Hydrogeological Characterization of the Thyspunt Area, Eastern Cape Province, South Africa
Abstract
1. Introduction
2. The Study Area
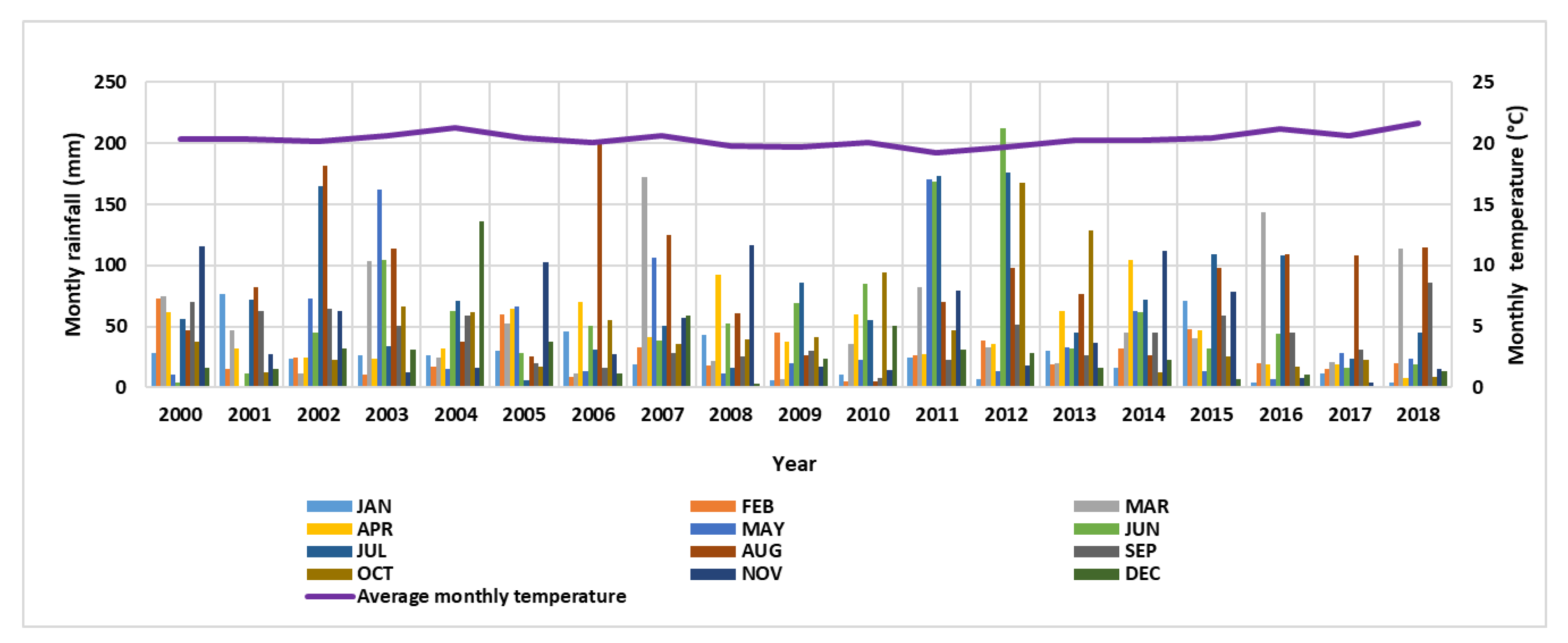
2.1. Location, Climate and Drainage
2.2. Geological and Hydrogeological Setting
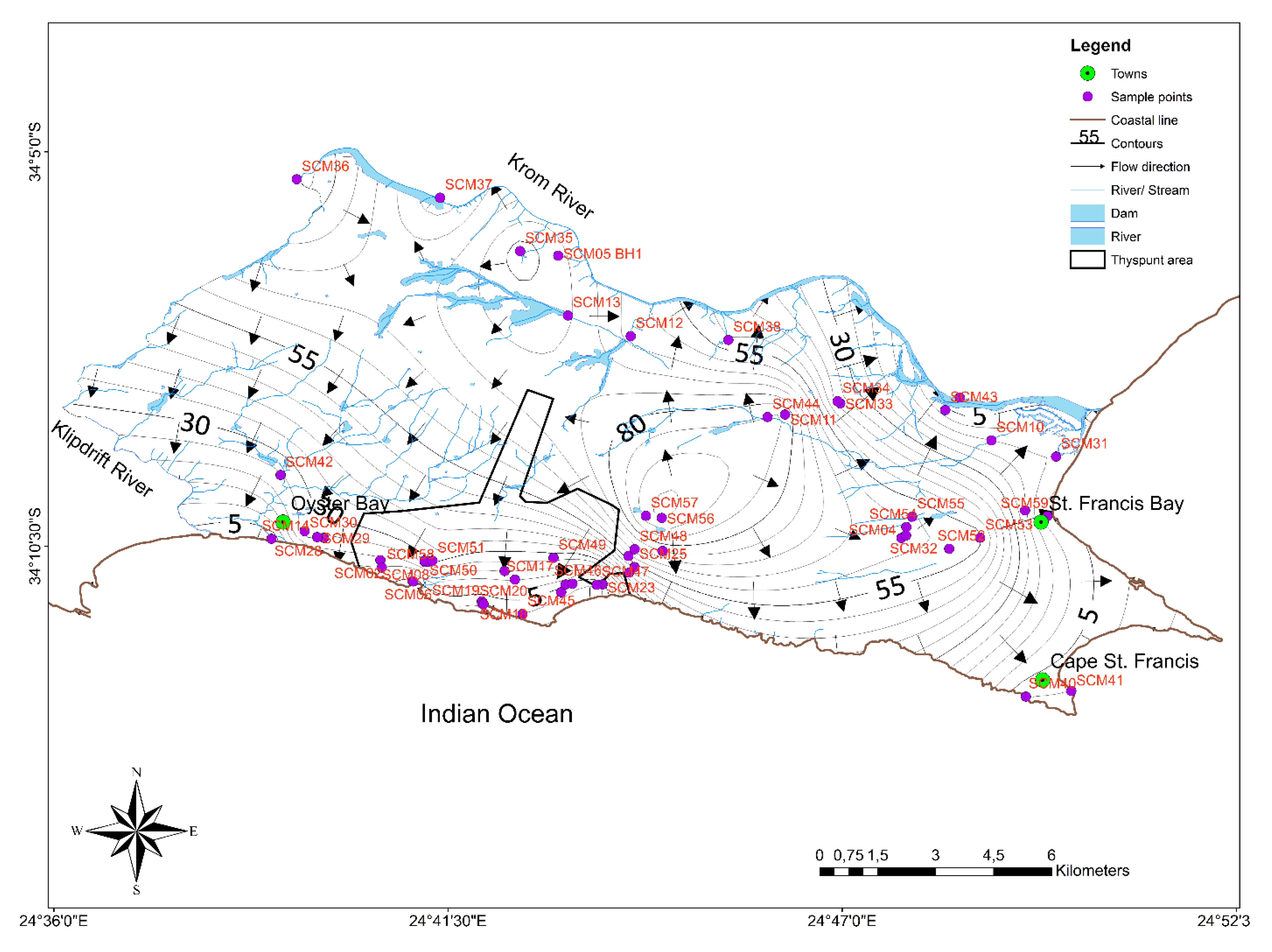
3. Materials and Methods
4. Results and Discussion
4.1. Groundwater Occurrence and Hydraulic Characteristics of Aquifers
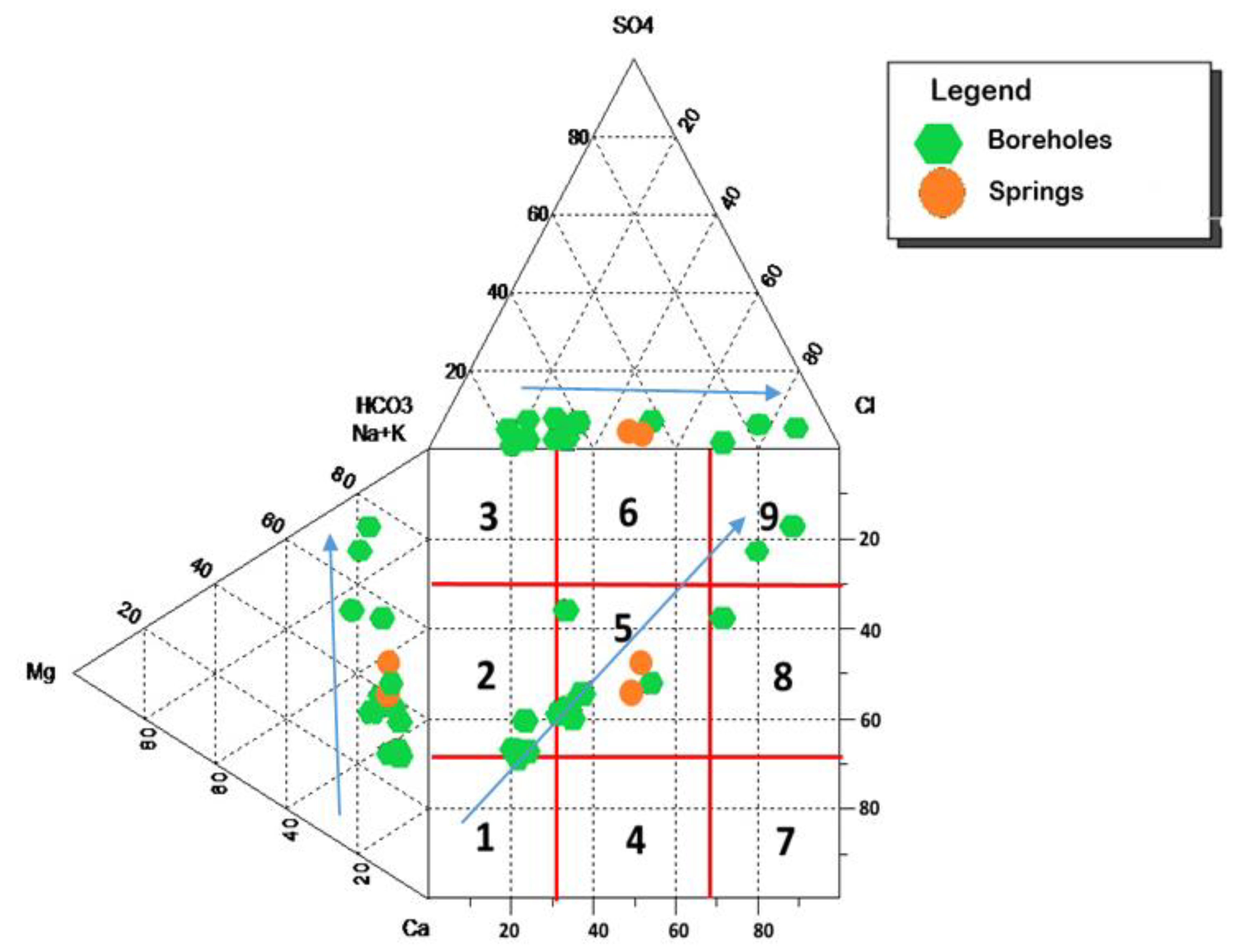
4.2. Hydrochemical and Stable Environmental Isotope Characteristics
4.3. Groundwater Residence Time and Implication for Its Management
4.4. Hydrogeological Conceptual Model of Groundwater Occurrence and Circulation in the Study Area
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, A.R.; Smith, S.D.; Lamontagne, S.; Suckow, A. Characterising alluvial aquifers in a remote ephemeral catchment (Flinders River, Queensland) using a direct push tracer approach. J. Hydrol. 2017, 556, 600–610. [Google Scholar] [CrossRef]
- Zhao, D.; Wanga, G.; Liaoa, F.; Yanga, N.; Jianga, W.; Guoa, L.; Liuc, C.; Shia, Z. Groundwater-surface water interactions derived by hydrochemical and isotopic (222Rn, deuterium, oxygen-18) tracers in the Nomhon area, Qaidam Basin, NW China. J. Hydrol. 2018, 565, 650–661. [Google Scholar] [CrossRef]
- Department of Water Affairs. Eastern Cape Groundwater Master Plan; DWA EC Office: Port Elizabeth, South Africa, 2010; p. 41.
- Department of Environmental Affairs. Nuclear-1 EIA, Draft Environmental Management Plan―Revision 2; Reference No.: 12/12/20/944; Department of Environmental Affairs: Pretoria, South Africa, 2016.
- Giacinto, J.; Barnhurst, D.; Tiruneh, N. Conceptual groundwater model development for new nuclear power plants. In Proceedings of the 2nd Joint Federal Interagency Conference, Las Vegas, NV, USA, 1–27 June 2010; pp. 1–12. [Google Scholar]
- Oxtobee, J.P.A.; Novakowski, K. A field investigation of groundwater/surface water interaction in a fractured bedrock environment. J. Hydrol. 2002, 269, 169–193. [Google Scholar] [CrossRef]
- Baskaran, S.; Ransle, T.; Brodie, R.S.; Baker, P. Investigating groundwater–river interactions using environmental tracers. Aust. J. Earth Sci. 2009, 56, 13–19. [Google Scholar] [CrossRef]
- Thamm, A.G.; Johnson, M.R. The Cape Supergroup. In The Geology of South Africa; Johnson, M.R., Anhaeusser, C.R., Thomas, R.J., Eds.; The Geological Society of South Africa and Council for Geosciences: Pretoria, South Africa, 2006; p. 691. [Google Scholar]
- Booth, P.W.K.; Munro, A.J.; Shone, R.W. Lithological and structural characteristics of Cape Supergroup rocks at Port Alfred, Eastern Cape, South Africa. S. Afr. J. Geol. 1999, 4, 391–404. [Google Scholar]
- Claassen, D. Geographical controls on sediment accretion of the Cenozoic Algoa group between oyster bay and ST. Francis, Eastern Cape Coastline. S. Afr. J. Geol. 2014, 117, 109–128. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Publishers: Boca Raton, FL, USA, 1997. [Google Scholar]
- Neuman, S.P. Effect of partial penetration on flow in unconfined aquifers considering delayed gravity response. Water Resour. Res. 1974, 10, 303–312. [Google Scholar] [CrossRef]
- Crosbie, R.S.; Binning, P.; Kalma, J.D. A time series approach to inferring groundwater recharge using the water table fluctuation method. Water Resour. Res. 2005, 41, 1–9. [Google Scholar] [CrossRef]
- Van Wyk, E. Correlations between rainwater and groundwater geochemistry signatures with reference to episodic rainfall events in semi-arid environments. In The Use of Isotope Hydrology to Characterize and Assess Water Resources in South Africa; WRC Report TT570/13; Abiye, T., Ed.; Water Research Commission: Pretoria, South Africa, 2013; pp. 102–110. [Google Scholar]
- Froehlich, K.; Gibson, J.J.; Aggarwal, P. deuterium excess in precipitation and its climatological significance. In C&S Papers Series, Proceedings of the International Conference on Study of Environmental Change Using Isotope Techniques, Vienna, Austria, 23–27 April 2001; International Atomic Energy Agency: Vienna, Austria, 2002; Volume 13/P, p. 13531. [Google Scholar]
- Abiye, T. (Ed.) The Use of Isotope Hydrology to Characterize and Assess Water Resources in South Africa; WRC Report No TT 570/13; Water Research Commission: Pretoria, South Africa, 2013; p. 211. ISBN 978-1-4312-0480-9. [Google Scholar]
- Harris, C.; Burger, C.; Miller, J.; Rawoot, F. O- and H-isotope record of Cape Town rainfall from 1991 to 2008, and its application to recharge studies of Table Mountain groundwater, South Africa. S. Afr. J. Geol. 2010, 113, 33–56. [Google Scholar] [CrossRef]
- Miller, J.A.; Dunforda, A.J.; Swanaa, K.A.; Palcsub, L.; Butlerc, M.; Clarked, C.E. Stable isotope and noble gas constraints on the source and residence time of spring water from the Table Mountain Group Aquifer, Paarl, South Africa and implications for large scale abstraction. J. Hydrol. 2017, 551, 100–115. [Google Scholar] [CrossRef]
- Kazemi, G.A.; Lehr, J.H.; Perrochet, P. Groundwater Age; John-Wiley and Sons, Inc.: Hoboken, NJ, USA, 2006; p. 9. [Google Scholar]
- Fritz, P.; Fonte, J.C.; Frape, S.K.; Louvat, D.; Michelot, J.L.; Balderer, W. The isotope geochemistry of carbon in groundwater at Stripa. Geochim. Cosmochim. Acta 1988, 53, 1765–1775. [Google Scholar] [CrossRef]







| ID | Fitts Geosolutions Determined Aquifer Properties | Aquifer Thickness | Aquifer Type | Aquifer Unit | |||
|---|---|---|---|---|---|---|---|
| T | K | ||||||
| (m2/d) | S | Sy | (m/d) | (m) | |||
| THY−RP1 | 1.45 | 1 × 10−3 | 0.012 | 125 | Confined | TMG | |
| THY−RP2 | 39.01 | 3.75 × 10−4 | 0.1 | 0.514 | 76 | Confined | TMG |
| THY−RP5 | 44.01 | 1.0 × 10−3 | 0.32 | 138 | Confined | TMG | |
| THY−RP6 | 37.99 | 5.0 × 10−5 | 1.58 | 24 | Confined | TMG | |
| THY−RP8 | 36.81 | 0.006 | 0.33 | 112 | Unconfined | TMG | |
| THY−RP14 | 15.04 | 1.69 × 10−3 | 0.84 | 18 | Confined | TMG | |
| THY−RP10 | 2.51 | 0.045 | 0.044 | 57 | Unconfined | TMG | |
| THY−RP9 | 4.56 | 3.0 × 10−2 | 0.067 | 68 | Confined | TMG | |
| THY−MR2 | 108.3 | 0.12 | 4.47 | 24 | Unconfined | ALGOA | |
| THY−MR5 | 275 | 1.47 × 10−2 | 19.13 | 13 | Unconfined | ALGOA | |
| THY−RP11 | 0.36 | 1.0 × 10−5 | 0.01 | 40 | Confined | TMG | |
| THY−MR11 | 125.1 | 0.1 | 7.72 | 16 | Unconfined | ALGOA | |
| Test Site | Latitude | Longitude | Altitude (m.a.s.l) | Soil Type | Infiltration Rate (mm/min) |
|---|---|---|---|---|---|
| St. Francis Bay Park | −34.16364 | 24.82639 | 10 | Sandy soil | 5.1 |
| Thyspunt | −34.17872 | 24.68634 | 54 | Sandy soil | 5.20 |
| Thyspunt | 34.19150 | 26.71325 | 9 | Sandy soil | 4.5 |
| Pennisands Farm | −34.15047 | 24.70840 | 130 | Sandy soil | 2.5 |
| Rosa Farm | −34.10791 | 24.71703 | 56 | Loamy | 1.1 |
| ID | Water Point | pH | EC (μS/cm) | TDS (mg/L) | ORP (mV) | Temp (°C) | Na (mg/L) | K (mg/L) | Mg (mg/L) | Ca (mg/L) | Cl (mg/L) | SO4 (mg/L) | HCO3 (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCM01 | Rain | 7.21 | 286 | 180 | 197.7 | 14.75 | |||||||
| SCM03 | Spring | 7.75 | 1341 | 856 | −69 | 119.54 | 3.13 | 16.39 | 96.88 | 183.2 | 15.7 | 300.1 | |
| SCM05 | Borehole | 6.56 | 1665 | 1048.95 | 5 | 18.5 | 200.55 | 7.24 | 31.95 | 35.83 | 324.8 | 31.2 | 131.8 |
| SCM07 | Borehole | 7.22 | 940 | 592.2 | −32 | 19.7 | 50.22 | 1.32 | 8.33 | 64.44 | 78.9 | 23.4 | 316.7 |
| SCM08 | Borehole | 7.18 | 779 | 490.77 | −30 | 20.8 | 52.04 | 2.35 | 10.15 | 75.61 | 79.7 | 11.5 | 310.6 |
| SCM09 | Borehole | 7.16 | 876 | 551.88 | −29 | 18.4 | 49.10 | 0.77 | 8.24 | 94.46 | 67.9 | 0.2 | 468.3 |
| SCM10 | River | 7.96 | 1284 | 808.92 | −32 | 11.5 | |||||||
| SCM11 | Spring | 6.82 | 416 | 262.08 | −76 | 14.2 | |||||||
| SCM12 | Wetland | 6.75 | 1367 | 861.21 | −5 | 12.9 | |||||||
| SCM13 | Wetland | 7.23 | 1186 | 747.18 | −26 | 13 | |||||||
| SCM14 | Ocean | 7.52 | 34,300 | 21,609 | −51 | 16.7 | |||||||
| SCM16 | Borehole | 7.2 | 908 | 572.04 | −30 | 18.5 | 45.35 | 1.94 | 11.49 | 91.37 | 65 | 32.2 | 402 |
| SCM17 | Borehole | 7.24 | 781 | 492.03 | −33 | 21.3 | |||||||
| SCM18 | Ocean | 7.91 | 33,500 | 21,105 | −75 | 19 | |||||||
| SCM19 | Spring | 7.7 | 1066 | 671.58 | −60 | 18.7 | |||||||
| SCM20 | Spring | 7.95 | 986 | 621.18 | −74 | 18.3 | |||||||
| SCM21 | Borehole | 7.3 | 923 | 581.49 | −36 | 19.2 | 118.02 | 4.84 | 15.65 | 59.60 | 202 | 3 | 140.1 |
| SCM22 | Borehole | 7.12 | 912 | 574.56 | −29 | 17.8 | 39.55 | 2.15 | 7.54 | 82.54 | 54.2 | 9.9 | 375.3 |
| SCM23 | Borehole | 7.09 | 1041 | 655.83 | −27 | 20.4 | |||||||
| SCM24 | Wetland | 7.8 | 787 | 495.81 | −72 | 17.5 | |||||||
| SCM25 | Borehole | 7.29 | 863 | 543.69 | −38 | 18.5 | |||||||
| SCM26 | Borehole | 7.08 | 1493 | 940.59 | −26 | 19.9 | 87.42 | 1.22 | 12.04 | 125.42 | 98.1 | 14.5 | 584.6 |
| SCM27 | Spring | 7.68 | 964 | 607.32 | −51 | 18.1 | |||||||
| SCM28 | Spring | 7.74 | 1885 | 1187.55 | −63 | 16.3 | |||||||
| SCM29 | Borehole | 7.4 | 1470 | 926.1 | −42 | 14.3 | |||||||
| SCM30 | Spring | 7.74 | 2800 | 1764 | −60 | 16.7 | |||||||
| SCM31 | Borehole | 7.38 | 1500 | 945 | −42 | 17 | |||||||
| SCM33 | Artesian BH | 6.14 | 985 | 620.55 | |||||||||
| SCM34 | Wetland | 7.58 | 1440 | 907.2 | −52 | 16 | |||||||
| SCM35 | Spring | 7.7 | 955 | 601.65 | −72 | 19.5 | |||||||
| SCM36 | Wetland | 7.6 | 1624 | 1023.12 | −43 | 14.5 | |||||||
| SCM38 | Spring | 7.69 | 866 | 545.58 | −49 | 19.6 | |||||||
| SCM39 | Ocean | 8.03 | 33,800 | 21,294 | −78 | 19.3 | |||||||
| SCM40 | Ocean | 7.9 | 35,100 | 22,113 | −72 | 17.6 | |||||||
| SCM42 | River | 7.01 | 1669 | 1051.47 | −26 | 14.4 | |||||||
| SCM43 | Wetland | 7.53 | 1453 | 915.39 | −49 | 13.4 | |||||||
| SCM44 | Artesian BH | 6.75 | 1224 | 771.12 | 98 | 17.2 | 154.14 | 4.91 | 19.10 | 16.21 | 345.3 | 28.1 | 63.4 |
| SCM45 | Borehole | 7.32 | 931 | 586.53 | −38 | 17.5 | 60.61 | 4.33 | 11.97 | 70.20 | 98.1 | 27.5 | 314.1 |
| SCM46 | Borehole | 7.9 | 580 | 365.4 | −71 | 17.6 | |||||||
| SCM47 | Borehole | 7.4 | 1093 | 688.59 | −41 | 18.6 | 113.34 | 13.66 | 26.68 | 50.95 | 100.3 | 12.2 | 363.8 |
| SCM48 | Borehole | 7.05 | 900 | 567 | 17.6 | 60.68 | 1.22 | 16.13 | 85.46 | 122.8 | 5.4 | 431.4 | |
| SCM50 | Borehole | 8.02 | 2460 | 1549.8 | −77 | 18.9 | |||||||
| SCM51 | Borehole | 7.85 | 430 | 270.9 | −70 | ||||||||
| SCM52 | Spring | 7.97 | 949 | 597.87 | −68 | 14.5 | |||||||
| SCM53 | Spring | 8.26 | 1236 | 778.68 | −77 | 13.9 | |||||||
| SCM54 | Wetland | 8.22 | 1768 | 1113.84 | −88 | 15.1 | |||||||
| SCM55 | Wetland | 7.73 | 1936 | 1219.68 | −60 | 13.2 | |||||||
| SCM56 | Wetland | 8.14 | 493 | 310.59 | −85 | 16.3 | |||||||
| SCM57 | Wetland | 7.63 | 7040 | 4435.2 | −55 | 15.8 | |||||||
| SCM58 | Spring | 7.92 | 1135 | 715.05 | −64 | 16.6 | 86.87 | 2.42 | 12.79 | 95.74 | 188.2 | 19.9 | 340.7 |
| SCM59 | Borehole | 7.51 | 1557 | 980.91 | −49 | 135.90 | 5.33 | 19.44 | 137.23 | 289.8 | 48.8 | 426.7 | |
| Minimum | Boreholes | 6.14 | 430 | 270.9 | −77 | 14.3 | 39.55 | 0.77 | 7.54 | 16.21 | 54.20 | 0.20 | 63.40 |
| Springs | 6.82 | 416 | 262.08 | −77 | 13.9 | 86.87 | 2.42 | 12.79 | 95.74 | 183.20 | 15.70 | 300.10 | |
| Wetlands | 6.75 | 493 | 310.59 | −88 | 12.9 | ||||||||
| River | 7.01 | 1284 | 808.92 | −32 | 11.5 | ||||||||
| Ocean | 7.52 | 33,500 | 21,105 | −78 | 16.7 | ||||||||
| Maximum | Boreholes | 8.02 | 2460 | 1549.8 | 98 | 21.3 | 200.55 | 13.66 | 31.95 | 137.23 | 345.30 | 48.80 | 584.60 |
| Springs | 8.26 | 2800 | 1764 | −49 | 19.6 | 119.54 | 3.13 | 16.39 | 96.88 | 188.20 | 19.90 | 340.70 | |
| Wetlands | 8.22 | 7040 | 4435.2 | −5 | 17.5 | ||||||||
| River | 7.96 | 1669 | 1051.47 | −26 | 14.4 | ||||||||
| Ocean | 8.03 | 35,100 | 22,113 | −51 | 19.3 | ||||||||
| Mean | Boreholes | 7.23 | 1116.57 | 703.44 | −31.53 | 18.56 | 89.76 | 3.95 | 15.29 | 76.10 | 148.22 | 19.07 | 332.98 |
| Springs | 7.72 | 1237.55 | 768.25 | −66.15 | 16.69 | 103.20 | 2.78 | 14.59 | 96.31 | 185.70 | 17.80 | 320.40 | |
| Wetlands | 7.62 | 1909.40 | 1202.92 | −53.50 | 14.77 | ||||||||
| River | 7.49 | 1476.50 | 930.20 | −29 | 12.95 | ||||||||
| Ocean | 7.84 | 34,175 | 21,530.25 | −69 | 18.15 |
| ID | Water Source | δ²H (‰) | δ18O (‰) | d−Excess |
|---|---|---|---|---|
| SCM01 | Rain | −3.3 ± 2.3 | −4.47 ± 0.3 | 22.65 |
| SCM02 | Rain | 0.3 ± 0.7 | −3.14 ± 0.2 | 18.50 |
| SCM03 | Spring | −13.4 ± 0.9 | −3.42 ± 0.1 | 6.46 |
| SCM04 | Rain | −12.5 ± 0.9 | −4.57 ± 0.2 | 13.99 |
| SCM05 BH1 | Borehole | −21.4 ± 0.4 | −5.37 ± 0.1 | 9.77 |
| SCM05−BH1.1 | Borehole | −22.4 ± 4.2 | −4.92 ± 0.3 | 6.18 |
| SCM07 | Borehole | −13.9 ± 0.2 | −3.94 ± 0.1 | 8.87 |
| SCM08 | Borehole | −14.3 ± 0.2 | −4.01 ± 0.1 | 8.95 |
| SCM09 | Borehole | −15.2 ± 0.5 | −4.28 ± 0.1 | 9.61 |
| SCM10 | River | −12.9 ± 0.2 | −3.62 ± 0.1 | 8.09 |
| SCM11 | Spring | −17.1 ± 0.5 | −4.82 ± 0.0 | 10.87 |
| SCM12 | Wetland | −9.9 ± 1.2 | −3.53 ± 0.1 | 10.50 |
| SCM15 | Borehole | −19.8 ± 0.3 | −4.95 ± 0.1 | 8.95 |
| SCM16 | Borehole | −20.6 ± 0.4 | −5.04 ± 0.1 | 8.60 |
| SCM17 | Borehole | −21.1 ± 0.5 | −4.94 ± 0.1 | 7.55 |
| SCM18 | Ocean water | 3.5 ± 0.9 | 0.73 ± 0.2 | −0.71 |
| SCM19 | Spring | −17.8 ± 1.4 | −4.07 ± 0.2 | 5.79 |
| SCM21 | Borehole | −13.2 ± 0.6 | −3.84 ± 0.1 | 9.11 |
| SCM22 | Borehole | −12.4 ± 1.0 | −4.24 ± 0.1 | 12.22 |
| SCM23 | Borehole | −10.5 ± 0.5 | −3.60 ± 0.1 | 10.33 |
| SCM24 | Wetland | −11.1 ± 0.4 | −3.53 ± 0.1 | 9.41 |
| SCM25 | Tap water | −8.5 ± 0.3 | −2.74 ± 0.1 | 7.38 |
| SCM26 | Borehole | −15.0 ± 1.4 | −4.36 ± 0.1 | 10.30 |
| SCM27 | Spring | −17.8 ± 0.4 | −4.69 ± 0.1 | 9.42 |
| SCM28 | Spring | −14.4 ± 0.8 | −4.94 ± 0.1 | 14.23 |
| SCM29 | Borehole | −18.6 ± 0.4 | −4.87 ± 0.1 | 9.59 |
| SCM30 | Spring | −14.8 ± 0.4 | −4.67 ± 0.1 | 12.30 |
| SCM31 | Borehole | −15.9 ± 0.7 | −4.08 ± 0.2 | 7.78 |
| SCM32 | Tap−Borehole | −19.2 ± 3.3 | −4.72 ± 0.3 | 8.18 |
| SCM33 | Artesian Borehole | −17.8 ± 1.5 | −4.58 ± 0.1 | 8.78 |
| SCM34 | Wetland | 16.5 ± 1.7 | 2.11 ± 0.2 | 4.29 |
| SCM36 | Wetland | −9.5 ± 0.8 | −3.57 ± 0.1 | 11.28 |
| SCM37 | Municipal dam | 6.0 ± 2.3 | 0.16 ± 0.3 | 5.11 |
| SCM38 | Spring | 9.8 ± 1.3 | 1.27 ± 0.2 | 2.43 |
| SCM39 | Estuary | 8.5 ± 0.8 | 1.07 ± 0.2 | 2.26 |
| SCM40 | Ocean water | 3.5 ± 0.7 | 0.59 ± 0.1 | 0.10 |
| SCM41 | Ocean water | 3.6 ± 0.8 | 0.60 ± 0.2 | 0.16 |
| SCM42 | stream | 5.8 ± 0.5 | −0.65 ± 0.1 | 9.58 |
| SCM43 | Wetland | −10.9 ± 2.4 | −3.25 ± 0.1 | 7.92 |
| SCM44 | Artesian Borehole | −17.1 ± 1.0 | −4.63 ± 0.2 | 9.72 |
| SCM45 | Borehole | −17.3 ± 3.2 | −4.64 ± 0.3 | 9.57 |
| SCM46 | Borehole | −13.8 ± 0.8 | −4.39 ± 0.2 | 11.60 |
| SCM47 | Borehole | −16.8 ± 0.4 | −4.64 ± 0.1 | 10.05 |
| SCM48 | Borehole | −14.1 ± 2.0 | −4.82 ± 0.3 | 13.82 |
| SCM50 | Borehole | −9.3 ± 1.4 | −3.83 ± 0.3 | 12.92 |
| SCM51 | Borehole | −13.1 ± 0.3 | −3.87 ± 0.1 | 9.34 |
| SCM52 | Pond | 14.5 ± 0.3 | 2.57 ± 0.1 | −0.34 |
| SCM53 | Quarry | 17.2 ± 1.2 | 2.11 ± 0.6 | 5.02 |
| SCM53 | Pond | −11.2 ± 1.0 | −4.10 ± 0.1 | 12.62 |
| SCM54 | Wetland | −3.60 ± 0.6 | −2.12 ± 0.2 | 8.69 |
| SCM55 | Pond | −4.43 ± 1.8 | −3.83 ± 0.2 | 17.76 |
| SCM56 | Wetland | −8.0 ± 0.5 | −3.30 ± 0.1 | 11.16 |
| SCM57 | Spring | −8.1 ± 1.4 | −3.53 ± 0.2 | 12.39 |
| SCM58 | Borehole | −14.5 ± 0.6 | −4.01 ± 0.1 | 8.73 |
| SCM59 | Borehole | −15.7 ± 0.4 | −4.10 ± 0.1 | 8.11 |
| ID | Water Source | Aquifer Type | Hydrochemical Facies | Tritium (T.U.) | 3H MRT (Years) | δ13C (‰) | 14C (pMC) | 14C MRT (Years) |
|---|---|---|---|---|---|---|---|---|
| SCM07 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 1.0 ± 0.3 | 20.86 | |||
| SCM08 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 1.0 ± 0.3 | 20.86 | |||
| SCM09 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | −12.34 | 94.99 ± 0.51 | 430 ± 5 | ||
| SCM15 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 2.0 ± 0.3 | 8.43 | |||
| SCM16 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 1.8 ± 0.3 | 10.32 | |||
| SCM17 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 2.3 ± 0.3 | 5.92 | |||
| SCM21 | Borehole | Intergranular: Algoa Group | Na+−Cl− | 1.3 ± 0.3 | 16.15 | |||
| SCM22 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 1.5 ± 0.3 | 13.59 | |||
| SCM23 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 0.7 ± 0.2 | 27.25 | |||
| SCM26 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 1.9 ± 0.3 | 9.35 | |||
| SCM29 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 0.8 ± 0.2 | 24.86 | |||
| SCM31 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 2.2 ± 0.3 | 6.72 | |||
| SCM33 | Artesian BH | Fractured: TMG | Na+−Cl− | −18.81 | 88.78 ± 0.45 | 980 ± 5 | ||
| SCM44 | Artesian BH | Fractured: TMG | Na+−Cl− | 0.8 ± 0.2 | 24.86 | |||
| SCM45 | Borehole | Fractured: TMG | Ca2+−Mg2+−HCO3− | −10.90 | 88.6 ± 0.62 | 1000 ± 10 | ||
| SCM46 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 1.6 ± 0.3 | 12.43 | |||
| SCM47 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 0.2 ± 0.2 | 49.71 | |||
| SCM48 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−HCO3− | 1.0 ± 0.3 | 20.86 | |||
| SCM50 | Borehole | Fractured: TMG | Ca2+−Mg2+−HCO3− | 0.8 ± 0.2 | 24.86 | |||
| SCM51 | Borehole | Fractured: TMG | Ca2+−Mg2+−Cl− | 0.8 ± 0.2 | 24.86 | |||
| SCM59 | Borehole | Intergranular: Algoa Group | Ca2+−Mg2+−Cl− | 1.7 ± 0.3 | 11.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohuba, S.C.; Abiye, T.A.; Demlie, M.B.; Modiba, M.J. Hydrogeological Characterization of the Thyspunt Area, Eastern Cape Province, South Africa. Hydrology 2020, 7, 49. https://doi.org/10.3390/hydrology7030049
Mohuba SC, Abiye TA, Demlie MB, Modiba MJ. Hydrogeological Characterization of the Thyspunt Area, Eastern Cape Province, South Africa. Hydrology. 2020; 7(3):49. https://doi.org/10.3390/hydrology7030049
Chicago/Turabian StyleMohuba, Seeke C., Tamiru A. Abiye, Molla B. Demlie, and Moneri. J. Modiba. 2020. "Hydrogeological Characterization of the Thyspunt Area, Eastern Cape Province, South Africa" Hydrology 7, no. 3: 49. https://doi.org/10.3390/hydrology7030049
APA StyleMohuba, S. C., Abiye, T. A., Demlie, M. B., & Modiba, M. J. (2020). Hydrogeological Characterization of the Thyspunt Area, Eastern Cape Province, South Africa. Hydrology, 7(3), 49. https://doi.org/10.3390/hydrology7030049





