Nature-Based Approaches for Managing Bioavailable Phosphorus in Aquatic Ecosystems
Abstract
1. Introduction
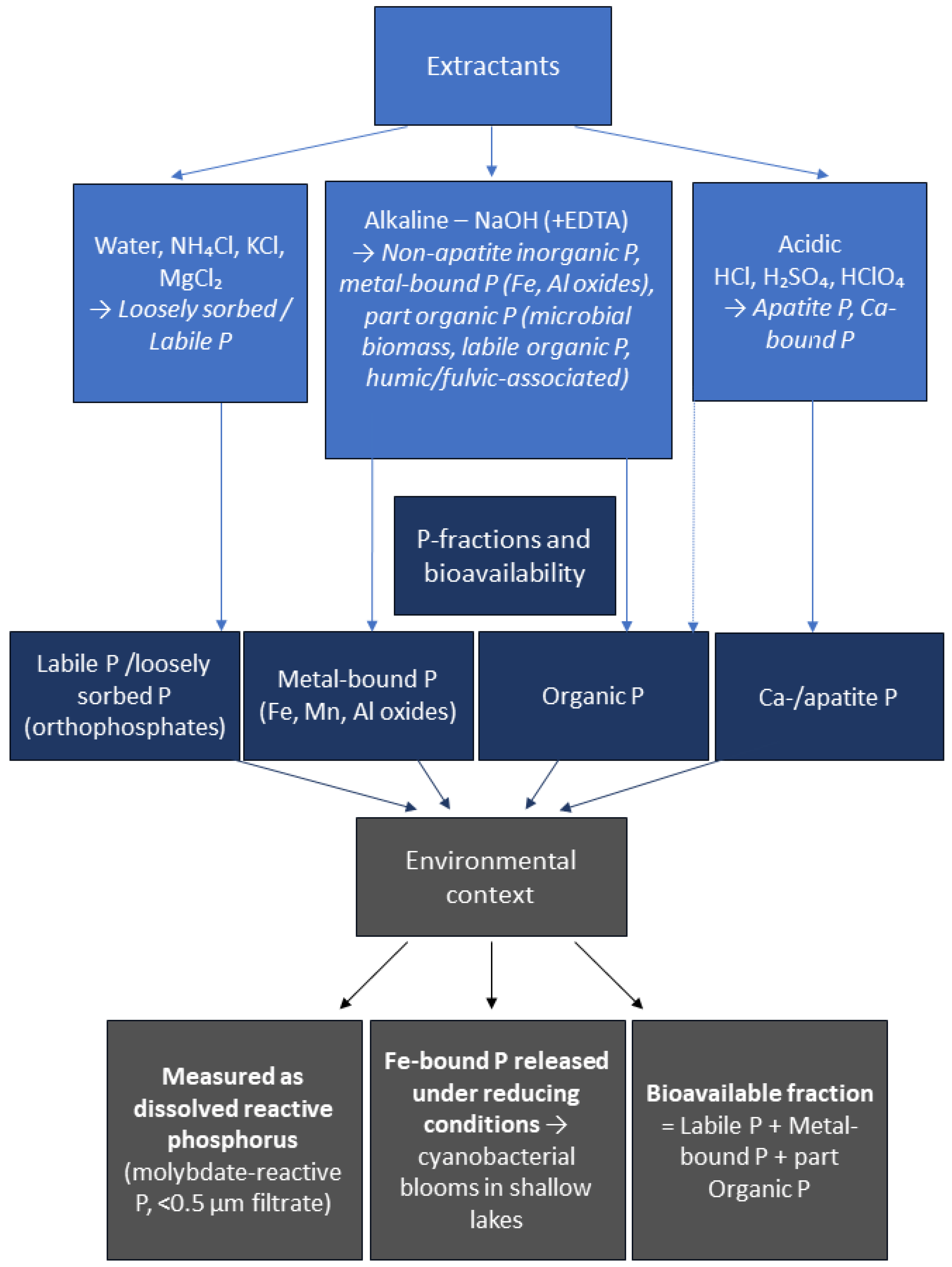
2. Bioavailable Forms of Phosphorus
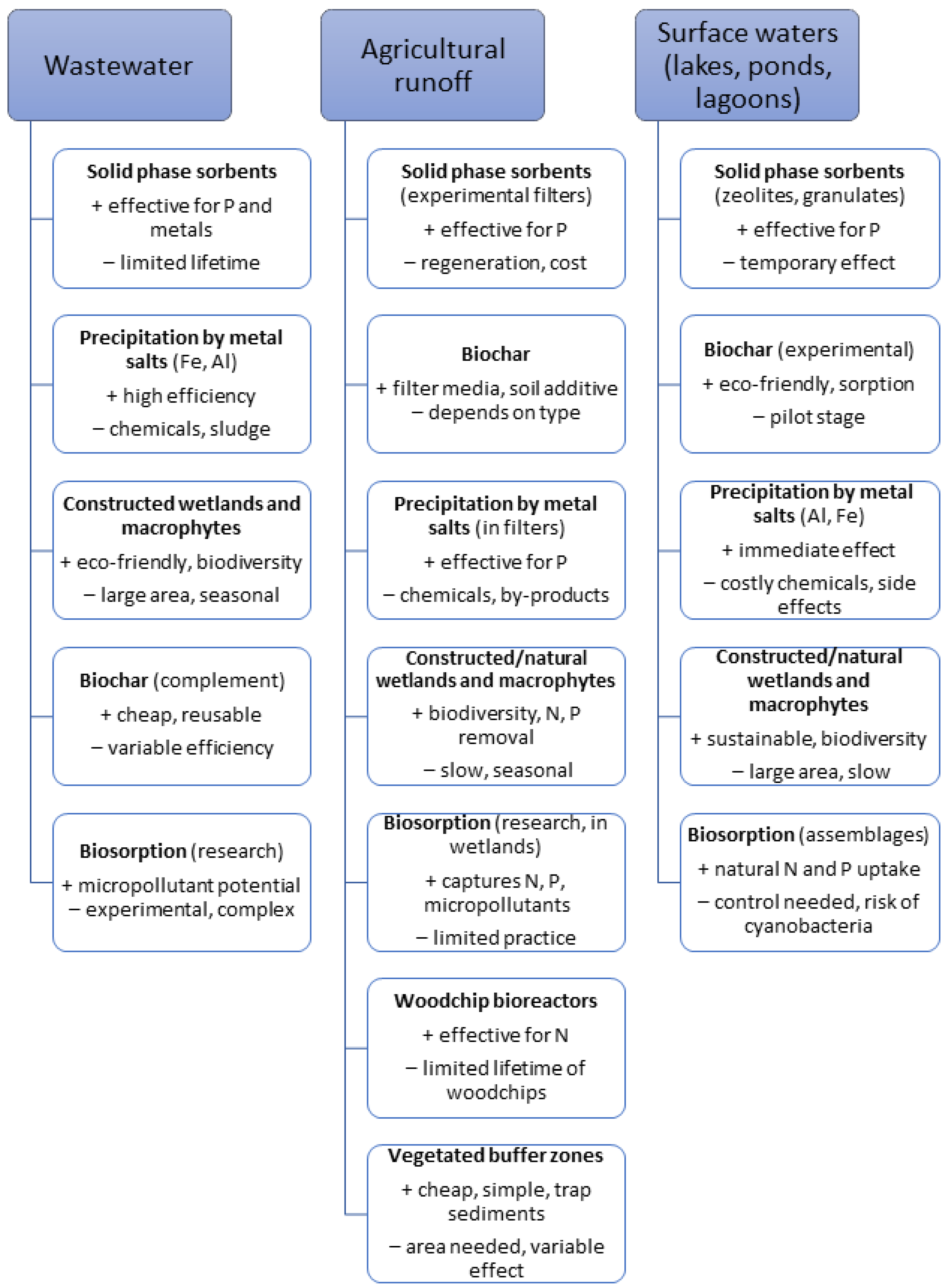
3. Methods and Technologies for Phosphorus Removal, Reduction, or Mitigation by Precipitation and (Bio)Sorption
3.1. Precipitation of Phosphorus by Metal Salts
3.2. Solid-Phase Sorbents
- -
- Naturally occurring minerals from soils (e.g., Fe-oxides/oxyhydroxides, allophone)
- -
- Naturally occurring (poly-mineral) soils or sands.
- -
- Derivatives from mineral deposits (e.g., wollastonite) or other natural materials (e.g., shale, serpentinite, marl).
- -
- Synthetic analogues of natural minerals produced on an experimental or industrial scale (e.g., polymeric hydrogels, hydrotalcites).
- -
- Expanded clay aggregates, including extruded clays fortified by nanomaterials, or metals.
- -
- Nanoscale zero-valent iron (nZVI).
- -
- Waste materials from industrial processes that adsorb phosphate or that may also be further modified to enhance their uptake capacity (e.g., electric arc furnace, steel slag, blast furnace slag, red mud).
| Method | Principles and Additional Information | Reference |
|---|---|---|
| Precipitation and sorption by metals or metal salts | Iron(II), iron(III), aluminum | [35,36] |
| Polyaluminium chloride (liquid) | [39] | |
| Solid-phase sorbents INORGANIC | Naturally occurring minerals from soils (Fe-oxides/hydroxides, allophone) | [39] |
| Sand filter amended with biochar coating with iron hydroxides | [38] | |
| Synthetic analogues of natural minerals produced on an industrial scale (polymeric hydrogels, hydrotalcites) | [39] | |
| Expanded clay aggregates, extruded clays fortified by nanomaterials or metals | [39] | |
| Waste materials from industrial processes (electric arc furnace, steel slag, blast furnace slag, red mud) and basic oxygen furnace slag and converter slag | [39] | |
| Other by-products, iron oxide tailing material; an industrial waste derived from a mineral processing industry; wollastonite tailing (calcium meta-silicate), iron-containing humic materials as iron humate by-product obtained from alkali humates wastewaters from manufacturing industry with iron salts (critical review) | [43] | |
| Metals, metal oxides, and hydroxides; Mn, Ti, Zr, lanthanum, iron oxide, nano-sized magnetite, Fe-Zr binary oxides, Ca-based layered double hydroxides, Mg-based layered double hydroxides, Mg-Ca-based layered double hydroxides, Zr-modified Mg-Fe-based layered double hydroxides | [43] | |
| Mesoporous silica doped with La, Zr, Ti, Fe, and Al oxides (critical review) | [43] | |
| Minerals and composite sorbents; goethite, akaganeite, lepidocrocite, hematite, magnetite, vesuvianite, limestone, dolomite, calcium and magnesium carbonates; bentonite, coal, and derived from mineral deposits (wollastonite) or other natural materials (shale, serpentinite, marl) (critical review) | [43] | |
| Clay with positive charges; layered double hydroxides or hydrotalcite adsorb oxyanions, e.g., phosphate | [43] | |
| Activated carbon prepared from shells, hulls, sobs, of plants, coal, lignite, wood (critical review) | [43] | |
| Solid-phase sorbents ORGANIC | Cellulose and its derivatives; grafted iron-coordinated amino-functionalized polymers on the cellulose (critical review) | [43] |
| Allepo pine saw dust containing lignocellulosic materials by acid prehydrolysis and urea addition (critical review) | [43] | |
| Mesostructural materials; ammonium functionalized mesoporous silica; Cu and Fe ethylene diamine complexes anchored inside mesoporous silica compound (critical review) | [43] | |
| Carboxylic acid-coated alumina and surfactant-coated nano-crystalline iron oxide, with a high surface area of the nano-sized material (critical review) | [43] | |
| Bamboo powder and rice husk powder; enrichment of P and N in sludge cake | [58] | |
| Mining, mineral processing by-products | [39] | |
| Orange juice plants, immobilized with zirconium | [14] | |
| Biochar and sorbents of biological origin | Biochar production methods; application, development (review) | [46] |
| Biochar modified by FeCl3 holds one-third of the agricultural superphosphate amount | [49] | |
| Overview of the preparation of biochar; adsorption of N, P by biochar | [53] | |
| Overview of the preparation and modification of biochar and adsorption of N, P | [54] | |
| Comparison of uncoated and Fe-coated biochars in sand filters in relation to the bacteria removal | [38] | |
| Sewage slug biochar modified by metals (lanthanum nitrate) | [47] | |
| Global meta-analysis; the most effective methods for activating the biochar to maximize heavy metal removal | [48] | |
| Biochar composites loaded with metals (La, Mn, Fe); recyclability by magnetisation | [50] | |
| Mulberry rods biochar composites with metal (Mg, Fe) layers | [49] | |
| Biochar from activated sewage sludge modified by MgCl2, KOH, or activated by CO2 | [55] | |
| Fecal-derived biochar (addition of undiluted human urine), MgO addition | [56] | |
| Biochar from canna aquatic plant waste, modified by MgO | [52] | |
| Wheat straw anion exchanger, reed, soybean hulls, saw dust from Allepo pine (critical review) | [43] | |
| Adsorbents produced from waste and biosorbents (sugarcane bagasse, rice hull, coconut shells, wheat straw charcoal, almond shell, sugar beet pulp, mustard straw charcoal) for nitrate removal (review) | [61] |
3.3. Biosorption by Microbial Assemblages Including Bacteria, Cyanobacteria, Microalgae, Fungi, or Filamentous Algae
| Method | Principles and Additional Information | Reference |
|---|---|---|
| Biosorption by bacteria and cyanobacteria | Phormidium bohneri; removal of dissolved inorganic nutrients from fish farm effluents | [63] |
| Rhodobacter cupsulatus; on cellulose beds for removal of N, P, and organic carbon from wastewater | [64] | |
| Bacillus subtilis, Pseudomonas, Achromobacter, Spirulina platensis, and Chlorella vulgaris for phosphates removal (review) | [61] | |
| Chlorella vulgaris, Bacillus subtillis in symbiotic particles using sol–gel method for removal of antibiotics from wastewater | [65] | |
| Bacillus subtillis, Paracoccus pantotrophus, and Pseudomonas putida for organic load and total N removal from surface water (poor removal of phosphates) | [66] | |
| Bacillus megaterium, Bacillus subtilis, and Bacillus coagulans for P, N and pathogen removal from aquaculture | [67] | |
| Proteobacteria, Actinobacteria, Comamonas, and Stenotrophomona for P and N removal to improve water quality in aquaculture | [67] | |
| Nano-bamboo charcoal powder, zeolite powder, and aquaculture pond sediment formed into nanospheres to observe the purification effect | [62] | |
| Spirulina platensis cultivated in human urine for wastewater treatment and biomass production | [68] | |
| Microalgae and filamentous algae | Scenedesmus sp., Arthrospira platensis for nutrients, metals, and organic materials removal from desalination systems wastewater | [70] |
| Chlorella vulgaris, Scenedesmus dimorphus for P removal from wastewaters, Spirulina platensis for nitrates, ammonia, and phosphates (review) | [16] | |
| Chlorella vulgaris for reducing colour, organic matter, ammonium, P, and chemical oxygen demand | [76] | |
| Tetradesmus obliquus and Chlorella vulgaris in hydrogel complex for N and phosphate removal from domestic wastewater | [71] | |
| Chlorella sp. in 3D-printed biocarriers for wastewater treatment, P and N removal | [72] | |
| Chlorella vulgaris ability to eliminate N and phosphates as biosorbent | [73] | |
| Chlorella vulgaris and Scenedesmus bijugatus in calcium alginate beads, removal of N and P | [74] | |
| Chlorella sorokiniana and activated sludge (Brevundimonas, Dokdonella, and Thermomonas); efficient removal of nutrients in wastewater | [78] | |
| Azospirillum spp. with microalgae in alginate; wastewater treatment | [79] | |
| Cladophora glomerata, Elodea Canadensis for P and N removal | [81] | |
| Cladophora sp., Oedogonium sp., Rhizoclonium sp., and Spirogyra sp.; P and N removal. Oedogonium sp. is recommended for all-year cultivation, as it is a good species for biomass production and bioremediation | [82] | |
| Cladophora sp., bacteria; algal–bacterial symbiosis improves N removal; wetland with filamentous algae; good ability for N removal; Cladophora resistant to ammonia N | [83] |
3.4. Woodchip Bioreactors for Nutrients Removal
| Method | Principles and Additional Information | Reference |
|---|---|---|
| Woodchip bioreactors | Combination of bioreactors with filters; dissolved P removal, pollution swapping by-products based on flow conditions | [84] |
| Study of N and P reduction efficiency in edge-of-field mitigations; constructed wetlands, aluminised zeolite filters; the highest reduction made using the catchment collective approach | [85] | |
| Pinus radiata woodchips and gravel; evaluation of pollution swapping | [86] | |
| Reduction of N in mesocosms by woodchip bioreactors, but soluble reactive P was higher | [87] | |
| Autochthonous P generated by reduction of iron; microbiological removal of P depends on P availability, and hydraulic retention efficiency may be comparable to nitrate removal | [89] | |
| Woodchip filters with P sorbent; ability to remove total suspended sediments, but the ability to remove P is minimal | [60] |
4. Wetlands and Macrophytes
5. Vegetated Buffer Zone
| Method | Principles and Additional Information | Reference |
|---|---|---|
| Wetlands | Biological uptake of bioavailable P forms; can remove 15 g/m2/year of TP, but can be a source of P | [3,9,20] |
| Constructed wetlands; (sub)surface flow systems; sink or source of P; removal rate of TP depends on loading rate; median of TP was 1.2 g/m2/year | [9,16,92] | |
| Floating wetlands; Lemna gibba and associated microorganisms; root system of macrophytes with the bacterial biofilm | [98] | |
| Constructed floating wetland for sewage treatment; P uptake rate by B. articulata was 12.9 g/m2 L. salicaria has the highest phosphorus removal compared with the other tested plants Agrostis alba, Canna spp., Iris hexagona, Juncus effusus, and Sagittaria lancifolia, P uptake rate was comparable for all plant species | [95,99,100] | |
| Vegetated buffer zones (type of constructed wetland); Setaria viridis (L.) Beauv., Humulus scandens (Lour.) Merr.; important to select species that are common to the local geography, climate, and conditions; Dichondra repens Forst > Cynodon dactylon (Linn.) Pers > Zoysia matrella > Festuca elata Keng ex E. Alexeev > Lolium perenne | [110] | |
| P in particulate form on the soil surface, plant stems, and roots intercepts soil particles during rapid runoff; buffer zone construction framework (small watershed) | [109] | |
| Buffer zones larger than 10 m show a total phosphorus intercept of 50%; large amounts of harmful substances are sorbed by plants (roots, stems, leaves) with microorganisms | [112] | |
| The reduction capacity of buffer strips depends on the differentiation of the biological structures and growth characteristics; high biomass was produced by Dichondra repens Forst and Cynodon dactylon (Linn.) Pers. | [113] | |
| Sequential Sedimentation–Biofiltration System; multi-zone constructed wetland with average removal efficiencies of 37% (TP), 46% (TN), and 45% (NO3−) | [93,94] | |
| Macrophytes | Typha latifolia, Phragmites australis, and Juncus effusus | [9] |
| Ceratophyllum demersum, Elodea canadensis, Potamogeton crispus, Myriophyllum spicatum, and Vallisneria spiralis | [17] | |
| C. demersum and C. vulgaris | [77] | |
| Lemna minuscula had no effect on nutrient removal from wastewater after wastewater treatment by Chlorella vulgaris | [76] | |
| Eichhornia crassipes, Lemna minor, Hydrocharis dubia, Trapa maximowiczii, Nymphoides peltata, three types of Potamogeton, Myriophyllum spicatum, Najas marina, and Elodea nuttallii | [96] | |
| Local wetland vegetation; Phalaris arundinacea L., Typha latifolia L., Iris pseudacorus L., Glyceria fluitans L., Sparganium erectum L. | [91] | |
| Water hyacinth | [107] |
6. Summarisation of P Removal Methods in Point Sources, Diffuse Sources, and Surface Waters
7. Conclusions, Key Findings, Determination of Research Gaps, and Recommendations for Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| P | Phosphorus |
| TP | Total Phosphorus |
| PO43−–P | Orthophosphate as phosphorus, soluble phosphate phosphorus |
| nZVI | Nanoscale Zero-Valent Iron |
| NH4-N | Ammonia Nitrogen |
| COD | Chemical Oxygen Demand |
| CWs | Constructed Wetlands |
| SSBS | Sequential Sedimentation–Biofiltration System |
| TN | Total Nitrogen |
| VBZs | Vegetated Buffer Zones |
| N-NO3 | Nitrate Nitrogen |
| WWTP | Wastewater Treatment Plan |
References
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Zhou, J.; Leavitt, P.R.; Zhang, Y.; Qin, B. Anthropogenic eutrophication of shallow lakes: Is it occasional? Water Res. 2022, 221, 118728. [Google Scholar] [CrossRef]
- Dunne, E.J.; Reddy, K.R.; Carton, O.T. Nutrient Management in Agricultural Watersheds: A Wetlands Solution Nutrient Management in Agricultural Watersheds; Wageningen Academic: Wageningen, The Netherlands, 2023; pp. 105–119. [Google Scholar]
- Schindler, D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. B 2012, 279, 4322–4333. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Jarvie, H.P. Delivery and cycling of phosphorus in rivers: A review. Sci. Total. Environ. 2008, 400, 379–395. [Google Scholar] [CrossRef]
- Johnston, A.E.; Steen, I.; Steén, I.; Association, E.F.M. Understanding Phosphorus and Its Use in Agriculture; EFMA: Brussels, Belgium, 2000. [Google Scholar]
- Schoumans, O.F.; Bouraoui, F.; Kabbe, C.; Oenema, O.; van Dijk, K.C. Phosphorus management in Europe in a changing world. Ambio 2015, 44, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Solangi, F.; Zhu, X.; Khan, S.; Rais, N.; Majeed, A.; Sabir, M.A.; Iqbal, R.; Ali, S.; Hafeez, A.; Ali, B.; et al. The Global Dilemma of Soil Legacy Phosphorus and Its Improvement Strategies under Recent Changes in Agro-Ecosystem Sustainability. ACS Omega 2023, 8, 23271–23282. [Google Scholar] [CrossRef] [PubMed]
- Dantas Mendes, L.R. Edge-of-Field Technologies for Phosphorus Retention from Agricultural Drainage Discharge. Appl. Sci. 2020, 10, 634. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Neal, C.; Withers, P.J.A.; Wescott, C.; Acornley, R.M. Nutrient hydrochemistry for a groundwater-dominated catchment: The Hampshire Avon, UK. Sci. Total. Environ. 2005, 344, 143–158. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Xu, L.; Yu, J.; Ren, S.; Yang, Y.; Wang, K.; López-Arredondo, D.; Herrera-Estrella, L.; Lambers, H.; et al. Engineering microalgae for water phosphorus recovery to close the phosphorus cycle. Plant Biotechnol. J. 2023, 21, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Cortez, V.; Quevedo-Nolasco, A.; del Valle-Paniagua, D.; Castro-Popoca, M.; Bravo-Vinaja, A.; Ramírez-Zierold, J. State of art: A current review of the mechanisms that make the artificial wetlands for the removal of nitrogen and phosphorus. Tecnol. Y Cienc. Del Agua 2019, 10, 319–342. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Removal and recovery of phosphorus from water by means of adsorption onto orange waste gel loaded with zirconium. Bioresour Technol. 2008, 99, 8685–8690. [Google Scholar] [CrossRef]
- Cao, L.; Yang, Y.; Xue, Y.; Ma, H.; Li, Y.; Hu, Y. A review of efficient nitrogen removal and phosphorus recovery by anammox-hydroxyapatite based processes: Challenges and opportunities. J. Environ. Chem. Eng. 2023, 11, 111103. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Gao, J.Q.; Xiong, Z.T.; Zhang, J.D.; Zhang, W.H.; Mba, F.O. Phosphorus removal from water of eutrophic Lake Donghu by five submerged macrophytes. Desalination 2009, 242, 193–204. [Google Scholar] [CrossRef]
- Heathwaite, A.L.; Dils, R.M. Characterising phosphorus loss in surface and subsurface hydrological pathways. Sci. Total. Environ. 2000, 251–252, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Sun, S.; Wang, S.; Yan, X.; Qian, J.; Pan, B. Phosphorus in water: A review on the speciation analysis and species specific removal strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 435–456. [Google Scholar] [CrossRef]
- Land, M.; Granéli, W.; Grimvall, A.; Hoffmann, C.C.; Mitsch, W.J.; Tonderski, K.S.; Verhoeven, J.T.A. How effective are created or restored freshwater wetlands for nitrogen and phosphorus removal? A systematic review. Environ. Evid. 2016, 5, 9. [Google Scholar] [CrossRef]
- Morse, G.K.; Brett, S.W.; Guy, J.A.; Lester, J.N. Review: Phosphorus removal and recovery technologies. Sci. Total. Environ. 1998, 212, 69–81. [Google Scholar] [CrossRef]
- Golterman, H. The Chemistry of Phosphate and Nitrogen Compounds in Sediments; Springer: Dordrecht, The Netherlands, 2004; p. 52. [Google Scholar]
- Ruttenberg, K.C. Phosphorus Cycle. In Encyclopedia of Ocean Sciences; Steele, J.H., Ed.; Academic Press: Oxford, UK, 2001; pp. 2149–2162. [Google Scholar]
- Reynolds, C.; Davies, P. Sources and bioavailability of phosphorus fractions in freshwaters: A British perspective. Biol. Rev. 2001, 76, 27–64. [Google Scholar] [CrossRef]
- Dijkstra, M.; Auer, M.; Kuczynski, A.; Lambert, R. Determination of bioavailable phosphorus in water samples using bioassay methods. MethodsX 2020, 7, 100807. [Google Scholar] [CrossRef]
- Lambert, R.; Auer, M.; Effler, S.; Greene, M.; Downer, B.; Kuczynski, A. Onondaga to Ontario: Management of bioavailable phosphorus in municipal wastewaters for control of Cladophora. J. Great Lakes Res. 2015, 41, 1106–1113. [Google Scholar] [CrossRef]
- Rönspieß, L.; Nausch, G.; Schulz-Bull, D. Bioavailability of Various Phosphorus Fractions and Their Seasonality in a Eutrophic Estuary in the Southern Baltic Sea—A Laboratory Approach. Front. Mar. Sci. 2021, 8, 715238. [Google Scholar] [CrossRef]
- Wang, X. Phosphorus Fractionation and Bio-availability in Surface Sediments from the Middle and Lower Reaches of the Yellow River. Procedia Environ. Sci. 2012, 12, 379–386. [Google Scholar] [CrossRef]
- Zhou, Q.; Gibson, C.E.; Zhu, Y. Evaluation of phosphorus bioavailability in sediments of three contrasting lakes in China and the UK. Chemosphere 2001, 42, 221–225. [Google Scholar] [CrossRef]
- Hupfer, M.; René, G.; Giovanoli, R. Transformation of Phosphorus Species in Settling Seston and During Early Sediment Diagenesis. Aquat. Sci. 1995, 57, 305–324. [Google Scholar] [CrossRef]
- Koch, S.; Rosewig, E.I.; Lennartz, B. Legacy Phosphorus in Sediments of Lowland Waterways. Environments 2023, 10, 43. [Google Scholar] [CrossRef]
- Psenner, R.; Puckso, R. Phosphorus fractionation: Advantages and limits of the method for the study of sediment P origins and interactions. Arch. Hydrobiol. 1988, 30, 43–59. [Google Scholar]
- Selig, U.; Schlungbaum, G. Longitudinal patterns of phosphorus and phosphorus binding in sediment of a lowland lake–river system. Hydrobiologia 2002, 472, 67–76. [Google Scholar] [CrossRef]
- Ramasahayam, S.K.; Guzman, L.; Gunawan, G.; Viswanathan, T. A Comprehensive Review of Phosphorus Removal Technologies and Processes. J. Macromol. Sci. Part A 2014, 51, 538–545. [Google Scholar] [CrossRef]
- Ping, Q.; Zhang, B.Q.; Zhang, Z.P.; Lu, K.X.; Li, Y.M. Speciation analysis and formation mechanism of iron-phosphorus compounds during chemical phosphorus removal process. Chemosphere 2023, 310, 136852. [Google Scholar] [CrossRef] [PubMed]
- Thistleton, J.; Clark, T.; Pearce, P.; Parsons, S.A. Mechanisms of chemical phosphorus removal—1-iron (II) salts. Process Saf. Environ. Prot. 2001, 79, 339–344. [Google Scholar] [CrossRef]
- Konadu-Amoah, B.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Noubactep, C. Metallic iron (Fe0)-based materials for aqueous phosphate removal: A critical review. J. Environ. Manag. 2022, 315, 115157. [Google Scholar] [CrossRef]
- Bolster, C.H. Comparing unamended and Fe-coated biochar on removal efficiency of bacteria, microspheres, and dissolved phosphorus in sand filters. Biochar 2021, 3, 329–338. [Google Scholar] [CrossRef]
- Douglas, G.B.; Hamilton, D.P.; Robb, M.S.; Pan, G.; Spears, B.M.; Lurling, M. Guiding principles for the development and application of solid-phase phosphorus adsorbents for freshwater ecosystems. Aquat. Ecol. 2016, 50, 385–405. [Google Scholar] [CrossRef]
- Johansson Westholm, L. Substrates for phosphorus removal—Potential benefits for on-site wastewater treatment? Water Res. 2006, 40, 23–36. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Sepúlveda, P.; Cáceres-Jensen, L.; Castro-Rojas, J.; Poblete-Grant, P.; Bolan, N.; Mora, M. nZVI-Based Nanomaterials Used for Phosphate Removal from Aquatic Systems. Nanomaterials 2023, 13, 399. [Google Scholar] [CrossRef]
- Valsami-Jones, E. Phosphorus in Environmental Technology: Principles and Applications; IWA Publishing: London, UK, 2005. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and Recovery of Phosphate From Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Vaiskunaite, R. An Investigation of the Batch Adsorption Capacity for the Removal of Phosphate from Wastewater Using Both Unmodified and Functional Nanoparticle-Modified Biochars. Processes 2024, 12, 2560. [Google Scholar] [CrossRef]
- Kofinas, P.; Kioussis, D. Reactive phosphorus removal from aquaculture and poultry productions systems using polymeric hydrogels. Environ. Sci. Technol. 2003, 37, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, N.S.; Dahalan, F.A.; Hasan, M.; An, O.S.; Parmin, N.A.; Ibrahim, N.; Hamdzah, M.; Zain, N.A.M.; Muda, K.; Wikurendra, E.A. Biochar: A Review of its History, Characteristics, Factors that Influence its Yield, Methods of Production, Application in Wastewater Treatment and Recent Development. Biointerface Res. Appl. Chem. 2022, 12, 7914–7926. [Google Scholar] [CrossRef]
- Mo, J.J.; Li, Q.; Sun, X.J.; Zhang, H.X.; Xing, M.Y.; Dong, B.; Zhu, H.X. Capacity and Mechanisms of Phosphate Adsorption on Lanthanum-Modified Dewatered Sludge-Based Biochar. Water 2024, 16, 418. [Google Scholar] [CrossRef]
- Pathy, A.; Pokharel, P.; Chen, X.; Balasubramanian, P.; Chang, S.X. Activation methods increase biochar’s potential for heavy-metal adsorption and environmental remediation: A global meta-analysis. Sci. Total. Environ. 2023, 865, 161252. [Google Scholar] [CrossRef]
- Kopecky, M.; Kolár, L.; Konvalina, P.; Strunecky, O.; Teodorescu, F.; Mráz, P.; Peterka, J.; Váchalová, R.; Bernas, J.; Bartos, P.; et al. Modified Biochar-A Tool for Wastewater Treatment. Energies 2020, 13, 5270. [Google Scholar] [CrossRef]
- Ouyang, E.R.; Xiang, H.R.; Zhao, R.; Yang, H.W.; He, W.Y.; Zhang, R.Y. Structural design of La2(CO3)3 loaded magnetic biochar for selective removal of phosphorus from wastewater. Environ Pollut. 2024, 345, 123510. [Google Scholar] [CrossRef]
- Liang, M.N.; Wu, Z.M.; Cao, H.Y.; Dong, K.; Bai, S.Y.; Wang, D.Q. The Phosphorus Adsorption and Recovery of Mg/Fe-LDHs Mulberry Rod Biochar Composite. Separations 2024, 11, 86. [Google Scholar] [CrossRef]
- Xiao, J.J.; Long, H.P.; He, X.M.; Chen, G.Y.; Yuan, T.; Liu, Y.; Xu, Q.L. Synthesis of MgO-Coated Canna Biochar and Its Application in the Treatment of Wastewater Containing Phosphorus. Water 2024, 16, 873. [Google Scholar] [CrossRef]
- Wu, X.C.; Quan, W.X.; Chen, Q.; Gong, W.; Wang, A.P. Efficient Adsorption of Nitrogen and Phosphorus in Wastewater by Biochar. Molecules 2024, 29, 1005. [Google Scholar] [CrossRef]
- Xu, Q.S.; Li, C.; Pang, W.H. Study on the removal efficacy and mechanism of phosphorus from wastewater by eggshell-modified biochar. Water Environ Res. 2024, 96, e10998. [Google Scholar] [CrossRef]
- Wystalska, K.; Grosser, A. Sewage Sludge-Derived Biochar and Its Potential for Removal of Ammonium Nitrogen and Phosphorus from Filtrate Generated during Dewatering of Digested Sludge. Energies 2024, 17, 1310. [Google Scholar] [CrossRef]
- Koulouri, M.E.; Templeton, M.R.; Fowler, G.D. Enhancing the nitrogen and phosphorus content of faecal-derived biochar via adsorption and precipitation from human urine. J. Environ. Manag. 2024, 352, 119981. [Google Scholar] [CrossRef]
- Hadroug, S.; Jellali, S.; Issaoui, M.; Kwapinska, M.; Jeguirim, M.; Leahy, J.; Kwapinski, W. Poultry manure conversion into eco-friendly materials: Synthesis of Mg-/Al-based biochars, characterization and application for phosphorus recovery from aqueous solutions. Biomass Convers. Biorefin. 2024, 14, 8103–8113. [Google Scholar] [CrossRef]
- Li, A.M.; Li, Y.Z.; Huang, K.W.; Song, L.; Shen, F.; Wang, S.; Li, J. Sustainable use of rice husk powder and bamboo powder as sludge deep dewatering conditioners in pilot-scale application: Feasibility for incineration and potential application for land use. Environ. Technol. Innov. 2023, 32, 103411. [Google Scholar] [CrossRef]
- Ospina-Montoya, V.; Pérez, S.; Muñoz-Saldan, J.; Forgionny, A.; Flórez, E.; Acelas, N. Performance of novel Ca-biocomposites produced from banana peel and eggshell for highly efficient removal and recovery of phosphate from domestic wastewater. J. Environ. Manag. 2024, 352, 120029. [Google Scholar] [CrossRef] [PubMed]
- Plach, J.M.; Pluer, W.T.; Kompanizare, M.; Brunke, R.; McKague, K.; Jarvie, H.P.; Macrae, M.L. Performance of simple low-cost edge-of-field filters for mitigating P losses in surface runoff from agricultural fields. Agric. Water Manag. 2023, 284, 108362. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.; Vo, D.; Sindhu, J.; Sneka, D.; Subhashini, B. Advanced techniques to remove phosphates and nitrates from waters: A review. Environ. Chem. Lett. 2021, 19, 3165–3180. [Google Scholar] [CrossRef]
- Shao, Y.L.; Zhong, H.; Wang, L.K.; Elbashier, M.M.A. Purification Effect of the Aquaculture Wastewater and Sediment by Microbial Nanospheres with Different Material Ratios and Dosing Methods. Sustainability 2020, 12, 1462. [Google Scholar] [CrossRef]
- Dumas, A.; Laliberté, G.; Lessard, P.; de la Noüe, J. Biotreatment of fish farm effluents using the cyanobacterium Phormidium bohneri. Aquac. Eng. 1998, 17, 57–68. [Google Scholar] [CrossRef]
- Sawayama, S.; Rao, K.K.; Hall, D.O. Immobilization of Rhodobacter capsulatus on cellulose beads and water treatment using a photobioreactor. J. Ferment. Bioeng. 1998, 86, 517–520. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, X.J.; Chen, J.Q.; Wang, F. Treatment of antibiotic-containing wastewater with self-suspended algae-bacteria symbiotic particles: Removal performance and reciprocal mechanism. Chemosphere 2023, 323, 138240. [Google Scholar] [CrossRef]
- Simon, M.; Joshi, H.; Hazra, M. Performance appraisal of microbial inoculants treating polluted surface water through bench-scale investigation. J. Chem. Technol. Biotechnol. 2023, 98, 2462–2476. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.J.; Fu, B.R.; Mu, X.Y. Improvement of aquaculture water quality by mixed Bacillus and its effects on microbial community structure. Environ. Sci. Pollut. Res. Int. 2022, 29, 69731–69742. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Wu, Z.C.; Bian, L.; Feng, D.L.; Leung, D.Y.C. Cultivation of Spirulina platensis for biomass production and nutrient removal from synthetic human urine. Appl. Energy 2013, 102, 427–431. [Google Scholar] [CrossRef]
- Fu, X.; Jiang, Q.; Yang, X.; Liu, L.; Liu, L.; Li, J.; Li, S.; Luo, Q.; Chen, J.; Zhao, Z.; et al. Nutrients’ Removal from Mariculture Wastewater by Algal-Bacterial Aggregates Developed from Spirulina platensis. Water 2023, 15, 396. [Google Scholar] [CrossRef]
- Mehdipour, N.; Hosseini, S.A.; Hedayati, A.; Zolfaghari, M.; Kordi, H. Determination of suitable compatible microalgae for bioremediation of effluent from desalination facilities. Biomass Convers. Biorefinery 2023, 14, 31233–31244. [Google Scholar] [CrossRef]
- Tarabukin, D.V.; Patova, E.N.; Novakovskaya, I.V. New hydrogel complex with immobilized microalgae cells for removal ammonium and phosphate ions from wastewater. Theor. Appl. Ecol. 2023, 4, 61–69. [Google Scholar] [CrossRef]
- Yoon, S.W.; Kim, S.Y.; Jeon, J.S.; Oh, S.; Chung, S.Y.; Kim, J.S.; Maeng, S.K. 3D-printed Chlorella vulgaris biocarriers: A novel approach to wastewater treatment. J. Water Process. Eng. 2024, 57, 104711. [Google Scholar] [CrossRef]
- Asaad, A.A.; Amer, A.S. Evaluation of Chlorella vulgaris biosorption capacity for phosphate and nitrate removal from wastewater. Sci. Rep. 2024, 14, 884. [Google Scholar] [CrossRef]
- Megharaj, M.; Pearson, H.W.; Venkateswarlu, K. Removal of nitrogen and phosphorus by immobilized cells of Chlorella vulgaris and Scenedesmus bijugatus isolated from soil. Enzym. Microb. Technol. 1992, 14, 656–658. [Google Scholar] [CrossRef]
- Yu, D.; Yan, L.; Shi, J.; Liu, Y.; Zhang, A.; Wang, Y.; Zhang, Y.; Xie, T. Phosphorus Removal and Recovery During Microalgae-Based Wastewater Treatment: A Mini-review. Int. J. Environ. Res. 2024, 18, 34. [Google Scholar] [CrossRef]
- Valderrama, L.T.; Del Campo, C.M.; Rodriguez, C.M.; de-Bashan, L.E.; Bashan, Y. Treatment of recalcitrant wastewater from ethanol and citric acid production using the microalga Chlorella vulgaris and the macrophyte Lemna minuscula. Water Res. 2002, 36, 4185–4192. [Google Scholar] [CrossRef] [PubMed]
- Siric, I.; Taher, M.; Kumar, P.; Abou Fayssal, S.; Bachheti, R.; Mioc, B.; Andabaka, Z.; Singh, J.; Eid, E. Comparative Assessment of Treatment of Mushroom Farm Wastewater Using Plant (Ceratophyllum demersum L.) and Algae (Chlorella vulgaris): Experimental and Kinetic Studies. Horticulturae 2023, 9, 1081. [Google Scholar] [CrossRef]
- Qv, M.X.; Dai, D.; Liu, D.Y.; Wu, Q.R.; Tang, C.M.; Li, S.X.; Zhu, L.D. Towards advanced nutrient removal by microalgae-bacteria symbiosis system for wastewater treatment. Bioresour. Technol. 2023, 370, 128574. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Hernandez, J.P.; Bashan, Y. Interaction of Azospirillum spp. with microalgae: A basic eukaryotic-prokaryotic model and its biotechnological applications. In Handbook for Azospirillum: Technical Issues and Protocols; Springer International Publishing: Cham, Switzerland, 2015; pp. 367–388. [Google Scholar]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gumbricht, T. Nutrient removal capacity in submersed macrophyte pond systems in a temperate climate. Ecol. Eng. 1993, 2, 49–61. [Google Scholar] [CrossRef]
- Hariz, H.B.; Lawton, R.J.; Craggs, R.J. Nutrient uptake and biomass productivity performance comparison among freshwater filamentous algae species on mesocosm-scale FANS under ambient summer and winter conditions. Ecol. Eng. 2023, 189, 106910. [Google Scholar] [CrossRef]
- Zhao, C.C.; Li, W.Y.; Shang, D.W.; Ma, Q.L.; Liu, L.X.; Xu, J.T.; Meng, J.S.; Zhang, T.; Wang, Q.; Wang, X.F.; et al. Influence of nitrogen sources on wastewater treatment performance by filamentous algae in constructed wetland system. Environ. Res. 2023, 235, 116638. [Google Scholar] [CrossRef]
- Christianson, L.E.; Lepine, C.; Sibrell, P.L.; Penn, C.; Summerfelt, S.T. Denitrifying woodchip bioreactor and phosphorus filter pairing to minimize pollution swapping. Water Res. 2017, 121, 129–139. [Google Scholar] [CrossRef]
- Weeber, M.P.; Tanner, C.C.; Rozemeijer, J.C.; Neal, M.B.; Thiange, C.X.O.; Van den Roovaart, J.C.; Burger, D.F. Modelling the feasibility and cost-effectiveness of edge-of-field mitigations for reducing nitrogen and phosphorus loads in the Waituna Lagoon Catchment, Southland. New Zealand J. Agric. Res. 2023, 66, 493–517. [Google Scholar] [CrossRef]
- Burbery, L.; Abraham, P.; Sutton, R.; Close, M. Evaluation of pollution swapping phenomena from a woodchip denitrification wall targetting removal of nitrate in a shallow gravel aquifer. Sci. Total. Environ. 2022, 820, 153194. [Google Scholar] [CrossRef]
- Payne, G.K.; Moore, M.T.; Krajcir, K.J.; Classen, R.; Farris, J.L. Evaluation of woodchip-bioditch reactors as a nutrient reduction conservation strategy. Agrosyst. Geosci. Environ. 2024, 7, e20455. [Google Scholar] [CrossRef]
- Wickramarathne, N.M.; Christianson, L.E.; Foltz, M.E.; Zilles, J.L.; Christianson, R.D.; Cooke, R.A.C. Biological Nitrate Removal With Emerald Ash Borer-Killed Ash and High-Tannin Oak Woodchips. Front. Environ. Sci. 2021, 9, e20455. [Google Scholar] [CrossRef]
- Perera, G.N.; Rojas, D.T.; Rivas, A.; Barkle, G.; Moorhead, B.; Schipper, L.A.; Craggs, R.; Hartland, A. Elucidating phosphorus removal dynamics in a denitrifying woodchip bioreactor. Sci. Total. Environ. 2024, 917, 170478. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Wang, R.G.; Yan, P.H.; Wu, S.B.; Chen, Z.B.; Zhao, Y.Q.; Cheng, C.; Hu, Z.; Zhuang, L.L.; Guo, Z.Z.; et al. Constructed wetlands for pollution control. Nat. Rev. Earth Environ. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Krzeminska, D.; Blankenberg, A.; Bechmann, M.; Deelstra, J. The effectiveness of sediment and phosphorus removal by a small constructed wetland in Norway: 18 years of monitoring and perspectives for the future. Catena 2023, 223, 170478. [Google Scholar] [CrossRef]
- Hoffmann, C.C.; Kjaergaard, C.; Uusi-Kämppä, J.; Hansen, H.C.; Kronvang, B. Phosphorus retention in riparian buffers: Review of their efficiency. J. Environ. Qual. 2009, 38, 1942–1955. [Google Scholar] [CrossRef]
- Nájera, A.; Serwecinska, L.; Szklarek, S.; Mankiewicz-Boczek, J. Characterization and comparison of microbial communities in sequential sedimentation-biofiltration systems for removal of nutrients in urban rivers. Ecol. Eng. 2020, 149, 105796. [Google Scholar] [CrossRef]
- Szklarek, S.; Wagner, I.; Jurczak, T.; Zalewski, M. Sequential Sedimentation-Biofiltration System for the purification of a small urban river (the Sokolowka, Lodz) supplied by stormwater. J. Environ. Manag. 2018, 205, 201–208. [Google Scholar] [CrossRef]
- Karstens, S.; Langer, M.; Nyunoya, H.; Caraite, I.; Stybel, N.; Razinkovas-Baziukas, A.; Bochert, R. Constructed floating wetlands made of natural materials as habitats in eutrophicated coastal lagoons in the Southern Baltic Sea. J. Coast. Conserv. 2021, 25, 44. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhang, L.M.; Chua, H.; Li, X.D.; Xia, M.F.; Pu, P.M. A mosaic community of macrophytes for the ecological remediation of eutrophic shallow lakes. Ecol. Eng. 2009, 35, 582–590. [Google Scholar] [CrossRef]
- Nie, Z.; Zheng, Z.; Zhu, H.; Sun, Y.; Gao, J.; Xu, P.; Xu, G. Effects of submerged macrophytes (Elodea nuttallii) on water quality and microbial communities of largemouth bass (Micropterus salmoides) ponds. Front. Microbiol. 2022, 13, 1050699. [Google Scholar] [CrossRef] [PubMed]
- Körner, S.; Vermaat, J.E. The relative importance of Lemna gibba L., bacteria and algae for the nitrogen and phosphorus removal in duckweed-covered domestic wastewater. Water Res. 1998, 32, 3651–3661. [Google Scholar] [CrossRef]
- Huth, I.; Walker, C.; Kulkarni, R.; Lucke, T. Using Constructed Floating Wetlands to Remove Nutrients from a Waste Stabilization Pond. Water 2021, 13, 1746. [Google Scholar] [CrossRef]
- White, S.A. Plant Nutrient Uptake in Full-Scale Floating Treatment Wetlands in a Florida Stormwater Pond: 2016–2020. Water 2021, 13, 569. [Google Scholar] [CrossRef]
- Winston, R.J.; Hunt, W.F.; Kennedy, S.G.; Merriman, L.S.; Chandler, J.; Brown, D. Evaluation of floating treatment wetlands as retrofits to existing stormwater retention ponds. Ecol. Eng. 2013, 54, 254–265. [Google Scholar] [CrossRef]
- Nawaz, N.; Ali, S.; Shabir, G.; Rizwan, M.; Shakoor, M.B.; Shahid, M.J.; Afzal, M.; Arslan, M.; Hashem, A.; Abd_Allah, E.F.; et al. Bacterial Augmented Floating Treatment Wetlands for Efficient Treatment of Synthetic Textile Dye Wastewater. Sustainability 2020, 12, 3731. [Google Scholar] [CrossRef]
- Pavlidis, G.; Zotou, I.; Karasali, H.; Marousopoulou, A.; Bariamis, G.; Tsihrintzis, V.A.; Nalbantis, I. Performance of Pilot-scale Constructed Floating Wetlands in the Removal of Nutrients and Pesticides. Water Resour. Manag. 2022, 36, 399–416. [Google Scholar] [CrossRef]
- Cule, N.; Lucic, A.; Nesic, M.; Veselinovic, M.; Mitrovic, S.; Sredojevic, Z.; Brasanac-Bosanac, L. Accumulation of chromium and nickel by Canna indica and decorative macrophytes grown in floating treatment wetland. Fresenius Environ. Bull. 2021, 30, 7881–7890. [Google Scholar]
- Tondera, K.; Chazarenc, F.; Chagnon, P.; Brisson, J. Bioaugmentation of treatment wetlands—A review. Sci. Total. Environ. 2021, 775, 145820. [Google Scholar] [CrossRef] [PubMed]
- Tara, N.; Iqbal, M.; Habib, F.E.; Khan, Q.M.; Iqbal, S.; Afzal, M.; Brix, H. Investigating degradation metabolites and underlying pathway of azo dye “Reactive Black 5” in bioaugmented floating treatment wetlands. Environ. Sci. Pollut. Res. Int. 2021, 28, 65229–65242. [Google Scholar] [CrossRef] [PubMed]
- Corman, J.R.; Roegner, A.; Ogari, Z.; Miller, T.R.; Aura, C.M. Local-scale impacts of water hyacinth on water quality in a hypereutrophic lake. Front. Water. 2023, 5, 917837. [Google Scholar] [CrossRef]
- Wu, J.Q.; Xiong, L.J.; Sha, C.Y. Removal of N, P from seepage and runoff by different vegetated and slope buffer strips. Water Sci. Technol. 2020, 82, 351–363. [Google Scholar] [CrossRef]
- Duan, Y.C.; Tang, J.; Li, Z.Y.; Yang, B.; Yan, Y.; Yang, Y. Vegetated Buffer Zone Restoration Planning in Small Urban Watersheds. Water 2021, 13, 3000. [Google Scholar] [CrossRef]
- Koenig, S.; Trémolières, M. Transfer of Nitrogen and Phosphorus Nutrients in Vegetated Buffer Zones Receiving Treatment Plant Effluent. Environ. Process. 2018, 5, 555–575. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Huang, J.L.; Dzakpasu, M.; De Silva, K.; Ohore, O.E.; Ajibade, F.O.; Li, X.Y.; Su, J.J.; Muyembe, D.K.; Huang, K.X. An updated review of the efficacy of buffer zones in warm/temperate and cold climates: Insights into processes and drivers of nutrient retention. J. Environ. Manag. 2023, 336, 117646. [Google Scholar] [CrossRef]
- Dai, T.T.; Liu, R.; Zhou, X.X.; Zhang, J.; Song, M.T.; Zou, P.; Bi, X.Y.; Li, S.B. Role of Lake Aquatic-Terrestrial Ecotones in the Ecological Restoration of Eutrophic Water Bodies. Toxics 2023, 11, 560. [Google Scholar] [CrossRef]
- Hu, Y.X.; Gao, L.; Ma, C.M.; Wang, H.; Zhou, C. The Comprehensive Reduction Capacity of Five Riparian Vegetation Buffer Strips for Primary Pollutants in Surface Runoff. Appl. Sci. 2023, 13, 3898. [Google Scholar] [CrossRef]
- Miao, K.X.; Dai, W.Q.; Xie, Z.J.; Li, C.H.; Ye, C. Effect of Grass Buffer Strips on Nitrogen and Phosphorus Removal from Paddy Runoff and Its Optimum Widths. Agronomy 2023, 13, 2980. [Google Scholar] [CrossRef]
- Auteri, N.; Saiano, F.; Scalenghe, R. Recycling Phosphorus from Agricultural Streams: Grey and Green Solutions. Agronomy 2022, 12, 2938. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlíková, M.; Odehnalová, K.; Zezulka, Š.; Maršálková, E.; Lamaczová, A.; Maršálek, B. Nature-Based Approaches for Managing Bioavailable Phosphorus in Aquatic Ecosystems. Hydrology 2025, 12, 236. https://doi.org/10.3390/hydrology12090236
Pavlíková M, Odehnalová K, Zezulka Š, Maršálková E, Lamaczová A, Maršálek B. Nature-Based Approaches for Managing Bioavailable Phosphorus in Aquatic Ecosystems. Hydrology. 2025; 12(9):236. https://doi.org/10.3390/hydrology12090236
Chicago/Turabian StylePavlíková, Marcela, Klára Odehnalová, Štěpán Zezulka, Eliška Maršálková, Adéla Lamaczová, and Blahoslav Maršálek. 2025. "Nature-Based Approaches for Managing Bioavailable Phosphorus in Aquatic Ecosystems" Hydrology 12, no. 9: 236. https://doi.org/10.3390/hydrology12090236
APA StylePavlíková, M., Odehnalová, K., Zezulka, Š., Maršálková, E., Lamaczová, A., & Maršálek, B. (2025). Nature-Based Approaches for Managing Bioavailable Phosphorus in Aquatic Ecosystems. Hydrology, 12(9), 236. https://doi.org/10.3390/hydrology12090236







