.; Guo, C.; Stetzenbach, K.J.; Hodge, V.F. Origin of rare earth element signatures in groundwaters of circumneutral pH from southern Nevada and eastern Califiornia, USA. Chem. Geol. 2000, 164, 239–257. [Google Scholar] [CrossRef]
- Möller, P.; Rosenthal, E.; Geyer, S.; Guttmann, J.; Dulski, P.; Rybakov, M.; Zilberbrand, M.; Jahnke, C.; Flexer, A. Hydrochemical processes in the lower Jordan valley and in the Dead Sea area. Chem. Geol. 2007, 239, 27–49. [Google Scholar] [CrossRef]
- Göb, S.; Loges, A.; Nolde, N.; Bau, M.; Jacob, D.E.; Markl, G. Major and trace element compositions (including REE) of mineral, thermal, mine and surface waters in SW Germany and implications for water rock interaction. Appl. Geochem. 2013, 33, 127–152. [Google Scholar] [CrossRef]
- Biddau, R.; Cidu, R.; Frau, F. Rare earth elements in water from the albite-bearing granodiorites of Central Sardinia, Italy. Chem. Geol. 2002, 182, 1–14. [Google Scholar] [CrossRef]
- Tesmer, M.; Möller, P.; Wieland, S.; Jahnke, C.; Pekdeger, A.; Voigt, H. Deep reaching fluid flow in the North-East German Basin. Origin and processes of groundwater salinisation. J. Hydrol. 2007, 15, 1291–1306. [Google Scholar] [CrossRef]
- Möller, P.; Weise, S.M.; Tesmer, M.; Dulski, P.; Pekdeger, A.; Bayer, U.; Magri, F. Salinization of groundwater in the North German Basin; Results from conjoint investigation of major, trace element and multi-isotope distribution. Int. J. Earth Sci. (Geol. Rundsch.) 2008, 97, 1057–1073. [Google Scholar] [CrossRef]
- Paces, T.; Möller, P.; Fuganti, A.; Morteani, G.; Pecek, J. Sparkling mineral water at western rim of the Doupovske hory Mountains (Czech Republic): Genesis by water-rock interaction and deep-seated CO2. Bull. Czech. Geol. Surv. 2001, 76, 189–202. [Google Scholar]
- Möller, P.; Dulski, P.; Morteani, G. Partitioning of rare earth elements, yttrium and major elements among source rocks, liquid and vapor of Larderello-Travale Geothermal Field, Tuscany (central Italy). Geochim. Cosmochim. Acta 2003, 67, 171–183. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Johannesson, K.H. Rare earth elements (REE) and yttrium in stream waters, stream sediments, and Fe-Mn oxyhydroxides: Fractionation, speciation and controls over REE+Y patterns in the surface environment. Geochim. Cosmochim. Acta 2008, 72, 5962–5983. [Google Scholar] [CrossRef]
- Möller, P.; Rosenthal, E.; Dulski, P.; Geyer, S.; Guttman, Y. Rare earths and yttrium hydrostratigraphy along the Lake Kinneret-Dead Sea-Arava transform fault, Israel and adjoining territories. Appl. Geochem. 2003, 18, 1613–1628. [Google Scholar] [CrossRef]
- Möller, P.; Morteani, G.; Dulski, P.; Reinfalk, C. Vapor/liquid fractionation of rare earths, Y3+, Na+, K+, NH4+, Cl−, HCO3−, SO42−, and borate in fluids from the Piancastagnaio geothermal field, Italy. Geothemics 2009, 38, 360–369. [Google Scholar] [CrossRef]
- Möller, P.; Dulski, P. Induced Rare earth element fractionation in brines by hydraulic fracturing of their aquifer rocks. Geothermics 2018, 72, 307–315. [Google Scholar] [CrossRef]
- Douville, E.; Bienvenue, P.; Charlou, J.L.; Donval, J.P.; Fouquet, Y.; Appriou, P.; Gamo, T. Yttrium and rare earth elements in fluids from various deep-sea hydrothermal systems. Geochim. Cosmochim. Acta 1999, 63, 627–643. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Comparing yttrium and rare earths in hydrothermal fluids from the Mid-Atlantic Ridge: Implications for Y and REE behaviour during near-vent mixing and for the Y/Ho ratio of Proterozoic seawater. Chem. Geol. 1999, 155, 77–90. [Google Scholar] [CrossRef]
- Möller, P.; Woith, H.; Dulski, P.; Lüders, V.; Erzinger, J.; Kämpf, H.; Pekdeger, A.; Hansen, B.; Lodemann, M.; Banks, D. Main and trace elements in KTB-VB fluid: Composition and hints to its origin. Geofluids 2005, 5, 28–41. [Google Scholar] [CrossRef]
- Barkan, E.; Luz, B.; Lazar, B. Dynamics of carbon dioxide system in the Dead Sea. Geochim. Cosmochim. Acta 2001, 65, 355–368. [Google Scholar] [CrossRef]
- Scherer, M.; Seitz, H. Rare-earth element distribution in Holocene and Pleistocene corals and their redistribution during diagenesis. Chem. Geol. 1980, 28, 279–289. [Google Scholar] [CrossRef]
- Tanaka, K.; Ohta, A.; Kawabe, I. Experimental REE partitioning between calcite and aqueous solution at 25 °C and 1 atm: Constraints on the incorporation of seawater REE into seamount-type limestones. Geochem. J. 2004, 38, 19–32. [Google Scholar] [CrossRef][Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenites. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Kikawada, Y.; Oi, T.; Honda, T.; Ossaka, T.; Kakihana, H. Lanthanoid abundances of acidic hot spring and crater lake waters in the Kusatsu-shirane volcano region, Japan. Geochem. J. 1993, 27, 19–33. [Google Scholar] [CrossRef]
- Lewis, A.J.; Komninou, A.; Yardley, B.W.D.; Palmer, M.R. Rare earth speciation in geothermal fluids from Yellowstone National Park, Wyoming, USA. Geochim. Cosmochim. Acta 1998, 62, 657–663. [Google Scholar] [CrossRef]
- Ohta, A.; Kawabe, I. RE(III) adsorption onto Mn dioxide (δ-MnO2) and Fe oxihydroxide: Ce(III) oxidation by δ-MnO2. Geochim. Cosmochim. Acta 2001, 65, 695–703. [Google Scholar] [CrossRef]
- Möller, P.; Siebert, C. Cycling of calcite and hydrous metal oxides and chemical changes of major element and REE chemistry in monomictic hardwater lake; Impact on sedimentation. Geochemistry 2016, 76, 133–148. [Google Scholar] [CrossRef]

| Intercepts | ||||
|---|---|---|---|---|
| Slope | X-Axis | Y-Axis | ||
| Seawater | 0.8 | 0.5 | ||
| deep basins | 0.072 | 0 | ||
| surface basin | 0.18 | 0.05 | ||
| Lakes and seas | 0.3–0.7 | 0–0.1 | ||
| Rivers | 0.5–1 | |||
| Springs | 0.5 | |||
| Groundwater | <20 C | 0.5 | ||
| 20–50 C | <0.9 | |||
| >50 C | 0.3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, P.; Dulski, P.; De Lucia, M. REY Patterns and Their Natural Anomalies in Waters and Brines: The Correlation of Gd and Y Anomalies. Hydrology 2021, 8, 116. https://doi.org/10.3390/hydrology8030116
Möller P, Dulski P, De Lucia M. REY Patterns and Their Natural Anomalies in Waters and Brines: The Correlation of Gd and Y Anomalies. Hydrology. 2021; 8(3):116. https://doi.org/10.3390/hydrology8030116
Chicago/Turabian StyleMöller, Peter, Peter Dulski, and Marco De Lucia. 2021. "REY Patterns and Their Natural Anomalies in Waters and Brines: The Correlation of Gd and Y Anomalies" Hydrology 8, no. 3: 116. https://doi.org/10.3390/hydrology8030116
APA StyleMöller, P., Dulski, P., & De Lucia, M. (2021). REY Patterns and Their Natural Anomalies in Waters and Brines: The Correlation of Gd and Y Anomalies. Hydrology, 8(3), 116. https://doi.org/10.3390/hydrology8030116






