Transport and Fate of Nitrate in the Streambed of a Low-Gradient Stream
Abstract
1. Introduction
2. Materials and Methods
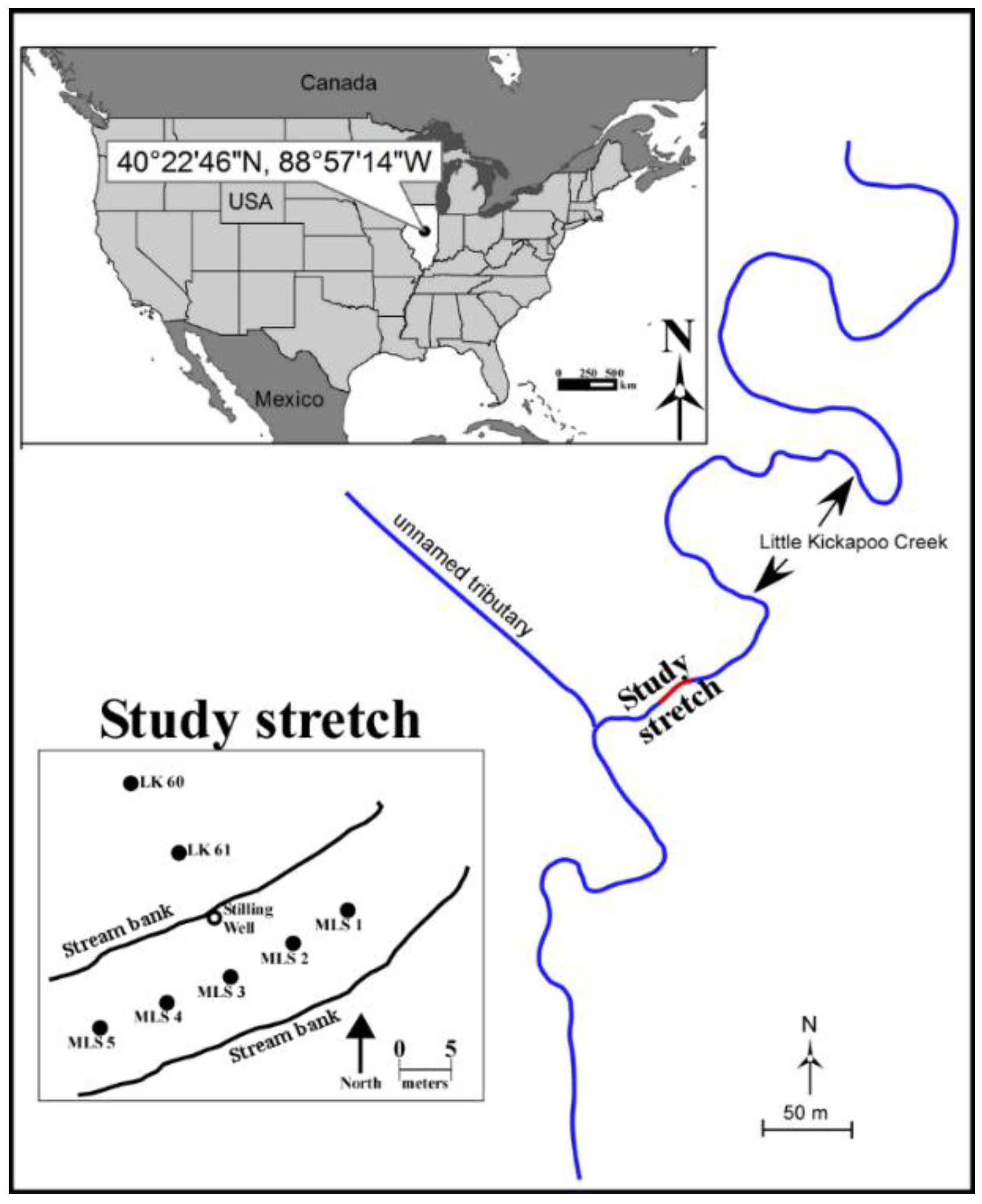
2.1. Site Description
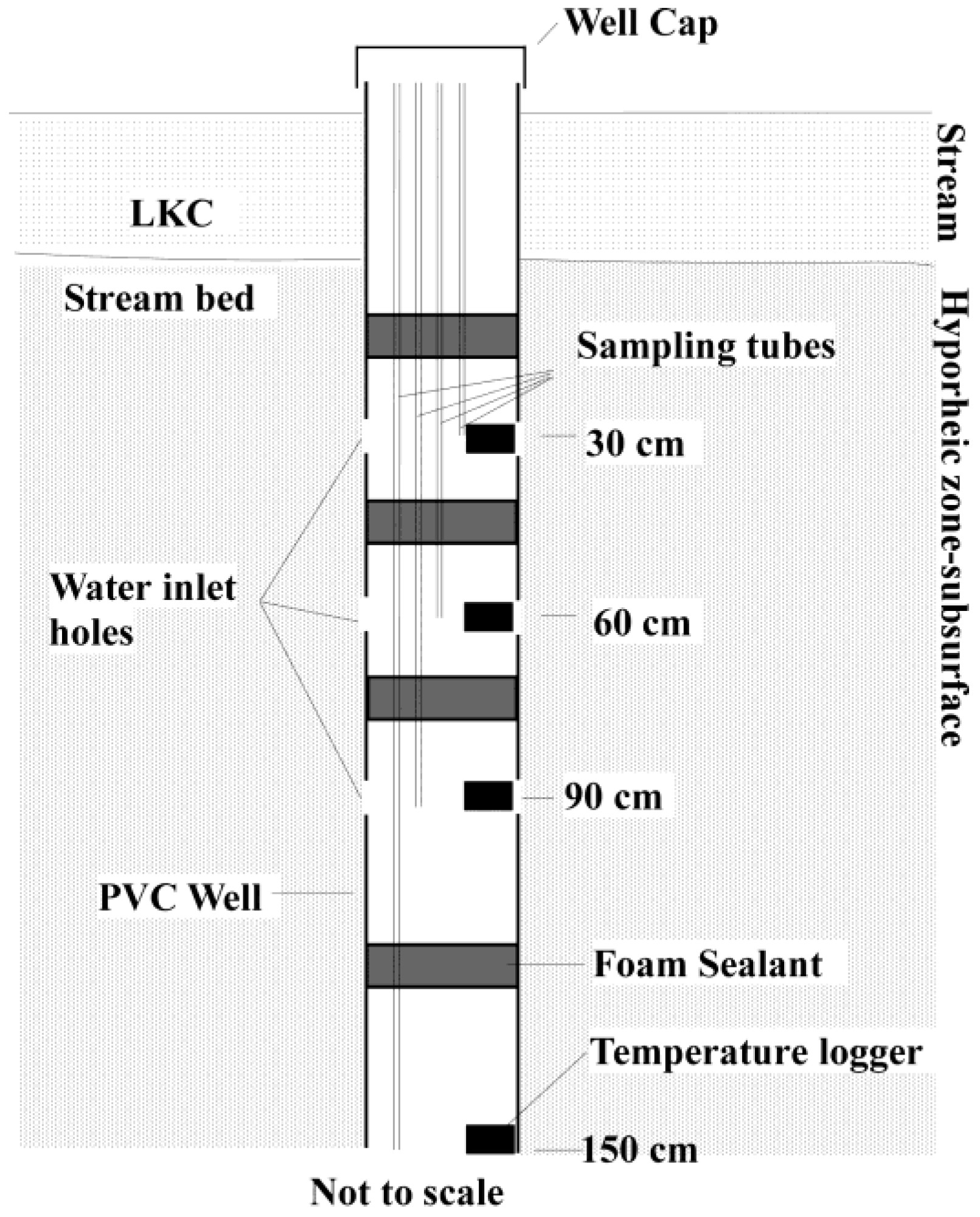
2.2. Sampling
2.3. Mixing Model
- %SW is the percentage of stream water within the streambed pore water (%),
- ClHZ is the chloride concentration in the streambed pore water at the depth of interest (mg/L),
- Clg is the chloride concentration in the groundwater (mg/L),
- Cls is the chloride concentration in the stream water (mg/L).
- NO3-N is the calculated concentration of NO3-N in the pore water as a given depth (mg/L),
- Ns is the NO3-N concentration of the stream water (mg/L),
- Ng is the NO3-N concentration of the groundwater (mg/L).
2.4. Statistical Analysis
3. Results
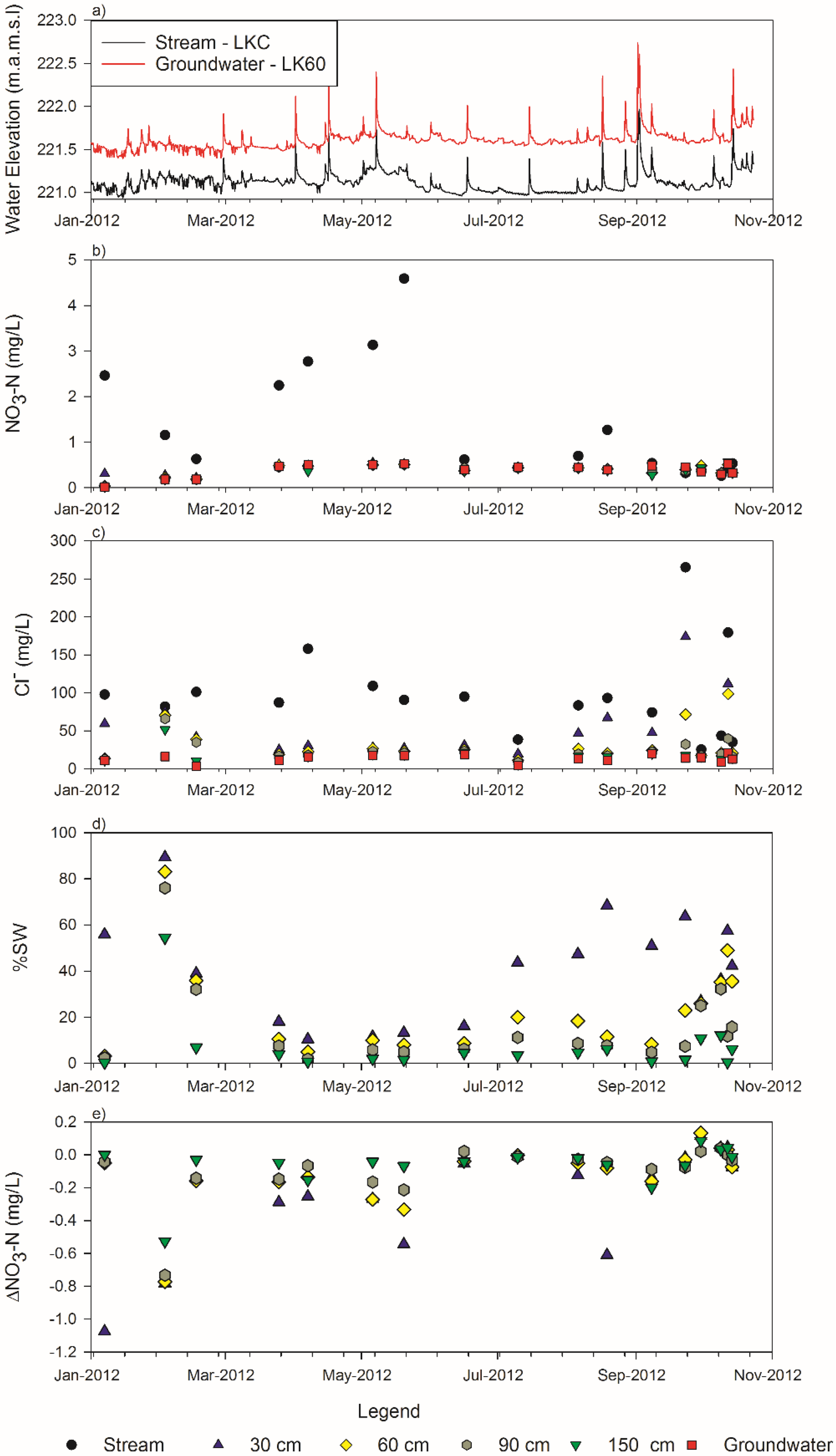
3.1. Stream and Groundwater
3.2. Nitrate
3.3. Chloride
3.4. Mixing Model
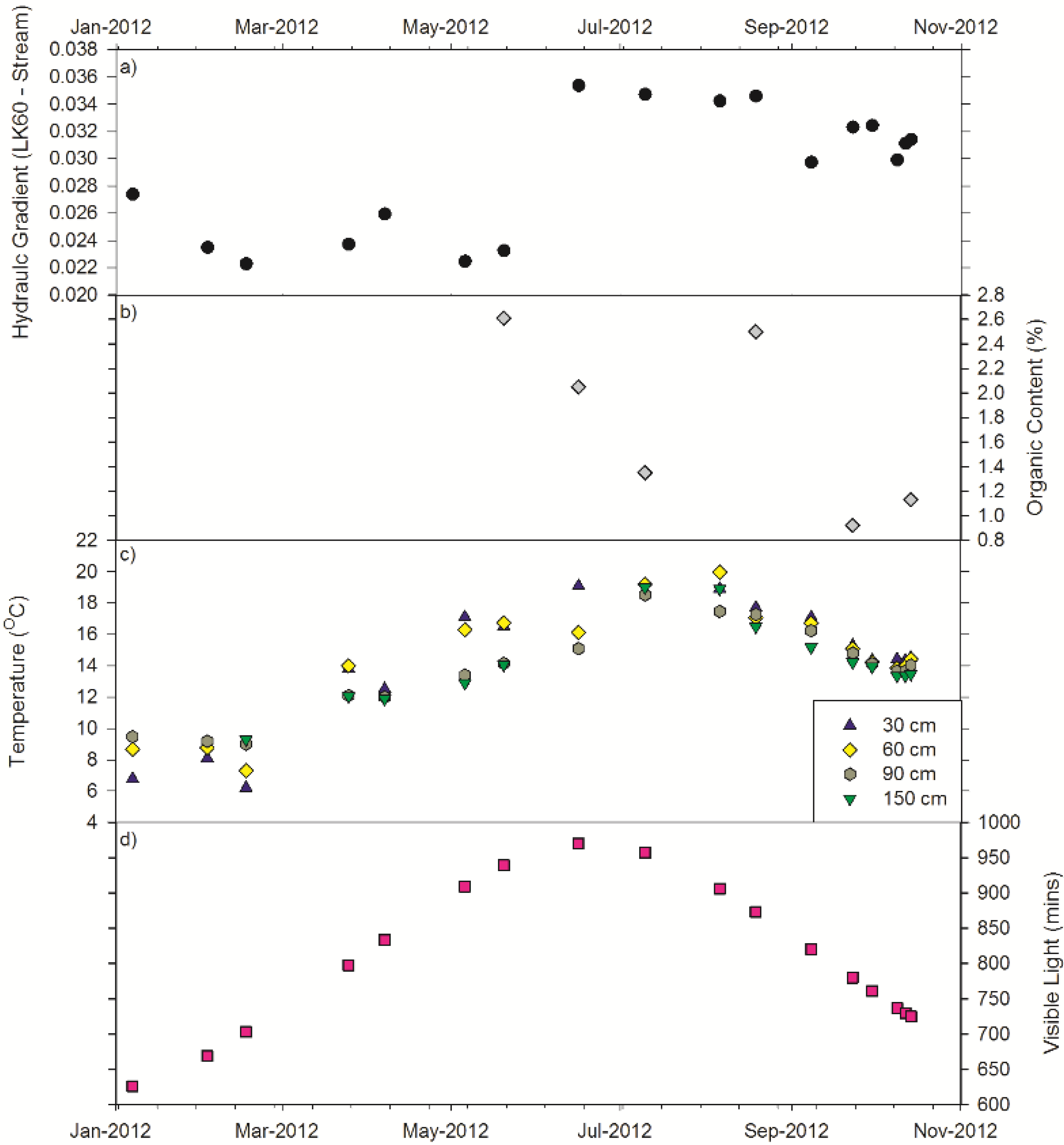
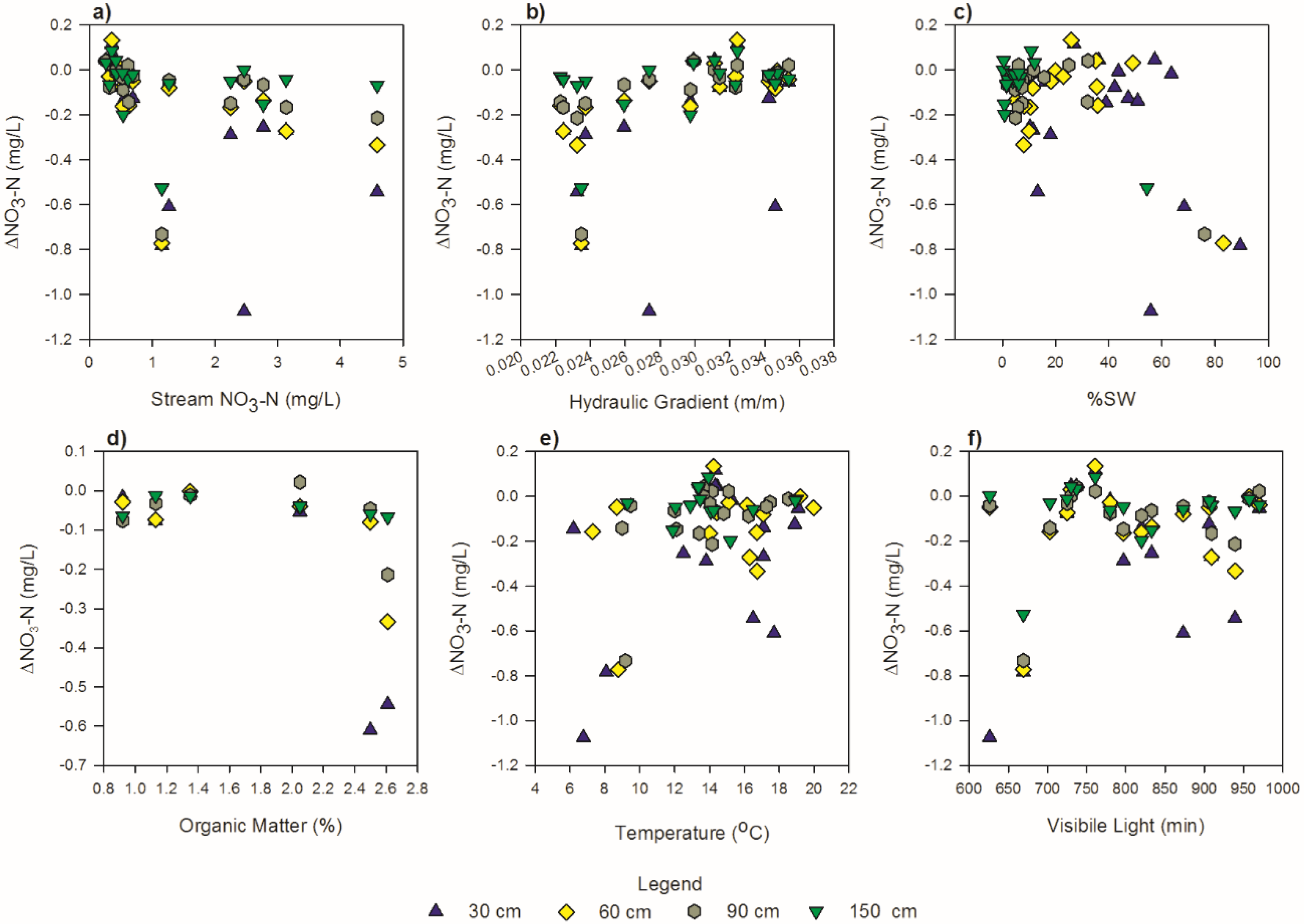
3.5. Controlling Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oberle, S.L.; Keeney, D.R. Factors influencing corn fertilizer N requirements in the northern US corn belt. J. Prod. Agric. 1990, 3, 527–534. [Google Scholar] [CrossRef]
- Gentry, L.E.; David, M.B.; Smith, K.M.; Kovacic, D.A. Nitrogen cycling and tile drainage nitrate loss in a corn/soybean watershed. Agric. Ecosyst. Environ. 1998, 68, 85–97. [Google Scholar] [CrossRef]
- Alexander, R.B.; Boyer, E.W.; Smith, R.A.; Schwarz, G.E.; Moore, R.B. The Role of headwater streams in downstream water quality. J. Am. Water Resour. Assoc. 2007, 43, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Dagg, M.J.; Breed, G.A. Biological effects of Mississippi River nitrogen on the northern gulf of Mexico—A review and synthesis. J. Mar. Syst. 2003, 43, 133–152. [Google Scholar] [CrossRef]
- Scavia, D.; Justic, D.; Bierman, V.J.J. Reducing hypoxia in the Gulf of Mexico: Advice from three models. Estuaries 2004, 27, 419–425. [Google Scholar] [CrossRef]
- Arango, C.P.; Tank, J.L.; Schaller, J.L.; Royer, T.V.; Bernot, M.J.; David, M.B. Benthic organic carbon influences denitrification in streams with high nitrate concentration. Freshw. Biol. 2007, 52, 1210–1222. [Google Scholar] [CrossRef]
- Christensen, P.B.; Nielsen, L.P.; Sorensen, J.; Revsbech, N.P. Denitrification in nitrate-rich streams: Diurnal and seasonal variation related to benthic oxygen metabolism. Limnol. Oceanogr. 1990, 35, 640–651. [Google Scholar] [CrossRef]
- Goolsby, D.A.; Battaglin, W.A.; Aulenbach, B.T.; Hooper, R.P. Nitrogen input to the Gulf of Mexico. J. Environ. Qual. 2001, 30, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Scavia, D.; Rabalais, N.N.; Turner, R.E.; Justić, D.; Wiseman, W.J., Jr. Predicting the response of Gulf of Mexico hypoxia to variations in Mississippi River nitrogen load. Limnol. Oceanogr. 2003, 48, 951–956. [Google Scholar] [CrossRef]
- Scott, D.; Harvey, J.; Alexander, R.; Schwarz, G. Dominance of organic nitrogen from headwater streams to large rivers across the conterminous United States. Glob. Biogeochem. Cycles 2007, 21, GB1003. [Google Scholar] [CrossRef]
- Boulton, A.J.; Findlay, S.; Marmonier, P. The functional significance of the hyporheic zone in streams and rivers (review). Ann. Rev. Ecol. Syst. 1998, 29, 59–81. [Google Scholar] [CrossRef]
- David, M.B.; Wall, L.G.; Royer, T.V.; Tank, J.L. Denitrification and the nitrogen budget of a reservoir in an agricultural landscape. Ecol. Appl. 2006, 16, 2177–2190. [Google Scholar] [CrossRef]
- Keeney, D.R.; Hatfield, J.L. The nitrogen cycle: Historical perspective, and current and potential future concerns. In Nitrogen in the Environment: Sources, Problems, and Solutions; Follett, R., Hatfield, J.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 3–16. [Google Scholar]
- Rabalais, N.; Turner, R.E.; Dortch, Q.; Justic, D.; Bierman, V., Jr.; Wiseman, W., Jr. Nutrient-enhanced productivity in the northern Gulf of Mexico: Past, present and future. In Nutrients and Eutrophication in Estuaries and Coastal Waters; Orive, E., Elliott, M., de Jonge, V., Eds.; Springer: Dordrecht, The Netherlands, 2002; Volume 164, pp. 39–63. [Google Scholar]
- Turner, R.E.; Rabalais, N.N.; Justic, D. Predicting summer hypoxia in the northern Gulf of Mexico: Riverine N, P, and Si loading. Mar. Pollut. Bull. 2006, 52, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.E.; Rabalais, N.N.; Justić, D. Predicting summer hypoxia in the northern Gulf of Mexico: Redux. Mar. Pollut. Bull. 2012, 64, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.; Olesen, J.E.; Hansen, A.L.; Børgesen, C.D.; Dalgaard, T. Spatially differentiated strategies for reducing nitrate loads from agriculture in two Danish catchments. J. Environ. Manag. 2018, 208, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.K.; Bali, S.K. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2018, 10. [Google Scholar] [CrossRef]
- Sieczka, A.; Koda, E. Kinetic and Equilibrium Studies of Sorption of Ammonium in the Soil-Water Environment in Agricultural Areas of Central Poland. Appl. Sci. 2016, 6, 269. [Google Scholar] [CrossRef]
- David, M.B.; Gentry, L.E. Anthropogenic inputs of nitrogen and phosphorus and riverine export for Illinois, USA. J. Environ. Qual. 2000, 29, 494–508. [Google Scholar] [CrossRef]
- Delgado, J.A. Quantifying the loss mechanisms of nitrogen. J. Soil Water Conserv. 2002, 57, 389–398. [Google Scholar]
- Follett, R.F.; Delgado, J.A. Nitrogen fate and transport in agricultural systems. J. Soil Water Conserv. 2002, 57, 402–408. [Google Scholar]
- Kovacic, D.A.; David, M.B.; Gentry, L.E.; Starks, K.M.; Cooke, R.A. Effectiveness of constructed wetlands in reducing nitrogen and phosphorus export from agricultural tile drainage. J. Environ. Qual. 2000, 29, 1262–1274. [Google Scholar] [CrossRef]
- Mehnert, E.; Hwang, H.-H.; Johnson, T.M.; Sanford, R.A.; Beaumont, W.C.; Holm, T.R. Denitrification in the shallow ground water of a tile-drained, agricultural watershed. J. Environ. Qual. 2007, 36, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.W.; Benning, C. Factors influencing nitrate within a low-gradient agricultural stream. Environ. Earth Sci. 2013, 68, 1233–1245. [Google Scholar] [CrossRef]
- Schilling, K.; Zhang, Y.-K. Baseflow contribution to nitrate-nitrogen export from a large, agricultural watershed, USA. J. Hydrol. 2004, 295, 305–316. [Google Scholar] [CrossRef]
- Bardini, L.; Boano, F.; Cardenas, M.B.; Revelli, R.; Ridolfi, L. Nutrient cycling in bedform induced hyporheic zones. Geochim. Cosmochim. Acta 2012, 84, 47–61. [Google Scholar] [CrossRef]
- Duff, J.H.; Triska, F.J. Denitrification in sediments from the hyporheic zone adjacent to a small forested stream. Can. J. Fish. Aquat. Sci. 1990, 47, 1140–1147. [Google Scholar] [CrossRef]
- Triska, F.J.; Kennedy, V.C.; Avanzino, R.J.; Zellweger, G.W.; Bencala, K.E. Retention and transport of nutrients in a third-order stream in northwestern California: Hyporheic processes. Ecology 1989, 70, 1893–1905. [Google Scholar] [CrossRef]
- Zarnetske, J.P.; Haggerty, R.; Wondzell, S.M.; Bokil, V.A.; Gonzalez-Pinzon, R. Coupled transport and reaction kinetics control the nitrate source-sink function of hyporheic zones. Water Resour. Res. 2012, 48, 15. [Google Scholar] [CrossRef]
- Storey, R.G.; Williams, D.D.; Fulthorpe, R.R. Nitrogen processing in the hyporheic zone of a pastoral stream. Biogeochemistry 2004, 69, 285–313. [Google Scholar] [CrossRef]
- Hinkle, S.R.; Duff, J.H.; Triska, F.J.; Laenen, A.; Gates, E.B.; Bencala, K.E.; Wentz, D.A.; Silva, S.R. Linking hyporheic flow and nitrogen cycling near the Willamette River—A large river in Oregon, USA. J. Hydrol. 2001, 244, 157–180. [Google Scholar] [CrossRef]
- Hill, A.R.; Labadia, C.F.; Sanmugadas, K. Hyporheic zone hydrology and nitrogen dynamics in relation to the streambed topography of a N-rich stream. Biogeochemistry 1998, 42, 285–310. [Google Scholar] [CrossRef]
- Findlay, S.; Strayer, D.; Goumbala, C.; Gould, K. Metabolism of streamwater dissolved organic carbon in the shallow hyporheic zone. Limnol. Oceanogr. 1993, 38, 1493–1499. [Google Scholar] [CrossRef]
- Fischer, H.; Kloep, F.; Wilzcek, S.; Pusch, M.T. A river’s liver—Microbial processes within the hyporheic zone of a large lowland river. Biogeochemistry 2005, 76, 349–371. [Google Scholar] [CrossRef]
- Opdyke, M.R.; David, M.B.; Rhoads, B.L. Influence of geomorphological variability in channel characteristics on sediment denitrification in agricultural streams. J. Environ. Qual. 2006, 35, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.J.; Wollheim, W.M.; Mulholland, P.J.; Webster, J.R.; Meyer, J.L.; Tank, J.L.; Martí, E.; Bowden, W.B.; Valett, H.M.; Hershey, A.E.; et al. Control of nitrogen export from watersheds by headwater streams. Science 2001, 292, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Pind, A.; Risgaard-Petersen, N.; Revsbech, N.P. Denitrification and microphytobenthic NO3− consumption in a Danish lowland stream: Diurnal and seasonal variation. Aquat. Microb. Ecol. 1997, 12, 275–284. [Google Scholar] [CrossRef]
- Brunke, M.; Gonser, T. The ecological significance of exchange processes between rivers and groundwater. Freshw. Biol. 1997, 37, 1–33. [Google Scholar] [CrossRef]
- Gu, C.; Hornberger, G.M.; Mills, A.L.; Herman, J.S.; Flewelling, S.A. Nitrate reduction in streambed sediments: Effects of flow and biogeochemical kinetics. Water Resour. Res. 2007, 43. [Google Scholar] [CrossRef]
- Kemp, M.J.; Dodds, W.K. Comparisons of nitrification and denitrification in prairie and agriculturally influenced streams. Ecol. Soc. Am. 2002, 12, 998–1009. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Helton, A.M.; Poole, G.C.; Hall, R.O.; Hamilton, S.K.; Peterson, B.J.; Tank, J.L.; Ashkenas, L.R.; Cooper, L.W.; Dahm, C.N.; et al. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 2008, 452, 202–205. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.M.; Dodds, W.K.; Wilson, K.C.; Murdock, J.N.; Eichmiller, J. Saturation of N cycling in Central Plains streams: 15N experiments across a broad gradient of nitrate concentrations. Biogeochemistry 2007, 84, 31–49. [Google Scholar] [CrossRef]
- Zarnetske, J.P.; Haggerty, R.; Wondzell, S.M.; Baker, M.A. Dynamics of nitrate production and removal as a function of residence time in the hyporheic zone. J. Geophys. Res. Biogeosci. 2011, 116, G01025. [Google Scholar] [CrossRef]
- Royer, T.V.; Tank, J.L.; David, M.B. Transport and fate of nitrate in headwater agricultural streams in Illinois. J. Environ. Qual. 2004, 33, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoven, S.J.; Fromm, N.J.; Peterson, E.W. Quantifying nitrogen cycling beneath a meander of a low gradient, N-impacted, agricultural stream using tracers and numerical modelling. Hydrol. Proc. 2008, 22, 1206–1215. [Google Scholar] [CrossRef]
- Chavan, P.V.; Dennett, K.E.; Marchand, E.A.; Spurkland, L.E. Potential of constructed wetland in reducing total nitrogen loading into the Truckee River. Wetl. Ecol. Manag. 2008, 16, 189–197. [Google Scholar] [CrossRef]
- Wollheim, W.M.; Harms, T.K.; Peterson, B.J.; Morkeski, K.; Hopkinson, C.S.; Stewart, R.J.; Gooseff, M.N.; Briggs, M.A. Nitrate uptake dynamics of surface transient storage in stream channels and fluvial wetlands. Biogeochemistry 2014, 120, 239–257. [Google Scholar] [CrossRef]
- Alexander, R.B.; Smith, R.A.; Schwarz, G.E. Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature 2000, 403, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, T.; Hill, A.R. Lateral hyporheic zone chemistry in an artificially constructed gravel bar and a re-meandered stream channel, southern Ontario, Canada. J. Am. Water Resour. Assoc. 2007, 43, 1257–1269. [Google Scholar] [CrossRef]
- Peterson, E.W.; Sickbert, T.B.; Moore, S.L. High frequency stream bed mobility of a low-gradient agricultural stream with implications on the hyporheic zone. Hydrol. Process. 2008, 22, 4239–4248. [Google Scholar] [CrossRef]
- Goodale, C.L.; Aber, J.D.; Vitousek, P.M.; McDowell, W.H. Long-term decreases in stream nitrate: Successional causes unlikely; Possible links to DOC? Ecosystem 2005, 8, 334–337. [Google Scholar] [CrossRef]
- Illinois State Geological Survey. Physiographic Division of Illinois. Available online: http://isgs.illinois.edu/sites/isgs/files/maps/statewide/physio-w-color-8x11.pdf (accessed on 8 March 2018).
- Ludwikowski, J.; Malone, D.H.; Peterson, E.W. Surficial geologic map, Bloomington East Quadrangle, McLean County, Illinois. Available online: http://isgs.illinois.edu/maps/isgs-quads/surficial-geology/student-map/bloomington-east (accessed on 8 March 2018).
- Peterson, E.W.; Sickbert, T.B. Stream water bypass through a meander neck, laterally extending the hyporheic zone. Hydrogeol. J. 2006, 14, 1443–1451. [Google Scholar] [CrossRef]
- Bastola, H.; Peterson, E.W. Heat tracing to examine seasonal groundwater flow beneath a low-gradient stream. Hydrogeol. J. 2016, 24, 181–194. [Google Scholar] [CrossRef]
- Beach, V.; Peterson, E.W. Variation of hyporheic temperature profiles in a low gradient third-order agricultural stream—A statistical approach. Open J. Modern Hydrol. 2013, 3, 55–66. [Google Scholar] [CrossRef]
- Sickbert, T.B.; Peterson, E.W. The effect of surface water velocity on hyporheic interchange. J. Water Resour. Prot. 2014, 6, 327–336. [Google Scholar] [CrossRef]
- Bastola, H. Identifying Seasonal Changes in Streambed Thermal Profile in a Third Order Agricultural Stream using 2D Thermal Modeling. Master’s Thesis, Illinois State University, Normal, IL, USA, 2011. [Google Scholar]
- Buyck, M.S. Tracking Nitrate Loss and Modeling Flow through the Hyporheic Zone of a Low Gradient Stream through the Use of Conservative Tracers. Master’s Thesis, Illinois State University, Normal, IL, USA, 2005. [Google Scholar]
- Lax, S.; Peterson, E.W. Characterization of chloride transport in the unsaturated zone near salted road. Environ. Geol. 2009, 58, 1041–1049. [Google Scholar] [CrossRef]
- Lax, S.M.; Peterson, E.W.; Van der Hoven, S. Quantifying Stream chloride concentrations as a function of land-use. Environ. Earth Sci. 2017, 76, 12. [Google Scholar] [CrossRef]
- Ludwikowski, J.J.; Peterson, E.W. Transport and fate of chloride from road salt within a mixed urban and agricultural watershed in Illinois (USA): Assessing the influence of chloride application rates. Hydrogeol. J. 2018. [Google Scholar] [CrossRef]
- Barcelona, M.J.; Gibb, J.P.; Helfrich, J.A.; Garske, E.E. Practical Guide for Ground-Water Sampling; Illinois State Water Survey, Ed.; Illinois State Water Survey: Champaign, IL, USA, 1985; Volume ISWS CR-374, p. 94. [Google Scholar]
- Hautman, D.P.; Munch, D.J.J.E.O. Method 300.1: Determination of Inorganic Anions in Drinking Water by Ion Chromatography; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1997.
- Stelzer, R.S.; Bartsch, L.A.; Richardson, W.B.; Strauss, E.A. The dark side of the hyporheic zone: Depth profiles of nitrogen and its processing in stream sediments. Freshw. Biol. 2011, 56, 2021–2033. [Google Scholar] [CrossRef]
- Stelzer, R.S.; Thad Scott, J.; Bartsch, L.A.; Parr, T.B. Particulate organic matter quality influences nitrate retention and denitrification in stream sediments: Evidence from a carbon burial experiment. Biogeochemistry 2014, 119, 387–402. [Google Scholar] [CrossRef]
- Schulte, E.E.; Hopkins, B.G. Estimation of soil organic matter by weight loss-on-ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F.R., Tabatabia, M.A., Hanlon, E.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 21–31. [Google Scholar]
- Weather Underground. Weather History for KBMI. Available online: https://www.wunderground.com/history/airport/KBMI/ (accessed on 6 March 2017).
- Hill, A.R.; Lymburner, D.J. Hyporheic zone chemistry and stream-subsurface exchange in two groundwater-fed streams. Can. J. Fish. Aquat. Sci. 1998, 55, 495–506. [Google Scholar] [CrossRef]
- Ackerman, J.R.; Peterson, E.W.; Van der Hoven, S.; Perry, W. Quantifying nutrient removal from groundwater seepage out of constructed wetlands receiving treated wastewater effluent. Environ. Earth Sci. 2015, 74, 1633–1645. [Google Scholar] [CrossRef]
- Maxwell, E.L.; Peterson, E.W.; O’Reilly, C.M. Enhanced nitrate reduction within a constructed wetland system: Nitrate removal within groundwater flow. Wetlands 2017, 37, 413–422. [Google Scholar] [CrossRef]
- Jaynes, D.B.; Isenhart, T.M. Reconnecting tile drainage to riparian buffer hydrology for enhanced nitrate removal. J. Environ. Qual. 2014, 43, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Kuusemets, V.; Mander, Ü.; Lõhmus, K.; Ivask, M. Nitrogen and phosphorus variation in shallow groundwater and assimilation in plants in complex riparian buffer zones. Water Sci. Technol. 2001, 44, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [PubMed]
- Zou, K.H.; Tuncali, K.; Silverman, S.G. Correlation and simple linear regression. Radiology 2003, 227, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Boano, F.; Arnon, S. Impact of losing and gaining streamflow conditions on hyporheic exchange fluxes induced by dune-shaped bed forms. Water Resour. Res. 2014, 50, 1895–1907. [Google Scholar] [CrossRef]
- Inwood, S.E.; Tank, J.L.; Bernot, M.J. Factors controlling sediment denitrification in midwestern streams of varying land use. Microb. Ecol. 2007, 53, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Runkel, R.L. Toward a transport-based analysis of nutrient spiraling and uptake in streams. Limnol. Oceanogr. Methods 2007, 5, 50–62. [Google Scholar] [CrossRef]
- García-Ruiz, R.; Pattinson, S.N.; Whitton, B.A. Denitrification in river sediments: Relationship between process rate and properties of water and sediment. Freshw. Biol. 1998, 39, 467–476. [Google Scholar] [CrossRef]
- García-Ruiz, R.; Pattinson, S.N.; Whitton, B.A. Denitrification and nitrous oxide production in sediments of the Wiske, a lowland eutrophic river. Sci. Total Environ. 1998, 210–211, 307–320. [Google Scholar] [CrossRef]
- Stow, C.A.; Walker, J.T.; Cardoch, L.; Spence, P.; Geron, C. N2O emissions from streams in the Neuse River Watershed, North Carolina. Environ. Sci. Technol. 2005, 39, 6999–7004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yuan, X.; Peng, S.; Yue, J.; Wang, X.; Liu, H.; Williams, D.D. Groundwater-surface water interactions in the hyporheic zone under climate change scenarios. Environ. Sci. Pollut. Res. 2014, 21, 13943–13955. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.J.; Mayer, P.M.; Kaushal, S.S.; Pennino, M.J.; Arango, C.P.; Balz, D.A.; Canfield, T.J.; Elonen, C.M.; Fritz, K.M.; Hill, B.H.; et al. Effects of urban stream burial on organic matter dynamics and reach scale nitrate retention. Biogeochemistry 2014, 121, 107–126. [Google Scholar] [CrossRef]
- Groffman, P.M.; Gold, A.J.; Simmons, R.C. Nitrate dynamics in riparian forests: Microbial studies. J. Environ. Qual. 1992, 21, 666–671. [Google Scholar] [CrossRef]
- Simmons, R.C.; Gold, A.J.; Groffman, P.M. Nitrate dynamics in riparian forests: Groundwater studies. J. Environ. Qual. 1992, 21, 659–665. [Google Scholar] [CrossRef]
- Mayer, P.M.; Reynolds, S.K.; McCutchen, M.D.; Canfield, T.J. Meta-Analysis of nitrogen removal in riparian buffers. J. Environ. Qual. 2007, 36, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.; Belitz, K.; Burton, C.A. Algal productivity and nitrate assimilation in an effluent dominated concrete lined stream. J. Am. Water Resour. Assoc. 2005, 41, 1109–1128. [Google Scholar] [CrossRef]
- Miller, J.; Peterson, E.W. Diurnal and seasonal variation in nitrate-nitrogen concentrations of groundwater in a saturated buffer zone. Hydrogeol. J. 2018. submitted. [Google Scholar]
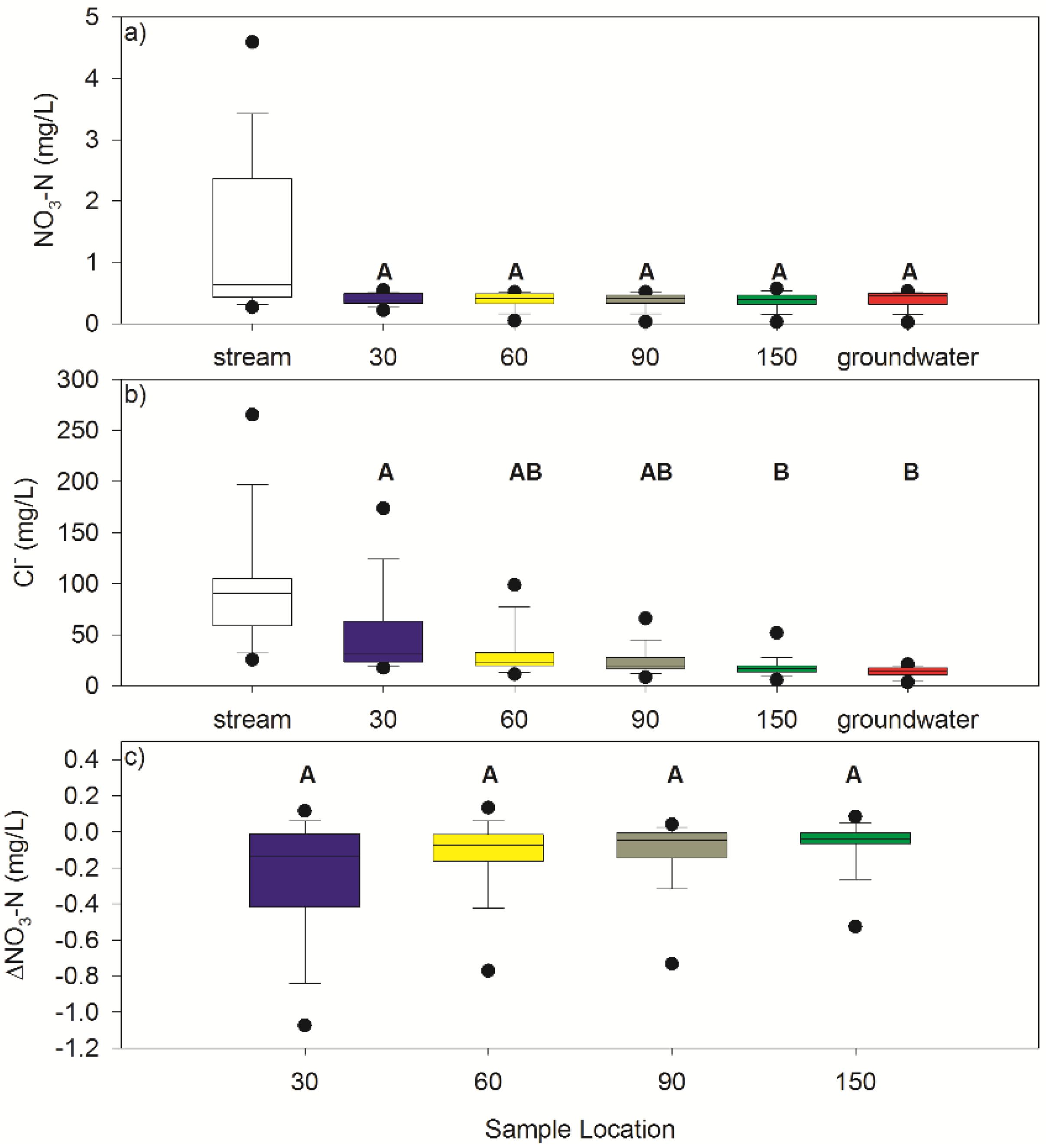

| Sample Location | n | NO3-N (mg/L) | Cl− (mg/L) | ΔNO3-N (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± σ | Max | Min | Mean ± σ | Max | Min | Mean ± σ | Max | Min | ||
| Stream | 17 | 1.32 ± 1.26 | 4.59 | 0.26 | 97.52 ± 58.80 | 265.29 | 25.36 | |||
| 30 cm | 17 | 0.40 ± 0.09 | 0.54 | 0.21 | 49.64 ± 40.48 | 173.82 | 17.60 | −0.25 ± 0.33 | 0.11 | −1.08 |
| 60 cm | 17 | 0.38 ± 0.13 | 0.51 | 0.04 | 32.83 ± 24.25 | 98.65 | 11.37 | −0.13 ± 0.20 | 0.13 | −0.77 |
| 90 cm | 17 | 0.37 ± 0.13 | 0.51 | 0.03 | 24.00 ± 13.36 | 66.00 | 8.41 | −0.10 ± 0.18 | 0.04 | −0.73 |
| 150 cm | 17 | 0.37 ± 0.14 | 0.57 | 0.02 | 17.89 ± 9.70 | 51.77 | 5.80 | −0.07 ± 0.14 | 0.08 | −0.53 |
| Groundwater | 34 | 0.38 ± 0.14 | 0.52 | 0.02 | 13.55 ± 4.93 | 21.06 | 3.49 | |||
| Sample Location | n | %SW | ||
|---|---|---|---|---|
| Mean ± σ | Max | Min | ||
| 30 cm | 17 | 40% ± 22% | 89% | 11% |
| 60 cm | 17 | 83% ± 20% | 83% | 3% |
| 90 cm | 17 | 15% ± 18% | 76% | 2% |
| 150 cm | 34 | 7% ± 12% | 54% | 0% |
| Factors | N | r | p-Value | Classification 1 |
|---|---|---|---|---|
| NO3-N stream: ΔNO3-N at 30 cm | 17 | −0.569 | 0.010 | moderately negative |
| NO3-N stream: ΔNO3-N at 60 cm | 17 | −0.409 | 0.017 | weakly negative |
| Hydraulic gradient: ΔNO3-N at 60 cm | 17 | 0.636 | <0.01 | moderately positive |
| Hydraulic gradient: ΔNO3-N at 90 cm | 17 | 0.584 | 0.01 | moderately positive |
| %SW: ΔNO3-N at 150 cm | 17 | −0.765 | <0.01 | moderately negative |
| OM: ΔNO3-N at 30 cm | 6 | −0.852 | 0.031 | strongly negative |
| %SW: ΔNO3-N at 90 cm | 17 | −0.723 | <0.01 | moderately negative |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, E.W.; Hayden, K.M. Transport and Fate of Nitrate in the Streambed of a Low-Gradient Stream. Hydrology 2018, 5, 55. https://doi.org/10.3390/hydrology5040055
Peterson EW, Hayden KM. Transport and Fate of Nitrate in the Streambed of a Low-Gradient Stream. Hydrology. 2018; 5(4):55. https://doi.org/10.3390/hydrology5040055
Chicago/Turabian StylePeterson, Eric W., and Kelly M. Hayden. 2018. "Transport and Fate of Nitrate in the Streambed of a Low-Gradient Stream" Hydrology 5, no. 4: 55. https://doi.org/10.3390/hydrology5040055
APA StylePeterson, E. W., & Hayden, K. M. (2018). Transport and Fate of Nitrate in the Streambed of a Low-Gradient Stream. Hydrology, 5(4), 55. https://doi.org/10.3390/hydrology5040055





