Hydrogeochemical and Stable Isotope Data of the Groundwater of a Multi-Aquifer System in the Maknessy Basin (Mediterranean Area, Central Tunisia)
Abstract
1. Introduction
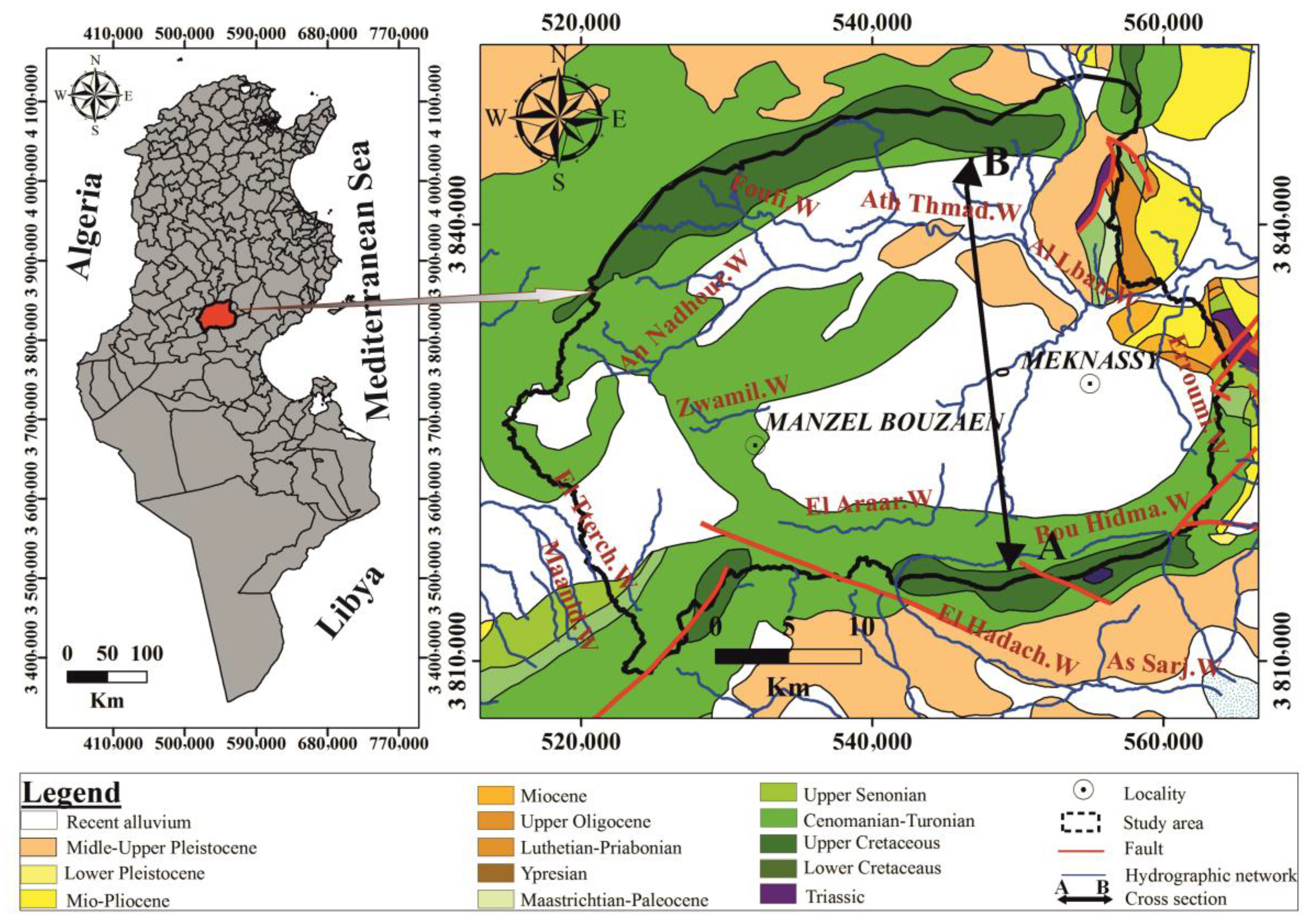
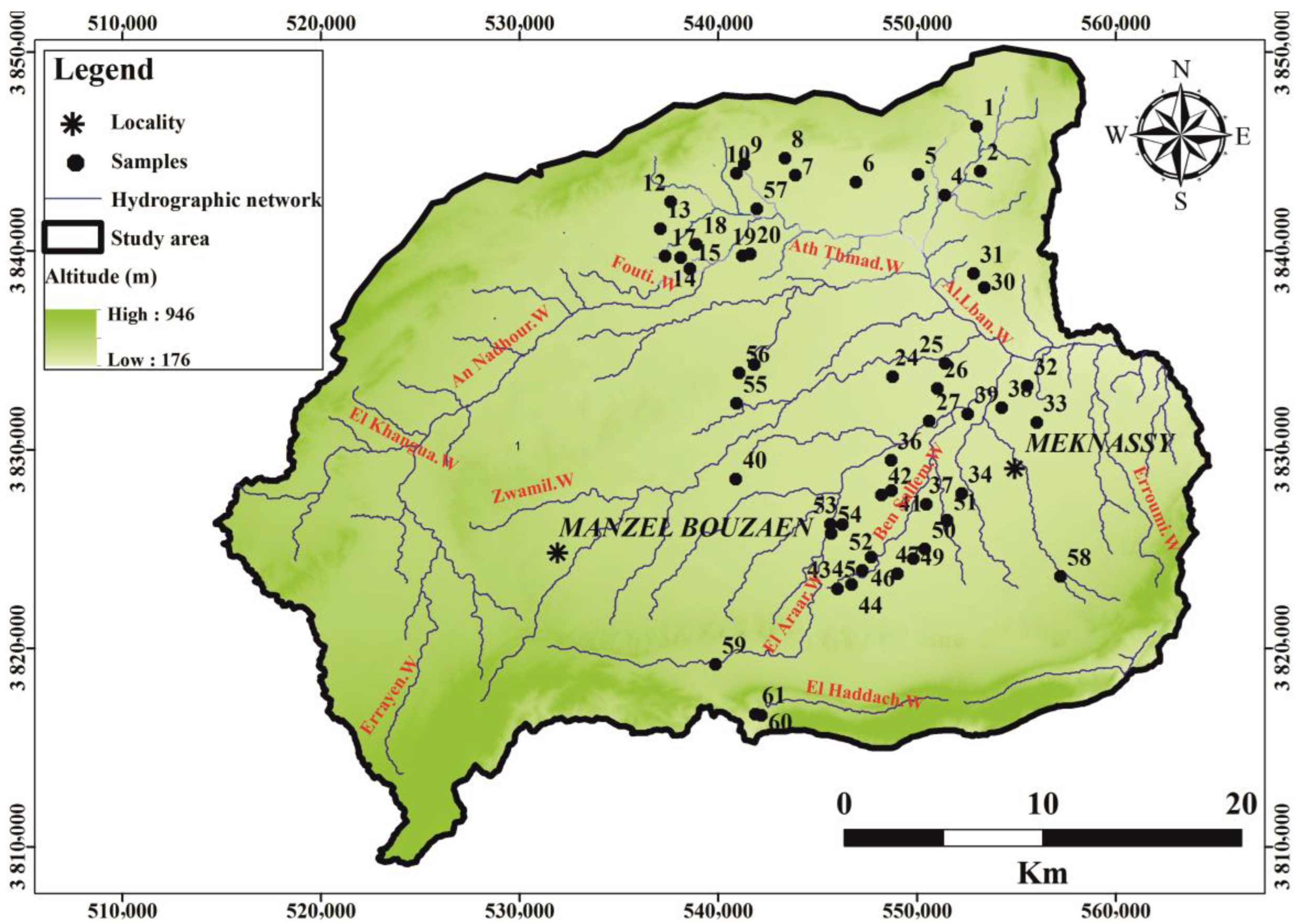
2. Study Area
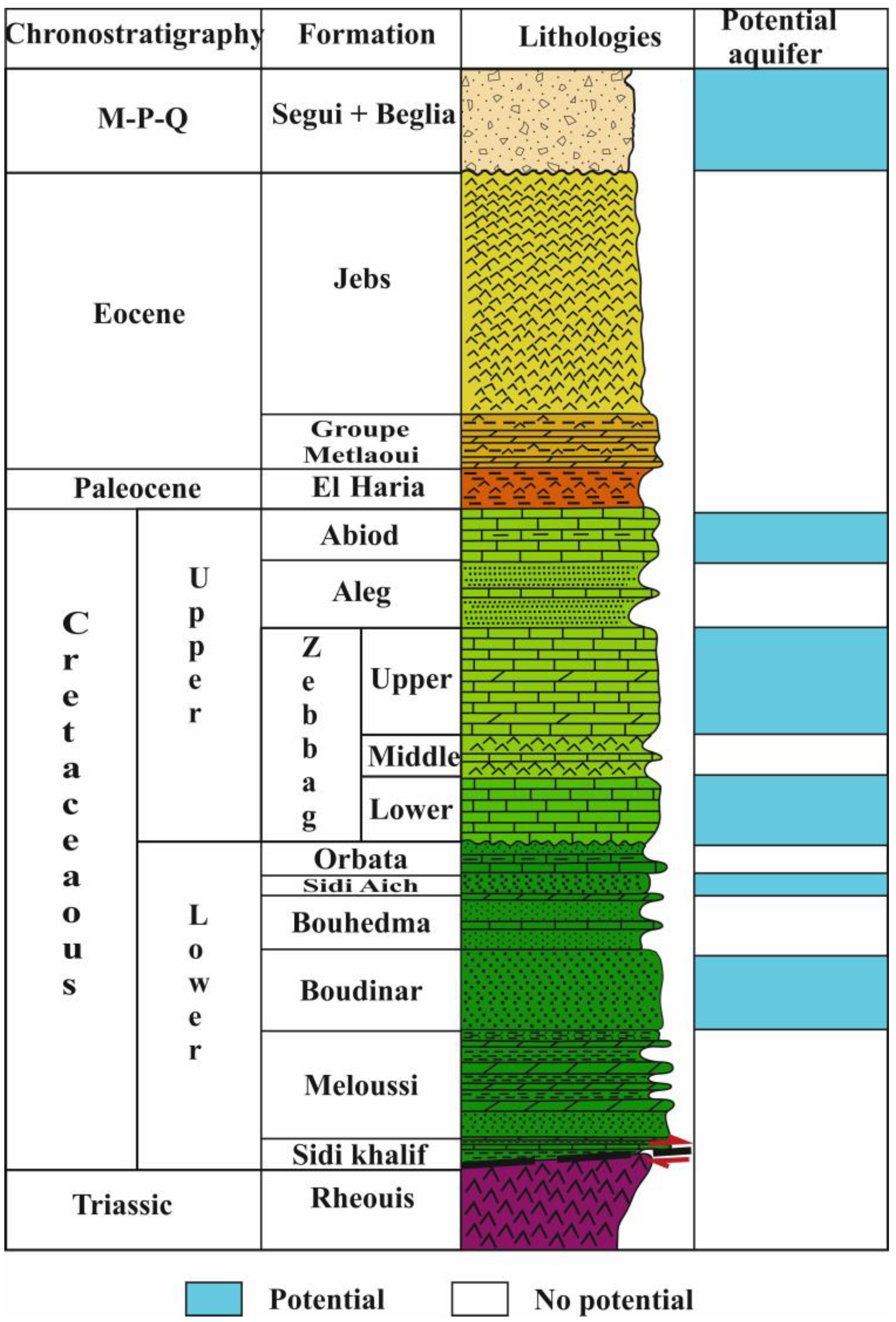
3. Geological and Tectonic Setting
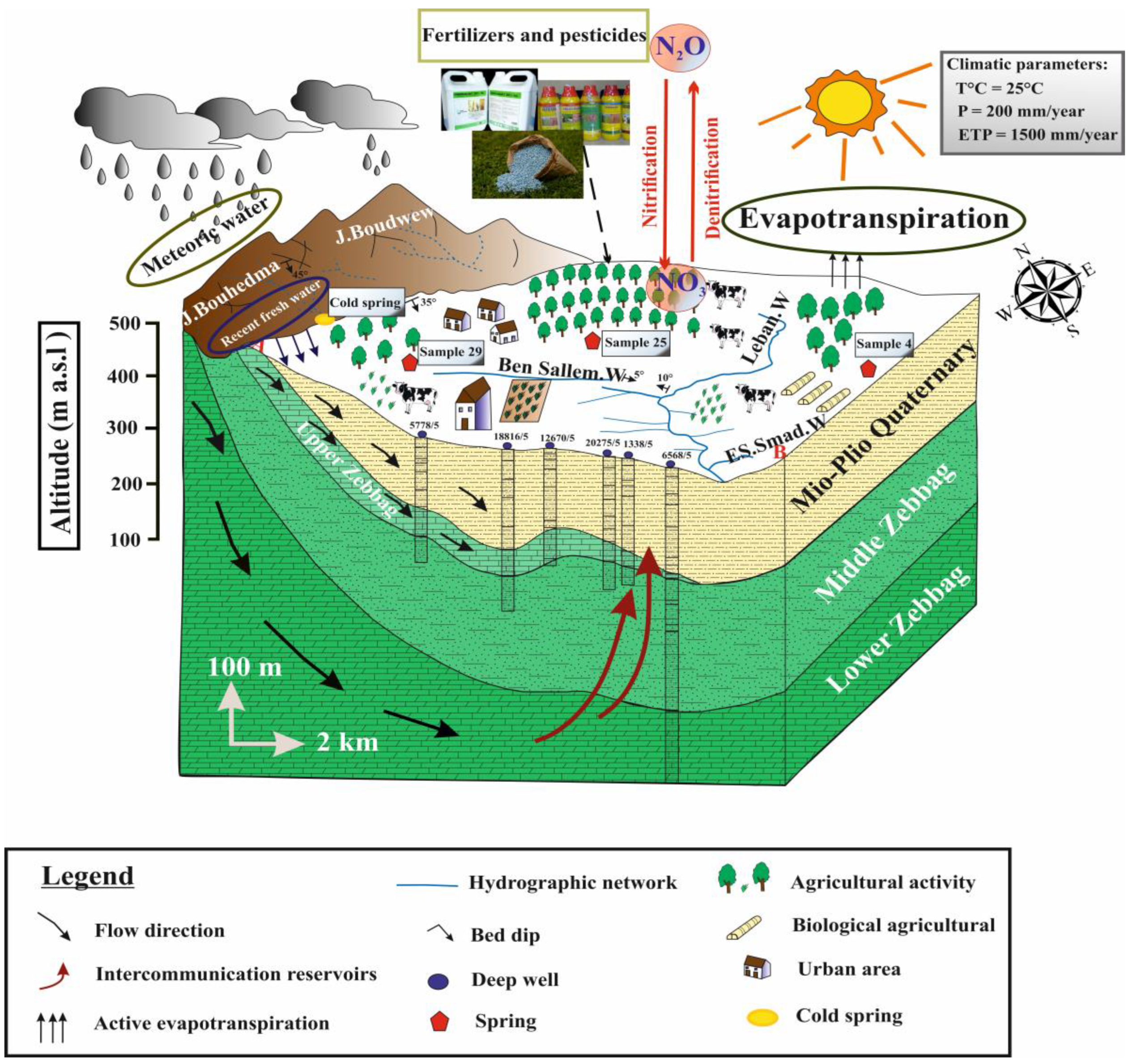
4. Hydrogeological Setting
5. Materials and Methods
5.1. Sampling and Analytical Procedure
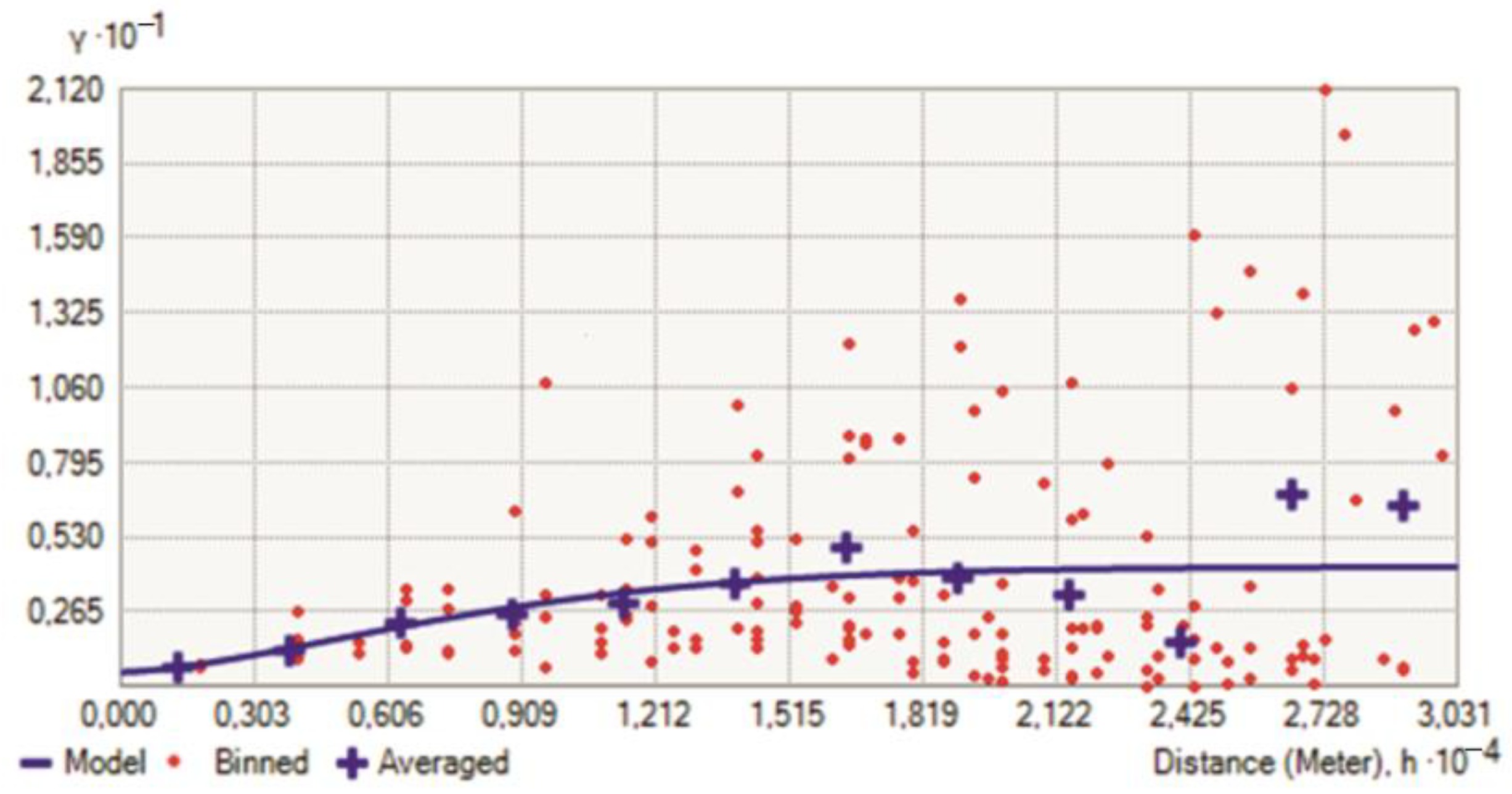
5.2. Graphical Methods
5.3. Multivariate Statistical Analysis
6. Results and Discussions
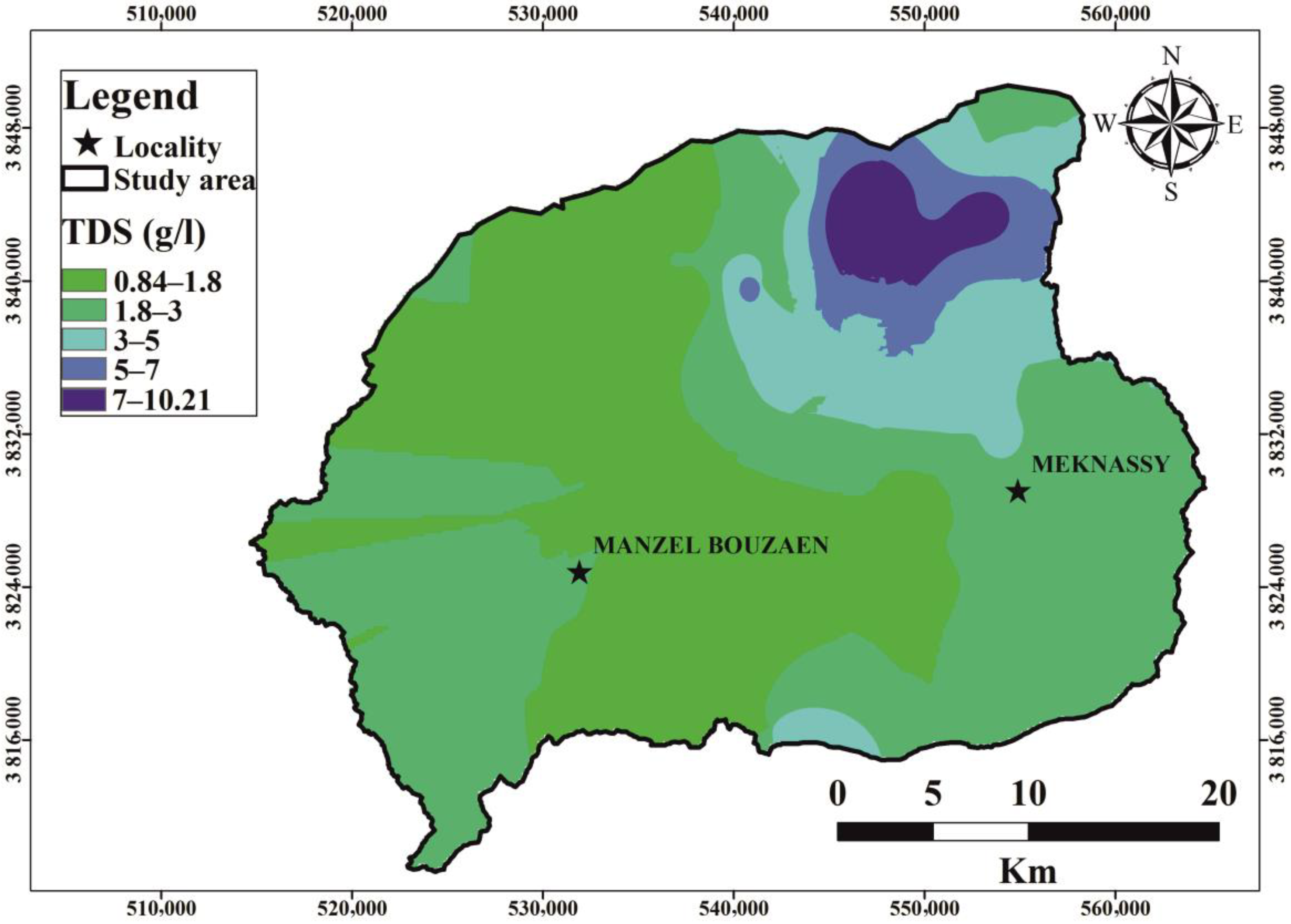
6.1. Groundwater Parameters
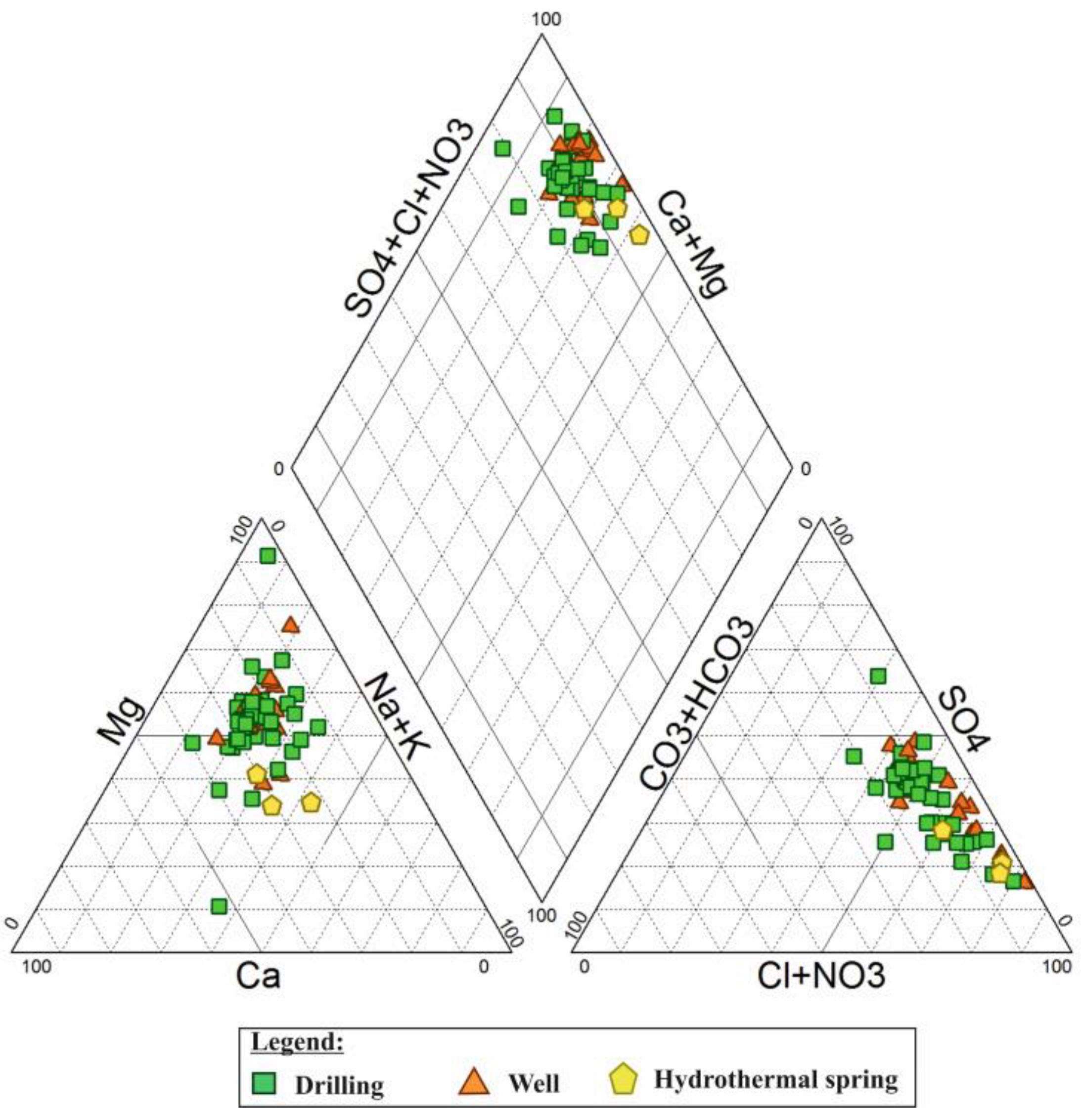
6.2. Hydrogeochemical Facies
6.3. Major Ion Ratios and Relationships
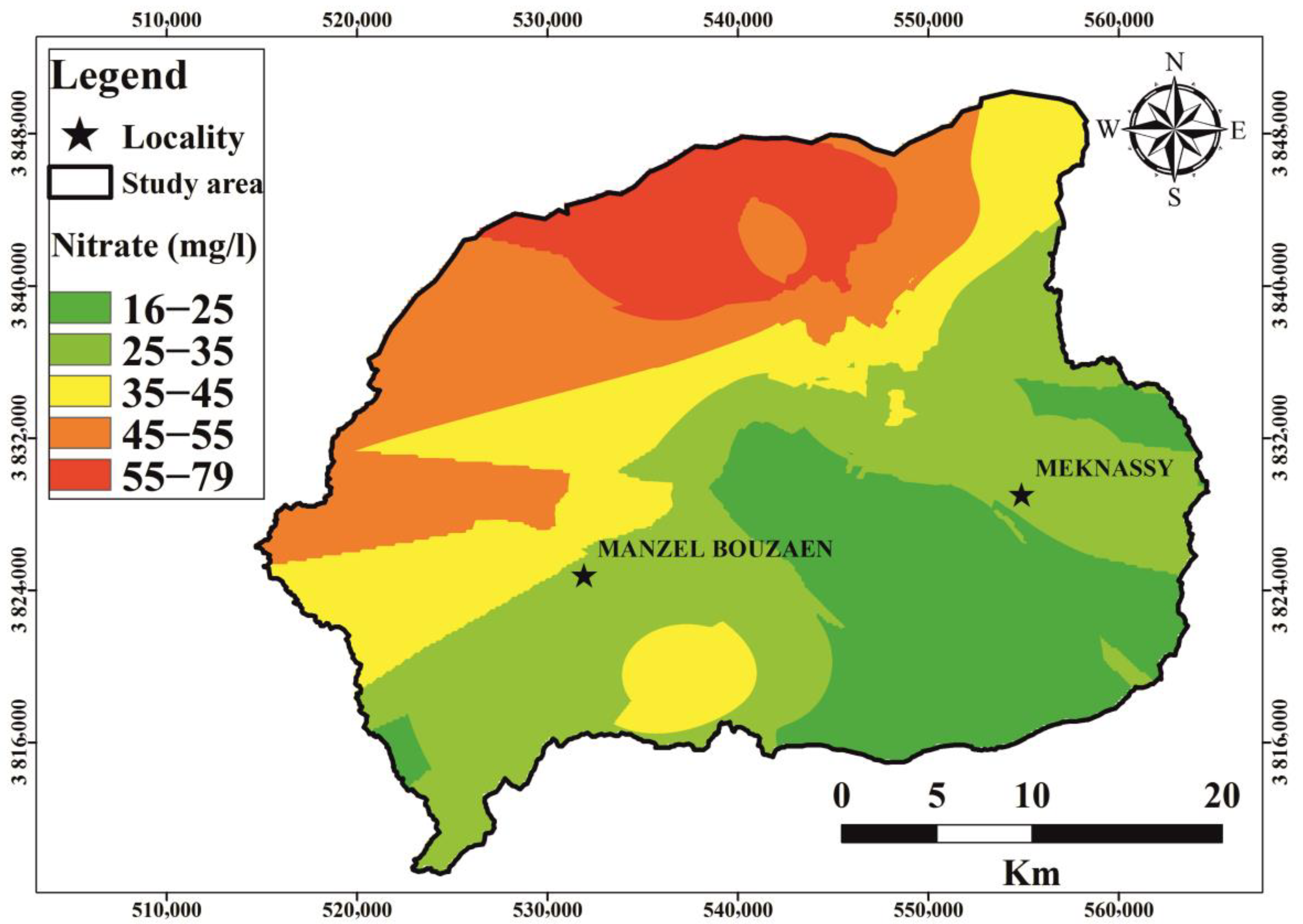
6.4. Nitrate Contamination
6.5. Multivariate Statistical Techniques
6.5.1. Correlation Coefficient Matrix
6.5.2. Principal Component Analysis: PCA
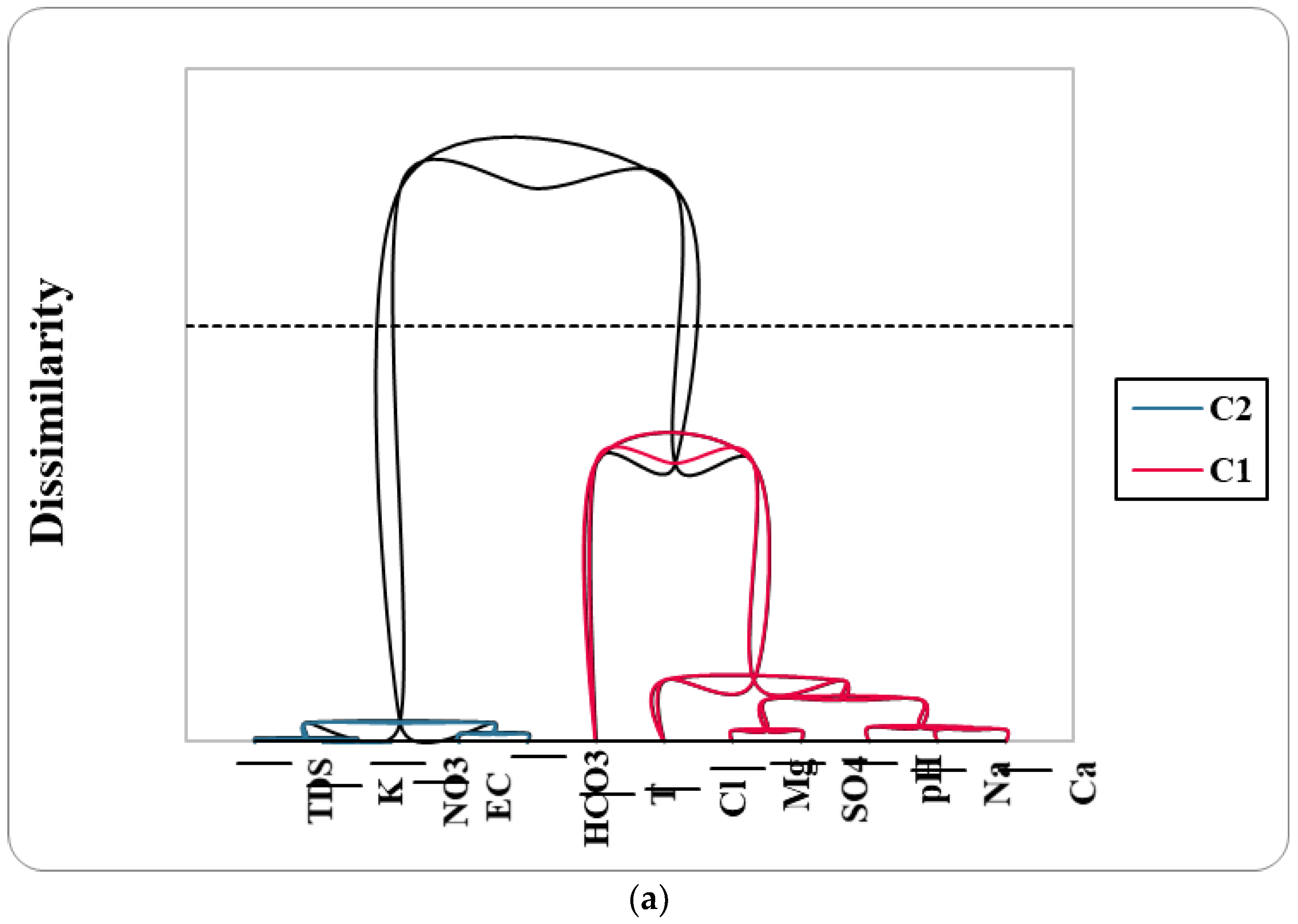
6.5.3. Hierarchical Cluster Analysis: HCA
6.6. Environmental Isotopes
7. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gasmi, M.; Andrieux, P.; Dhia, B.H.; Amri, F. Electric prospection contribution to the hydrogeological study of groundwater in the Mech and South Sned plain groundwater (Central Tunisia). Houille Blanche 2001, 87, 97–111. [Google Scholar] [CrossRef]
- Hamed, Y.; Hadji, R.; Redhaounia, B.; Zighmi, K.; Bâali, F.; El Gayar, A. Climate impact on surface and groundwater in North Africa: A global synthesis of findings and recommendations. Euro-Mediterr. J. Environ. Integr. 2018, 3, 25. [Google Scholar] [CrossRef]
- El Gayar, A.; Hamed, Y. Climate change and water resources management in Arab countries. In Proceedings of the Euro-Mediterranean Conference for Environmental Integration, Sousse, Tunisia, 20–25 November 2017; pp. 89–91. [Google Scholar]
- Chandrasekar, N.; Selvakumar, S.; Srinivas, Y.; John Wilson, J.S.; Simon Peter, T.; Magesh, N.S. Hydrogeochemical assessment of groundwater quality along the coastal aquifers of southern Tamil Nadu, India. Environ. Earth Sci. 2013, 71, 4739–4750. [Google Scholar] [CrossRef]
- Qian, C.; Wu, X.; Mu, W.; Fu, R.; Zhu, G.; Wang, Z.; Wang, D. Hydrogeochemical characterization and of groundwater in an agro-pastoral area, Ordos Basin, NW China. Environ. Earth Sci. 2016, 75, 1356. [Google Scholar] [CrossRef]
- Hamzaoui-Azaza, F.; Tlili-Zrelli, B.; Bouhlila, R.; Gueddari, M. An integrated statistical methods and modeling mineral–water interaction to identifying hydrogeochemical processes in groundwater in Southern Tunisia. Chem. Speciat. Bioavailab. 2013, 25, 165–178. [Google Scholar] [CrossRef]
- Cloutier, V.; Lefebvre, R.; Therrien, R.; Savard, M.M. Multivariate statistical analysis of geochemical data as indicative of the hydro-geochemical evolution of groundwater in a sedimentary rock aquifer system. J. Hydrol. 2008, 353, 294–313. [Google Scholar] [CrossRef]
- Amiri, V.; Nakagawa, K. Using a linear discriminant analysis (LDA)-based nomenclature system and self-organizing maps (SOM) for spatiotemporal assessment of groundwater quality in a coastal aquifer. J. Hydrol 2021, 603, 127082. [Google Scholar] [CrossRef]
- Al-Hmani, A.; Ben Jamaa, N.; Kharroubi, A.; Agoubi, B. Assessment of groundwater in Sana’a Basin aquifers, Yemen, using hydrogeochemical modeling and multivariate statistical analysis. Arab. J. Geosci. 2022, 15, 684. [Google Scholar] [CrossRef]
- Hamed, H.; Awad, S.; Ben Saad, A. Nitrate contamination in groundwater in the Sidi Aïch-Gafsa oases region, Southern Tunisia. Environ. Earth Sci. 2013, 70, 2335–2348. [Google Scholar] [CrossRef]
- Ncibi, K.; Chaar, H.; Hadji, R.; Baccari, N.; Sebei, A.; Khelifi, F.; Abbes, M.; Hamed, Y. A GIS-based statistical model for assessing groundwater susceptibility index in shallow aquifer in Central Tunisia (Sidi Bouzid basin). Arab. J. Geosci. 2020, 13, 98. [Google Scholar] [CrossRef]
- Nematollahi, M.J.; Ebrahimi, P.; Razmara, M.; Ghasemi, A. Hydrogeochemical investigations and groundwater quality assessment of Torbat-Zaveh plain, Khorasan Razavi, Iran. Environ. Monit. Assess. 2016, 188, 2. [Google Scholar] [CrossRef]
- Rajmohan, N.; Elango, L. Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ. Geol. 2004, 46, 47–61. [Google Scholar] [CrossRef]
- AL-Hussein, A.A.M.; Khan, S.; Ncibi, K.; Hamdi, N.; Hamed, Y. Flood Analysis Using HEC-RAS and HEC-HMS: A Case Study of Khazir River (Middle East-Northern Iraq). Water 2022, 14, 3779. [Google Scholar] [CrossRef]
- Mokadem, N.; Demdoum, A.; Hamed, Y.; Bouri, S.; Hadji, R.; Boyce, A.; Laouar, R.; Sâad, S. Hydrogeochemical and stable isotope data of groundwater of a multi-aquifer system: Northern Gafsa basin—Central Tunisia. J. Afr. Earth Sci. 2016, 114, 174–191. [Google Scholar] [CrossRef]
- Chenini, I.; Msaddek, M.H.; Dlala, M. Hydrogeological characterization and aquifer recharge mapping for groundwater resources management using multicriteria analysis and numerical modeling: A case study from Tunisia. J. Afr. Earth Sci. 2019, 154, 59–69. [Google Scholar] [CrossRef]
- Smida, H. Apports des Systèmes d’Informations Géographiques (SIG) pour Une Approche intégrée Dans L’étude et la Gestion des Ressources en Eau des Systèmes Aquifères de la Région de Sidi Bouzid (Tunisie centrale). Ph.D. Thesis, Université de Sfax-Tunisie, Sfax, Tunisia, 2008. [Google Scholar]
- Smida, H.; Dassi, L.; Boukhachem, K.; Masrouhi, A. Satellite remote sensing and GIS-based multi-criteria analysis for the assessment of groundwater potentiality in fractured limestone aquifer: Case study of Maknassy Basin, central Tunisia. J. Afr. Earth Sciences. 2022, 104, 643. [Google Scholar] [CrossRef]
- Hamed, Y.; Ahmadi, R.; Hadji, R.; Mokadem, N.; Ben Dhia, H.; Ali, W. Groundwater evolution of the Continental Intercalaire aquifer of Southern Tunisia and a part of Southern Algeria: Use of geochemical and isotopic indicators. Desalin. Water Treat. 2013, 52, 1990–1996. [Google Scholar] [CrossRef]
- Besser, H.; Hamed, Y. Causes and risk evaluation of oil and brine contamination in the Lower Cretaceous Continental Intercalaire aquifer in the Kebili region of southern Tunisia using chemical fingerprinting techniques. Environ. Pollut. 2019, 253, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Jang, C.S.; Chen, C.P.; Lin, C.N.; Lou, K.L. Characterization of groundwater quality in Kinmen Island using multivariate analysis and geochemical modelling. Hydrol. Process. 2008, 22, 376–383. [Google Scholar] [CrossRef]
- Singh, A.K.; Mondal, G.; Kumar, S.; Singh, T.; Tewary, B.; Sinha, A. Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ. Geol. 2008, 54, 745–758. [Google Scholar] [CrossRef]
- Rajmohan, N.; Al-Futaisi, A.; Al-Touqi, S. Geochemical process regulating groundwater quality in a coastal region with complex contamination sources: Barka, Sultanate of Oman. Environ. Earth Sci. 2009, 59, 385–398. [Google Scholar] [CrossRef]
- Tirumalesh, K.; Shivanna, K.; Sriraman, A.; Tyagi, A. Assessment of quality and geochemical processes occurring in groundwaters near central air conditioning plant site in Trombay, Maharashtra, India. Environ. Monit. Assess. 2010, 163, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.; Brindha, K.; Murugan, R.; Elango, L. Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda District, Andhra Pradesh, India. Environ. Earth Sci. 2012, 65, 1203–1213. [Google Scholar] [CrossRef]
- Chenini, I.; Ben Mammou, A. Hydrochimie et caractérisation qualitative des ressources en eaux d’un systéme aquifére multicouche en zone aride: Application au bassin de Maknassy (Tunisie centrale). Sécheresse 2009, 20, 217–222. [Google Scholar] [CrossRef]
- Hamed, Y.; Ahmadi, R.; Demdoum, D.; Bouri, S.; Gargouri, I.; Ben Dhia, H.; Al-Gamal, S.; Laouar, R.; Choura, A. Use of geochemical, isotopic, and age tracer data to develop models of groundwater flow: A case study of Gafsa mining basin-Southern Tunisia. J. Afr. Earth Sci. 2014, 100, 428–436. [Google Scholar] [CrossRef]
- Hamed, Y.; Dassi, L.; Ahmadi, R.; Ben Dhia, H. Geochemical and isotopic study of the multilayer aquifer system in the Moulares- Redayef basin, southern Tunisia (Etude géochimique et isotopique du système aquifère multicouche du bassin de Moulares- Redayef, sud tunisien). Hydrol. Sci. J. 2008, 53, 1241–1252. [Google Scholar] [CrossRef]
- Regional Agency of Agricultural Development (CRDA). The Main Characteristics of the Agricultural Sector in Sidi Bouzid; Internal Report; Regional Agency of Agricultural Development: Sidi Bouzid, Tunisia, 2019. [Google Scholar]
- Missaoui, R.; Abdelkarim, B.; Ncibi, K.; Hamed, Y.; Choura, A.; Essalami, L. Assessment of Groundwater Vulnerability to Nitrate Contamination Using an Improved Model in the Regueb Basin, Central Tunisia. Water Air Soil Pollut. 2022, 233, 320. [Google Scholar] [CrossRef]
- Ncibi, K.; Hadji, R.; Hamdi, M.; Mokadem, N.; Abbes, M.; Khelifi, F.; Zighmi, K.; Hamed, Y. Application of the analytic hierarchy process to weight the criteria used to determine the Water Quality Index of groundwater in the northeastern basin of the Sidi Bouzid region, Central Tunisia. Euro-Mediterr. J. Environ. Integr. 2020, 5, 19. [Google Scholar] [CrossRef]
- Ben Haj Ali, M.; Jedoui, Y.; Dali, T.; Ben Salem, H.; Memmi, L. Carte Géologique de la Tunisie 1/500000; Office Nationale des Mines: Tunis, Tunisia, 1985. [Google Scholar]
- Zouaghi, T. Subsurface Distribution of Cretaceous (Aptien-Maastrichtian) Deposition Sequences: Tectonic Deformation Role, Halocienese and Geodynamic Evolution (Atlas of Central Tunisia). Ph.D. Thesis, Tunis El Manar University, Tunis, Tunisia, 2008. [Google Scholar]
- Bouri, S.; Gasmi, M.; Jaouadi, M.; Souissi, I.; Lahlou Mimi, A.; Ben Dhia, H. Integrated study of surface and subsurface data for the prospecting of hydroelectric thermal basins: Case of the Maknassy basin (Central Tunisia). J. Hydrol. Sci. 2007, 52, 1298–1315. [Google Scholar] [CrossRef]
- Zouaghi, T.; Ferhi, I.; Bédir, M.; Ben Youssef, M.; Gasmi, M.; Inoubli, M.H. Analysis of Cretaceous (Aptian) strata in central Tunisia, using 2D seismic data and well logs. J. Afr. Earth Sci. 2011, 61, 38–61. [Google Scholar] [CrossRef]
- Ouda, B. Isotopic Palaeohydrology of the Maknassy Basin (Central Tunisia) during the Quaternary Recent. Ph.D. Thesis, Faculty of Sciences of Tunis, Master of Science Faculty of Sfax, University of Sfax, Sfax, Tunisia, 2000; pp. 121–234. [Google Scholar]
- Piper, A.M. A graphical procedure in the geochemical interpretation of water analysis. Am. Geophys. Union Trans. 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Bakhara, B.; Mahanta, N.; Sahoo, H.K. Interpretation of Groundwater Chemistry using Piper AND Chadha‘s Diagrams: A Comparative Study from Balangir and Puintala Block of Balangir District, Odisha, India. ETIR J. 2019, 6, 35–39. [Google Scholar]
- Ren, X.; Li, P.; He, X.; Su, F.; Elumalai, V. Hydrogeochemical processes affecting groundwater chemistry in the central part of the Guanzhong Basin, China. Arch. Environ. Contam. Toxicol. 2021, 80, 74–91. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Sci. J. 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Chebbah, M.; Allia, Z. Geochemistry and hydrogeochemical processes of groundwater in the Souf Valley of low Septentrional Sahara, Algeria. Afr. J. Environ. Sci. Technol. 2015, 9, 261–273. [Google Scholar] [CrossRef]
- Islam, S.M.D.; Majumder, R.K.; Uddin, M.J.; Khalil, M.I.; Alam, M.F. Hydrochemical characteristics and quality assessment of groundwater in Patuakhali district, southern coastal region of Bangladesh. Expo. Health 2017, 9, 43–60. [Google Scholar] [CrossRef]
- Maliki, M.A. Etude Hyrogéologique, Hydrochimique et Isotopique du Systéme Aquifeére de Sfax (Tunisie). Ph.D. Thesis, Tunis II University, Tunis, Tunisia, 2000. [Google Scholar]
- Rana, R.; Ganguly, R.; Gupta, A.K. Indexing method for assessment of pollution potential of leachate from non-engineered landfill sites and its effect on ground water quality. Environ. Monit. Assess. 2018, 190, 46–69. [Google Scholar] [CrossRef]
- SBWMP. Hydrogeochemistry of Sana’a Basin. Sana’a Basin Water Management Project (SBWMP); Final Report; Alderwish Consultants Ltd.: Sana’a, Yemen, 2009. [Google Scholar]
- Kharroubi, A.; Tlahigue, F.; Agoubi, B.; Azri, C.; Bouri, S. Hydrochemical and statistical studies of the groundwater salinization in Mediterranean arid zones, Jerba coastal aquifer in southeast Tunisia. Environ. Earth Sci. 2012, 67, 2089–2100. [Google Scholar] [CrossRef]
- Jmal, I.; Ayed, B.; Boughariou, E.; Allouche, N.; Saidi, S.; Hamdi, M.; Bouri, S. Assessing groundwater vulnerability to nitrate pollution using statistical approaches: A case study of Sidi Bouzid shallow aquifer, Central Tunisia. Arab. J. Geosci. 2017, 10, 364. [Google Scholar] [CrossRef]
- Ncibi, K.; Hadji, R.; Hajji, S.; Besser, H.; Hajlaoui, H.; Hamad, A.; Mokadem, N.; Ben Saad, A.; Hamdi, M.; Hamed, Y. Spatial variation of groundwater vulnerability to nitrate pollution under excessive fertilization using index overlay method in central Tunisia (Sidi Bouzid basin). Irrig. Drain. 2021, 70, 1209–1226. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Wang, D.; Ren, X.; Wei, M. Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum. Ecol. Risk Assess. 2020, 26, 1603–1621. [Google Scholar] [CrossRef]
- Amiri, V.; Nakhaei, M.; Lak, R.; Kholghi, M. Investigating the salinization and freshening processes of coastal groundwater resources in Urmia aquifer, NW Iran. Environ. Monit Assess 2016, 188, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Craig, H. Isotopic variation in meteoric waters. Sciences 1964, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1961, 16, 436e468. [Google Scholar]
- Mokadem, N.; Younes, H.; Hfaid, M.; Ben Dhia, H. Hydrogeochemical and Isotope Evidence of Groundwater Evolution in El Guettar Oasis Area, Southwest Tunisia. Carbonates Evaporites. 2015, 30, 417–437. [Google Scholar] [CrossRef]
- Hamed, Y. Stable isotope ratios in meteoric waters in El Kef Region, Northwestern Tunisia: Implications for changes of moisture sources. J. Earth Sci. Clim. Change 2014, 5, 203. [Google Scholar]
- Hamed, Y.; Redhaounia, B.; Sâad, A.; Hadji, R.; Zahri, F.; Zighmi, K. Hydrothermal waters from karst aquifer: Case study of the Trozza basin (Central Tunisia). J. Tethys 2017, 5, 33–44. [Google Scholar]
- Edmunds, W.M.; Guendouz, A.; Mamou, A.; Moulla, A.; Shand, P.; Zouari, K. Groundwater evolution in the Continental Intercalaire aquifer of southern Algeria and Tunisia: Trace element and isotopic indicators. Appl. Geochem. 2003, 18, 805–822. [Google Scholar] [CrossRef]
- Andreoa, B.; Linana, C.; Carrascob, F.; Jiménez de Cisnerosb, C.; Caballerob, F.; Mudryc, J. Influence of rainfall quantity on the isotopic composition (18O and 2H) of water in mountainous areas. Application for groundwater research in the Yunquera-Nieves karst aquifers (S Spain). Appl. Geochem. 2004, 19, 561–574. [Google Scholar] [CrossRef]
- Hamed, Y. The hydrogeochemical characterization of groundwater in Gafsa-Sidi Boubaker region (Southwestern Tunisia). Arab. J. Geosci. 2013, 6, 697–710. [Google Scholar]
- Mokadem, N.; Hamed, Y.; Ben Sâad, A.; Gargouri, I. Atmospheric pollution in North Africa (Ecosystems-Atmosphere interactions): A case study in the mining basin of El Guettar-M’Dilla (Southwestern Tunisia). Arab. J. Geosci. 2012, 7, 2071–2079. [Google Scholar] [CrossRef]



| T °C | EC (µS/cm) | TDS (g/L) | pH | Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | SO42− | NO3− (mg/L) | Error % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (meq/L) | |||||||||||||
| 1 | 22.5 | 4.4 | 3.1 | 8.1 | 14.7 | 0.5 | 12.8 | 20.4 | 26 | 4.4 | 14.7 | 32 | 2.8 |
| 2 | 21 | 10.3 | 7.2 | 8.1 | 27.1 | 0.8 | 23.2 | 39.2 | 64 | 4.4 | 26.3 | 48 | 2.7 |
| 3 | 21.9 | 5.5 | 3.8 | 8.2 | 17.5 | 0.5 | 12 | 26.4 | 30 | 4 | 17.4 | 50 | 3.8 |
| 4 | 21.7 | 9.1 | 6.4 | 8.2 | 26.2 | 0.8 | 21.2 | 34.8 | 48 | 4.8 | 35.1 | 61 | 3.3 |
| 5 | 23.2 | 7.7 | 5.4 | 8.2 | 22.4 | 1.0 | 18.8 | 26 | 50 | 4 | 19.5 | 57 | 4.3 |
| 6 | 22.5 | 14.6 | 10.2 | 7.7 | 37.5 | 3.2 | 24.8 | 22.8 | 80 | 3.2 | 13.5 | 67 | 5.1 |
| 7 | 23.2 | 5.9 | 4.1 | 8.3 | 16.9 | 1.1 | 5.6 | 38.4 | 20 | 5.6 | 30.3 | 60 | 4.3 |
| 8 | 23.9 | 3.9 | 2.8 | 8.4 | 13.9 | 1.2 | 10 | 14.8 | 12,5 | 5.2 | 18. | 61 | 4.2 |
| 9 | 24.6 | 3.5 | 2.4 | 8.3 | 13.5 | 0.4 | 11.8 | 9.2 | 12 | 5.2 | 13.8 | 55 | 4.5 |
| 10 | 24.4 | 3.2 | 2.2 | 8.5 | 13.8 | 0.4 | 6 | 16.4 | 14 | 4 | 15.7 | 53 | 2.8 |
| 11 | 23 | 2.3 | 1.3 | 8.5 | 9.2 | 0.4 | 6.4 | 11.2 | 10 | 4.8 | 10.6 | 62 | 1.4 |
| 12 | 24.9 | 1.6 | 1.1 | 8.5 | 5.6 | 0.3 | 6.8 | 8.4 | 8 | 5.2 | 8.2 | 55 | 2.5 |
| 13 | 23.9 | 2.9 | 2.1 | 8.5 | 12.9 | 0.3 | 7.2 | 9.8 | 10 | 5.6 | 11.4 | 64 | 3.8 |
| 14 | 23.4 | 1.6 | 1.1 | 8.5 | 6.8 | 0.2 | 4.8 | 4.8 | 6 | 4 | 7.2 | 56 | 4.3 |
| 15 | 23.3 | 2.1 | 1.4 | 8.4 | 7.6 | 0.2 | 5.6 | 12.4 | 8 | 4.8 | 11 | 52 | 2.4 |
| 16 | 24.1 | 1.7 | 1.2 | 8.5 | 8 | 0.2 | 4 | 7.2 | 4 | 4.8 | 8.2 | 53 | 4.2 |
| 17 | 23.3 | 1.7 | 1.1 | 8.5 | 7.8 | 0.2 | 4 | 8.6 | 6 | 5.6 | 8.2 | 72 | 2.3 |
| 18 | 23.6 | 3.5 | 2.4 | 8.4 | 15.2 | 0.7 | 5.6 | 12.2 | 14 | 3.6 | 12.4 | 79 | 3.6 |
| 19 | 23.1 | 8.7 | 6.1 | 8.3 | 28.8 | 1.8 | 13.8 | 23.2 | 54 | 4 | 15.2 | 59 | 4.7 |
| 20 | 24.1 | 2.6 | 1.8 | 8.5 | 11.2 | 0.4 | 5.6 | 11.6 | 14 | 4.8 | 9.03 | 59 | 0.2 |
| 21 | 45.6 | 4.9 | 3.4 | 8.3 | 19.6 | 1.4 | 9.6 | 8.8 | 28 | 2.4 | 10.5 | 41 | 2.5 |
| 22 | 26.1 | 2.6 | 1.8 | 8.2 | 7. 9 | 0.2 | 9.6 | 12.8 | 10 | 5.2 | 12.04 | 42 | 4.4 |
| 23 | 22.8 | 2.4 | 1.7 | 8.3 | 8.5 | 0.2 | 7.6 | 12.8 | 12 | 4 | 10.75 | 4.3 | 4.1 |
| 24 | 22.9 | 5.3 | 3.7 | 8.2 | 12.5 | 0.3 | 10.4 | 22.8 | 24 | 4.4 | 12.6 | 51.6 | 4.8 |
| 25 | 22.9 | 5 | 3.5 | 8.2 | 14.2 | 0.3 | 18.4 | 21.6 | 18 | 3.2 | 27.9 | 25.8 | 4.8 |
| 26 | 22.8 | 5.6 | 3.9 | 8.2 | 14.9 | 0.3 | 16.8 | 22.8 | 26 | 3.6 | 20.2 | 35.4 | 4.2 |
| 27 | 23 | 3.3 | 2.3 | 8.3 | 10.8 | 0.2 | 10.8 | 6.8 | 12 | 2.4 | 12.8 | 25.8 | 1.8 |
| 28 | 23.2 | 4.4 | 3.1 | 8 | 11.9 | 0.2 | 12 | 19.8 | 21 | 4 | 14.9 | 21.6 | 4.3 |
| 29 | 22.5 | 2.8 | 2 | 8.3 | 9.4 | 0.34 | 8.4 | 12.8 | 14 | 4.4 | 9.6 | 23.4 | 4.1 |
| 30 | 23 | 5.4 | 3.8 | 8.1 | 16.2 | 1 | 8.8 | 29.2 | 32 | 3.2 | 16.2 | 19.8 | 3.3 |
| 31 | 22.4 | 5 | 3.5 | 8.1 | 14.6 | 0.9 | 12 | 18 | 28 | 4.4 | 9.1 | 10 | 4.4 |
| 32 | 24.2 | 3.4 | 2.3 | 8.3 | 12.7 | 0.4 | 9.6 | 12.4 | 18 | 4 | 9.2 | 10 | 5.7 |
| 33 | 22.6 | 2.9 | 2.1 | 8.3 | 9.7 | 0.3 | 9.6 | 11 | 14 | 4.8 | 8.7 | 39.6 | 4.3 |
| 34 | 24.5 | 2.8 | 1.9 | 8.3 | 9.1 | 0.3 | 8.2 | 11.8 | 14 | 3.6 | 9.1 | 3.9 | 4.9 |
| 35 | 22.1 | 2.2 | 1.6 | 8.4 | 6,8 | 0.2 | 7.4 | 8.8 | 12 | 3.6 | 5.1 | 21.6 | 4.7 |
| 36 | 23.8 | 2.2 | 1.5 | 8.2 | 7.6 | 0.1 | 9.8 | 1.2 | 8 | 3.2 | 5.8 | 17.7 | 3.9 |
| 37 | 23.2 | 1.9 | 1.3 | 7.9 | 6.8 | 0.2 | 5.2 | 7.8 | 9 | 4 | 5.3 | 19.8 | 3.6 |
| 38 | 22.4 | 5.9 | 4.1 | 8.1 | 15.1 | 0.4 | 11.2 | 25.4 | 22 | 3.6 | 21.3 | 41.4 | 4.6 |
| 39 | 23.6 | 4.1 | 2.8 | 8.2 | 12.1 | 0.2 | 13.6 | 16.8 | 16 | 4 | 17.2 | 45.6 | 5.3 |
| 40 | 23.4 | 2.1 | 1.5 | 8.3 | 6 | 0.2 | 8.4 | 9.2 | 8 | 4.6 | 8.6 | 29.7 | 4.6 |
| 41 | 24.4 | 2.3 | 1.6 | 7.9 | 7.5 | 0.2 | 9.2 | 12.4 | 8 | 4.4 | 13.3 | 25.8 | 5.8 |
| 42 | 23.5 | 2.2 | 1.5 | 7.6 | 7.4 | 0.2 | 8 | 9.6 | 8 | 4.4 | 10.8 | 15.6 | 3.5 |
| 43 | 22 | 2 | 1.4 | 7.5 | 6.3 | 0.2 | 8.8 | 7.8 | 8 | 4 | 9.3 | 23.4 | 3.3 |
| 44 | 24.9 | 2.2 | 1.5 | 7.4 | 6.9 | 0.2 | 10.6 | 9.2 | 10 | 3.6 | 10.5 | 17.7 | 4.9 |
| 45 | 25.1 | 2 | 1.4 | 7.4 | 6.4 | 0.2 | 7.2 | 10 | 8 | 4 | 9.7 | 33.6 | 3.2 |
| 46 | 23.6 | 2 | 1.4 | 7.4 | 6.5 | 0.2 | 8.4 | 10 | 8 | 4.4 | 10.4 | 29.7 | 3.7 |
| 47 | 24.5 | 2.2 | 1.5 | 7.3 | 7.2 | 0.2 | 11.2 | 6.4 | 8 | 4 | 10.7 | 11.7 | 4.4 |
| 48 | 22.4 | 2.2 | 1.5 | 7.4 | 7.16 | 0.2 | 7.6 | 10.2 | 8 | 4 | 10.8 | 19.8 | 4.2 |
| 49 | 23.8 | 2.1 | 1.5 | 7.3 | 6,9 | 0.2 | 9.2 | 9 | 10 | 4.8 | 10.9 | 23.4 | 1.3 |
| 50 | 23.9 | 2.2 | 1.5 | 7.4 | 7.1 | 0.2 | 8.2 | 10.2 | 8 | 4.8 | 10.3 | 21.6 | 4.5 |
| 51 | 26.4 | 2.8 | 1.9 | 7.3 | 9.5 | 0.3 | 11.2 | 11.2 | 12 | 4.4 | 12.9 | 27.6 | 4 |
| 52 | 24 | 2.4 | 1.6 | 7.3 | 7.4 | 0.2 | 10 | 11.4 | 10 | 4 | 12.3 | 23.4 | 4.1 |
| 53 | 24 | 2.2 | 1.5 | 7.3 | 7.3 | 0.2 | 7.8 | 11.8 | 9 | 4.4 | 11.6 | 7.8 | 3.8 |
| 54 | 24 | 1.23 | 0.9 | 7.4 | 7.1 | 0.2 | 8.2 | 9.8 | 8 | 3.6 | 11.7 | 7.8 | 3.9 |
| 55 | 21 | 3.1 | 2.2 | 7.5 | 8.2 | 0.2 | 15.2 | 13.2 | 12 | 4.4 | 17.4 | 15.6 | 3.9 |
| 56 | 23.7 | 3.8 | 2.7 | 7.2 | 7.7 | 0.27 | 22.8 | 16.8 | 10 | 4 | 29.2 | 1.8 | 4.7 |
| 57 | 24 | 3.7 | 2.6 | 7.4 | 12.4 | 0.5 | 10 | 16.8 | 16 | 4.8 | 19 | 27.6 | 0.6 |
| 58 | 25 | 4 | 2.8 | 7.4 | 7 | 0.2 | 9.1 | 9 | 11 | 4.7 | 10.8 | 23.2 | 3 |
| 59 | 23 | 1.2 | 0.8 | 7.1 | 3.5 | 0.2 | 4 | 8.4 | 6 | 5.6 | 4.5 | 61.6 | 3.2 |
| 60 | 45 | 5.3 | 3.7 | 7.4 | 19.2 | 1.2 | 15.4 | 10.2 | 35 | 4 | 11.4 | 11.7 | 4.6 |
| 61 | 20 | 2.4 | 1.7 | 7.4 | 18 | 1.5 | 17.6 | 14.4 | 27 | 9.2 | 17.3 | 23.4 | 2.2 |
| Parameter | Minimum | Maximum | Mean | S.D |
|---|---|---|---|---|
| T | 20.00 | 45.60 | 24.10 | 4.09 |
| EC | 1.27 | 20.30 | 4.00 | 3.19 |
| TDS | 0.88 | 10.21 | 2.58 | 1.68 |
| pH | 7.10 | 8.53 | 7.99 | 0.45 |
| Na+ | 3.54 | 37.52 | 11.83 | 6.45 |
| K+ | 0.02 | 3.28 | 0.50 | 0.52 |
| Ca2+ | 4.00 | 24.80 | 10.44 | 4.78 |
| Mg2+ | 1.20 | 39.20 | 14.39 | 7.90 |
| Cl− | 4.00 | 80.00 | 17.64 | 14.84 |
| NO3− | 0.03 | 1.31 | 0.58 | 0.33 |
| SO42− | 4.58 | 35.07 | 13.43 | 6.22 |
| HCO3− | 2.40 | 9.20 | 4.33 | 0.94 |
| T | EC | TDS | pH | Na+ | K+ | Ca2+ | Mg2+ | Cl− | NO3− | SO42− | HCO3− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | 1 | |||||||||||
| EC | −0.045 | 1 | ||||||||||
| TDS | −0.006 | 0.684 | 1 | |||||||||
| pH | −0.061 | −0.001 | 0.136 | 1 | ||||||||
| Na+ | −0.102 | 0.621 | 0.935 | 0.228 | 1 | |||||||
| K+ | −0.206 | 0.533 | 0.784 | 0.087 | 0.870 | 1 | ||||||
| Ca2+ | −0.033 | 0.525 | 0.744 | −0.187 | 0.654 | 0.515 | 1 | |||||
| Mg2+ | −0.227 | 0.494 | 0.764 | 0.180 | 0.701 | 0.452 | 0.549 | 1 | ||||
| Cl− | −0.045 | 0.654 | 0.953 | 0.114 | 0.951 | 0.818 | 0.734 | 0.712 | 1 | |||
| NO3− | −0.085 | 0.175 | 0.304 | 0.510 | 0.380 | 0.311 | −0.088 | 0.261 | 0.260 | 1 | ||
| SO42− | −0.154 | 0.369 | 0.577 | 0.008 | 0.530 | 0.284 | 0.660 | 0.804 | 0.461 | 0.137 | 1 | |
| HCO3− | −0.256 | −0.192 | −0.212 | −0.079 | −0.086 | 0.015 | −0.084 | 0.007 | −0.130 | 0.232 | 0.046 | 1 |
| Parameters | PC1 | PC2 |
|---|---|---|
| T | 0.013 | −0.315 |
| EC | 0.728 | −0.085 |
| TDS | 0.973 | 0.029 |
| pH | 0.096 | 0.751 |
| Na+ (meq/L) | 0.950 | 0.141 |
| K+ (meq/L) | 0.797 | 0.075 |
| Ca2+ (meq/L) | 0.801 | −0.345 |
| Mg2+ (meq/L) | 0.803 | 0.187 |
| Cl− (meq/L) | 0.951 | 0.013 |
| NO3− (meq/L) | 0.271 | 0.829 |
| SO42− (meq/L) | 0.673 | 0.003 |
| HCO3− (meq/L) | −0.133 | 0.400 |
| Variance% | 47.980 | 14.153 |
| Cumulative % | 47.980 | 62.133 |
| Samples | δ18O % | δ2H % | d % |
|---|---|---|---|
| 1 | −5.31 | −36.9 | 5.55 |
| 3 | −7.52 | −52.2 | 7.98 |
| 5 | −4.99 | −36.5 | 3.41 |
| 7 | −5.65 | −34.0 | 11.19 |
| 9 | −5.02 | −35.3 | 4.90 |
| 12 | −5.30 | −38.9 | 3.42 |
| 14 | −7.06 | −52.4 | 4.05 |
| 18 | −7.26 | −47.5 | 10.51 |
| 21 | −5.77 | −39.8 | 6.35 |
| 22 | −5.28 | −37.1 | 5.12 |
| 23 | −7.62 | −53.6 | 7.38 |
| 25 | −4.90 | −32.2 | 6.99 |
| 26 | −6.32 | −44.5 | 6.00 |
| 28 | −5.76 | −44.1 | 1.93 |
| 29 | −5.28 | −37.7 | 4.55 |
| 30 | −5.35 | −38.1 | 4.78 |
| 32 | 6.35 | −47.1 | 3.66 |
| 35 | −4.89 | −32.7 | 6.42 |
| 37 | −7.36 | −51.7 | 7.13 |
| 40 | −5.34 | −34.0 | 8.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussaoui, Z.; Gentilucci, M.; Wederni, K.; Hidouri, N.; Hamedi, M.; Dhaoui, Z.; Hamed, Y. Hydrogeochemical and Stable Isotope Data of the Groundwater of a Multi-Aquifer System in the Maknessy Basin (Mediterranean Area, Central Tunisia). Hydrology 2023, 10, 32. https://doi.org/10.3390/hydrology10020032
Moussaoui Z, Gentilucci M, Wederni K, Hidouri N, Hamedi M, Dhaoui Z, Hamed Y. Hydrogeochemical and Stable Isotope Data of the Groundwater of a Multi-Aquifer System in the Maknessy Basin (Mediterranean Area, Central Tunisia). Hydrology. 2023; 10(2):32. https://doi.org/10.3390/hydrology10020032
Chicago/Turabian StyleMoussaoui, Zouhour, Matteo Gentilucci, Khyria Wederni, Naima Hidouri, Monji Hamedi, Zahra Dhaoui, and Younes Hamed. 2023. "Hydrogeochemical and Stable Isotope Data of the Groundwater of a Multi-Aquifer System in the Maknessy Basin (Mediterranean Area, Central Tunisia)" Hydrology 10, no. 2: 32. https://doi.org/10.3390/hydrology10020032
APA StyleMoussaoui, Z., Gentilucci, M., Wederni, K., Hidouri, N., Hamedi, M., Dhaoui, Z., & Hamed, Y. (2023). Hydrogeochemical and Stable Isotope Data of the Groundwater of a Multi-Aquifer System in the Maknessy Basin (Mediterranean Area, Central Tunisia). Hydrology, 10(2), 32. https://doi.org/10.3390/hydrology10020032








