Hydrochemical Variability in Karst Hypothermal Mineral Springs of Greece
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area Description
2.1.1. General Overview
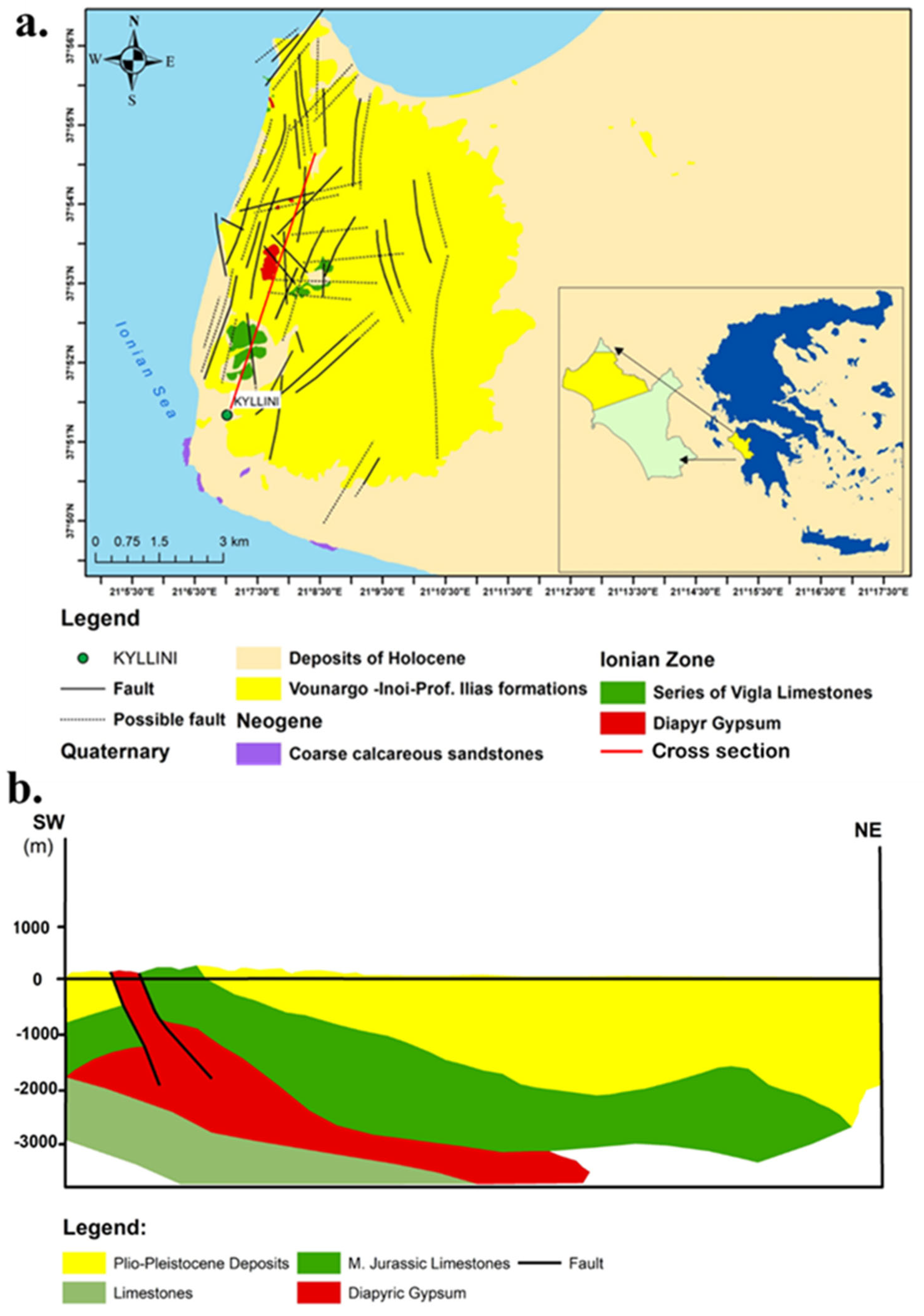
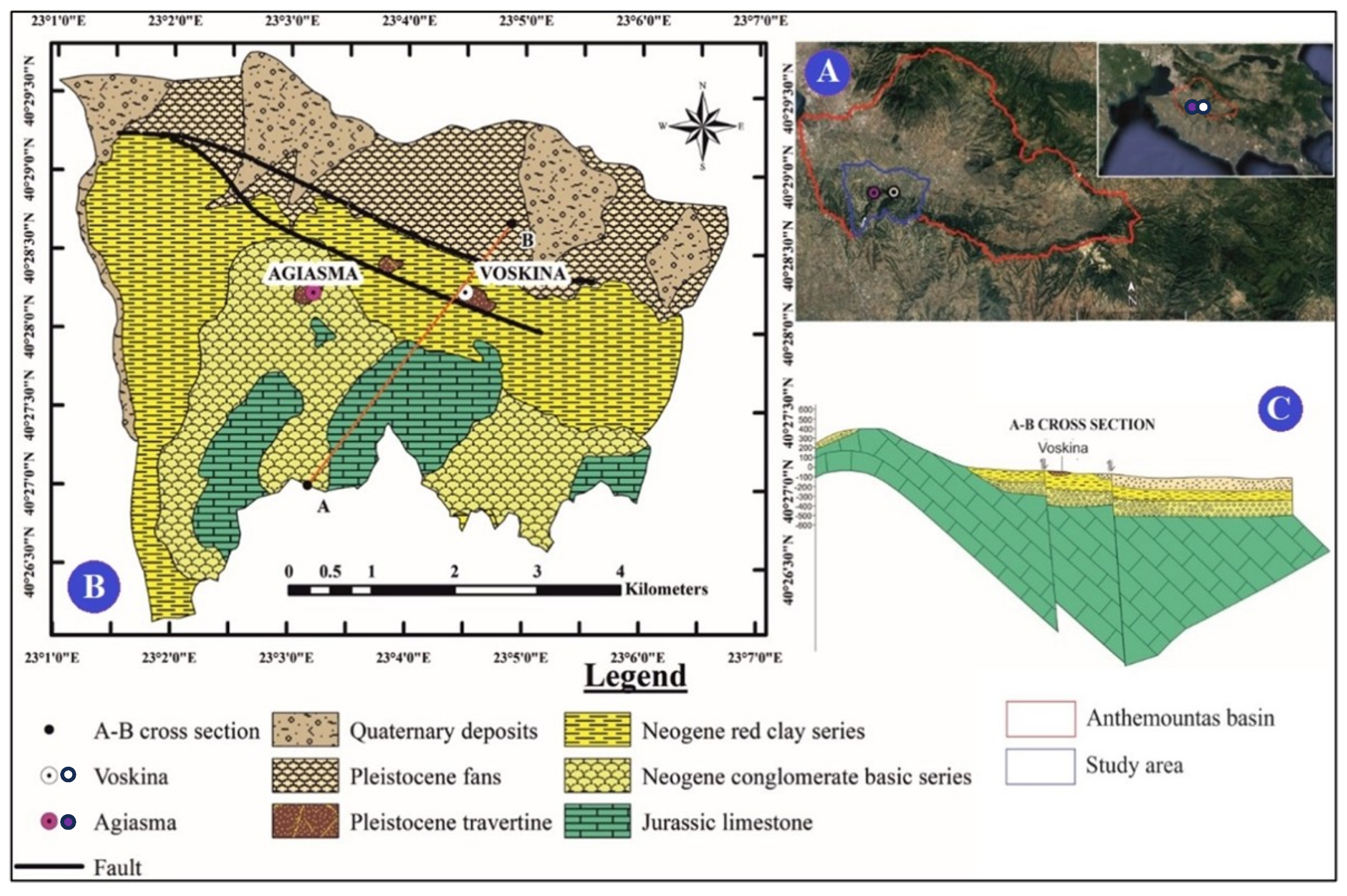
2.1.2. Geological Setting
2.2. Hydrochemical Parameters and Analysis Methods
3. Results and Discussion
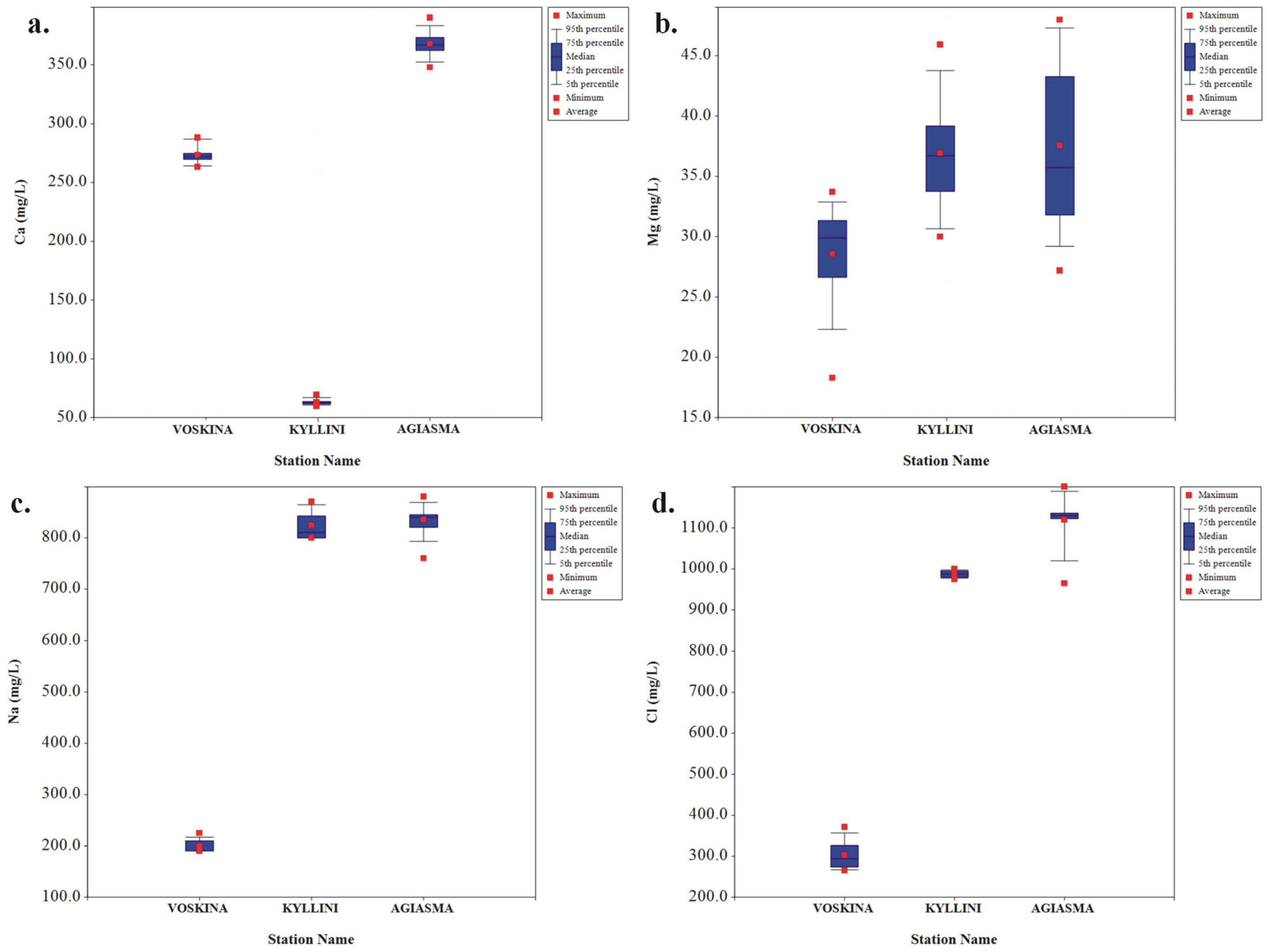
3.1. Hydrochemical Characteristics of Groundwater
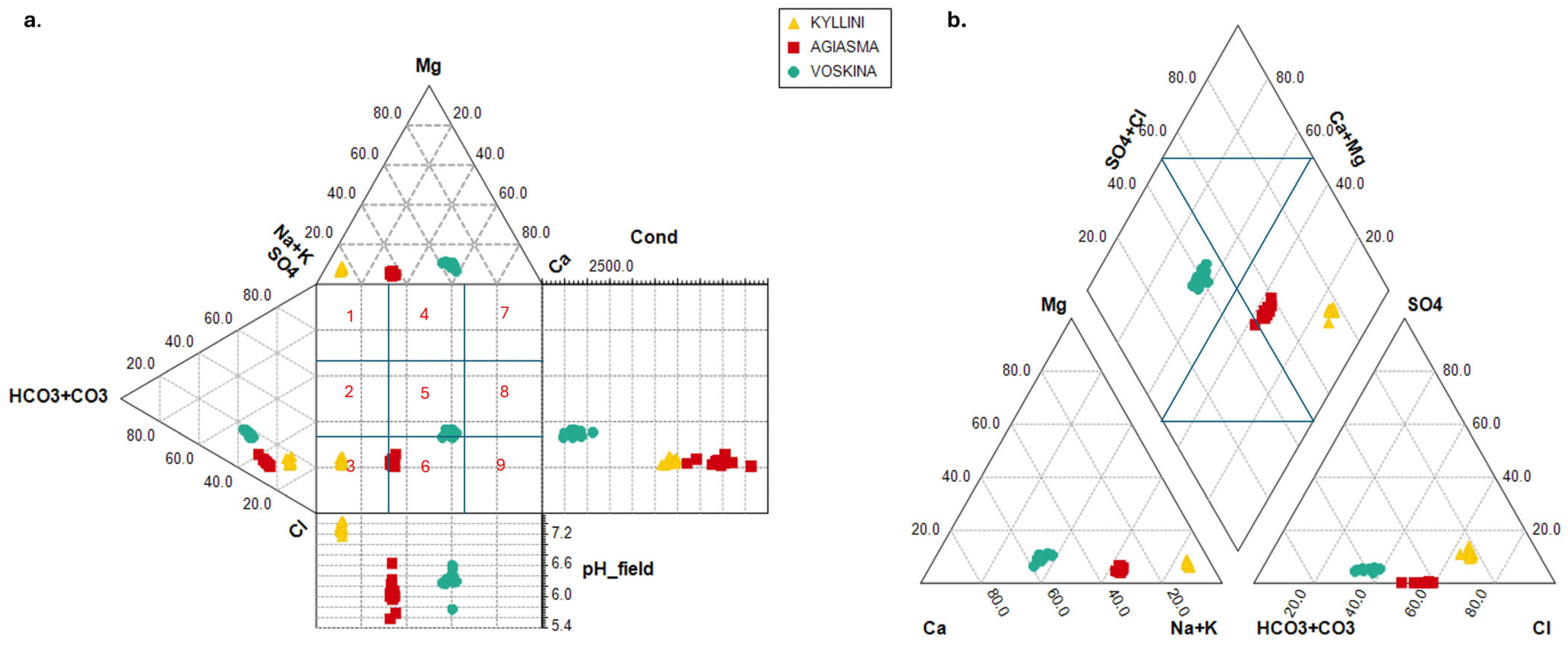
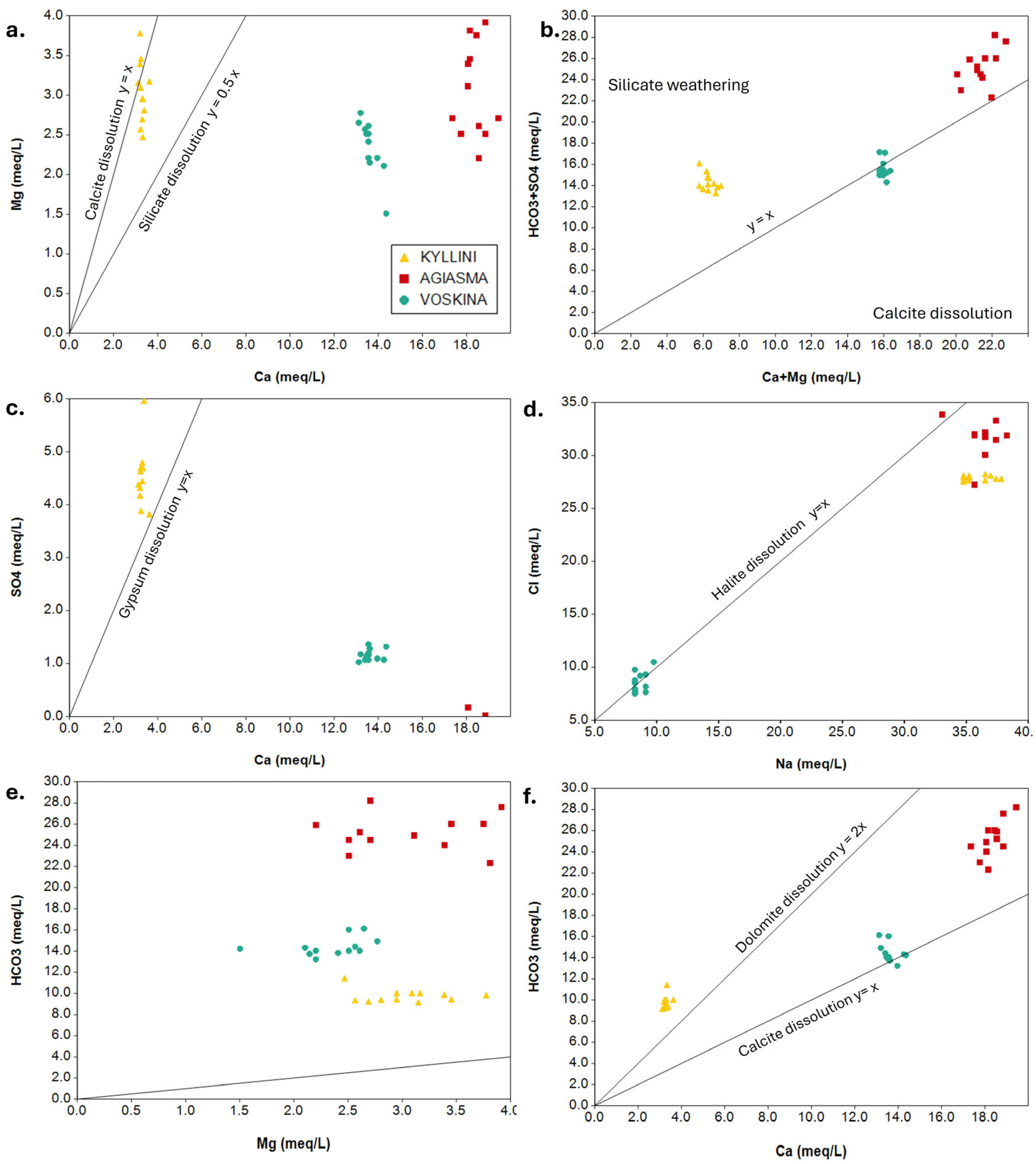
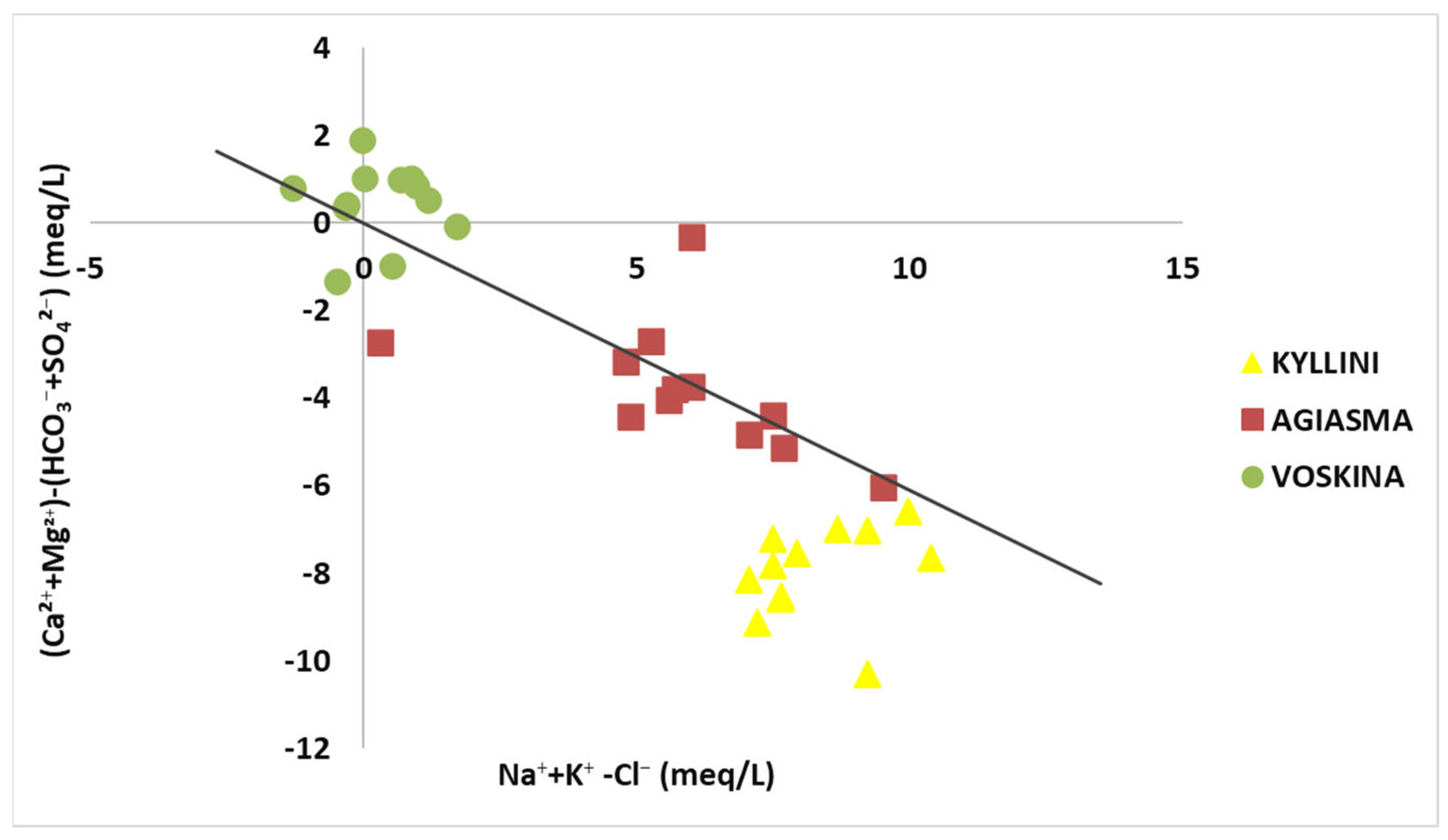
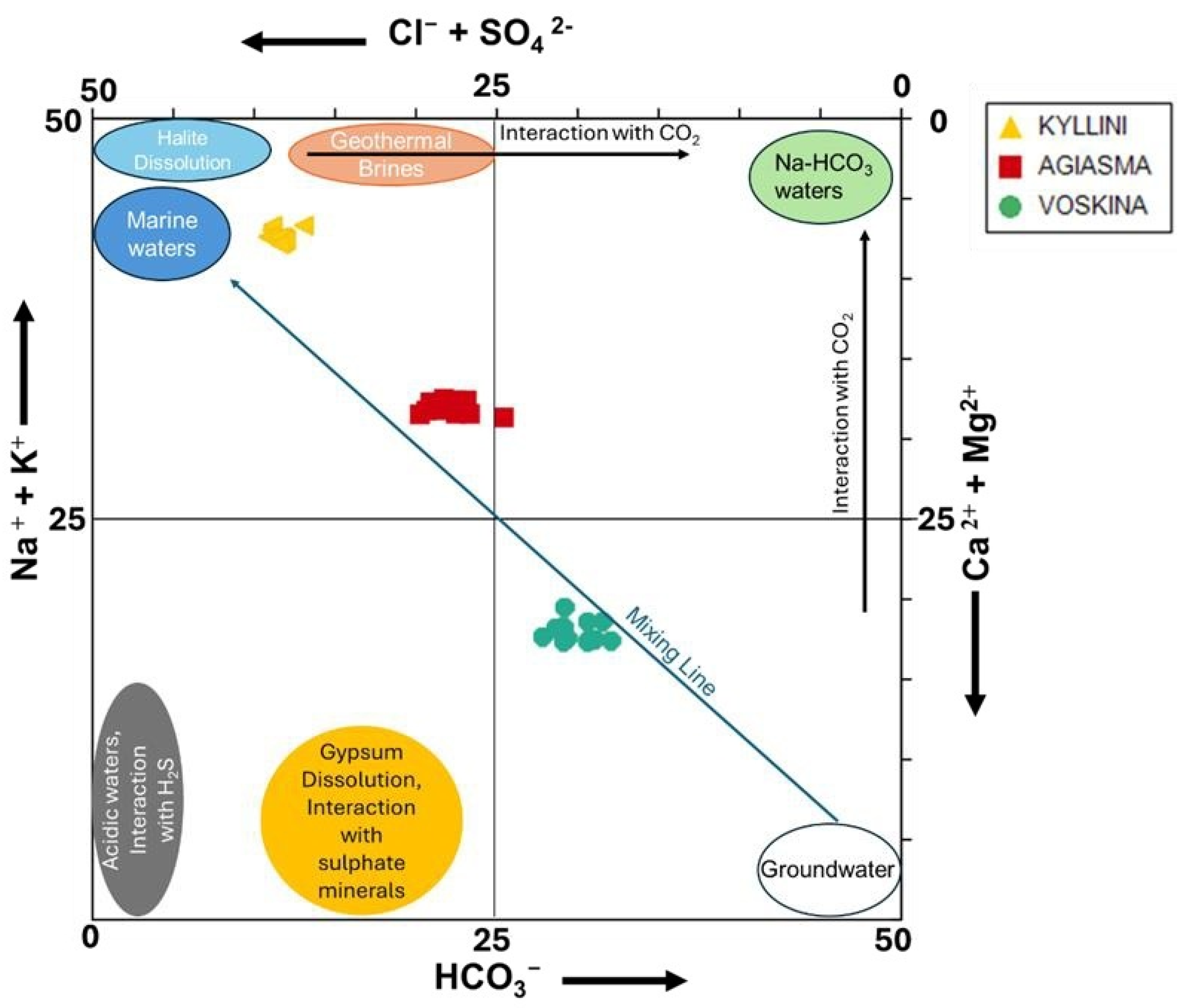
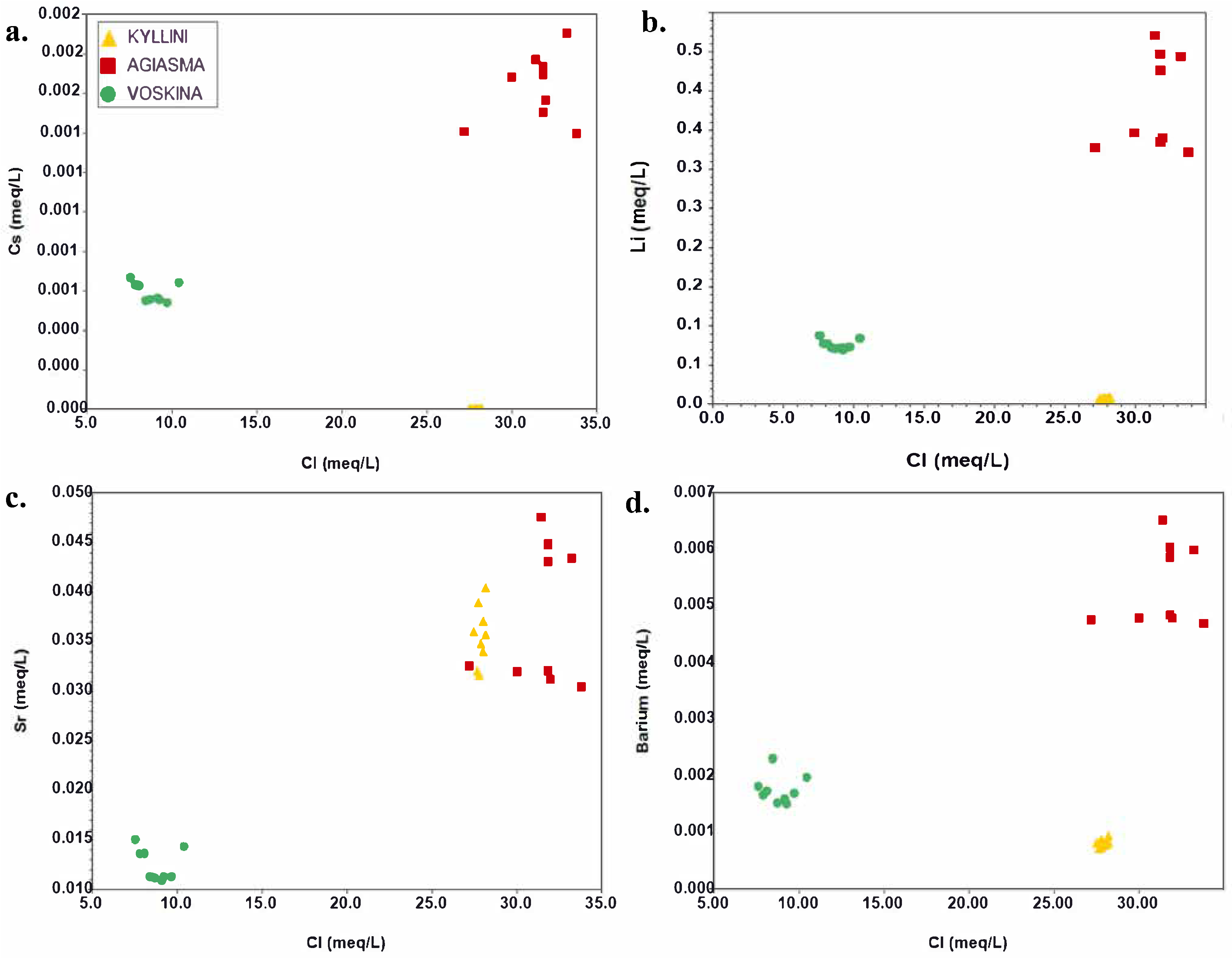
3.2. Ion Ratio Analysis
3.3. Saturation Indices
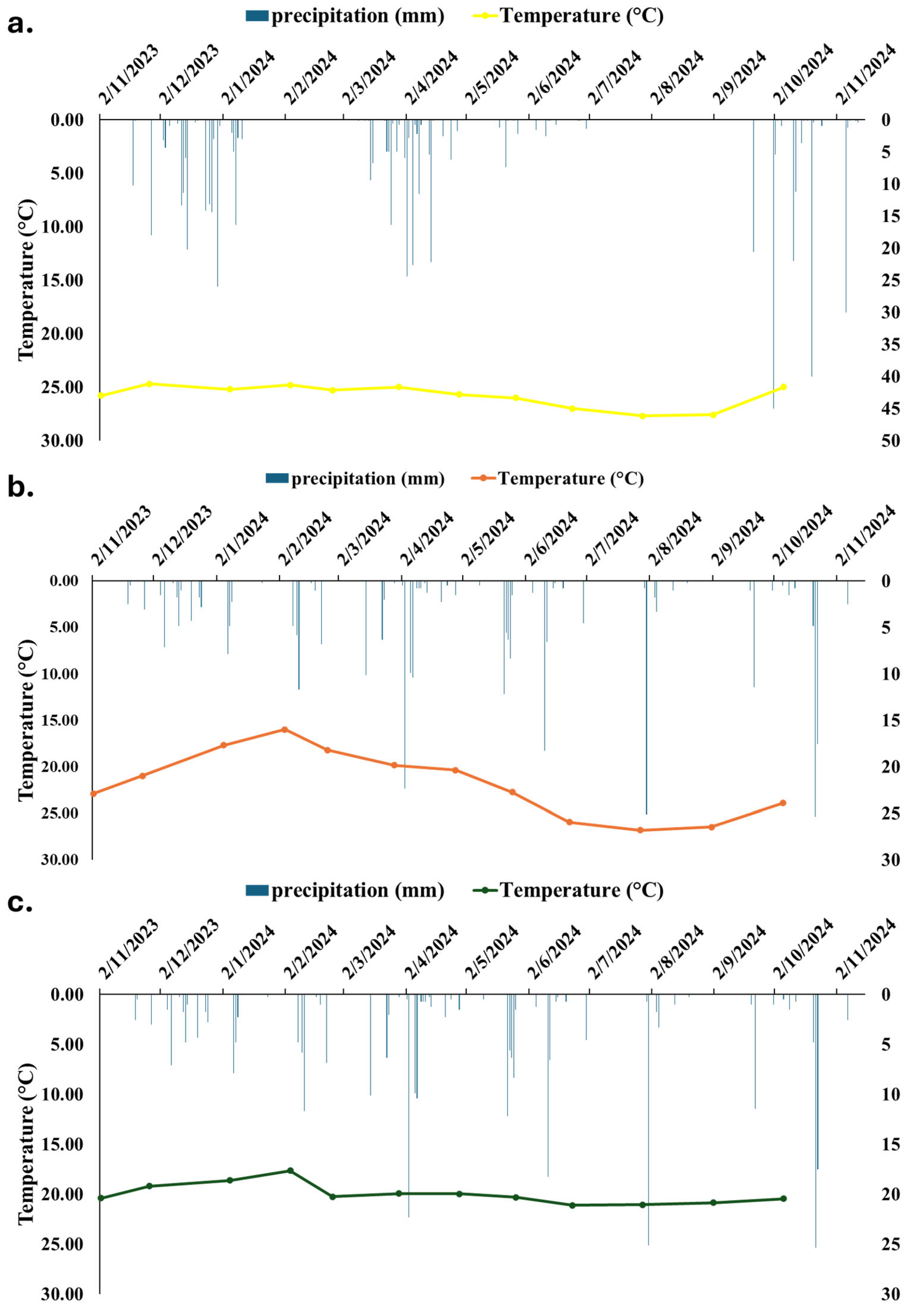
3.4. Time Series Data
3.5. Hydrochemical Relationship of the Studied Springs of Greece with Other Regions
3.6. Future Work and Challenges
4. Conclusions
- a.
- Kyllini: Characterized by high salinity, with Cl− and Na+ dominance, likely indicative of geothermal or hydrothermal activity.
- b.
- Agiasma: Exhibits a mixed water type with moderate salinity, representing an intermediate geochemical environment.
- c.
- Voskina: Marked by bicarbonate-rich groundwater, lower salinity, and a dominance of Ca2+/Mg2+, indicative of a more dilute system.
- Dominance of carbonate dissolution processes: All springs had elevated concentrations of Ca2+, Mg2+, and HCO3−, indicating that water–rock interactions are primarily governed by the dissolution of calcite and dolomite.
- Redox conditions and trace element mobilization: Negative ORP values in all sites suggest reducing environments, which favor the mobilization and transport of trace elements, such as Mn, Fe, and As.
- Consistent saturation indices for carbonate minerals: The waters of all three springs show near-equilibrium to over-saturation states with respect to carbonate phases (calcite, dolomite), indicating a periodically shared geochemical tendency towards carbonate precipitation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gleeson, T.; Befus, K.M.; Jasechko, S.; Luijendijk, E.; Cardenas, M.B. The Global Volume and Distribution of Modern Groundwater. Nat. Geosci. 2016, 9, 161–167. [Google Scholar] [CrossRef]
- Waring, G.A. Thermal Springs of the United States and Other Countries: A Summary; Geological Survey Professional Paper 492; U.S. Government Printing Office: Washington, DC, USA, 1965. [Google Scholar]
- Piscopo, V.; Barbieri, M.; Monetti, V.; Pagano, G.; Pistoni, S.; Ruggi, E.; Stanzione, D. Hydrogeology of Thermal Waters in Viterbo Area, Central Italy. Hydrogeol. J. 2006, 14, 1508–1521. [Google Scholar] [CrossRef]
- Alçiçek, H.; Bülbül, A.; Alçiçek, M.C. Hydrogeochemistry of the Thermal Waters from the Yenice Geothermal Field (Denizli Basin, Southwestern Anatolia, Turkey). J. Volcanol. Geotherm. Res. 2016, 309, 118–138. [Google Scholar] [CrossRef]
- Goldscheider, N.; Mádl-Szőnyi, J.; Erőss, A.; Schill, E. Review: Review: Thermal Water Resources in Carbonate Rock Aquifers. Hydrogeol. J. 2010, 18, 1303–1318. [Google Scholar] [CrossRef]
- Guo, Y.; Yeh, T.-C.J.; Hao, Y. Investigation of Karst Spring Flow Cessation Using Grey System Models. Water 2019, 11, 1927. [Google Scholar] [CrossRef]
- Nhu, V.-H.; Rahmati, O.; Falah, F.; Shojaei, S.; Al-Ansari, N.; Shahabi, H.; Shirzadi, A.; Górski, K.; Nguyen, H.; Ahmad, B.B. Mapping of Groundwater Spring Potential in Karst Aquifer System Using Novel Ensemble Bivariate and Multivariate Models. Water 2020, 12, 985. [Google Scholar] [CrossRef]
- Chang, Y.; Hartmann, A.; Liu, L.; Jiang, G.; Wu, J. Identifying More Realistic Model Structures by Electrical Conductivity Observations of the Karst Spring. Water Resour. Res. 2021, 57, e2020WR028587. [Google Scholar] [CrossRef]
- Kiyani, V.; Esmaili, A.; Alijani, F.; Samani, S.; Vasić, L. Investigation of Drainage Structures in the Karst Aquifer System through Turbidity Anomaly, Hydrological, Geochemical and Stable Isotope Analysis (Kiyan Springs, Western Iran). Environ. Earth Sci. 2022, 81, 517. [Google Scholar] [CrossRef]
- Iacurto, S.; Grelle, G.; De Filippi, F.M.; Sappa, G. Karst Spring Recharge Areas and Discharge Relationship by Oxygen-18 and Deuterium Isotopes Analyses: A Case Study in Southern Latium Region, Italy. Appl. Sci. 2020, 10, 1882. [Google Scholar] [CrossRef]
- De Filippi, F.M.; Iacurto, S.; Grelle, G.; Sappa, G. Magnesium as Environmental Tracer for Karst Spring Baseflow/Overflow Assessment—A Case Study of the Pertuso Karst Spring (Latium Region, Italy). Water 2021, 13, 93. [Google Scholar] [CrossRef]
- Olarinoye, T.; Gleeson, T.; Hartmann, A. Karst Spring Recession Curve Analysis: Efficient, Accurate Methods for Both Fast and Slow Flow Components. Hydrol. Earth Syst. Sci. Discuss. 2021, 26, 5431–5447. [Google Scholar] [CrossRef]
- Iván, V.; Stevenazzi, S.; Pollicino, L.C.; Masetti, M.; Mádl-Szőnyi, J. An Enhanced Approach to the Spatial and Statistical Analysis of Factors Influencing Spring Distribution on a Transboundary Karst Aquifer. Water 2020, 12, 2133. [Google Scholar] [CrossRef]
- Salvadori, M.; Pennisi, M.; Masciale, R.; Fidelibus, M.D.; Frollini, E.; Ghergo, S.; Parrone, D.; Preziosi, E. Passarella, GIsotopic Study for Evaluating Complex Groundwater Circulation Patterns, Hydrogeological Zoning, and Water-Rock Interaction in a Mediterranean Coastal Karst Environment. Sci. Total Environ. 2024, 955, 176850. [Google Scholar] [CrossRef]
- Gao, Z.; Hao, M.; Liu, J.; Li, Q.; Tan, M.; Niu, Y. A Comprehensive Study on the Hydrogeochemical and Isotope Characteristics and Genetic Mechanism of Geothermal Water in the Northern Jinan Region. Energies 2023, 16, 7658. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Ataie-Ashtiani, B. Conceptualization of Karstic Aquifer with Multiple Outlets Using a Dual Porosity Model. Groundwater 2017, 55, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Labat, D.; Hoang, C.T.; Masbou, J.; Mangin, A.; Tchiguirinskaia, I.; Lovejoy, S.; Schertzer, D. Multifractal Behaviour of Long-Term Karstic Discharge Fluctuations. Hydrol. Process. 2013, 27, 3708–3717. [Google Scholar] [CrossRef]
- Dursun, O.F.; Celiker, M.; Firat, M. Hydrological Properties of the Derme Karstic Springs by Using Hydrogeochemical Analyses and Environmental Isotope Techniques. CLEAN—Soil Air Water 2016, 44, 143–153. [Google Scholar] [CrossRef]
- Moldovan, O.T.; Baricz, A.; Szekeres, E.; Kenesz, M.; Hoaghia, M.A.; Levei, E.A.; Mirea, I.C.; Năstase-Bucur, R.; Brad, T.; Chiciudean, I.; et al. Testing Different Membrane Filters for 16S rRNA Gene-Based Metabarcoding in Karstic Springs. Water 2020, 12, 3400. [Google Scholar] [CrossRef]
- Nikolaidis, N.P.; Bouraoui, F.; Bidoglio, G. Hydrologic and Geochemical Modeling of a Karstic Mediterranean Watershed. J. Hydrol. 2013, 477, 129–138. [Google Scholar] [CrossRef]
- Voudouris, K.S. Status and Codification of Karst Aquifer Systems in Greece. Bull. Geol. Soc. Greece 2021, 57, 23–51. [Google Scholar] [CrossRef]
- Capraro, F.; Bizzotto, A.; Masiol, M.; Pavoni, B. Chemical Analyses of Spring Waters and Factor Analysis to Monitor the Functioning of a Karstic System. The Role of Precipitations Regimen and Anthropic Pressures. J. Environ. Monit. 2011, 13, 2543. [Google Scholar] [CrossRef]
- Graça, I.; Lopes, J.M.; Cerqueira, H.S.; Ribeiro, M.F. Bio-Oils Upgrading for Second Generation Biofuels. Ind. Eng. Chem. Res. 2013, 52, 275–287. [Google Scholar] [CrossRef]
- Kazakis, N. Groundwater Pollution Risk Assessment in Anthemountas Basin. Ph.D. Thesis, Department of Geology, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2013. [Google Scholar]
- Zega, M.; Rožič, B.; Gaberšek, M.; Kanduč, T.; Rožič, P.Ž.; Verbovšek, T. Mineralogical, Hydrogeochemical and Isotopic Characteristics of the Žveplenica Sulphide Karstic Spring (Trebuša Valley, NW Slovenia). Environ. Earth Sci. 2015, 74, 3287–3300. [Google Scholar] [CrossRef]
- Calo, G.C.; Tinelli, R. Hydrogeological Study of a Hypothermal Spring (S. Cesarea Terme, Apulia), Italy. J. Hydrol. 1995, 165, 185–205. [Google Scholar] [CrossRef]
- Corniello, A.; Guida, M.; Stellato, L.; Trifuoggi, M.; Carraturo, F.; Del Gaudio, E.; Del Giudice, C.; Forte, G.; Giarra, A.; Iorio, M.; et al. Hydrochemical, Isotopic and Microbiota Characterization of Telese Mineral Waters (Southern Italy). Environ. Geochem. Health 2022, 44, 1949–1970. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, Y.; Huang, J.; Yang, S.; Wang, Y.; Wang, Y.; Zhang, J. Hydrochemical Characterisation and Genesis Mechanism of Li-Rich Geothermal Waters in the High-Temperature Geothermal Areas of Western Sichuan, China. Geol. J. 2024, 60, 2033–2048. [Google Scholar] [CrossRef]
- Gori, F.; Barberio, M.D.; Barbieri, M.; Boschetti, T.; Cardello, G.L.; Petitta, M. Groundwater–Rock Interactions and Mixing in Fault–Controlled Karstic Aquifers: A Structural, Hydrogeochemical and Multi-Isotopic Review of the Pontina Plain (Central Italy). Sci. Total Environ. 2024, 951, 175439. [Google Scholar] [CrossRef]
- Malik, N.A.; Taran, Y.A.; Svirid, I.Y.; Tskhovrebova, A.R. Nizhne-Shchapinsky Thermal Springs (Kamchatka) as an Example of Magnesium Carbon Dioxide Waters. Russ. J. Pac. Geol. 2024, 18, S28–S43. [Google Scholar] [CrossRef]
- Arifin; Taylor, R.G.; Shamsudduha, M.; Ramdhan, A.M.; Iskandar, I.; Setiawan, T.; Iman, M.I.; Noor, R.A. Hydrochemistry of a Coastal Sedimentary Basin: Evidence from the Lower Kutai Basin, Indonesia. Appl. Geochem. 2025, 190, 106496. [Google Scholar] [CrossRef]
- Kobare, N.D.; Kashiwaya, K.; Koike, K.; Mahecha, A. Circulation Process of Geothermal Fluids and Potential Assessment of Geothermal Resources in the Songwe Half-Graben and Kiejo-Mbaka Prospects in Southwestern Tanzania: Insight from Hydrochemistry and Stable Isotopes. Geothermics 2025, 130, 103347. [Google Scholar] [CrossRef]
- Stober, I.; Grimmer, J.C.; Kraml, M. Origin and Development of the Geothermal Fluids of the Baden-Baden Area (SW-Germany): Implications for Geothermal Systems of Granitic Reservoirs. Swiss J. Geosci. 2025, 118, 8. [Google Scholar] [CrossRef]
- Pasternáková, B.; Kuchovský, T.; Chroustová, K.; Říčka, A.; Nehyba, S.; Rüde, T.R. The Hydrochemistry and Geothermometry of Thermal Waters from a Deep Jurassic Aquifer in Lower Austria–South Moravia Region. Geothermics 2025, 125, 103173. [Google Scholar] [CrossRef]
- Reinoso Carbonell, V.V.; Campodonico, V.A.; Alasino, P.H. Origin of Thermal Waters in the Fiambalá Basin (Argentina): Preliminary Insights from Hydrochemistry and Isotopic Tracers. Geothermics 2025, 132, 103434. [Google Scholar] [CrossRef]
- Ueda, A.; Yang, H.; Hoshino, Y.; Satake, S.; Mao, D.; Terai, A. Isotope Geochemical Study of the Origin and Formation Mechanism of Carbonate Minerals in Geothermal Wells and Surrounding Hot Spring Waters in the Western Unzen Area. Appl. Geochem. 2025, 185, 106384. [Google Scholar] [CrossRef]
- Benmarce, K.; Hadji, R.; Hamed, Y.; Zahri, F.; Zighmi, K.; Hamad, A.; Gentilucci, M.; Ncibi, K.; Besser, H. Hydrogeological and Water Quality Analysis of Thermal Springs in the Guelma Region of North-Eastern Algeria: A Study Using Hydrochemical, Statistical, and Isotopic Approaches. J. Afr. Earth Sci. 2023, 205, 105011. [Google Scholar] [CrossRef]
- Lunardi, M.; Bonotto, D.M. Hydrochemistry of Hot Springs from Caldas Novas Thermal Complex, Brazil. Geoenergy Sci. Eng. 2025, 245, 213502. [Google Scholar] [CrossRef]
- Lambrakis, N.; Katsanou, K.; Siavalas, G. Geothermal Fields and Thermal Waters of Greece: An Overview. Geotherm. Syst. Energy Resour. 2014, 63–84. [Google Scholar]
- Voudouris, K.; Yapijakis, C.; Georgaki, Μ.-Ν.; Angelakis, A.N. Historical Issues of Hydrotherapy in Thermal–Mineral Springs of the Hellenic World. Sustain. Water Resour. Manag. 2023, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Castany, G. Traité Pratique Des Eaux Souterraines. Dumond Paris Fr. 1963, 657. [Google Scholar]
- Stavropoulou, V.; Pyrgaki, A.; Zagana, E.; Pouliaris, C.; Kazakis, N. The Contributions of Tectonics, Hydrochemistry and Stable Isotopes to the Water Resource Management of a Thermal–Mineral Aquifer: The Case Study of Kyllini, Northwest Peloponnese. Geosciences 2024, 14, 205. [Google Scholar] [CrossRef]
- Fountoulis, I.; Mariolakos, I. Neotectonic Folds in the Central-Western Peloponnese, Greece. Z. Der Dtsch. Ges. Fur Geowiss. 2008, 159, 485–494. [Google Scholar] [CrossRef]
- Skourlis, K.; Doutsos, T. The Pindos Fold-and-Thrust Belt (Greece): Inversion Kinematics of a Passive Continental Margin. Int. J. Earth Sci. 2003, 92, 891–903. [Google Scholar] [CrossRef]
- Davis, G.H. Stylolitic Limestone, the Stone of Choice for Ancient Sanctuaries and Temples, Southwestern Peloponnese, Greece. Geoarchaeology 2018, 33, 708–722. [Google Scholar] [CrossRef]
- Piper, D.J.W. Sedimentology and Tectonic Setting of the Pindos Flysch of the Peloponnese, Greece. Geol. Soc. Lond. Spec. Publ. 2006, 260, 493–505. [Google Scholar] [CrossRef]
- Roumelioti, Z.; Theodoulidis, N.; Bouchon, M. Constraints on the Location of the 2008, MW 6.4 Achaia-Ilia Earthquake Fault from Strong Motion Data. Geosociety 2016, 47, 1231. [Google Scholar] [CrossRef]
- Mavroulis, S.; Fountoulis, I.; Lekkas, E. Environmental Effects Caused by the Andravida (08-06-2008, ML = 6.5, NW Peloponnese, Greece) Earthquake. In Proceedings of the Geologically Active: 11th IAEG Congress, Auckland, New Zealand, 5–10 September 2010; Taylor & Francis Group: Milton Park, UK, 2010; pp. 451–459. [Google Scholar]
- Karkani, A.; Evelpidou, N.; Tzouxanioti, M.; Petropoulos, A.; Gogou, M.; Mloukie, E. Tsunamis in the Greek Region: An Overview of Geological and Geomorphological Evidence. Geosciences 2021, 12, 4. [Google Scholar] [CrossRef]
- Obrocki, L.; Vött, A.; Wilken, D.; Fischer, P.; Willershäuser, T.; Koster, B.; Lang, F.; Papanikolaou, I.; Rabbel, W.; Reicherter, K. Tracing Tsunami Signatures of the Ad 551 and Ad 1303 Tsunamis at the Gulf of Kyparissia (Peloponnese, Greece) Using Direct Push in Situ Sensing Techniques Combined with Geophysical Studies. Sedimentology 2020, 67, 1274–1308. [Google Scholar] [CrossRef]
- Syrides, G. Neogene Mollusk Faunas fromStrymon Basin, Macedonia, Greece. First Results for Biochronology and Palaeoenvironment. Geobios 1995, 28, 381–388. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.a.J. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Reston, VA, USA, 1999. [Google Scholar]
- Piper, A.M. Graphic Procedure in the Geochemical Interpretation of Water Analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Durov, S.A. Natural Waters and Graphic Representation of Their Composition. Dokl. Akad. 1948, 59, 87–90. [Google Scholar]
- Official Recognition of the Thermal Spring “Loutra Kyllinis” as a Natural Therapeutic Resource. 2982/B. Hellenic Republic Government Gazette, 11 April 2014.
- Wynn, J.G.; Sumrall, J.B.; Onac, B.P. Sulfur Isotopic Composition and the Source of Dissolved Sulfur Species in Thermo-Mineral Springs of the Cerna Valley, Romania. Chem. Geol. 2010, 271, 31–43. [Google Scholar] [CrossRef]
- Stavropoulou, V.; Zagana, E.; Pouliaris, C.; Kazakis, N. Assessing the Interaction Between Geologically Sourced Hydrocarbons and Thermal–Mineral Groundwater: An Overview of Methodologies. Water 2025, 17, 1940. [Google Scholar] [CrossRef]
- Ding, L.; Wang, F.; Yuan, J.; Liu, H.; Cheng, Z.; Cao, Y. Spatial Variability of Hydrochemistry in Coal-Bearing Karst Areas Considering Sulfur Pollution and Underground Engineering Effects. Environ. Pollut. 2025, 371, 125957. [Google Scholar] [CrossRef]
- Luo, H.; Yuan, X.; Zhao, X.; Wang, Y.; Wu, H.; Zhou, P.; Zhang, H.; Liu, G.; Zhang, Y. A Conceptual Model and Changing Trends for the Yangbajing Geothermal Field in the Tibetan Plateau: New Insights from Hydrochemistry and Multi-Isotopes. Geothermics 2025, 131, 103391. [Google Scholar] [CrossRef]
- Kazakis, N.; Matiatos, I.; Ntona, M.-M.; Bannenberg, M.; Kalaitzidou, K.; Kaprara, E.; Mitrakas, M.; Ioannidou, A.; Vargemezis, G.; Voudouris, K. Origin, Implications and Management Strategies for Nitrate Pollution in Surface and Ground Waters of Anthemountas Basin Based on a δ15N-NO3− and δ18O-NO3− Isotope Approach. Sci. Total Environ. 2020, 724, 138211. [Google Scholar] [CrossRef]
- Al-Bassam, A.M.; Khalil, A.R. DurovPwin: A New Version to Plot the Expanded Durov Diagram for Hydro-Chemical Data Analysis. Comput. Geosci. 2012, 42, 1–6. [Google Scholar] [CrossRef]
- Arnórsson, S.; Gunnlaugsson, E.; Svavarsson, H. The Chemistry of Geothermal Waters in Iceland. III. Chemical Geothermometry in Geothermal Investigations. Geochim. Cosmochim. Acta 1983, 47, 567–577. [Google Scholar] [CrossRef]
- Liu, B.; Xu, C.; Sun, J.; Yuan, H. Analysis of the Migration of Carbon Dioxide in Deep Saline Fractured Aquifer. Int. J. Energy 2023, 2, 49–52. [Google Scholar] [CrossRef]
- Yingkai, X.; Dapeng, S.; Yunhui, W.; Hairing, Q.; Lin, J. Boron Isotopic Compositions of Brine, Sediments, and Source Water in Da Qaidam Lake, Qinghai, China. Geochim. Cosmochim. Acta 1992, 56, 1561–1568. [Google Scholar] [CrossRef]
- Kong, F.; Yang, Y.; Luo, X.; Sha, Z.; Wang, J.; Ma, Y.; Ling, Z.; He, B.; Liu, W. Deep Hydrothermal and Shallow Groundwater Borne Lithium and Boron Loadings to a Mega Brine Lake in Qinghai Tibet Plateau Based on Multi-Tracer Models. J. Hydrol. 2021, 598, 126313. [Google Scholar] [CrossRef]
- Dreybrodt, W. Kinetics of the Dissolution of Calcite and Its Applications to Karstification. Chem. Geol. 1980, 31, 245–269. [Google Scholar] [CrossRef]
- Schäffer, R.; Bär, K.; Fischer, S.; Fritsche, J.-G.; Sass, I. Mineral, Thermal and Deep Groundwater of Hesse, Germany. Earth Syst. Sci. Data 2021, 13, 4847–4860. [Google Scholar] [CrossRef]
- Kazakis, N.; Mattas, C.; Pavlou, A.; Patrikaki, O.; Voudouris, K. Multivariate Statistical Analysis for the Assessment of Groundwater Quality under Different Hydrogeological Regimes. Environ. Earth Sci. 2017, 76, 349. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Jiang, H.; Ma, Z.; Fang, X. Constraining Hydrocarbon Migration and Accumulation by Two-Dimensional Numerical Simulation: Ordovician Carbonate Reservoirs of the Daniudi Area, Ordos Basin. Energy Explor. Exploit. 2023, 41, 309–325. [Google Scholar] [CrossRef]
- Manaka, T.; Araoka, D.; Yoshimura, T.; Hossain, H.M.Z.; Nishio, Y.; Suzuki, A.; Kawahata, H. Downstream and Seasonal Changes of Lithium Isotope Ratios in the Ganges-Brahmaputra River System. Geochem. Geophys. Geosyst. 2017, 18, 3003–3015. [Google Scholar] [CrossRef]
- Trefry, J.H.; Metz, S. Role of Hydrothermal Precipitates in the Geochemical Cycling of Vanadium. Nature 1989, 342, 531–533. [Google Scholar] [CrossRef]
- Seyedali, M.; Coogan, L.A.; Gillis, K.M. The Effect of Solution Chemistry on Elemental and Isotopic Fractionation of Lithium during Inorganic Precipitation of Calcite. Geochim. Cosmochim. Acta 2021, 311, 102–118. [Google Scholar] [CrossRef]
- Zhou, J.; Li, B.; He, M.; Jiao, J.; Tang, Z.; Li, Z. Hydrochemical Characteristics and Sources of Lithium in Carbonate-Type Salt Lake in Tibet. Sustainability 2023, 15, 16235. [Google Scholar] [CrossRef]
- Tian, H.; Ma, Z.; Chen, X.; Zhang, H.; Bao, Z.; Wei, C.; Xie, S.; Wu, S. Geochemical Characteristics of Selenium and Its Correlation to Other Elements and Minerals in Selenium-Enriched Rocks in Ziyang County, Shaanxi Province, China. J. Earth Sci. 2016, 27, 763–776. [Google Scholar] [CrossRef]
- Loftus-Hills, G.; Solomon, M. Cobalt, Nickel and Selenium in Sulphides as Indicators of Ore Genesis. Miner. Depos. 1967, 2, 228–242. [Google Scholar] [CrossRef]
- Hoque, M.A.; Amponsah, K.B.; Blum, A.; Walton, N.; Dennis, P.; Butler, A.P.; Hugman, S.; Bamberger, A.; Fowler, M. The Origin and Water Quality of Spring Systems in Monchique, Portugal: A Focus on Long-Term Sustainability and Elevated Sodium Levels. J. Hydrol. 2024, 637, 131363. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Chemical Aspects of Uranium Behavior in Soils: A Review. Eurasian Soil Sci. 2011, 44, 862–873. [Google Scholar] [CrossRef]
- O’Day, P.; Vlassopoulos, D.; Root, R.; Rivera, N. The Influence of Sulfur and Iron on Dissolved Arsenic Concentrations in the Shallow Subsurface under Changing Redox Conditions. Proc. Natl. Acad. Sci. USA 2004, 101, 13703–13708. [Google Scholar] [CrossRef]
- White, A.F.; Brantley, S.L. The Effect of Time on the Weathering of Silicate Minerals: Why Do Weathering Rates Differ in the Laboratory and Field? Chem. Geol. 2003, 202, 479–506. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- McArthur, J.M.; Ravenscroft, P.; Safiulla, S.; Thirlwall, M.F. Arsenic in Groundwater: Testing Pollution Mechanisms for Sedimentary Aquifers in Bangladesh. Water Resour. Res. 2001, 37, 109–117. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater Chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Templ, M.; Gozzi, C.; Buccianti, A. A New Version of the Langelier-Ludwig Square Diagram under a Compositional Perspective. J. Geochem. Explor. 2022, 242, 107084. [Google Scholar] [CrossRef]
- Madonia, P.; Campilongo, G.; Cangemi, M.; Carapezza, M.L.; Inguaggiato, S.; Ranaldi, M.; Vita, F. Hydrogeological and Geochemical Characteristics of the Coastal Aquifer of Stromboli Volcanic Island (Italy). Water 2021, 13, 417. [Google Scholar] [CrossRef]
- Nyirenda, T.M.; Zhou, J.; Mapoma, H.W.T.; Xie, L.; Li, Y. Hydrogeochemical Characteristics of Groundwater at the Xikuangshan Antimony Mine in South China. Mine Water Environ. 2016, 35, 86–93. [Google Scholar] [CrossRef]
- Vahidipour, M.; Raeisi, E.; Van Der Zee, S.E.A.T.M. Temporal Dynamics of Inundation Area, Hydrochemistry and Brine in Bakhtegan Lake, South-Central Iran. J. Hydrol. Reg. Stud. 2024, 52, 101714. [Google Scholar] [CrossRef]
- Besser, H.; Mokadem, N.; Redhaounia, B.; Hadji, R.; Hamad, A.; Hamed, Y. Groundwater Mixing and Geochemical Assessment of Low-Enthalpy Resources in the Geothermal Field of Southwestern Tunisia. Euro-Mediterr. J. Environ. Integr. 2018, 3, 16. [Google Scholar] [CrossRef]
- Marques, J.M.; Carreira, P.M. Geosciences in the Assessment of Thermal and Mineral Groundwater Systems in N-Portugal: A Review. Sustain. Water Resour. Manag. 2019, 5, 1511–1523. [Google Scholar] [CrossRef]
- Lebedeva, E.G.; Bragin, I.V.; Pavlov, A.A.; Rusakova, D.A. Hydrochemistry, Microbial Ecology and Physiological-Biochemical Properties of Isolated Bacteria of Tyrma Hot Spring (Far East of Russia). Limnologica 2025, 112, 126255. [Google Scholar] [CrossRef]
- Nouali, H.; Bouroubi-Ouadfel, Y.; Moulla, A.S.; Mutlu, H.; Vaselli, O.; Dinar, H.; Khiari, A. Hydrogeochemical and Isotopic Characterization of the El-Tarf Geothermal Aquifer (Algerian−Tunisian Border): Implications of the Regional Geodynamic Structure and the Water−rock Interactions. J. Afr. Earth Sci. 2025, 223, 105523. [Google Scholar] [CrossRef]
- Cherchali, M.E.-H.; Liégeois, J.-P.; Mesbah, M.; Moulla, A.S.; Ouarezki, S.-A.; Daas, N.; Achachi, A. Interconnected Multi-Layer Aquifer with Evaporitic Fossil Waters in Chott-El-Gharbi Endorheic Basin (Western High Plateaus, Algeria): Hydrochemistry, Environmental and Strontium Isotopes. Appl. Geochem. 2023, 148, 105537. [Google Scholar] [CrossRef]
- Temovski, M.; Túri, M.; Futó, I.; Braun, M.; Molnár, M.; Palcsu, L. Multi-Method Geochemical Characterization of Groundwater from a Hypogene Karst System. Hydrogeol. J. 2021, 29, 1129–1152. [Google Scholar] [CrossRef]
- Bournaris, T.; Papathanasiou, J.; Manos, B.; Kazakis, N.; Voudouris, K. Support of Irrigation Water Use and Eco-Friendly Decision Process in Agricultural Production Planning. Oper. Res. Int. J. 2015, 15, 289–306. [Google Scholar] [CrossRef]
- Gountôh Douagui, A.; Oi Mangoua, J.M.; Kouamé Kouassi, A.; Coulibaly, B.; Savané, I. Assessment of Groundwater Physicochemical Quality in Gbêkê Region of Côte d’Ivoire Using Water Quality Indices and Multivariate Analysis. Curr. J. Appl. Sci. Technol. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Su, C.; Li, Z.; Wang, W.; Cheng, Z.; Zheng, Z.; Chen, Z. Key Factors Dominating the Groundwater Chemical Composition in a Grain Production Base: A Case Study of Muling–Xingkai Plain, Northeast China. Water 2022, 14, 2222. [Google Scholar] [CrossRef]
- Goné, D.L.; Douagui, A.G.; Bai, L.; Kamagaté, B.; Ligban, R. Using Graphical and Multivariate Statistical Methods for Geochemical Assessment of Groundwater Quality in Oumé Department (Côte d’Ivoire). J. Environ. Prot. 2014, 05, 1255. [Google Scholar] [CrossRef]
- Paul, R.; Prasanna, M.V.; Gantayat, R.R.; Singh, M.K. Groundwater Quality Assessment in Jirania Block, West District of Tripura, India, Using Hydrogeochemical Fingerprints. SN Appl. Sci. 2019, 1, 1055. [Google Scholar] [CrossRef]
- Han, D.; Cao, G.; Currell, M.J.; Priestley, S.C.; Love, A.J. Groundwater Salinization and Flushing During Glacial-Interglacial Cycles: Insights From Aquitard Porewater Tracer Profiles in the North China Plain. Water Resour. Res. 2020, 56, e2020WR027879. [Google Scholar] [CrossRef]
- Jiménez, J.; Gasco Cavero, S.; Marazuela, M.Á.; Baquedano, C.; Laspidou, C.; Santamarta, J.C.; García-Gil, A. Effects of the 2021 La Palma Volcanic Eruption on Groundwater Hydrochemistry: Geochemical Modelling of Endogenous CO2 Release to Surface Reservoirs, Water-Rock Interaction and Influence of Thermal and Seawater. Sci. Total Environ. 2024, 929, 172594. [Google Scholar] [CrossRef]
- Paikaray, S.; Mahajan, T. Hydrogeochemical Processes, Mobilization Controls, Soil-Water-Plant-Rock Fractionation and Origin of Fluoride around a Hot Spring Affected Tropical Monsoonal Belt of Eastern Odisha, India. Appl. Geochem. 2023, 148, 105521. [Google Scholar] [CrossRef]
- Jourde, H.; Wang, X. Advances, Challenges and Perspective in Modelling the Functioning of Karst Systems: A Review. Environ. Earth Sci. 2023, 82, 396. [Google Scholar] [CrossRef]
- Dong, S.; Li, L.; Zhou, Z.; Fu, Q.; Li, M.; Xue, P. Groundwater Drought Propagation and the Drought Resistance Capacity in Different Climatic Regions of China. Agric. Water Manag. 2025, 312, 109425. [Google Scholar] [CrossRef]
- Kazakis, N.; Karakatsanis, D.; Ntona, M.M.; Polydoropoulos, K.; Zavridou, E.; Voudouri, K.A.; Busico, G.; Kalaitzidou, K.; Patsialis, T.; Perdikaki, M.; et al. Groundwater Depletion. Are Environmentally Friendly Energy Recharge Dams a Solution? Water 2024, 16, 1541. [Google Scholar] [CrossRef]
- Patrikaki, O.; Kazakis, N.; Voudouris, K. Vulnerability Map: A Useful Tool for Groundwater Protection: An Example from Mouriki Basin, North Greece. In Proceedings of the Fresenius Environmental Bulletin, Mykonos Island, Greece, 14–18 June2012; Volume 21, pp. 2516–2521. [Google Scholar]



| Parameters | Units | Min. | Max. | Average | Median | Standard Deviation |
|---|---|---|---|---|---|---|
| pH | 7.2 | 7.4 | 7.3 | 7.3 | 0.08 | |
| T | °C | 24.7 | 27.7 | 25.8 | 25.5 | 1.0 |
| Conductivity | μS/cm | 4183.0 | 4457.0 | 4313.8 | 4276.5 | 92.8 |
| TDS | mg/L | 2697.3 | 2865.8 | 2752.5 | 2747.3 | 45.7 |
| Ca2+ | mg/L | 62.8 | 72.8 | 66.1 | 65.8 | 2.5 |
| Mg2+ | mg/L | 30.0 | 45.9 | 36.7 | 35.8 | 4.7 |
| Na+ | mg/L | 800 | 870 | 824 | 810 | 26.1 |
| K+ | mg/L | 13.0 | 13.6 | 13.4 | 13.4 | 0.2 |
| HCO3− | mg/L | 556.3 | 695.4 | 593.9 | 585.6 | 37.7 |
| SO42− | mg/L | 183.0 | 286.0 | 217.7 | 217.5 | 26.5 |
| Cl− | mg/L | 975 | 1000 | 987 | 987.5 | 7.8 |
| NO3− | mg/L | 2.00 | 6.0 | 4.3 | 4.5 | 1.3 |
| NO2− | mg/L | 0.00 | 0.03 | 0.01 | 0.01 | 0.01 |
| NH4+ | mg/L | 6.57 | 9.51 | 8.56 | 8.66 | 0.8 |
| PO43− | mg/L | 0.30 | 0.50 | 0.38 | 0.37 | 0.07 |
| Parameters | Units | Min. | Max. | Average | Median | Standard Deviation |
|---|---|---|---|---|---|---|
| pH | 5.6 | 6.6 | 6.1 | 6.0 | 0.28 | |
| T | °C | 16.0 | 26.8 | 21.8 | 21.9 | 3.6 |
| ORP | −46.3 | −16.5 | −31.6 | −32 | 9.5 | |
| Conductivity | μS/cm | 4843 | 5519 | 5224 | 5313 | 253.9 |
| TDS | mg/L | 3795 | 4135 | 3952 | 3958 | 97.6 |
| Ca2+ | mg/L | 348.0 | 390.0 | 368.0 | 367 | 11.1 |
| Mg2+ | mg/L | 26.4 | 47.6 | 37.1 | 35.4 | 7.2 |
| Na+ | mg/L | 760 | 880 | 835 | 840 | 29.7 |
| K+ | mg/L | 41.0 | 47.0 | 44.4 | 44.0 | 2.3 |
| HCO3− | mg/L | 1360.3 | 1720.2 | 1535.7 | 1528.1 | 104.5 |
| SO42− | mg/L | 0.0 | 8.0 | 0.8 | 0.00 | 2.3 |
| Cl− | mg/L | 965 | 1200 | 1120 | 1130 | 58.8 |
| NO3− | mg/L | 1.0 | 5.3 | 3.8 | 4.0 | 1.2 |
| NO2− | mg/L | 0.00 | 0.02 | 0.00 | 0.0 | 0.01 |
| NH4+ | mg/L | 6.90 | 8.60 | 7.60 | 7.6 | 0.5 |
| PO43− | mg/L | 0.03 | 1.66 | 0.47 | 0.35 | 0.5 |
| Parameters | Units | Min. | Max. | Average | Median | Standard Deviation |
|---|---|---|---|---|---|---|
| pH | 5.8 | 6.6 | 6.3 | 6.3 | 0.2 | |
| T | °C | 17.6 | 21.1 | 19.9 | 20.3 | 1.0 |
| ORP | −46.3 | −3.6 | −17.3 | −12.3 | 14.6 | |
| Conductivity | μS/cm | 1983 | 2635 | 2251 | 2423 | 165.9 |
| TDS | mg/L | 1674.7 | 1937.3 | 1755.8 | 1754 | 73.1 |
| Ca2+ | mg/L | 263.2 | 286 | 272.5 | 272 | 6.1 |
| Mg2+ | mg/L | 25.61 | 33.7 | 29.2 | 29.9 | 2.8 |
| Na+ | mg/L | 190.0 | 225 | 198.8 | 190 | 12.1 |
| K+ | mg/L | 6.3 | 7.8 | 6.7 | 6.5 | 0.5 |
| HCO3− | mg/L | 805.2 | 982.1 | 877.4 | 860.1 | 53.7 |
| SO42− | mg/L | 49.0 | 65.0 | 55.4 | 54.5 | 5.2 |
| Cl− | mg/L | 265.0 | 371.0 | 302.2 | 294 | 34.2 |
| NO3− | mg/L | 6.3 | 13.0 | 11.3 | 12.0 | 2.3 |
| NO2− | mg/L | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 |
| NH4+ | mg/L | 1.0 | 2.6 | 2.0 | 2.2 | 0.4 |
| PO43− | mg/L | 0.07 | 0.46 | 0.17 | 0.13 | 0.1 |
| Kyllini Spring (n = 12) | Voskina Spring (n = 12) | Agiasma Spring (n = 12) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Minimum | Maximum | Average | Std. Dev. | Minimum | Maximum | Average | Std. Dev. | Minimum | Maximum | Average | Std. Dev. |
| Li | 37.1 | 88.8 | 53.1 | 16.6 | 429.5 | 991.9 | 567.9 | 187.1 | 1985 | 4851 | 2834 | 936 |
| Be | 0.00 | 0.08 | 0.03 | 0.02 | 0.04 | 0.11 | 0.07 | 0.03 | 0.37 | 0.85 | 0.58 | 0.19 |
| B | 1289 | 1968 | 1566 | 201 | 9969 | 16,381 | 12,229 | 2042 | 36,675 | 58,879 | 46,411 | 8011 |
| Al | 1.3 | 454.0 | 60.3 | 125 | 1.27 | 43.8 | 12.4 | 16.5 | 4.92 | 11.8 | 7.99 | 2.78 |
| P | 0.0 | 110 | 45.1 | 32.8 | 0.01 | 39.0 | 18.3 | 12.3 | 0.06 | 284.4 | 188.8 | 115 |
| Ti | 209 | 295 | 245 | 32.6 | 980 | 1391 | 1136 | 176 | 1094 | 1820 | 1363 | 309 |
| V | 6.4 | 11.4 | 8.3 | 1.5 | 1.39 | 3.53 | 2.33 | 0.83 | 5.5 | 13.1 | 8.3 | 3.28 |
| Cr | 15.4 | 213.9 | 39.1 | 55.3 | 3.83 | 16.3 | 9.92 | 4.05 | 6.8 | 30.8 | 15.0 | 8.26 |
| Mn | 9.5 | 15.9 | 12.8 | 2.4 | 1.20 | 8.9 | 4.08 | 2.12 | 898 | 2166 | 1521 | 450 |
| Fe | 12.6 | 269.9 | 80.0 | 71.5 | 29.3 | 166.9 | 76.9 | 44.6 | 9571 | 21,695 | 15,505 | 4341 |
| Co | 0.1 | 0.3 | 0.2 | 0.1 | 0.55 | 1.09 | 0.78 | 0.20 | 7.06 | 12.8 | 10.0 | 1.87 |
| Ni | 0.6 | 2.0 | 1.2 | 0.4 | 10.4 | 22.2 | 15.1 | 4.0 | 17.8 | 31.7 | 23.1 | 4.54 |
| Cu | 12.3 | 88.1 | 30.9 | 23.8 | 4.96 | 55.0 | 22.5 | 15.7 | 11.8 | 118.2 | 54.2 | 37.3 |
| Zn | 0.64 | 20.6 | 7.42 | 6.79 | 11.2 | 687 | 156 | 180 | 31.6 | 146.3 | 72.5 | 36.4 |
| As | 0.63 | 4.33 | 2.13 | 1.40 | 9.27 | 21.3 | 13.4 | 4.22 | 314 | 651 | 418 | 105 |
| Se | 2.46 | 7.07 | 4.40 | 1.46 | 1.34 | 6.73 | 2.71 | 1.69 | 3.08 | 11.6 | 5.44 | 2.83 |
| Rb | 5.22 | 6.99 | 6.13 | 0.62 | 32.8 | 42.5 | 36.4 | 3.43 | 129 | 183 | 154 | 20.5 |
| Sr | 1386 | 2317 | 1711 | 305 | 474 | 889 | 613 | 145 | 1331 | 2684 | 1838 | 461 |
| Nb | 0.00 | 0.11 | 0.03 | 0.04 | 0.00 | 0.13 | 0.03 | 0.04 | 0.03 | 0.14 | 0.07 | 0.04 |
| Mo | 0.03 | 0.33 | 0.13 | 0.14 | 0.11 | 0.74 | 0.39 | 0.20 | 0.02 | 0.60 | 0.31 | 0.19 |
| Cd | 0.00 | 0.23 | 0.06 | 0.07 | 0.01 | 0.05 | 0.03 | 0.01 | 0.00 | 0.04 | 0.02 | 0.01 |
| In | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.09 | 0.03 | 0.04 | n.d | n.d | n.d | n.d |
| Sn | 0.00 | 0.40 | 0.11 | 0.12 | 0.00 | 26.1 | 3.99 | 8.56 | 0.00 | 0.78 | 0.28 | 0.25 |
| Sb | 0.01 | 0.22 | 0.07 | 0.07 | 0.12 | 0.32 | 0.19 | 0.06 | 0.01 | 0.14 | 0.06 | 0.05 |
| Cs | 0.08 | 0.24 | 0.13 | 0.04 | 35.8 | 61.4 | 43.6 | 8.71 | 92.5 | 189.1 | 126.2 | 34.9 |
| Ba | 49.0 | 81.3 | 61.0 | 10.7 | 103.4 | 179.9 | 133.8 | 28.0 | 321.6 | 558.4 | 406.1 | 83.3 |
| Pb | 0.00 | 3.56 | 0.92 | 1.47 | 0.00 | 0.52 | 0.16 | 0.20 | n.d | n.d | n.d | n.d |
| Bi | 0.00 | 0.05 | 0.02 | 0.01 | 0.00 | 0.07 | 0.02 | 0.03 | 0.00 | 0.05 | 0.01 | 0.01 |
| U | 0.00 | 0.05 | 0.01 | 0.01 | 3.84 | 6.35 | 4.72 | 0.94 | 0.32 | 0.64 | 0.45 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakis, N.; Stavropoulou, V.; Ntona, M.M.; Pouliaris, C.; Papailiopoulou, M.; Nanou, E.-A.; Tsoutanis, A.; Lambropoulou, D.; Zagana, E. Hydrochemical Variability in Karst Hypothermal Mineral Springs of Greece. Hydrology 2025, 12, 237. https://doi.org/10.3390/hydrology12090237
Kazakis N, Stavropoulou V, Ntona MM, Pouliaris C, Papailiopoulou M, Nanou E-A, Tsoutanis A, Lambropoulou D, Zagana E. Hydrochemical Variability in Karst Hypothermal Mineral Springs of Greece. Hydrology. 2025; 12(9):237. https://doi.org/10.3390/hydrology12090237
Chicago/Turabian StyleKazakis, Nerantzis, Vasiliki Stavropoulou, Maria Margarita Ntona, Christos Pouliaris, Maria Papailiopoulou, Eleni-Anna Nanou, Apostolis Tsoutanis, Dimitra Lambropoulou, and Eleni Zagana. 2025. "Hydrochemical Variability in Karst Hypothermal Mineral Springs of Greece" Hydrology 12, no. 9: 237. https://doi.org/10.3390/hydrology12090237
APA StyleKazakis, N., Stavropoulou, V., Ntona, M. M., Pouliaris, C., Papailiopoulou, M., Nanou, E.-A., Tsoutanis, A., Lambropoulou, D., & Zagana, E. (2025). Hydrochemical Variability in Karst Hypothermal Mineral Springs of Greece. Hydrology, 12(9), 237. https://doi.org/10.3390/hydrology12090237







