Investigation of Sediment Characteristics and Nutrient Content in Relation to Pilot Dredging at Kis-Balaton Water Protection System (Hungary)
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling and Experiments
2.3. Determination of the Physical and Chemical Parameters of Sediments and Leachates
| Leachate Samples | |||
|---|---|---|---|
| Properties | Standard | Method/Equipment | Investigated Samples |
| Total phosphorus (TP; mg/L) | MSZ EN ISO 6878:2024 [56] | Skalár SA5000—S25 Elemental analyzer | Water leaching from artificial sediment columns I–VI |
| Total nitrogen (TN; mg/L) | MSZ EN 12260:2004 [57] | Elementar VarioTOC S-20-0402 TOC + TN analyzer | |
| Total potassium (TK; mg/L) | MSZ 448-10:1977 [58] | BWB XP Flame photometer | |
| Sediment samples | |||
| Particle size distribution (PSD—clay, silt, and sand content; %) | ISO 11277:2009(E) [59] | Laser diffraction (Malvern Mastersizer 3000) | Disturbed samples I–VII * |
| Water retention (pF; vol%) /hygroscopic moisture content | ISO 11274:2019 [60] | Sand/kaolin box/pressure membrane apparatus/ at CaCl2x6H2O environment | Disturbed samples I–III ** |
| Bulk density (g/cm3) | ISO 11274:2019 [60] | Calculated (dry weight (105 °C)/volume) | Disturbed samples I–III ** |
| Chemistry (pH(Dw) in distilled water) | MSZ-08-0206-2:1978 [61] | MultiLine P4, WTW Multi 350i | Disturbed samples IV–VII ** |
| Ammonium-lactate-soluble P and K (AL-P; AL-K; mg/kg) | MSZ 20135:1999 [62] | UV/VIS Thermo Scientific Genesys spectrophotometer | Disturbed * and core samples I–VII |
| Total nitrogen (TN; mg/kg) | MSZ 20135:1999 [62] | Elementar vario Macro Cube Elemental analyzer | |
| Total organic nitrogen (TON; mg/kg) | MSZ 20135:1999 [62] | Disturbed * and core samples I–VII | |
| Total organic carbon (TOC; %) | MSZ 20135:1999 [62] | Disturbed samples IV–VII * and core samples I–VII | |
| Ca and Mg content (mg/kg) | MSZ-21470-50:1998 [63] | Aqua regia microwave digestion/ICP-OES | Disturbed samples IV–VII ** |
| Carbonates (CaCO3; %) | MSZ-08-0206-2:1978 [61] | Scheibler method, calcimeter | Disturbed samples IV–VI ** |
| CDB and HCl extractable-P (CDB-P and HCl-P; mg/kg) | [64] | UV/VIS Thermo Scientific Genesys spectrophotometer | Disturbed samples IV–VII * |
| Total phosphorus and potassium (TP, TK; mg/kg) | MSZ-21470-50:1998 [63] | Aqua regia microwave digestion/ICP-OES | Disturbed samples IV–VII ** |
2.4. Statistical Analysis and Calculation
2.5. Comparison of Column Experiment Leachate, Experimental Drying, and Relevant Monitoring Points of Water Quality Data
3. Results
3.1. Physical Properties of the Sediment (Disturbed Samples of I–VII Sampling Sites)
3.2. Chemical Properties of the Sediment
3.2.1. Core Samples
3.2.2. Comparison of Some Chemical Characteristics of Disturbed and Core Samples
3.2.3. Column Experiment
3.3. Evaluation of the Nutrient Loss in the Columns and Settling Pits
3.4. Relationships Between P Fractions, Loss, and Sediment Properties
4. Discussion
4.1. Physical Properties of the Sediment
4.2. Basic Chemical Properties of the Sediments
4.3. Amount of Nutrients in the Sediments
4.4. Phosphorus Fractions in the Sediment
4.5. Evaluation of the Nutrient Loss
4.6. Relationships Between P Loss and Sediment Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TP | Total phosphorus content (mg/kg or mg/L); |
| TK | Total potassium content (mg/kg or mg/L); |
| TN | Total nitrogen (mg/kg or mg/L); |
| AL-P | Ammonium-lactate-soluble phosphorus (mg/kg); |
| AL-K | Ammonium-lactate-soluble potassium; |
| TOC | Total organic carbon content (%); |
| DOC | Dissolved organic carbon content (%); |
| TSS | Total suspended solid (mg/L); |
| CDB-P | Citrate–dithionite–bicarbonate-soluble phosphorus (mg/kg); |
| HCl-P | Hydrochloric-acid-soluble phosphorus content (mg/kg); |
| PWP | Permanent wilting point (vol%); |
| NaOH-P | Natrium-hydroxide-soluble phosphorus content (mg/kg); |
| FC | Field capacity (vol%); |
| NH4Cl-P | Ammonium-chloride-soluble phosphorus (mg/kg); |
| TPl | Phosphate loss by leaching (mg/L); |
| TKl | Potassium loss by leaching (mg/L); |
| TNl | Nitrogen loss by leaching (mg/L); |
| SRP | Soluble reactive phosphorus (mg/L); |
| EC | Electrical conductivity (µS/cm). |
Appendix A
| Properties | Mean | Std. Dev. | |
|---|---|---|---|
| pH | - | 8.2 | 0.3 |
| EC | µS/s | 730.8 | 117.9 |
| HCO3 | mg/L | 394.1 | 87.5 |
| CO3 | 6.0 | 11.4 | |
| TSS | 39.1 | 40.1 | |
| TP | 0.2 | 0.1 | |
| PO4-P | 0.1 | 0.0 | |
| Na | 28.5 | 8.9 | |
| K | 4.6 | 0.8 | |
| TOC | 10.0 | 3.4 | |
| DOC | 9.4 | 2.5 | |
| TN | 2.4 | 0.9 | |

| Sample | Depth | Clay (<7 µm) | Silt (7–50 µm) | Sand (>50 µm) | ||||
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | |||
| Column experiment 1. (at the beginning) | I | 0–180 | 17.9 | 0.5 | 54.3 | 1.1 | 27.8 | 1.6 |
| II | 0–180 | 28.7 | 0.9 | 59.9 | 0.3 | 11.4 | 0.7 | |
| III | 0–180 | 30.6 | 0.2 | 59.4 | 0.3 | 10.0 | 0.4 | |
| IV | 0–180 | 26.3 | 3.6 | 61.1 | 0.5 | 12.7 | 3.2 | |
| V | 0–180 | 28.9 | 0.1 | 59.1 | 0.7 | 11.9 | 0.6 | |
| VI | 0–180 | 25.9 | 2.7 | 61.0 | 0.8 | 13.2 | 1.9 | |
| VII | 0–180 | 25.3 | 0.1 | 55.6 | 0.2 | 19.2 | 0.3 | |
| I | 0–20 | 20.5 | 0.8 | 56.8 | 1.1 | 22.7 | 1.8 | |
| 40–60 | 20.7 | 0.7 | 57.5 | 0.9 | 21.8 | 1.4 | ||
| 80–100 | 20.9 | 1.0 | 57.6 | 1.1 | 21.5 | 2.1 | ||
| II | 0–20 | 33.5 | 1.0 | 59.0 | 0.3 | 7.6 | 0.8 | |
| 40–60 | 33.7 | 1.5 | 59.4 | 0.6 | 7.0 | 0.9 | ||
| 80–100 | 33.2 | 1.9 | 59.0 | 1.1 | 7.9 | 2.3 | ||
| III | 0–20 | 35.4 | 0.6 | 58.1 | 0.6 | 6.6 | 1.2 | |
| 40–60 | 35.5 | 0.5 | 58.4 | 0.8 | 6.2 | 1.3 | ||
| 80–100 | 35.1 | 0.3 | 58.2 | 0.4 | 6.8 | 0.2 | ||
| IV | 0–20 | 29.5 | 0.4 | 59.0 | 0.3 | 11.5 | 0.2 | |
| 40–60 | 29.8 | 0.4 | 59.2 | 0.5 | 10.9 | 0.8 | ||
| 80–100 | 29.3 | 0.2 | 58.7 | 0.7 | 11.9 | 0.8 | ||
| V | 0–20 | 34.7 | 0.7 | 56.3 | 0.7 | 9.0 | 1.2 | |
| 40–60 | 34.2 | 1.0 | 55.9 | 0.9 | 9.8 | 1.8 | ||
| 80–100 | 34.5 | 1.4 | 55.4 | 1.5 | 10.1 | 2.3 | ||
| VI | 0–20 | 33.2 | 0.7 | 53.3 | 3.2 | 13.6 | 3.8 | |
| 40–60 | 33.3 | 0.6 | 54.9 | 2.1 | 11.8 | 2.7 | ||
| 80–100 | 33.7 | 0.9 | 55.7 | 1.5 | 10.6 | 2.3 | ||
| VII | 0–20 | 29.5 | 0.1 | 55.1 | 0.5 | 15.4 | 0.6 | |
| 40–60 | 29.4 | 0.1 | 54.9 | 0.5 | 15.8 | 0.5 | ||
| 80–100 | 29.5 | 0.4 | 54.9 | 0.4 | 15.5 | 0.6 | ||
| Sample | TP | TK | Ca | Mg | AL_P | AL_K | TN | TOC | CDB-P | HCl-P | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg | % | mg/kg | ||||||||||||||||||||
| Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | |||
| Column experiment 1. beginning) | I | 0–130 | 102.3 | 14.9 | 324.7 | 5.1 | 2252.3 | 121.9 | ||||||||||||||
| II | 0–130 | 113.1 | 18.0 | 345.5 | 10.7 | 2803.7 | 376.3 | |||||||||||||||
| III | 0–130 | 182.1 | 25.4 | 238.4 | 9.8 | 2029.5 | 53.8 | |||||||||||||||
| IV | 0–130 | 854.0 | 9.0 | 10,644.0 | 339.0 | 82,148.0 | 792.0 | 10708.0 | 17.0 | 328.5 | 24.2 | 2794.0 | 35.1 | 5.3 | 0.1 | 222.7 | 41.6 | 188.7 | 84.4 | |||
| V | 0–130 | 879.0 | 9.0 | 7824.0 | 1623.0 | 133,064.0 | 2147.0 | 9323.0 | 140.0 | 234.0 | 7.8 | 3213.0 | 55.1 | 7.1 | 0.0 | 182.3 | 19.2 | 139.5 | 36.7 | |||
| VI | 0–130 | 876.0 | 8.0 | 7047.0 | 149.0 | 128,661.0 | 1218.0 | 9370.0 | 49.0 | 266.0 | 1.6 | 2858.0 | 139.2 | 6.8 | 0.1 | 158.4 | 13.3 | 84.5 | 13.8 | |||
| VII | 0–130 | 971.0 | 9.0 | 7767.0 | 376.0 | 66,471.0 | 619.0 | 10,616.0 | 92.0 | 411.2 | 52.2 | 270.8 | 18.6 | 3463.7 | 75.0 | 5.0 | 0.0 | 215.9 | 44.8 | 194.5 | 68.9 | |
| Column experiment 2. (at the end) | I | 0–20 | 95.1 | 34.2 | 302.2 | 3.9 | 2793.3 | 111.5 | ||||||||||||||
| 40–60 | 71.9 | 59.6 | 295.2 | 6.6 | 2793.3 | 51.3 | ||||||||||||||||
| 80–100 | 47.1 | 7.8 | 303.2 | 8.3 | 2746.7 | 35.1 | ||||||||||||||||
| II | 0–20 | 181.6 | 184.3 | 296.3 | 41.7 | 3132.5 | 498.3 | |||||||||||||||
| 40–60 | 155.2 | 143.2 | 297.1 | 40.0 | 3177.5 | 460.0 | ||||||||||||||||
| 80–100 | 135.0 | 45.6 | 305.5 | 38.4 | 3177.5 | 467.1 | ||||||||||||||||
| III | 0–20 | 372.9 | 105.2 | 217.7 | 4.6 | 2410.0 | 70.7 | |||||||||||||||
| 40–60 | 393.0 | 66.9 | 215.6 | 19.0 | 2430.0 | 0.0 | ||||||||||||||||
| 80–100 | 328.5 | 44.2 | 222.0 | 10.4 | 2410.0 | 28.3 | ||||||||||||||||
| IV | 0–20 | 316.6 | 9.4 | 224.1 | 11.3 | 2501.3 | 96.0 | 5.2 | 0.0 | 101.3 | 213.4 | 0.0 | ||||||||||
| 40–60 | 331.7 | 9.1 | 226.8 | 28.1 | 2570.0 | 40.0 | 5.3 | 0.1 | 120.6 | 194.3 | 0.0 | |||||||||||
| 80–100 | 353.4 | 37.2 | 213.9 | 14.1 | 2514.0 | 61.6 | 5.3 | 0.0 | 121.7 | 211.3 | 0.0 | |||||||||||
| V | 0–20 | 225.2 | 49.7 | 216.5 | 12.8 | 3082.0 | 290.8 | 7.2 | 0.1 | 113.7 | 70.2 | 0.0 | ||||||||||
| 40–60 | 232.1 | 25.8 | 209.5 | 6.3 | 2902.0 | 50.5 | 7.1 | 0.0 | 124.3 | 84.0 | 0.0 | |||||||||||
| 80–100 | 241.4 | 12.0 | 217.4 | 12.2 | 2958.7 | 137.4 | 7.2 | 0.1 | 170.5 | 106.8 | 0.0 | |||||||||||
| VI | 0–20 | 289.4 | 16.2 | 224.4 | 5.0 | 2569.3 | 134.4 | 6.4 | 0.8 | 195.4 | 114.2 | 0.0 | ||||||||||
| 40–60 | 252.6 | 37.9 | 250.1 | 7.4 | 2471.3 | 96.7 | 6.7 | 0.1 | 174.7 | 72.3 | 0.0 | |||||||||||
| 80–100 | 258.5 | 37.9 | 227.6 | 11.7 | 2566.0 | 218.2 | 6.7 | 0.1 | 216.1 | 123.8 | 0.0 | |||||||||||
| VII | 0–20 | 977.0 | 7366.0 | 66,593.0 | 10,676.0 | 0.0 | 522.8 | 16.9 | 301.5 | 13.7 | 3590.3 | 303.0 | 5.1 | 0.1 | 240.3 | 43.1 | 73.9 | 86.4 | ||||
| 40–60 | 961.0 | 7822.0 | 6,5800.0 | 10,510.0 | 0.0 | 440.5 | 57.3 | 294.7 | 8.2 | 3480.3 | 121.8 | 5.0 | 0.1 | 275.2 | 40.3 | 184.1 | 99.2 | |||||
| 80–100 | 974.0 | 8112.0 | 67,020.0 | 10,661.0 | 0.0 | 448.4 | 70.0 | 288.2 | 12.5 | 3320.3 | 93.5 | 4.9 | 0.1 | 238.0 | 9.0 | 107.7 | 16.9 | |||||
| Statistical Analysis | Sampling Site | Significance | Tamhane’s Test Sample Types * | |||||
|---|---|---|---|---|---|---|---|---|
| Properties | Levene’s Test | Univariate Anova | Core | Dist. 1. | Dist. 2. | |||
| Column experiment 1. ** (at the beginning) | A | I–VII | sand | 0.001 | 0.001 | |||
| Column experiment 2. ** (at the end) | sand | 0.001 | 0.001 | |||||
| Core samples | B | I–VII | AL-P | 0.083 | 0.001 | |||
| Column experiment 1.** (at the beginning) | C | I–VII | AL-P, AL-K, TN, TOC | 0.001 | 0.001 | |||
| Comparison of the chemical properties of the disturbed (column experiment) and undisturbed (core) samples | D | I | ALP | 0.006 | 0.095 | a | a | a |
| II | 0.309 | 0.651 | a | a | a | |||
| III | 0.026 | 0.270 | a | ab | b | |||
| IV | 0.056 | 0.029 | a | b | b | |||
| V | 0.306 | 0.001 | a | b | b | |||
| VI | 0.003 | 0.129 | a | a | a | |||
| VII | 0.229 | 0.037 | a | a | a | |||
| I | AL-K | 0.001 | 0.001 | b | a | a | ||
| II | 0.001 | 0.001 | b | a | a | |||
| III | 0.233 | 0.001 | b | ab | a | |||
| IV | 0.007 | 0.896 | a | a | ||||
| V | 0.002 | 0.008 | b | a | ||||
| VI | 0.126 | 0.001 | b | a | ||||
| VII | 0.042 | 0.001 | b | a | a | |||
| I | TN | 0.002 | 0.398 | b | a | a | ||
| II | 0.001 | 0.035 | b | a | a | |||
| III | 0.004 | 0.320 | b | a | a | |||
| IV | 0.010 | 0.001 | b | a | a | |||
| V | 0.001 | 0.006 | b | a | a | |||
| VI | 0.051 | 0.571 | a | a | a | |||
| VII | 0.168 | 1.00 | a | a | a | |||
| IV | TOC | 0.008 | 0.557 | a | a | a | ||
| V | 0.001 | 0.037 | a | a | a | |||
| VI | 0.001 | 0.092 | a | a | a | |||
| VII | 0.295 | 0.993 | a | a | a | |||
References
- Tátrai, I.; Mátyás, K.; Korponai, J.; Paulovits, G.; Pomogyi, P. The role of the Kis-Balaton Water Protection System in the control of water quality of Lake Balaton. Ecol. Eng. 2000, 16, 73–78. [Google Scholar] [CrossRef]
- Szilágyi, F.; Somlyódy, L.; Koncsos, L. Operation of the Kis-Balaton reservoir: Evaluation of nutrient removal rates. Hydrobiologia 1990, 191, 297–306. [Google Scholar] [CrossRef]
- Herodek, S.; Istvánovics, V.; Jolánkai, G.; Csathó, P.; Németh, T.; Várallyay, G. P-cycle in the Balaton catchment—A Hungarian case study. In Phosphorus in the Global Environment; Tiessen, H., Ed.; John Wiley and Sons Ltd.: Chichester, UK, 1995; pp. 275–300. [Google Scholar]
- Hatvani, I.G.; Clement, A.; Kovács, J.; Kovács Székely, I.; Korponai, J. Assessing water-quality data: The relationship between the water quality amelioration of the Lake Balaton and the construction its mitigation wetland. J. Great Lakes Res. 2014, 40, 115–125. [Google Scholar] [CrossRef]
- Somlyódy, L. Eutrophication modeling, management and decision making: The Kis–Balaton Case. Water Sci. Technol. 1998, 37, 165–176. [Google Scholar] [CrossRef]
- Istvánovics, V.; Somlyódy, L. Changes in the cycling of phosphorus in the Upper Kis-Balaton Reservoir following external load reduction. Freshwater Biol. 1999, 41, 147–165. [Google Scholar] [CrossRef]
- Honti, M.; Gao, C.; Istvánovics, V.; Clement, A. Lessons learnt from the long-term management of a large (re)constructed wetland, the Kis-Balaton protection system (Hungary). Water 2020, 12, 659. [Google Scholar] [CrossRef]
- Sisák, I.; Máté, F. A foszfor mozgása a Balaton vízgyűjtőjén (Movement of phosphorus in the catchment area of Lake Balaton). Agrokem Talajtan 1993, 42, 257–270. (In Hungarian) [Google Scholar]
- Sisák, I.; Pomogyi, P. A Zala tápanyag terhelésének vizsgálata (Investigation of the nutrient load of the River Zala). Vízügyi Közlemények 1994, LXXVI, 417–434. (In Hungarian) [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Meerhoff, M.; Lauridsen, T.L.; Jensen, J.P. Shallow lake restoration by nutrient loading reduction—Some recent findings and challenges ahead. Hydrobiologia 2007, 584, 239–252. [Google Scholar] [CrossRef]
- Hatvani, I.G.; Kovács, J.; Márkus, L.; Clement, A.; Hoffmann, R.; Korponai, J. Assessing the relationship of background factors governing the water quality of an agricultural watershed with changes in catchment property (W-Hungary). J. Hydrol. 2015, 521, 460–469. [Google Scholar] [CrossRef]
- Gelencsér, P.; Szilágyi, F.; Somlyódy, L.; Lijklema, L. A Study on the Influence of Sediment in the Phosphorus Cycle in Lake Balaton; IIASA: Laxenburg, Austria, 1982. [Google Scholar]
- Somlyódy, L.; van Traten, G. (Eds.) Modelling and Managing Shallow Lake Eutrophication; Springer: Laxenburg, Austria, 1986; ISBN 3-540-16227-5. [Google Scholar]
- Istvánovics, V.; Somlyódy, L. The role of sediments in P retention of the Kis-Balaton reservoir. Internat. Rev. Hydrobiol. 1998, 83, 225–234. [Google Scholar]
- Istvánovics, V.; Osztoics, A.; Honti, M. Dynamics and ecological significance of daily internal load of phosphorus in shallow Lake Balaton. Hungary. Freshwater Biol. 2004, 49, 232–252. [Google Scholar] [CrossRef]
- de Vicente, I.; Cattaneo, K.; Cruz-Pizarro, L.; Brauer, A.; Guilizzoni, P. Sedimentary phosphate fractions related to calcite precipitation in an eutrophic hardwater lake (Lake Alserio, northern Italy). J. Paleolimnol. 2006, 35, 55–64. [Google Scholar] [CrossRef]
- Orihel, D.M.; Blauch, H.M.; Casson, N.J.; North, R.L.; Parsons, C.T.; Seckar, D.C.M.; Venkiteswaran, J.J. Internal phosphorus loading in Canadian fresh waters: A critical review and data analysis. Can. J. Fish. Aquat. Sci. 2017, 74, 2005–2029. [Google Scholar] [CrossRef]
- Wu, X.; Ma, T.; Du, Y.; Jiang, Q.; Shen, S.; Liu, W. Phosphorus cycling in freshwater lake sediments: Influence of seasonal water level fluctuations. Sci. Total Environ. 2021, 792, 148383. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, M.; Yin, H. Phosphorus release from the sediment of a drinking water reservoir under the influence of seasonal hypoxia. Sci. Total Environ. 2024, 917, 170490. [Google Scholar] [CrossRef]
- Chen, N.; Peng, B.; Hong, H.; Turyaheebwa, N.; Cui, S.; Mo, X. Nutrient enrichment and N:P ratio decline in a coastal bay-river system in southeast China: The need for dual nutrient (N and P) management strategy. Ocean Coast. Manage. 2013, 81, 7–13. [Google Scholar] [CrossRef]
- Horváth, H.; Mátyás, K.; Süle, G.; Présing, M. Contribution of nitrogen fixation to external nitrogen load of water quality control reservoir (Kis-Balaton Water Protection System, Hungary). Hydrobiologia 2013, 702, 255–265. [Google Scholar] [CrossRef][Green Version]
- Ding, S.; Chen, M.; Gong, M.; Fan, X.; Qin, B.; Xu, H.; Gao, S.; Jin, Z.; Tsang, D.C.W.; Zhang, C. Internal phosphorus loading from sediments causes seasonal nitrogen limitation for harmful algal blooms. Sci. Tot. Environ. 2018, 625, 872–884. [Google Scholar] [CrossRef]
- Kovács, J.; Hatvani, I.G.; Korponai, J.; Székely Kovács, I. Morlet wavelet and autocorrelation analysis of long-term data series of the Kis-Balaton water protection system (KBWPS). Ecol. Eng. 2010, 36, 1469–1477. [Google Scholar] [CrossRef]
- Kowalczewska-Madura, K.; Gołdy, R. Internal loading of phosphorus from sediments of Swarzędzkie Lake (Western Poland). Polish J. Environ. Stud. 2009, 18, 635–643. [Google Scholar] [CrossRef]
- Coppens, J.; Özen, A.; Tavşanoğlu, Ü.N.; Erdoğan, S.; Levi, E.E.; Yozgatlıgil, C.; Jeppesen, E.; Beklioğlu, M. Impact of alternating wet and dry periods on long-term seasonal phosphorus and nitrogen budgets of two shallow Mediterranean lakes. Sci. Total Environ. 2016, 563–564, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Liu, L.; Zhang, J.; Cooper, M.; Mortimer, R.J.G.; Pan, G. Effects of elevated sulfate in eutrophic waters on the internal phosphate release under oxic conditions across the sediment water interface. Sci. Total Environ. 2021, 790, 1480102021. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, P.; Søndergaard, M.; Jeppesen, E. Resuspension in a shallow eutrophic lake. Hydrobiologia 1992, 228, 101–109. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of the sediment and internal loading of phosphorus in lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Förster, W.; Scholten, J.C.; Schubert, M.; Knoeller, K.; Classen, N.; Lechelt, M.; Richard, J.-H.; Rochweder, U.; Zunker, I.; Wanner, S.C. Phosphorus supply to a eutrophic artificial lake: Sedimentary versus groundwater sources. Water 2021, 13, 563. [Google Scholar] [CrossRef]
- Andrieux-Loyer, F.; Aminot, A. Assessing exchangeable phosphate and related data in coastal sediments: Theoretical and practical considerations. Estuar. Coast. Shelf Sci. 2023, 281, 108218. [Google Scholar] [CrossRef]
- Jensen, H.S.; Andersen, F.O. Importance of temperature, nitrate, and pH for phosphate release from aerobic sediments of four shallow, eutrophic lakes. Limnol. Oceanogr. 1992, 37, 577–589. [Google Scholar] [CrossRef]
- Istvánovics, V.; Herodek, S.; Szilágyi, F. Phosphate adsorption by different sediment fractions in Lake Balaton and its protecting reservoirs. Water Res. 1989, 23, 1357–1366. [Google Scholar] [CrossRef]
- Özen, A.; Karapinar, B.; Kucuk, I.; Jeppesen, E.; Beklioglu, M. Drought-induced changes in nutrient concentrations and retention in two shallow Mediterranean lakes subjected to different degrees of management. Hydrobiologia 2010, 646, 61–72. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, Q.; Buyang, S.; Cui, H.; Zhang, S. Enrichment of bioavailable phosphorus in fine particles when sediment resuspension hinders the ecological restoration of shallow eutrophic lakes. Sci. Total Environ. 2020, 710, 135672. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Retention and internal loading of phosphorus in shallow, eutrophic lakes. Review. Sci. World J. 2001, 1, 427–442. [Google Scholar] [CrossRef]
- Toner, J.D.; Catling, D.C. A carbonate-rich lake solution to the problem of the origin of life. Proc. Natl. Acad. Sci. USA 2019, 117, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.Ø.; Jensen, H.S. Regeneration of inorganic phosphorus and nitrogen from decomposition of seston in a freshwater sediment. Hydrobiologia 1992, 228, 71–81. [Google Scholar] [CrossRef]
- Cooke, G.D.; Heath, R.T.; Kennedy, R.H.; McComas, M.R. Effects of Diversion and Alum Application on Two Eutrophic Lakes; Ecological Research Series; Environmental Research Laboratory. U.S. Environmental Protection Agency: Corvallis, OR, USA, 1978. [Google Scholar]
- Balázs, L.; Istvánovics, V. P removal by reed harvest in the Kis-Balaton reservoir—dream or reality? Int. Rev. Hydrobiol. 1998, 83, 635–638. [Google Scholar]
- McAuliffe, T.F.; Lukatelich, R.J.; McComb, A.J.; Qiu, S. Nitrate applications to control phosphorus release from sediments of a shallow eutrophic estuary: An experimental evaluation. Mar. Freshwater Res. 1998, 49, 463–473. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.P. Hypolimnetic nitrate treatment to reduce internal phosphorus loading in a stratified lake. Lakes Reserv. Manage. 2000, 16, 195–204. [Google Scholar] [CrossRef]
- Murphy, T.P.; Hall, K.G.; Northcote, T.G. Lime treatment of a hardwater lake to reduce eutrophication. Lake Reserv. Manage. 1988, 4, 51–62. [Google Scholar] [CrossRef]
- Vörös, L.; Tóth, G.I.; Látrányi-Lovász, Z.; Somogyi, B. A Balaton szalinitásának hosszútávú változása (1891-2022) (Long term changes of salinity in Lake Balaton (1891-2022). Hidrológiai Közlöny. 2024, 104, 48–60. [Google Scholar] [CrossRef]
- Bormans, M.; Maršálek, B.; Jančula, D. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: A review. Aquatic Ecology. 2015, 50, 407–422. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Kristensen, P.; Søndergaard, M.; Mortensen, E.; Sortkjær, O.; Olrik, K. Fish manipulation as a lake restoration tool in shallow, eutrophic, temperate lakes 2: Threshold levels, long-term stability and conclusions. Hydrobiologia 1990, 200, 219–227. [Google Scholar] [CrossRef]
- Søndergaard, M.; Lauridsen, T.L.; Johansson, L.S.; Jeppessen, E. Repeated fish removal to restore Lakes: Case study of Lake Væng, Denmark—Two biomanipulations during 30 years of monitoring. Water 2017, 9, 43. [Google Scholar] [CrossRef]
- Dushyantha, N.; Ratnayake, N.; Panagoda, H.; Jayawardena, C.; Ratnayake, A.S. Phosphate mineral accumulation in lake sediment to form a secondary phosphate source: A case study in lake sediment around Eppawala Phosphate Deposit (EPD) in Sri Lanka. Int. J. Sed. Res. 2021, 36, 532–541. [Google Scholar] [CrossRef]
- Máté, F. A Balaton Meder Recens Üledékeinek Térképezése (Mapping of Modern Lake Balaton Bottom Sediments); Annual report of 1985; Hungarian Geological Institute: Budapest, Hungary, 1987; pp. 367–379. (In Hungarian) [Google Scholar]
- Istvánovics, V. Fractional composition, adsorption and release of sediment phosphorus in the Kis-Balaton reservoir. Water Res. 1994, 28, 717–726. [Google Scholar] [CrossRef]
- Istvánovics, V.; Kovács, A.; Vörös, L.; Herodek, S.; Pomogyi, P. Phosphorus cycling in a large, reconstructed wetland, the lower Kis-Balaton Reservoir (Hungary). Verh. int. Ver. Limnol. 1997, 26, 323–329. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Seasonal response of nutrients to reduced phosphorus loading in 12 Danish lakes. Freshwater Biology 2005, 50, 1605–1615. [Google Scholar] [CrossRef]
- Máté, F. Kis-Balaton Vízvédelmi Rendszer Talajviszonyai (Soils of the Kis-Balaton Water Protection System); Hungarian Academy of Sciences, Research Institute of Soil Science and Agrochemistry: Budapest, Hungary, 1985. (In Hungarian) [Google Scholar]
- Kosári-Tarnik, E. Sediment Removal Design in the Kis-Balaton Reservoir. Bachelor’s Thesis, Budapest University of Technology and Economics, Budapest, Hungary, 2019. (In Hungarian). [Google Scholar]
- Labancz, V.; Hernádi, H.; Barna, G.; Bakacsi, Z.; Szegi, T.; Kocsis, M.; Makó, A. The effect of different water types commonly applied during laser diffraction measurement on the particle size distribution of soils. Hun. Geo. Bull. 2024, 73, 355–377. [Google Scholar] [CrossRef]
- Makó, A.; Szabó, B.; Rajkai, K.; Szabó, J.; Bakacsi, Z.; Labancz, V.; Hernádi, H.; Barna, G. Evaluation of soil texture determination using soil fraction data resulting from laser diffraction method. Int. Agrophys. 2019, 33, 445–454. [Google Scholar] [CrossRef]
- MSZ EN ISO 6878:2024; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method (ISO 6878:2004 English Version). Hungarian Standards Institution: Budapest, Hungary, 2024.
- MSZ EN ISO 12260:2024; Water Quality—Determination of Nitrogen—Determination of Bound Nitrogen (TNb), Following Oxidation to Nitrogen Oxides. Hungarian Standards Institution: Budapest, Hungary, 2024.
- MSZ 448-10:1977; Water Quality—Determination of Sodium and Potassium Ion in Drinking Water by Flame Photometry. Hungarian Standards Institution: Budapest, Hungary, 1977. (In Hungarian)
- ISO 11277:2009(E2); Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 11274:2019; Soil Quality—Determination of the Water-Retention Characteristic—Laboratory Methods. International Organization for Standardization: Geneva, Switzerland, 2019.
- MSZ-08-0206-2:1978; Evaluation of Some Chemical Properties of the Soil. Laboratory Tests. Hungarian Standards Institution: Budapest, Hungary, 1978. (In Hungarian)
- MSZ 20135:1999; Determination of the Soluble Nutrient Element Content of the Soil. Hungarian Standards Institution: Budapest, Hungary, 1999. (In Hungarian)
- MSZ 21470-50:1998; Environment Protection. Testing of Soils. Determination of Total and Soluble Toxic Element, Heavy Metal and Chromium(VI) Content. Hungarian Standards Institution: Budapest, Hungary, 1998; (In Hungarian with English Summary).
- Anschutz, P.; Deborde, J. Spectrophotometric determination of phosphate in matrices from sequential leaching of sediments. Limnol. Oceanogr. Meth. 2016, 14, 245–256. [Google Scholar] [CrossRef]
- Di Gléria, J.; Klimes-Szmik, A.; Dvoracsek, M. Bodenphysik und Bodenkolloidik; Akadémiai Kiadó: Budapest, Hungary, 1962; p. 795. [Google Scholar]
- Murphey, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Rostási, Á.; Rácz, K.; Fodor, M.A.; Topa, B.; Molnár, Z.; Weiszburg, T.G.; Pósfai, M. Pathways of carbonate sediment accumulation in a large, shallow lake. Front. Earth Sci. 2022, 10, 1067105. [Google Scholar] [CrossRef]
- Istvánovics, V. Seasonal variation of phosphorus release from the sediment of shallow Lake Balaton (Hungary). Water Res. 1988, 22, 1473–1482. [Google Scholar] [CrossRef]
- Andrieux-Loyer, F.; Aminot, A. Phosphorus forms related to sediment grain size and geochemical characteristics in French coastal areas. Estuar. Coast. Shelf Sci. 2001, 52, 617–629. [Google Scholar] [CrossRef]
- Molnár, Z.; Pekker, P.; Dódony, I.; Pósfai, M. Clay minerals affect calcium (magnesium) carbonate precipitation and aging. Earth Planet. Sc. Lett. 2021, 567, 116971. [Google Scholar] [CrossRef]
- Molnár, Z.; Dódony, I.; Pósfai, M. Transformation of amorphous calcium carbonate in the presence of magnesium, phosphate, and mineral surfaces. Geochim. Cosmochim. Acta 2023, 345, 90–101. [Google Scholar] [CrossRef]
- Debreczeni, B. Kis Agrokémiai Útmutató (Agrochemical Guide); Mezőgazdasági Kiadó: Budapest, Hungary, 1979. (In Hungarian) [Google Scholar]
- Debreczeni, B.; Németh, T. (Eds.) Az Országos Műtrágyázási Tartamkísérletek (OMTK) Kutatási Eredményei (1967–2001). (Research Results of the National Fertilization Experiments (1967–2001)); Akadémia Kiadó: Budapest, Hungary, 2009. (In Hungarian) [Google Scholar]
- Deng, P.; Yi, Q.; Zhang, J.; Wang, C.; Chen, Y.; Zhang, T.; Shi, W. Phosphorus partitioning in sediments by particle size distribution in shallow lakes: From its mechanisms and patterns to its ecological implications. Sci. Tot. Environ. 2022, 814, 152753. [Google Scholar] [CrossRef]
- Pettersson, K.; Istvánovics, V. Sediment phosphorus in Lake Balaton—Forms and mobility. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1988, 30, 25–41. [Google Scholar]
- Fraters, D.; Boom, G.J.F.L.; Boumans, L.J.M.; de Weerd, H.; Wolters, M. Extraction of soil solution by drainage centrifugation-effects of centrifugal force and time of centrifugation on soil moisture recovery and solute concentration in soil moisture of loss subsoils. Environ. Monit. Assess. 2017, 189, 83. [Google Scholar] [CrossRef]
- Tyler, G. Effects of sample pretreatment and sequential fractionation by centrifuge drainage on concentration of minerals in a calcareous soil solution. Geoderma 2000, 94, 59–70. [Google Scholar] [CrossRef]
- Istvánovics, V.; Somlyódy, L.; Clement, A. Cyanobacteria-mediated internal eutrophication in shallow Lake Balaton after load reduction. Water Res. 2002, 36, 3314–3322. [Google Scholar] [CrossRef]
- Jensen, H.S.; Kristensen, P.; Jeppesen, E.; Skytthe, A. Iron:phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiologia 1992, 235, 731–743. [Google Scholar] [CrossRef]
- Vink, S.; Chambers, R.M.; Smith, S.V. Distribution of phosphorus in sediments from Tomales Bay, California. Mar. Geol. 1997, 139, 157–179. [Google Scholar] [CrossRef]
- Liu, L.; Tang, W.; Huang, J.; Teasdale, P.R.; Shu, L.; Zhang, H. In situ, high-resolution measurement of labile phosphate in sediment porewater using the DET technique coupled with optimized imaging densitometry. Environ. Res. 2020, 191, 110107. [Google Scholar] [CrossRef]
- Søndergaard, M.; Windolf, J.; Jeppesen, E. Phosphorus fractions and profiles in the sediment of shallow Danish lakes as related to phosphorus load, sediment composition and lake chemistry. Water Res. 1996, 30, 992–1002. [Google Scholar] [CrossRef]
- Søndergaard, M. Seasonal variation in the loosely sorbed phosphorus fraction of the sediment of shallow and hypereutrophic lake. Envir. Geol. Water Sci. 1988, 11, 115–121. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Bu, Q.; Zhou, X.; Wu, F. Effects of particle size, organic matter and ionic strength on the phosphate sorption in different trophic lake sediment. J. Hazard. Mater. 2006, 128, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, Q.; Ma, T. Geochemical processes of phosphorus-iron on sediment-water interface during discharge of groundwater to freshwater lakes: Kinetic and mechanistic insights. Sci. Tot. Environ. 2023, 901, 165962. [Google Scholar] [CrossRef] [PubMed]
- Füleky, G. Tápanyaggazdálkodás (Nutrient Management); Mezőgazdasági Kiadó: Budapest, Hungary, 1999. (In Hungarian) [Google Scholar]
- Li, J.; Zuo, Q. Forms of nitrogen and phosphorus in suspended solids: A case study of Lihu Lake, China. Sustainability 2020, 12, 5026. [Google Scholar] [CrossRef]
- Oláh, J. Structural and functional quantification in a series of Hungarian hypertrophic shallow lakes. In Hypertrophic Ecosystems. Developments in Hydrobiology; Barica, J., Mur, L.R., Eds.; Springer: Dordrecht, Switzerland, 1980; Volume 2, pp. 191–202. [Google Scholar] [CrossRef]
- Sasaki, K.; Noriki, S.; Tsunogai, S. Vertical distribution of interstitial phosphate and fluoride in anoxic sediment: Insight into the formation of an authigenic fluoro-phosphorus compound. Geochem. J. 2001, 35, 295–306. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Guo, M.; Duan, X.; Zhao, J.; Hua, Y.; Zhou, Y.; Liu, G.; Dionysiou, D.D. Distribution of organic phosphorus species in sediment profiles of shallow lakes and its effect on photo-release of phosphate during sediment. Environ. Int. 2019, 130, 104916. [Google Scholar] [CrossRef]
- Herodek, S.; Istvánovics, V. Mobility of phosphorus fractions in the sediments of Lake Balaton. Hydrobiologia 1986, 135, 149–154. [Google Scholar] [CrossRef]
- Hieltjes, A.H.M.; Lijklema, L. Fractionation of inorganic phosphates in calcareous sediments. J. Environ. Qual. 1980, 9, 405–407. [Google Scholar] [CrossRef]
- Boers, P.C.M. The influence of pH on phosphate release from lake sediments. Water Res. 1991, 25, 309–311. [Google Scholar] [CrossRef]
- Gerke, J. Humic (organic matter)–Al(Fe)–phosphate complexes: An underestimated phosphate form in soils and source of plant-available phosphate. Soil Sci. 2010, 175, 417–425. [Google Scholar] [CrossRef]
- Turner, B.L.; Frossard, E.; Baldwin, D.S. (Eds.) Organic Phosphorus in the Environment; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Li, Z.; Tang, H.; Xiao, Y.; Zhao, H.; Li, Q.; Ji, F. Factors influencing phosphorus adsorption onto sediment in a dynamic environment. J. Hydro-Environ. Res. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Meng, J.; Yao, Q.; Yu, Z. Particulate phosphorus speciation and phosphate adsorption characteristics associated with sediment grain size. Ecol. Eng. 2014, 70, 140–145. [Google Scholar] [CrossRef]
- Bridghem, S.C.; Johnston, C.A.; Schubauer-Berigan, J.P.; Weishamples, P. Phosphorus sorption dynamics in soils and coupling with surface and pore water in riverine wetlands. Soil Sci. Am. J. 2001, 65, 577–588. [Google Scholar] [CrossRef]
- Wen, S.; Lu, Y.; Luo, C.; An, S.; Dai, J.; Liu, Z.; Zhong, J.; Du, Y. Adsorption of humic acids to lake sediments: Compositional fractionation inhibitory effect of phosphate, and implications for lake eutrophication. J. Hazard. Mater. 2022, 433, 128791. [Google Scholar] [CrossRef]
- Sundman, A.; Karlsson, T.; Sjöberg, S.; Persson, P. Impact of iron-organic matter complexes on aqueous phosphate concentrations. Chem. Geol. 2016, 426, 109–117. [Google Scholar] [CrossRef]
- Murphy, T.P.; Hall, K.J.; Yesaki, I. Coprecipitation of phosphate with calcite in a naturally eutrophic lake. Limnol. Oceanogr. 1983, 28, 58–69. [Google Scholar] [CrossRef]
- Gächter, R.; Meyer, J.S.; Mares, A. Contribution of bacteria to release and fixation of phosphorus in lake sediments. Limnol. Oceanogr. 1988, 33, 1542–1558. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikkuta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Petticrew, E.L.; Arocena, J.M. Evaluation of iron-phosphate as a source on internal lake phosphorus loadings. Sci. Total Environ. 2001, 266, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, P.; Li, S.; Tang, H.; Liu, H. The low TN:TP ratio, a cause or a result of Microcystis blooms? Water Res. 2003, 37, 2073–2080. [Google Scholar] [CrossRef]
- Li, H.; Song, C.; Yang, L.; Qin, H.; Cao, X.; Zhou, Y. Phosphorus supply pathways and mechanisms in shallow lakes with different regime. Water Res. 2021, 193, 116886. [Google Scholar] [CrossRef] [PubMed]
- Csermák, K.; Máté, F. A Balaton Talaja (Soil of Lake Balaton); Pannon University, Georgicon Faculty of Agriculture: Keszthely, Hungary, 2004; ISBN 9639495441. (In Hungarian) [Google Scholar]
- Kocsis, M.; Pásztor, L.; Makó, A.; Kassai, P.; Csermák, K.; Csermák, A.; Aradvári-Tóth, E.; Szatmári, G. Geospatial data on the sediments of Lake Balaton. Sci. Data 2024, 11, 91. [Google Scholar] [CrossRef]
- Szatmári, G.; Kocsis, M.; Makó, A.; Pásztor, L.; Bakacsi, Z. Joint spatial modelling of nutrients and their ratio in the sediments of Lake Balaton (Hungary): A multivariate geostatistical approach. Water 2022, 14, 361. [Google Scholar] [CrossRef]




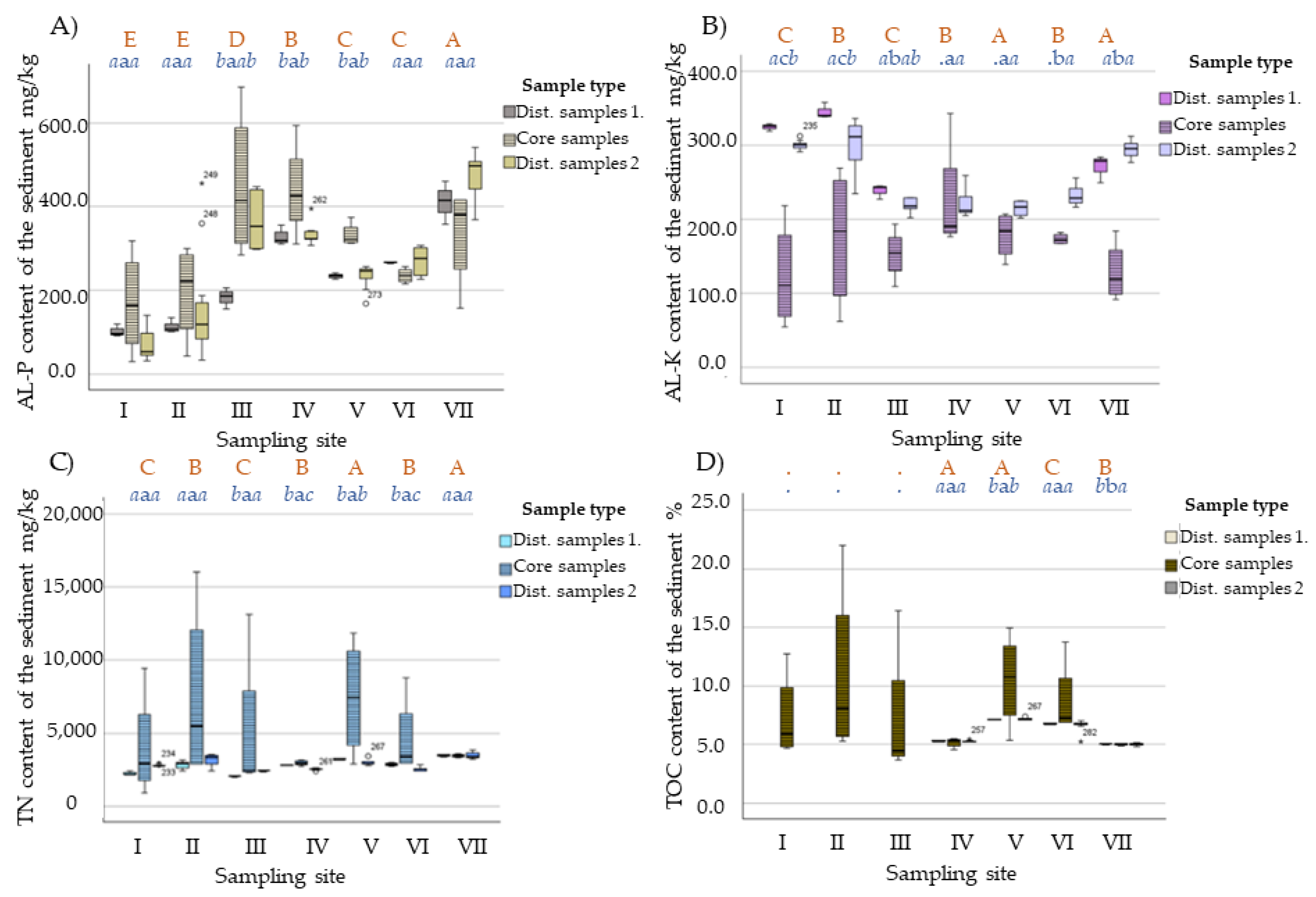


| Core Sample | Depth (cm) | AL-P | AL-K | TN | TOC | Core Sample | Depth (cm) | AL-P | AL-K | TN | TOC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg | % | mg/kg | % | ||||||||
| I | 0–15 | 211.8 | 218.4 | 3121.0 | 7.0 | IV | 0–15 | 430.6 | 342.8 | 3143.0 | 5.5 |
| 15–30 | 116.2 | 138.6 | 9448.0 | 12.7 | 15–30 | 521.2 | 194.6 | 2878.0 | 5.4 | ||
| 30–60 | 318.6 | 83.0 | 2647.0 | 4.7 | 30–60 | 311.2 | 176.6 | 2736.0 | 5.2 | ||
| 60– | 28.7 | 54.7 | 908.0 | 4.8 | 60- | 594.4 | 186.5 | 3045.0 | 4.5 | ||
| II | 0–15 | 268.9 | 269.2 | 2886.0 | 6.1 | V | 0–15 | 312.4 | 167.3 | 5466.0 | 9.6 |
| 15–30 | 299.7 | 235.7 | 8104.0 | 10.1 | 15–30 | 373.6 | 201.1 | 2875.0 | 5.4 | ||
| 30–60 | 42.7 | 131.8 | 16,054.0 | 22.0 | 30–60 | 315.7 | 207.1 | 9369.0 | 11.9 | ||
| 60– | 172.8 | 62.1 | 2899.0 | 5.3 | 60- | 326.4 | 139.0 | 11,857.0 | 14.9 | ||
| III | 0–15 | 340.7 | 156.9 | 2299.0 | 4.6 | VI | 0–15 | 241.8 | 167.6 | 3869.0 | 7.6 |
| 15–30 | 490.1 | 109.4 | 2344.0 | 4.4 | 15–30 | 226.6 | 176.4 | 2925.0 | 6.9 | ||
| 30–60 | 686.4 | 193.4 | 2591.0 | 3.7 | 30–60 | 255.3 | 182.3 | 2933.0 | 6.9 | ||
| 60– | 284.5 | 151.5 | 13,119.0 | 16.4 | 60- | 215.3 | 167.2 | 8779.0 | 13.7 | ||
| VII | 0–15 | 412.0 | 105.9 | 3463.7 | 5.0 | ||||||
| 15–30 | 415.8 | 91.7 | 3320.3 | 4.9 | |||||||
| 30–60 | 345.6 | 184.0 | 3480.3 | 5.0 | |||||||
| 60- | 156.0 | 132.4 | 3590.3 | 5.1 | |||||||
| Site | Depth | TP | TK | TN | AL-P | AL-K | Ca | Mg | AL-P/TP * | CDB-P | HCl-P | pH | TOC | CaCO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg | - | mg/kg | - | % | ||||||||||
| IV | 0–130 | 854.0 | 10,644.0 | 2794.0 | 328.5 | 221.6 | 82,148 | 10708 | 26 (33) | 222.7 | 188.7 | 7.7 | 5.3 | 20.3 |
| 0–20 | 2501.3 | 316.6 | 224.1 | 101.3 | 213.4 | 5.2 | ||||||||
| 40–60 | 2570.0 | 331.7 | 226.8 | 120.6 | 194.4 | 5.3 | ||||||||
| 80–100 | 2514.0 | 353.4 | 213.9 | 121.7 | 211.3 | 5.3 | ||||||||
| V | 0–130 | 879.4 | 7824.3 | 3213.0 | 234.0 | 214.5 | 133,064 | 9323 | 18 (22) | 182.3 | 139.0 | 7.9 | 7.1 | 33.5 |
| 0–20 | 3082.0 | 225.2 | 216.5 | 113.7 | 70.2 | 7.2 | ||||||||
| 40–60 | 2902.0 | 232.1 | 209.5 | 124.3 | 84.0 | 7.1 | ||||||||
| 80–100 | 2958.7 | 241.4 | 217.4 | 170.5 | 106.8 | 7.2 | ||||||||
| VI | 0–130 | 875.8 | 7047.1 | 2858.0 | 266.0 | 234.0 | 128,661 | 9353 | 21 (19) | 158.4 | 84.5 | 8.0 | 6.8 | 32.0 |
| 0–20 | 2569.3 | 289.4 | 224.4 | 195.4 | 114.2 | 6.4 | ||||||||
| 40–60 | 2471.3 | 252.6 | 250.1 | 174.7 | 72.3 | 6.7 | ||||||||
| 80–100 | 2566.0 | 258.5 | 227.6 | 216.1 | 123.8 | 6.7 | ||||||||
| VII | 0–130 | 970.5 | 7766.6 | 3463.7 | 270.79 | 270.8 | 66,471 | 10,599 | 30 (30) | 215.9 | 194.5 | 8.2 | 5.0 | |
| 0–20 | 977.0 | 7366.0 | 3590.3 | 289.77 | 301.5 | 240.3 | 73.9 | 5.1 | ||||||
| 40–60 | 961.0 | 7822.0 | 3480.3 | 297.93 | 294.7 | 275.2 | 184.1 | 5.0 | ||||||
| 80–100 | 974.0 | 8112.0 | 3320.3 | 296.73 | 288.2 | 238.0 | 107.7 | 4.9 | ||||||
| Drained Water | P Loss | K Loss | N Loss | |
|---|---|---|---|---|
| L | % | |||
| Mean | 4796 | 1.17 | 0.39 | 1.14 |
| Minimum | 3610 | 0.44 | 0.25 | 0.27 |
| Maximum | 5845 | 3.13 | 0.61 | 3.12 |
| Sampling Site | TP min. | TP max. |
TP mean | TN min. | TN max. | TN mean | TK min. | TK max. | TK mean |
|---|---|---|---|---|---|---|---|---|---|
| Leachate of dehydrating pad (Site I) + PAA * | 0.07 | 0.49 | 0.28 | 3.20 | 6.70 | 4.95 | |||
| Leachate of the northern settling pit (Site I) | 0.07 | 0.65 | 0.20 | 0.85 | 9.30 | 5.00 | |||
| Leachates of the southern settling pit (Site VII) | 0.07 | 0.56 | 0.25 | 3.40 | 10.70 | 5.70 | |||
| Column experiments (I–VI.) | 0.10 | 9.30 | 2.50 | 1.50 | 21.50 | 8.30 | 1.40 | 18.60 | 7.50 |
| Monitoring points (z15, kb4, and kb10) | 0.04 | 0.55 | 0.15 | 0.66 | 5.20 | 2.45 | 2.90 | 6.60 | 4.60 |
| Regulatory values | 0.70 | 5.00 |
| Principal Components | ||||
|---|---|---|---|---|
| Quality of Sediment | CDB-P | HCl-P | Communality | |
| TPl mg/L | 0.427 | 0.115 | 0.478 | 0.425 |
| clay% | 0.487 | 0.864 | −0.013 | 0.983 |
| silt% | −0.714 | −0.416 | −0.469 | 0.903 |
| sand% | −0.241 | −0.910 | 0.283 | 0.965 |
| TP mg/kg | 0.674 | 0.567 | −0.382 | 0.922 |
| TK mg/kg | −0.836 | 0.349 | 0.165 | 0.848 |
| TN mg/kg | 0.697 | 0.405 | 0.523 | 0.923 |
| Al-P mg/kg | −0.902 | 0.273 | −0.257 | 0.955 |
| TOC % | 0.986 | −0.082 | −0.025 | 0.980 |
| CDB-P mg/kg | −0.626 | 0.634 | −0.003 | 0.793 |
| HCl-P mg/kg | −0.544 | −0.061 | 0.646 | 0.716 |
| Ca mg/kg | 0.965 | −0.165 | −0.125 | 0.973 |
| Mg mg/kg | −0.963 | 0.207 | 0.125 | 0.985 |
| TON mg/kg | 0.743 | −0.415 | −0.068 | 0.729 |
| Variance | 53.6 | 22.1 | 10.7 | |
| Σ Variance | 53.6 | 75.8 | 86.4 | |
| TP | TN | TK | AL-P | TOC | Ca | pHDW (pHKCl **) | Loss of Ignition | CaCO3 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Site * | mg/kg | - | % | |||||||
| [53] | 1, 2, 9 | ~1000 | 6.6–7.9 | 1.5–69 | 0.4–10.6 | |||||
| [50] | 1, 2 | 1720–1780 | 8.12–8.19 | 12.6–19.2 | 20.4 *** | |||||
| Our measurements in 2023 | I–VII | 854–977 | 2471.0–3890.3 | 7074–10,644 | 47–393 | 4.9–7.2 | 7.7–8.2 | 20.3–33.5 **** | ||
| Data from an accredited laboratory | I and VII | 990–1090 | 1000–1100 | 2.7–3.1 | 236–286 | 7.57 ** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernádi, H.; Makó, A.; Lovász, Z.; Szoboszlay, S.; Harkai, P.; Háhn, J.; Kocsis, M.; Schöphen, E.; Tóth, Z.; Bidló, A.; et al. Investigation of Sediment Characteristics and Nutrient Content in Relation to Pilot Dredging at Kis-Balaton Water Protection System (Hungary). Hydrology 2025, 12, 112. https://doi.org/10.3390/hydrology12050112
Hernádi H, Makó A, Lovász Z, Szoboszlay S, Harkai P, Háhn J, Kocsis M, Schöphen E, Tóth Z, Bidló A, et al. Investigation of Sediment Characteristics and Nutrient Content in Relation to Pilot Dredging at Kis-Balaton Water Protection System (Hungary). Hydrology. 2025; 12(5):112. https://doi.org/10.3390/hydrology12050112
Chicago/Turabian StyleHernádi, Hilda, András Makó, Zsófia Lovász, Sándor Szoboszlay, Péter Harkai, Judit Háhn, Mihály Kocsis, Eszter Schöphen, Zoltán Tóth, András Bidló, and et al. 2025. "Investigation of Sediment Characteristics and Nutrient Content in Relation to Pilot Dredging at Kis-Balaton Water Protection System (Hungary)" Hydrology 12, no. 5: 112. https://doi.org/10.3390/hydrology12050112
APA StyleHernádi, H., Makó, A., Lovász, Z., Szoboszlay, S., Harkai, P., Háhn, J., Kocsis, M., Schöphen, E., Tóth, Z., Bidló, A., Rékási, M., Ferincz, Á., Csitári, G., & Barna, G. (2025). Investigation of Sediment Characteristics and Nutrient Content in Relation to Pilot Dredging at Kis-Balaton Water Protection System (Hungary). Hydrology, 12(5), 112. https://doi.org/10.3390/hydrology12050112








