The Integrated Use of Heavy-Metal Pollution Indices and the Assessment of Metallic Health Risks in the Phreatic Groundwater Aquifer—The Case of the Oued Souf Valley in Algeria
Abstract
:1. Introduction
2. Materials and Methods
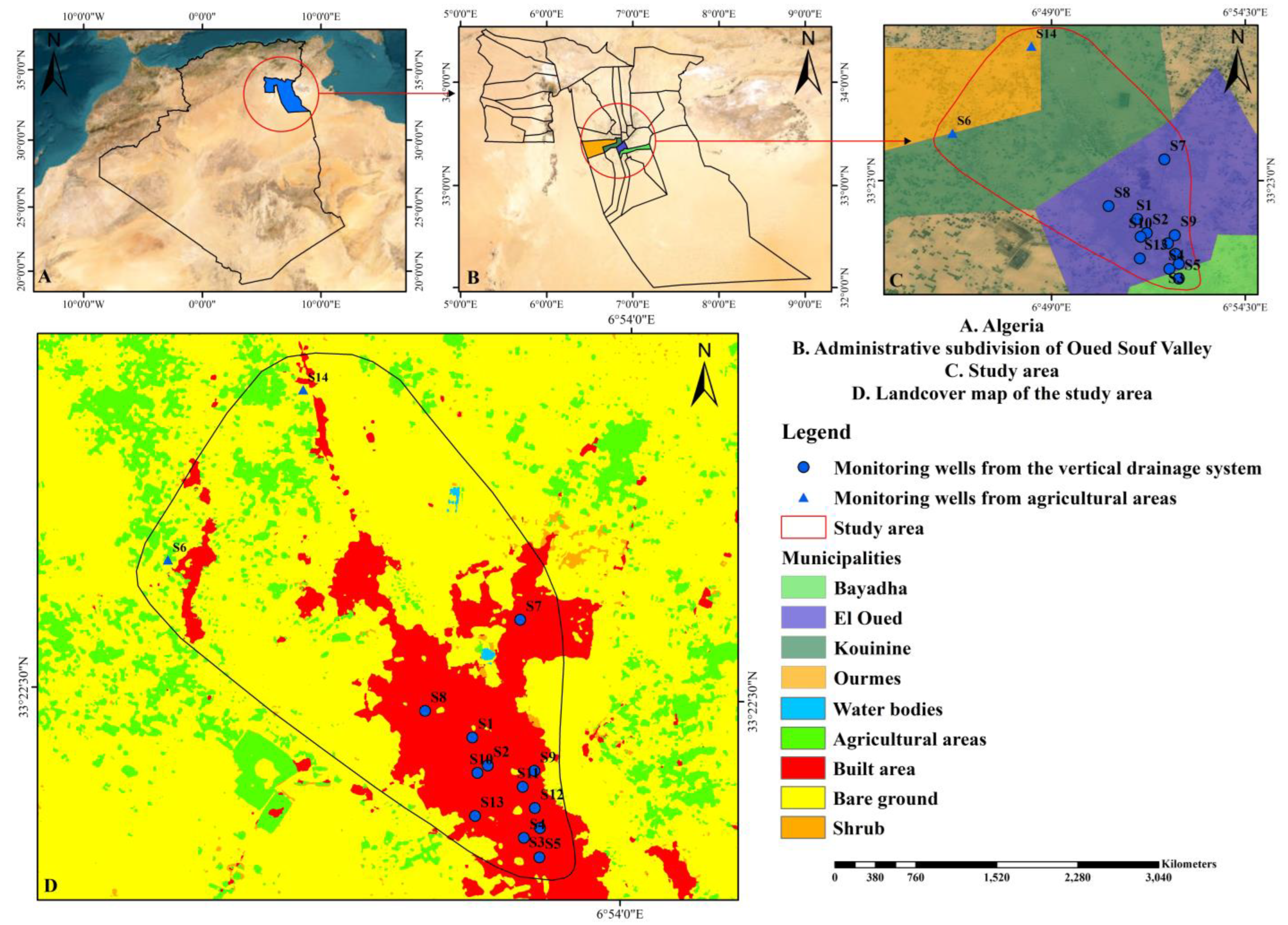
2.1. Background of the Study Area
2.1.1. Geographical Location of the Study Area
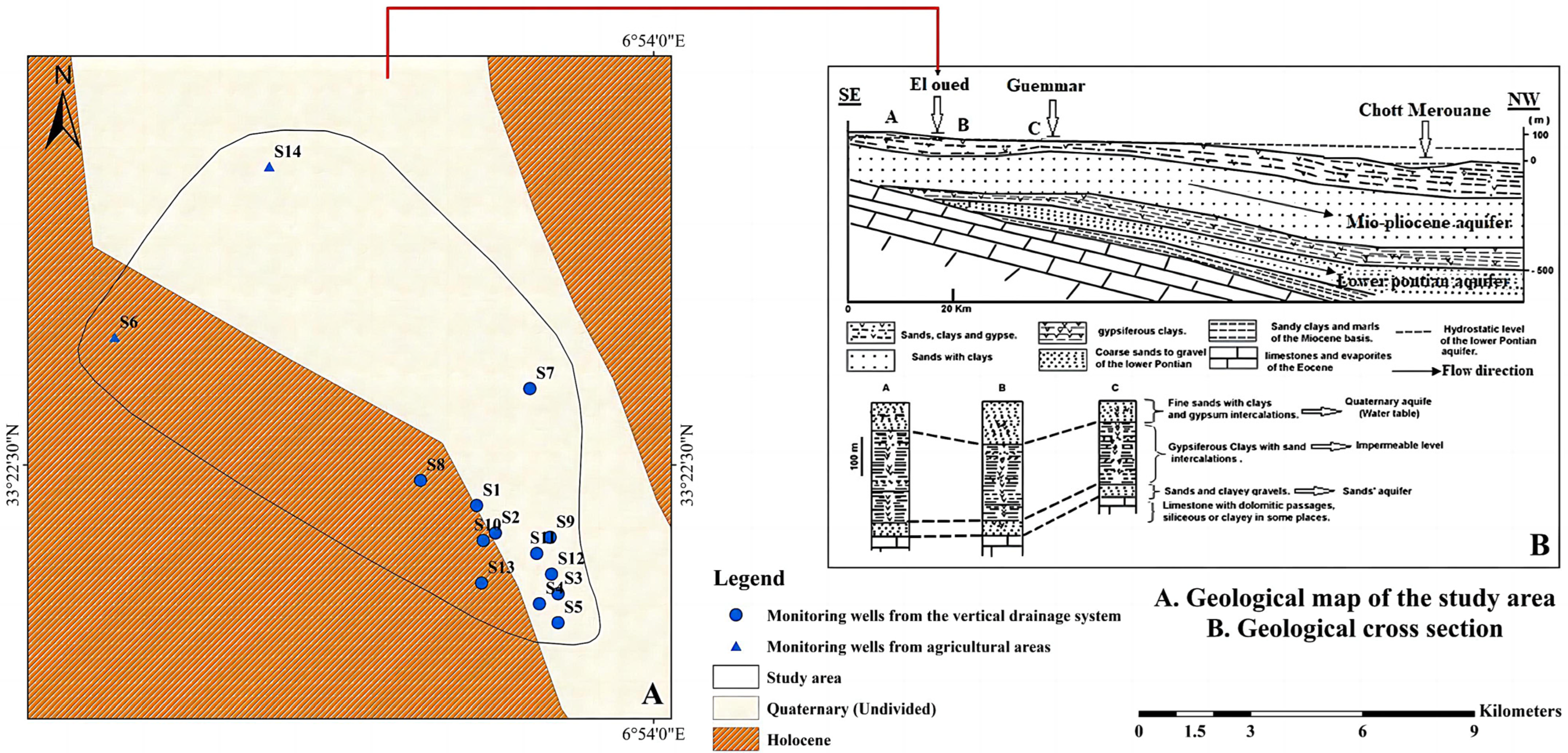
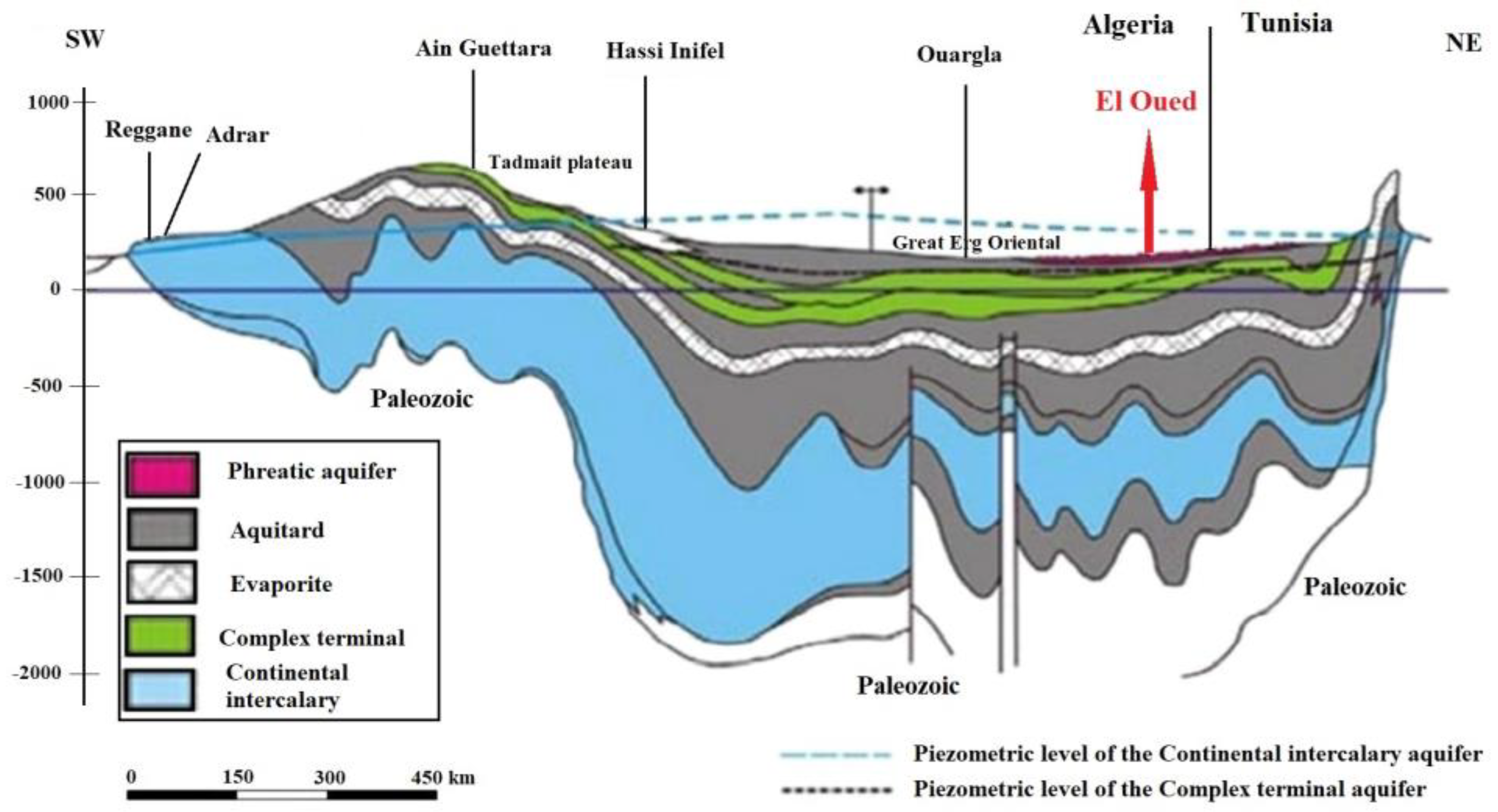
2.1.2. Geology and Hydrogeology
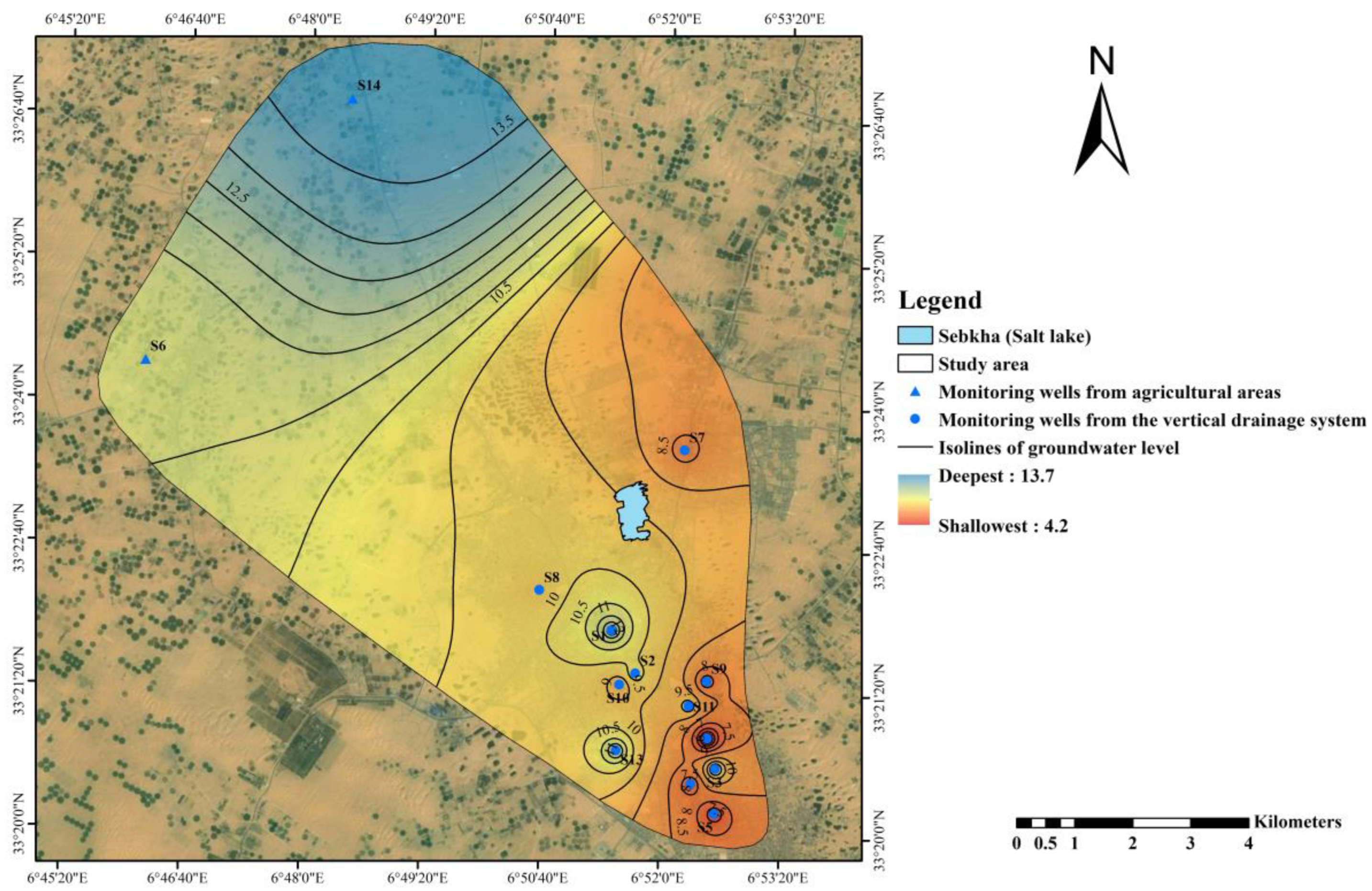
2.1.3. Phreatic Groundwater Static Level Fluctuation
2.2. Sampling and Measurements
2.3. Assessment of Environmental Hazards to Groundwater Vulnerability to Heavy Metals
2.3.1. The Degree of Contamination (Cdeg)
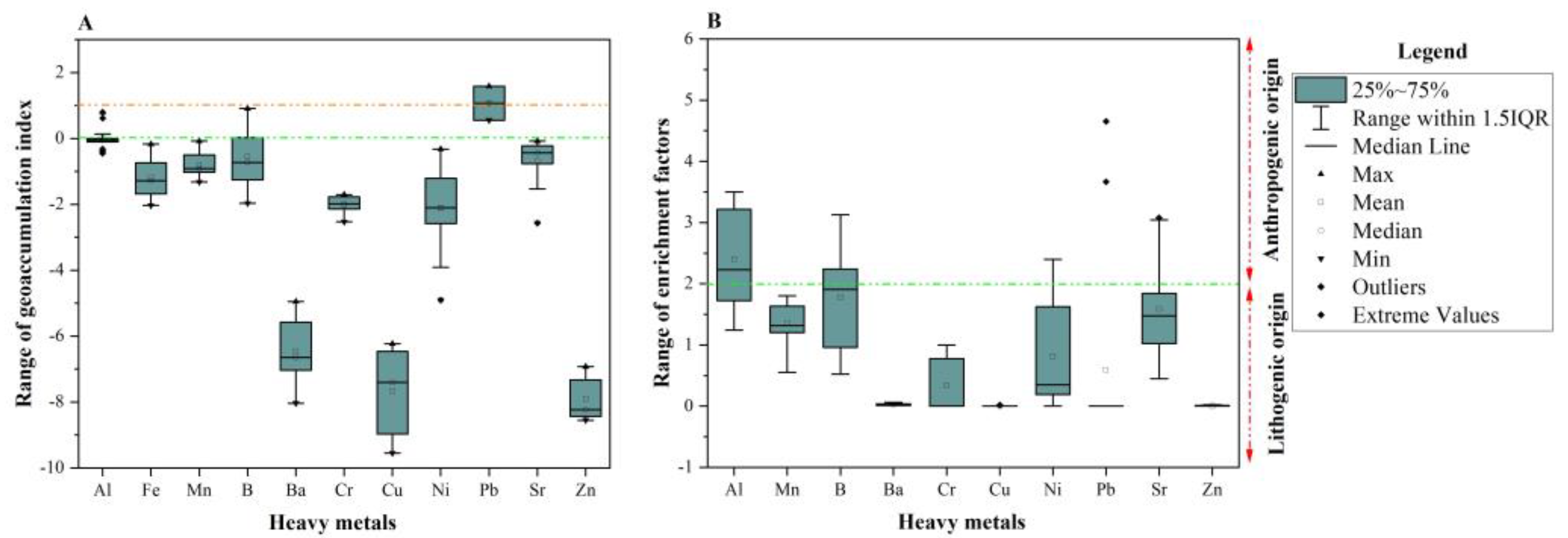
2.3.2. Geo-Accumulation Index (Igeo)
2.3.3. Enrichment Factor (EF)
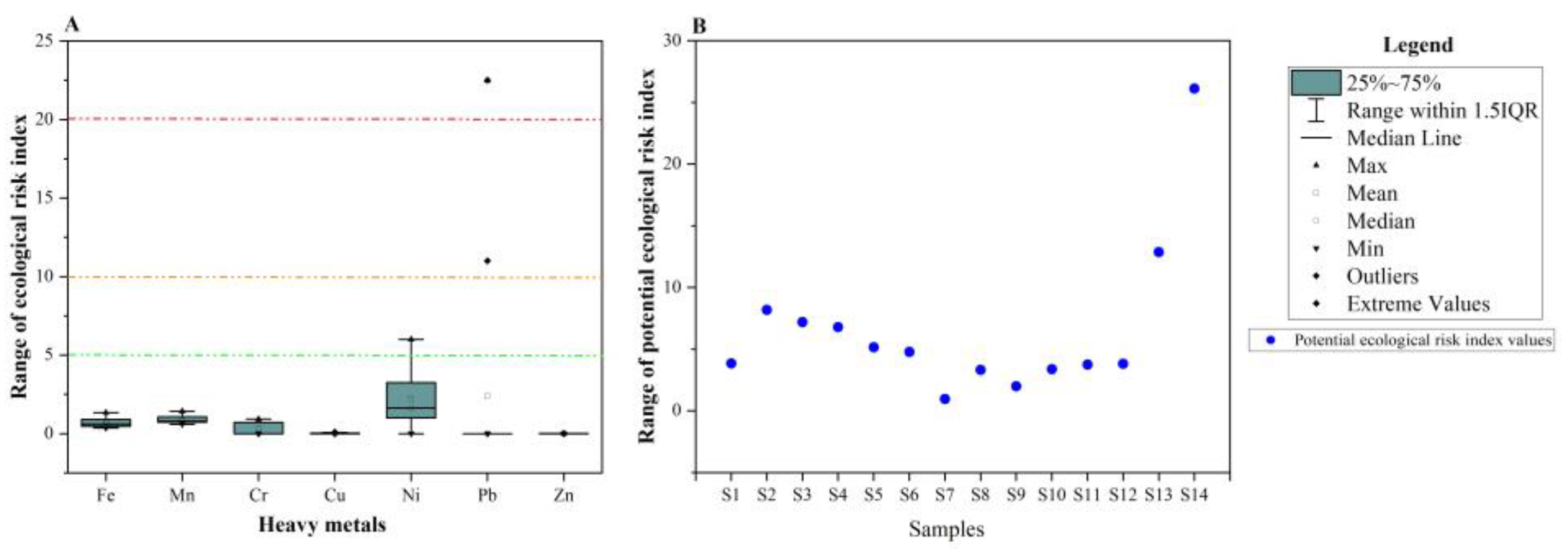
2.4. Ecological Risk (ER) and Potential Ecological Risk (PERI) Indices
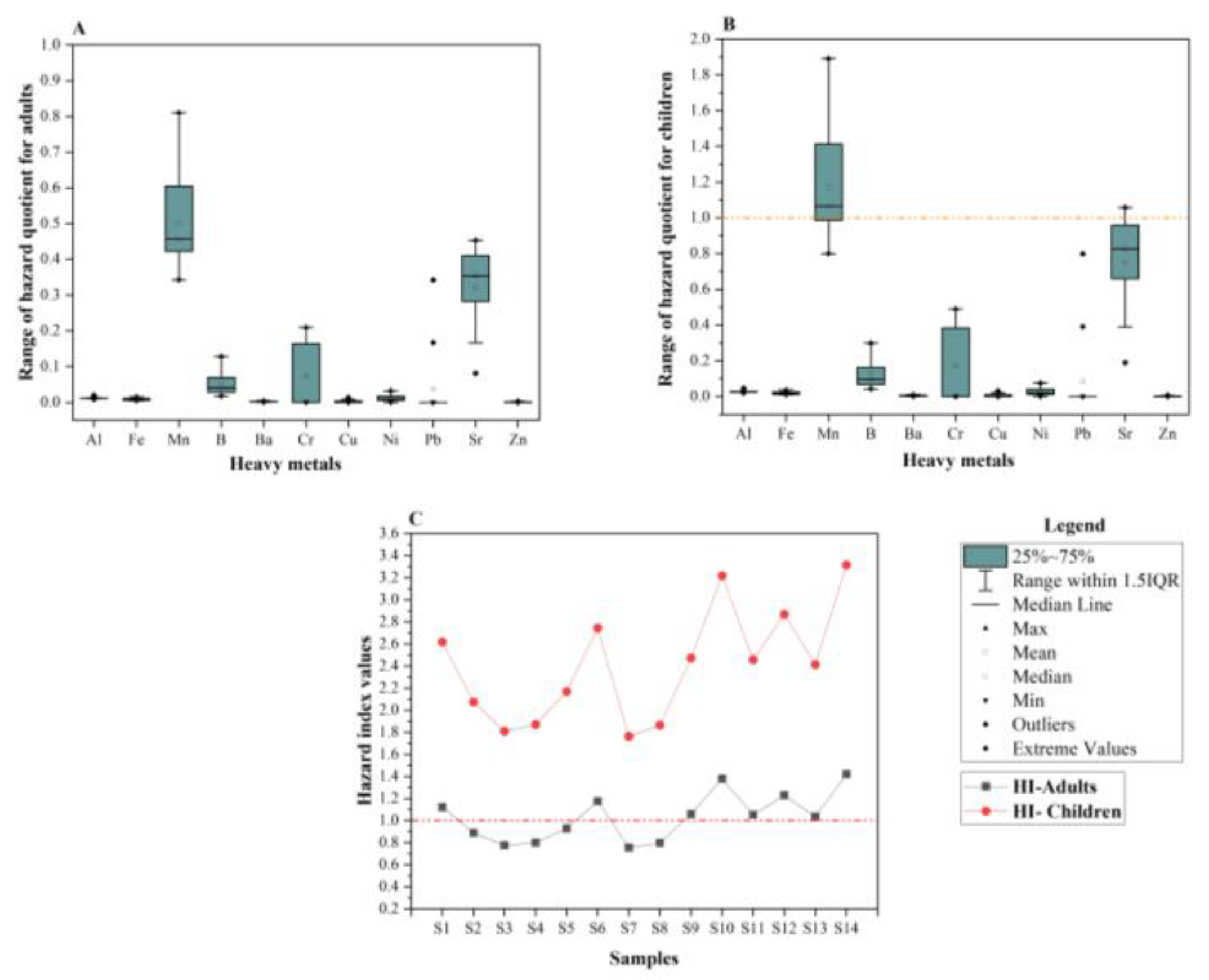
Human Health Risk Assessment from the Groundwater
2.5. Spatial Analysis
2.6. Hierarchical Clustering Analysis (HCA)
3. Results and Discussion
3.1. Physico-Chemical Data
3.2. Variations in Heavy Metals in the Phreatic Aquifer
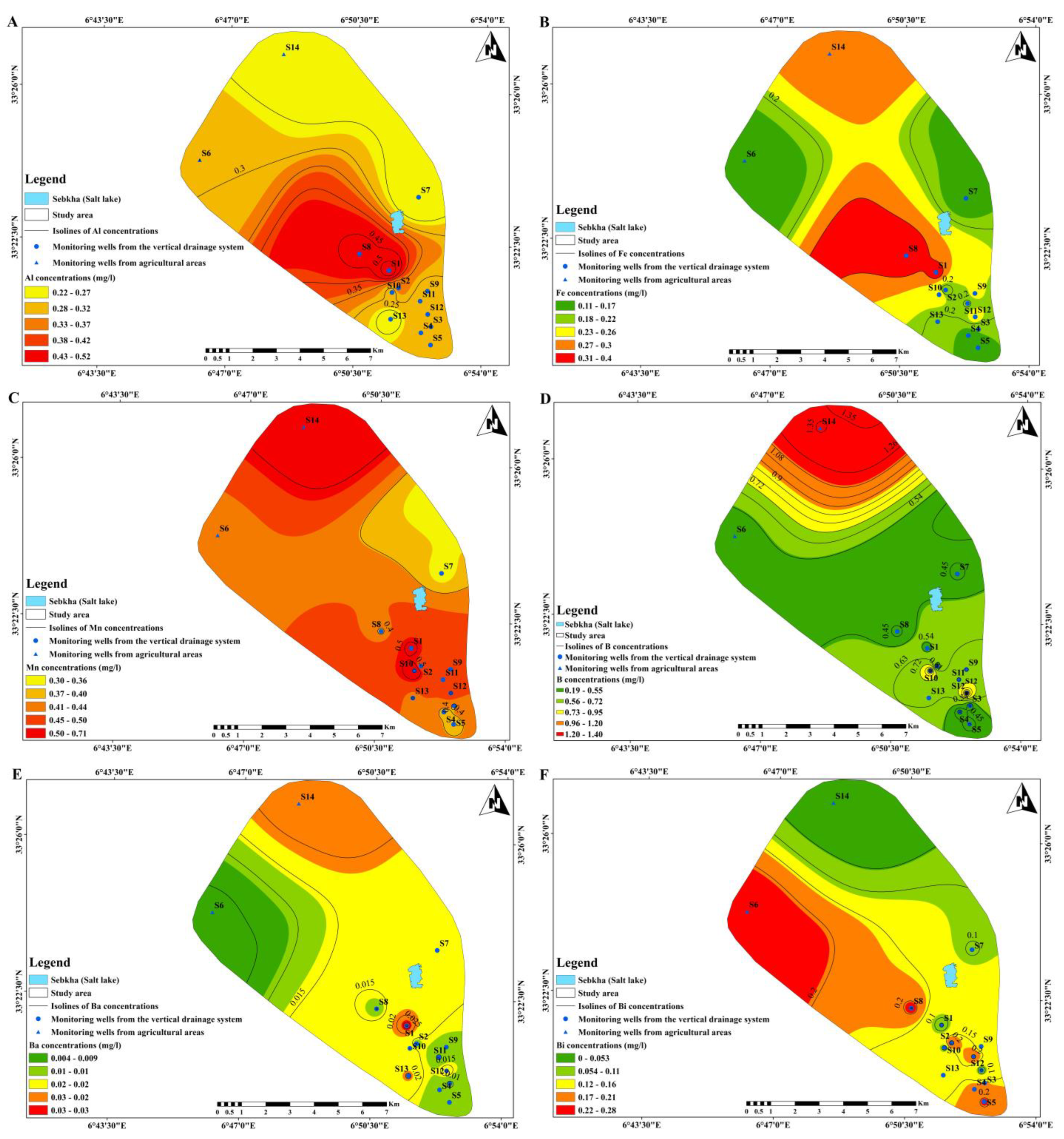
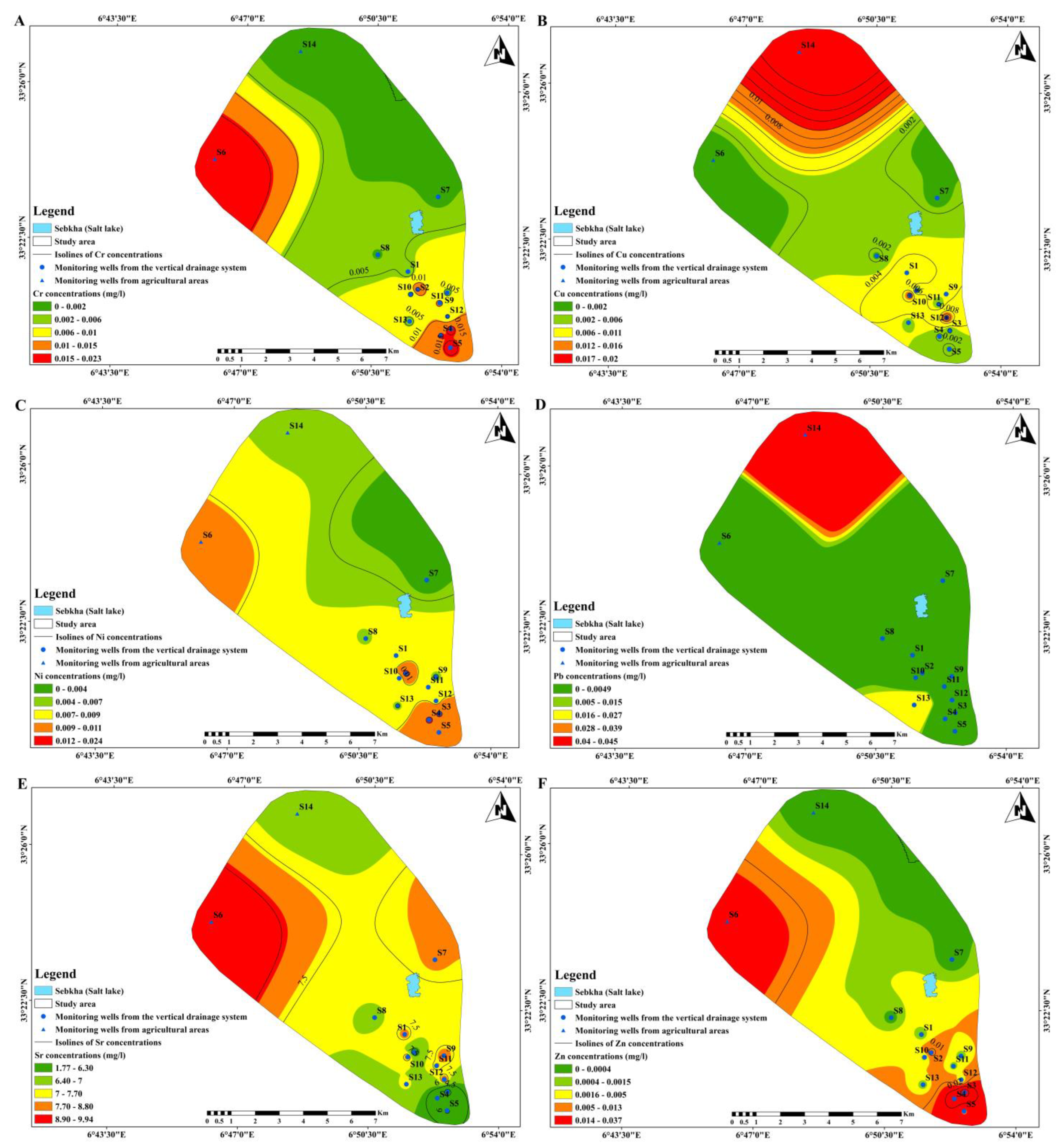
3.3. Spatial Patterns Detection
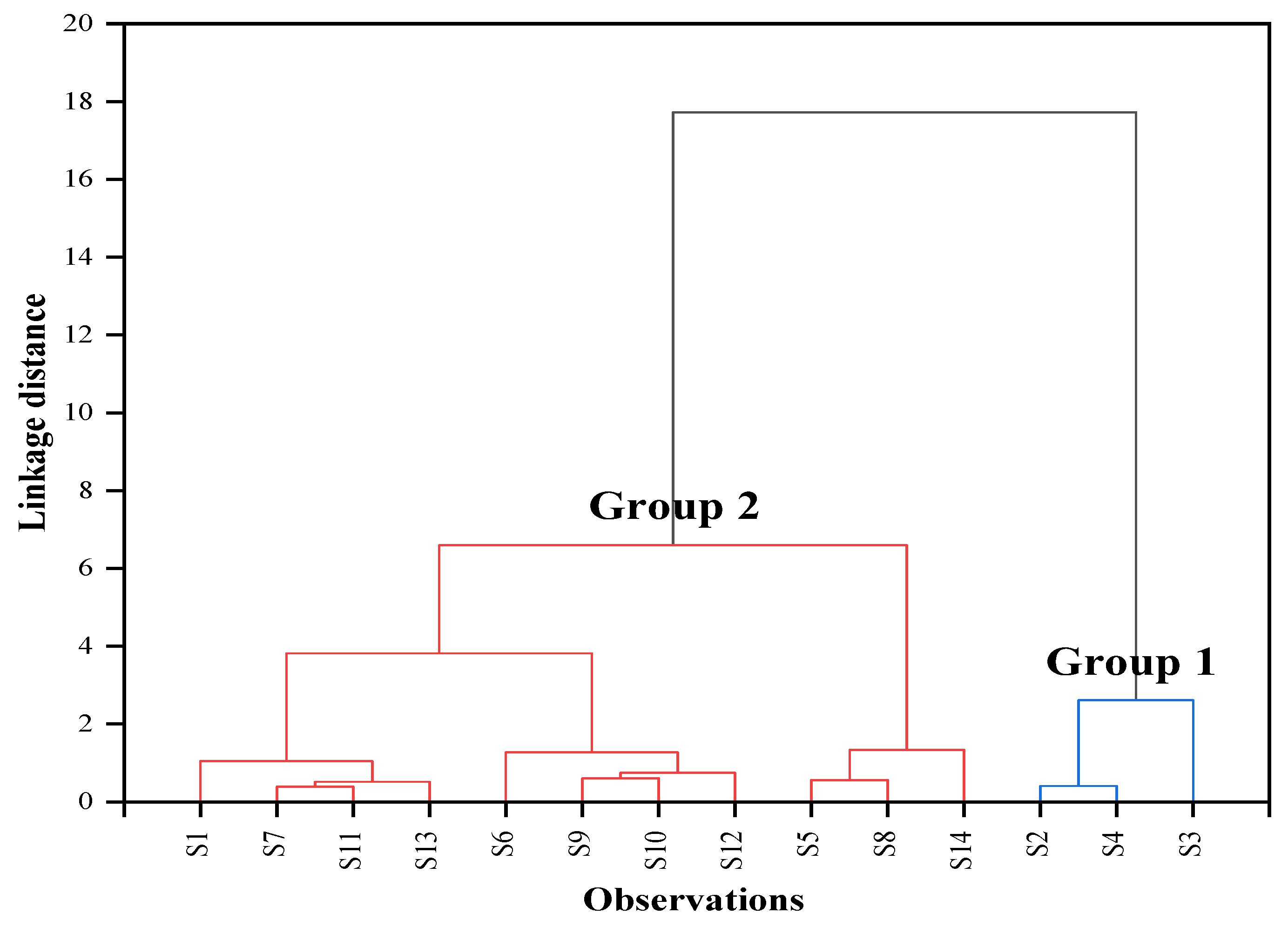
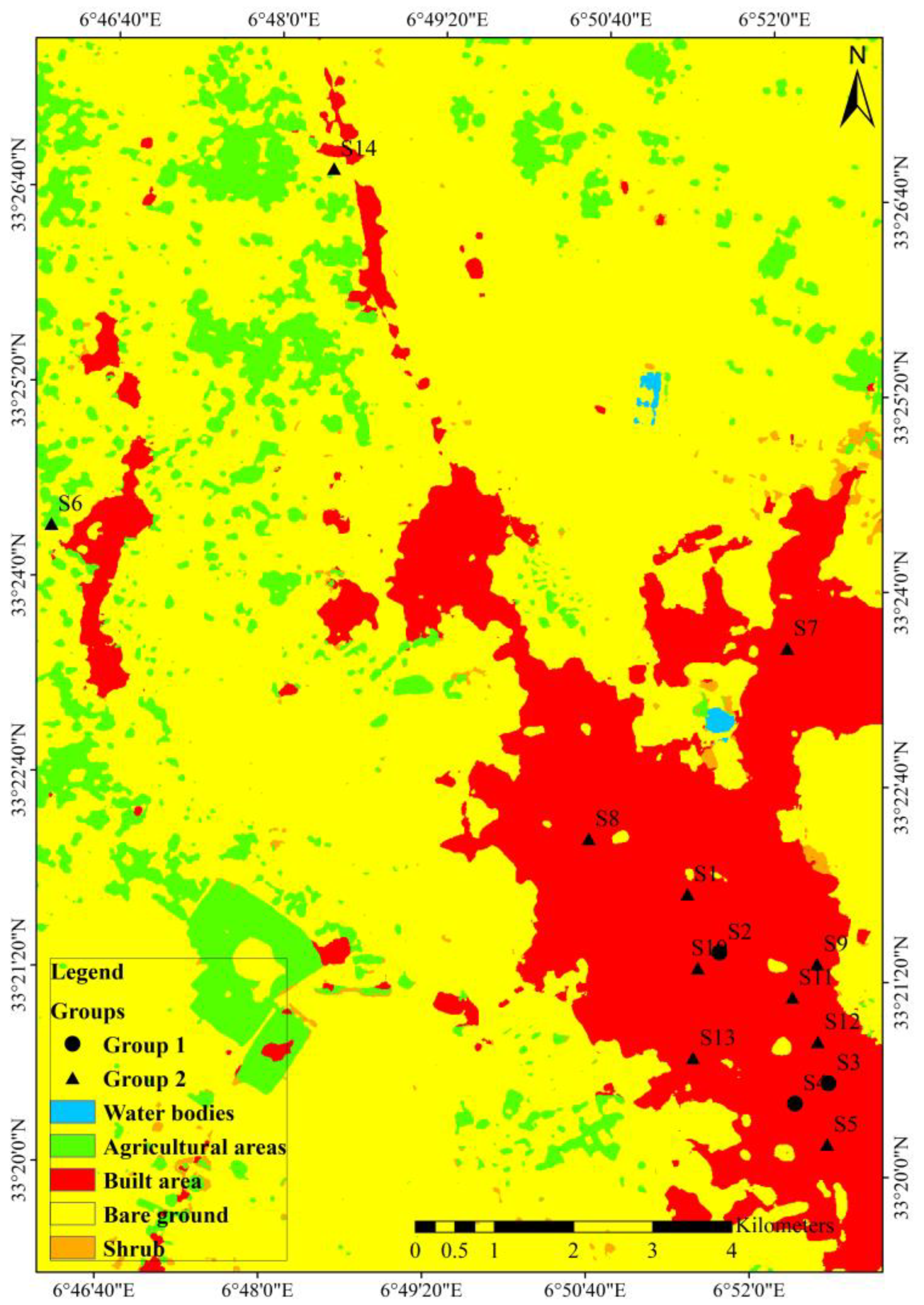
3.4. Cluster Analysis
3.5. Evaluation of the Heavy-Metal pollution in the Phreatic Aquifer
3.6. Risk Evaluation of Human Health from the Heavy Metals of the Phreatic Aquifer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jasrotia, A.S.; Taloor, A.K.; Andotra, U.; Kumar, R. Monitoring and Assessment of Groundwater Quality and Its Suitability for Domestic and Agricultural Use in the Cenozoic Rocks of Jammu Himalaya, India: A Geospatial Technology Based Approach. Groundw. Sustain. Dev. 2019, 8, 554–566. [Google Scholar] [CrossRef]
- Adimalla, N.; Dhakate, R.; Kasarla, A.; Taloor, A.K. Appraisal of Groundwater Quality for Drinking and Irrigation Purposes in Central Telangana, India. Groundw. Sustain. Dev. 2020, 10, 100334. [Google Scholar] [CrossRef]
- Momodu, M.A.; Anyakora, C.A. Heavy Metal Contamination of Ground Water: The Surulere Case Study. Res. J. Environ. Earth Sci. 2010, 2, 39–43. [Google Scholar]
- UNESCO; UN-Water; World Water Assessment Programme. WWAP (The United Nations World Water Development Report 3): Water in a Changing World; UNESCO: Paris, France, 2009. [Google Scholar]
- UNESCO; UN-Water; World Water Assessment Programme. WWAP (The United Nations World Water Development Report 6): Water for a Sustainable World; UNESCO: Paris, France, 2015. [Google Scholar]
- Brassington, R. Field Hydrogeology; The Geological Field Guide Series; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kavcar, P.; Sofuoglu, A.; Sofuoglu, S.C. A Health Risk Assessment for Exposure to Trace Metals via Drinking Water Ingestion Pathway. Int. J. Hyg. Environ. Health 2009, 212, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Suvarapu, L.N.; Baek, S.-O. Determination of Heavy Metals in the Ambient Atmosphere: A Review. Toxicol. Ind. Health 2017, 33, 79–96. [Google Scholar] [CrossRef]
- Visnjic-Jeftic, Z.; Jaric, I.; Jovanovic, L.; Skoric, S.; Smederevac-Lalic, M.; Nikcevic, M.; Lenhardt, M. Heavy Metal and Trace Element Accumulation in Muscle, Liver and Gills of the Pontic Shad (Alosa Immaculata Bennet 1835) from the Danube River (Serbia). Microchem. J. 2010, 95, 341–344. [Google Scholar] [CrossRef]
- Cabral-Pinto, M.M.S.; Inácio, M.; Neves, O.; Almeida, A.A.; Pinto, E.; Oliveiros, B.; Ferreira da Silva, E.A. Human Health Risk Assessment Due to Agricultural Activities and Crop Consumption in the Surroundings of an Industrial Area. Expo. Health 2020, 12, 629–640. [Google Scholar] [CrossRef]
- Rahman, M.A.T.M.T.; Paul, M.; Bhoumik, N.; Hassan, M.; Alam, M.K.; Aktar, Z. Heavy Metal Pollution Assessment in the Groundwater of the Meghna Ghat Industrial Area, Bangladesh, by Using Water Pollution Indices Approach. Appl. Water Sci. 2020, 10, 186. [Google Scholar] [CrossRef]
- Wagh, V.M.; Panaskar, D.B.; Mukate, S.V.; Gaikwad, S.K.; Muley, A.A.; Varade, A.M. Health Risk Assessment of Heavy Metal Contamination in Groundwater of Kadava River Basin, Nashik, India. Model. Earth Syst. Environ. 2018, 4, 969–980. [Google Scholar] [CrossRef]
- Singh, K.P.; Malik, A.; Sinha, S.; Singh, V.K.; Murthy, R.C. Estimation of Source of Heavy Metal Contamination in Sediments of Gomti River (India) Using Principal Component Analysis. Water. Air Soil Pollut. 2005, 166, 321–341. [Google Scholar] [CrossRef]
- Ahmed, S.; Khurshid, S.; Qureshi, F.; Hussain, A.; Bhattacharya, A. Heavy Metals and Geo-Accumulation Index Development for Groundwater of Mathura City, Uttar Pradesh. Desalin. Water Treat. 2019, 138, 291–300. [Google Scholar] [CrossRef]
- Loska, K.; Wiechulła, D.; Korus, I. Metal Contamination of Farming Soils Affected by Industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Rieumont, S.; De La Rosa, D.; Lima, L.; Graham, D.W.; D’Alessandro, K.; Borroto, J.; Martínez, F.; Sánchez, J. Assessment of Heavy Metal Levels in Almendares River Sediments—Havana City, Cuba. Water Res. 2005, 39, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Egbueri, J.C. Groundwater Quality Assessment Using Pollution Index of Groundwater (PIG), Ecological Risk Index (ERI) and Hierarchical Cluster Analysis (HCA): A Case Study. Groundw. Sustain. Dev. 2020, 10, 100292. [Google Scholar] [CrossRef]
- Fei, J.C.; Min, X.B.; Wang, Z.X.; Pang, Z.H.; Liang, Y.J.; Ke, Y. Health and Ecological Risk Assessment of Heavy Metals Pollution in an Antimony Mining Region: A Case Study from South China. Environ. Sci. Pollut. Res. 2017, 24, 27573–27586. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, J.S.; Chon, H.T.; Sager, M. Heavy Metal Contamination and Health Risk Assessment in the Vicinity of the Abandoned Songcheon Au-Ag Mine in Korea. J. Geochem. Explor. 2008, 96, 223–230. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Y.; Zhang, X.; Cheng, S. Health Risk from Exposure of Organic Pollutants through Drinking Water Consumption in Nanjing, China. Bull. Environ. Contam. Toxicol. 2010, 84, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Eyankware, M.O.; Akakuru, O.C. Appraisal of Groundwater to Risk Contamination near an Abandoned Limestone Quarry Pit in Nkalagu, Nigeria, Using Enrichment Factor and Statistical Approaches. Int. J. Energy Water Resour. 2022, 7, 1–19. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Wang, Y.; Fang, Y.; Martín, J.D. Multivariate Statistical Analysis of Hydrochemical Data for Shallow Ground Water Quality Factor Identification in a Coastal Aquifer. Pol. J. Environ. Stud. 2016, 24, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Helstrup, T.; Jørgensen, N.O.; Banoeng-Yakubo, B. Investigation of Hydrochemical Characteristics of Groundwater from the Cretaceous-Eocene Limestone Aquifer in Southern Ghana and Southern Togo Using Hierarchical Cluster Analysis. Hydrogeol. J. 2007, 15, 977–989. [Google Scholar] [CrossRef]
- Arslan, H.; Ayyildiz Turan, N. Estimation of Spatial Distribution of Heavy Metals in Groundwater Using Interpolation Methods and Multivariate Statistical Techniques; Its Suitability for Drinking and Irrigation Purposes in the Middle Black Sea Region of Turkey. Environ. Monit. Assess. 2015, 187, 516. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Natasha; Shah, A.H.; Saeed, F.; Ali, M.; Qaisrani, S.A.; Dumat, C. Heavy Metal Contamination and Exposure Risk Assessment via Drinking Groundwater in Vehari, Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 39852–39864. [Google Scholar] [CrossRef]
- Long, X.; Liu, F.; Zhou, X.; Pi, J.; Yin, W.; Li, F.; Huang, S. Chemosphere Estimation of Spatial Distribution and Health Risk by Arsenic and Heavy Metals in Shallow Groundwater around Dongting Lake Plain Using GIS Mapping. Chemosphere 2021, 269, 128698. [Google Scholar] [CrossRef]
- Mehrjardi, R.T.; Jahromi, M.Z.; Heidari, A. Spatial Distribution of Groundwater Quality with Geostatistics (Case Study: Yazd-Ardakan Plain). Appl. Sci. 2008, 4, 9–17. [Google Scholar]
- Bataillon, C. Le Souf, Étude de Géographie Humaine; Université d’Alger: Alger Ctre, Aljeria, 1955. [Google Scholar]
- Côte, M. Si Le Souf m’était Conté: Comment Se Fait et Se Défait Un Paysage; Hannachi, S., Ed.; Média-Plus: Constantine, Algeria, 2006; ISBN 9961-922-42-5. [Google Scholar]
- Guendouz, A.; Moulla, A.S.; Edmunds, W.M.; Zouari, K.; Shand, P.; Mamou, A. Hydrogeochemical and Isotopic Evolution of Water in the Complexe Terminal Aquifer in the Algerian Sahara. Hydrogeol. J. 2003, 11, 483–495. [Google Scholar] [CrossRef]
- Slimani, R.; Guendouz, A.; Trolard, F.; Souffi Moulla, A.; Hamdi-Aïssa, B.; Bourrié, G. Identification of Dominant Hydrogeochemical Processes for Groundwaters in the Algerian Sahara Supported by Inverse Modeling of Chemical and Isotopic Data. Hydrol. Earth Syst. Sci. 2017, 21, 1669–1691. [Google Scholar] [CrossRef]
- Bouchahm, N.; Achour, S. Hydrochimie Des Eaux Souterraines de La Région Orientale Du Sahara Septentrional Algérien—Identification d’un Risque de Fluorose Endémique. La Houille Blanche 2008, 94, 76–82. [Google Scholar] [CrossRef]
- Kholladi, M. SIG Pour Le Suivi de La Remontée Des Eaux de La Wilaya d’El Oued Souf. In Proceedings of the Congrès Internationale en Informatique Appliquée CiiA, Bordj Bou Arréridj, Algerie, 19–21 November 2005. [Google Scholar]
- Drouiche, A.; Chaab, S.; Khechana, S. Impact Du Deversement Direct Des Eaux Usees et de Drainage Dans La Nappe Libre de l’Oued Souf et Son Influence Sur La Qualite Des Eaux Souterraines. Synthèse Rev. Des Sci. La Technol. 2013, 27, 50–62. [Google Scholar]
- Barkat, A.; Bouaicha, F.; Bouteraa, O.; Ata, B.; Balla, D.; Rahal, Z.; Szabó, G. Assessment of Complex Terminal Groundwater Aquifer for Different Use of Oued Souf Valley (Algeria) Using Multivariate Statistical Methods, Geostatistical Modeling, and Water Quality Index. Water 2021, 13, 1609. [Google Scholar] [CrossRef]
- Khezzani, B.; Bouchemal, S. Variations in Groundwater Levels and Quality Due to Agricultural Over-Exploitation in an Arid Environment: The Phreatic Aquifer of the Souf Oasis (Algerian Sahara). Environ. Earth Sci. 2018, 77, 142. [Google Scholar] [CrossRef]
- Barkat, A.; Bouaicha, F.; Rahal, Z.; Mester, T.; Szabó, G. Evaluation of Climatic Conditions from 1978 to 2020 of Oued Souf Valley (Southern East of Algeria). Landsc. Environ. 2023, 17, 1–10. [Google Scholar]
- Messekher, I.; Chabour, N.; Menani, R. Remontée de La Nappe Phréatique Du Souf. Conséquences et Solutions Envisagées. Ann. Univ. Buchar. Geogr. Ser. 2012, LXI, 179–197. [Google Scholar]
- Kadri, S.R.; Chaouche, S. La Remontée Des Eaux Dans La Région Du Souf: Une Menace Sur Un Écosystème Oasien. Les Cah. D’emam 2018, 30, 1–20. [Google Scholar] [CrossRef]
- Côte, M. Dynamique Urbaine Au Sahara. Rev. Algérienne D’anthropologie Sci. Soc. 1998, 5, 85–92. [Google Scholar] [CrossRef]
- Djennane, A. Constat de Situation Dans Des Zones Sud Des Oasis Algériennes. In Les systèmes Agric. Oasiens; Dollé, V., Toutain, G., Eds.; CIHEAM: Montpellier, France, 1990; Volume 11, pp. 29–40. [Google Scholar]
- Burri, J.; Burri, J. Vallée Du Souf: Etudes d’assainissement Des Eaux Résiduaires, Pluviales et d’irrigation. In Mesures Complémentaires de Lutte Contre La Remontée de La Nappe Phréatique, Mission II, Rapport; Ministere Des Ressources En Eau Office National De L’assainissement (ONA): Tunis, Tunisia, 2004. [Google Scholar]
- Ballais, J.; Côte, M.; Bensaad, A. L’influence de La Géomorphologie Sur Le Comportement de La Nappe Phréatique Du Souf. Ph.D. Thesis, Université de Provence, Marseille, France, 2002. [Google Scholar]
- Fabre, J. Introduction à La Géologie Du Sahara Algérien et Des Régions Voisines: La Couverture Phanérozoïque. Vol. 1; Société Nationale d’Édition et de Diffusion: Algiers, Algeria, 1976; ISBN 84-499-2264-X. [Google Scholar]
- Nesson, C. L’évolution Des Ressources Hydrauliques Dans Les Oasis Du Bas-Sahara Algérien. GéoProdig Portail D’information Géographique 1975, 17, 7–99. [Google Scholar]
- Meziani, A.; Dridi, H. The Aquifer System of the Souf Valley: Algerian Northern Sahara. Eur. J. Sci. Res. 2011, 65, 416–423. [Google Scholar]
- Cornet, A. Introduction à l’hydrogéologie Saharienne. Géorg. Phys. Géol. Dyn 1946, 6, 5–72. [Google Scholar]
- Gonçalvès, J.; Petersen, J.; Deschamps, P.; Hamelin, B.; Baba-Sy, O. Quantifying the Modern Recharge of the “Fossil” Sahara Aquifers. Geophys. Res. Lett. 2013, 40, 2673–2678. [Google Scholar] [CrossRef]
- Guendouz, A.; Michelot, J.L. Chlorine-36 Dating of Deep Groundwater from Northern Sahara. J. Hydrol. 2006, 328, 572–580. [Google Scholar] [CrossRef]
- Castany, G. Sedimentary Basin of the Northern Sahara (Algeria, Tunisia). Intercalary Continental and Terminal Complex Aquifers [Confined Groundwater, Multilevel System, Hydrodynamics, Management of Water Resources, and Environment. Bull. Du Bur. Rech. Geol. Min. Sect. 3 Hydrogeol. Geol. L’ingenieur 1982, 2, 127–147. [Google Scholar]
- Najah, A. Le Souf Des Oasis.; La Maison des Livres Alger, Ed.; La Maison des Livres Alger: Alger, Algeria, 1970. [Google Scholar]
- Dervierux, F. La Nappe Phréatique Du Souf (Région d’El Oued, Algérie)-Etude Du Renouvellement de La Nappe. Terres Eaux OCRS 3trim 1957, 56, 12–39. [Google Scholar]
- UNESCO. Etude Des Ressources En Eau de Sahara Septentrional; UNESCO: Paris, France, 1972. [Google Scholar]
- Barkat, A.; Bouaicha, F.; Mester, T.; Debabeche, M.; Szabó, G. Assessment of Spatial Distribution and Temporal Variations of the Phreatic Groundwater Level Using Geostatistical Modelling: The Case of Oued Souf Valley—Southern East of Algeria. Water 2022, 14, 1415. [Google Scholar] [CrossRef]
- Sajtos, Z.; Herman, P.; Harangi, S.; Baranyai, E. Elemental Analysis of Hungarian Honey Samples and Bee Products by MP-AES Method. Microchem. J. 2019, 149, 103968. [Google Scholar] [CrossRef]
- Backman, B.; Bodiš, D.; Lahermo, P.; Rapant, S.; Tarvainen, T. Application of a Groundwater Contamination Index in Finland and Slovakia. Environ. Geol. 1998, 36, 55–64. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Muller, G. Index of Geoaccumulation in Sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Egbueri, J.C.; Unigwe, C.O. Understanding the Extent of Heavy Metal Pollution in Drinking Water Supplies from Umunya, Nigeria: An Indexical and Statistical Assessment. Anal. Lett. 2020, 53, 2122–2144. [Google Scholar] [CrossRef]
- Ukah, B.U.; Ameh, P.D.; Egbueri, J.C.; Unigwe, C.O.; Ubido, O.E. Impact of Effluent-Derived Heavy Metals on the Groundwater Quality in Ajao Industrial Area, Nigeria: An Assessment Using Entropy Water Quality Index (EWQI). Int. J. Energy Water Resour. 2020, 4, 231–244. [Google Scholar] [CrossRef]
- Adimalla, N.; Wang, H. Distribution, Contamination, and Health Risk Assessment of Heavy Metals in Surface Soils from Northern Telangana, India. Arab. J. Geosci. 2018, 11, 684. [Google Scholar] [CrossRef]
- Sinex, S.A.; Helz, G.R. Regional Geochemistry of Trace Elements in Chesapeake Bay Sediments. Environ. Geol. 1981, 3, 315–323. [Google Scholar] [CrossRef]
- Bhutiani, R.; Kulkarni, D.B.; Khanna, D.R.; Gautam, A. Geochemical Distribution and Environmental Risk Assessment of Heavy Metals in Groundwater of an Industrial Area and Its Surroundings, Haridwar, India. Energy Ecol. Environ. 2017, 2, 155–167. [Google Scholar] [CrossRef]
- Hakima, Z.; Mohamed, M.; Aziza, M.; Mehdi, M.; Meryem, E.B.; Bendahhou, Z.; Jean-Francois, B. Environmental and Ecological Risk of Heavy Metals in the Marine Sediment from Dakhla Bay, Morocco. Environ. Sci. Pollut. Res. 2017, 24, 7970–7981. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.L. Riverine Composition and Estuarine Geochemistry of Particulate Metals in China—Weathering Features, Anthropogenic Impact and Chemical Fluxes. Estuar. Coast. Shelf Sci. 2002, 54, 1051–1070. [Google Scholar] [CrossRef]
- Fang, H.; Wang, X.; Xia, D.; Zhu, J.; Yu, W.; Su, Y.; Zeng, J.; Zhang, Y.; Lin, X.; Lei, Y.; et al. Improvement of Ecological Risk Considering Heavy Metal in Soil and Groundwater Surrounding Electroplating Factories. Processes 2022, 10, 1267. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the Assessment of Heavy-Metal Levels in Estuaries and the Formation of a Pollution Index. Helgoländer Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Rezaei, H.; Zarei, A.; Kamarehie, B. Levels, Distributions and Health Risk Assessment of Lead, Cadmium and Arsenic Found in Drinking Groundwater of Dehgolan’ s Villages, Iran. Toxicol. Environ. Health Sci. 2019, 11, 54–62. [Google Scholar] [CrossRef]
- USEPA (US Environmental Protection Agency). Risk Assessment Guidance for Superfund Volume 1. Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); EPA/540/R/99/005; Office of Superfund Remediation and Technology Innovation: Washington, DC, USA, 2004. [Google Scholar]
- USEPA (US Environmental Protection Agency). Guidelines for Carcinogenic Risk Assessment; Risk Assessment Forum: Washington, DC, USA, 2006. [Google Scholar]
- USEPA (US Environmental Protection Agency). Guidance for Performing Aggregate Exposure and Risk Assessments; Office of Pesticide Programs: Washington, DC, USA, 1999. [Google Scholar]
- Qiu, H.; Gui, H. Human and Ecological Risk Assessment: An International Heavy Metals Contamination in Shallow Groundwater of a Coal-Mining District and a Probabilistic Assessment of Its Human Health Risk. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 548–563. [Google Scholar] [CrossRef]
- Murray, F.J. A Human Health Risk Assessment of Boron (Boric Acid and Borax) in Drinking Water. Regul. Toxicol. Pharmacol. 1995, 22, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.A.; Oyadeyi, A.T.; Adeyemi, A.A. Assessment of Heavy Metals Contamination and Associated Risks in Shallow Groundwater Sources from Three Different Residential Areas within Ibadan Metropolis, Southwest Nigeria. Appl. Water Sci. 2021, 11, 81. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Human Health Risk Assessment via Drinking Water Pathway Due to Metal Contamination in the Groundwater of Subarnarekha River Basin, India. Environ. Monit. Assess. 2015, 187, 63. [Google Scholar] [CrossRef]
- Nematollahi, M.J.; Dehdaran, S.; Moore, F.; Keshavarzi, B. Potentially Toxic Elements and Polycyclic Aromatic Hydrocarbons in Street Dust of Yazd, a Central Capital City in Iran: Contamination Level, Source Identification, and Ecological—Health Risk Assessment; Springer: Dordrecht, The Netherlands, 2021; Volume 43, ISBN 0123456789. [Google Scholar]
- Paustenbach, D. Human and Ecological Risk Assessment: Theory and Practice (Wiley Classics Library); John Wiley and Sons: New York, NY, USA, 2015; ISBN 0-471-14747-8. [Google Scholar]
- Charizopoulos, N.; Zagana, E.; Psilovikos, A. Assessment of Natural and Anthropogenic Impacts in Groundwater, Utilizing Multivariate Statistical Analysis and Inverse Distance Weighted Interpolation Modeling: The Case of a Scopia Basin (Central Greece). Environ. Earth Sci. 2018, 77, 380. [Google Scholar] [CrossRef]
- Loh, Y.S.A.; Akurugu, B.A.; Manu, E.; Aliou, A.S. Assessment of Groundwater Quality and the Main Controls on Its Hydrochemistry in Some Voltaian and Basement Aquifers, Northern Ghana. Groundw. Sustain. Dev. 2020, 10, 100296. [Google Scholar] [CrossRef]
- Ren, X.; Li, P.; He, X.; Su, F.; Elumalai, V. Hydrogeochemical Processes Affecting Groundwater Chemistry in the Central Part of the Guanzhong Basin, China. Arch. Environ. Contam. Toxicol. 2021, 80, 74–91. [Google Scholar] [CrossRef]
- Carrera-Villacrés, D.; Guevara-García, P.; Hidalgo-Hidalgo, A.; Teresa-Vivero, M.; Maya-Carrillo, M. Removal of Physical Information Chemistry of Spa That Is Utilizing Geothermal Water in Ecuador. Procedia Earth Planet. Sci. 2015, 15, 367–373. [Google Scholar] [CrossRef]
- Rusydi, A.F. Correlation between Conductivity and Total Dissolved Solid in Various Type of Water: A Review. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012019. [Google Scholar] [CrossRef]
- Ahmedat, C.; Dabi, S.; Zahraoui, M.; El Hassani, I.E.E.A. Spatial Distribution of Stream Sediment Pollution by Toxic Trace Elements at Tourtit and Ichoumellal Abandoned Mining Areas (Central Morocco). Arab. J. Geosci. 2018, 11, 55. [Google Scholar] [CrossRef]
- Batayneh, A.T. Toxic (Aluminum, Beryllium, Boron, Chromium and Zinc) in Groundwater: Health Risk Assessment. Int. J. Environ. Sci. Technol. 2012, 9, 153–162. [Google Scholar] [CrossRef]
- Mgbenu, C.N.; Egbueri, J.C. The Hydrogeochemical Signatures, Quality Indices and Health Risk Assessment of Water Resources in Umunya District, Southeast Nigeria. Appl. Water Sci. 2019, 9, 22. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Umar, R.; Lateh, H. Study of Trace Elements in Groundwater of Western Uttar Pradesh, India. Sci. Res. Essays 2010, 5, 3175–3182. [Google Scholar]
- Mondal, N.C.; Singh, V.S.; Puranik, S.C.; Singh, V.P. Trace Element Concentration in Groundwater of Pesarlanka Island, Krishna Delta, India. Environ. Monit. Assess. 2010, 163, 215–227. [Google Scholar] [CrossRef]
- White, A.F.; Benson, S.M.; Yee, A.W.; Wollenberg, H.A., Jr.; Flexser, S. Groundwater Contamination at the Kesterson Reservoir, California 2. Geochemical Parameters Influencing Selenium Mobility. Water Resour. Res. 1991, 27, 1085–1098. [Google Scholar] [CrossRef]
- Miller, R.W.; Donahue, R.L. Soils in Our Environment; Prentice Hall: Upper Saddle River, NJ, USA, 1995. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Volume 1: Recommendations; World Health Organization, Ed.; World Health Organization: Geneva, Switzerland, 1984. [Google Scholar]
- USEPA (US Environmental Protection Agency). Guidelines for Carcinogen Risk Assessment; Risk Assessment Forum: Washington, DC, USA, 2005. [Google Scholar]
- Jiang, Z.; Xu, N.; Liu, B.; Zhou, L.; Wang, J.; Wang, C.; Dai, B.; Xiong, W. Metal Concentrations and Risk Assessment in Water, Sediment and Economic Fish Species with Various Habitat Preferences and Trophic Guilds from Lake Caizi, Southeast China. Ecotoxicol. Environ. Saf. 2018, 157, 1–8. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, Q.; Wu, H.; Liu, J.; Ning, Y.; Xie, S.; Huang, W.; Bi, X. Enrichment of Trace Elements in Red Swamp Crayfish: Influences of Region and Production Method, and Human Health Risk Assessment. Aquaculture 2021, 535, 736366. [Google Scholar] [CrossRef]
- Rattan, R.K.; Datta, S.P.; Chhonkar, P.K.; Suribabu, K.; Singh, A.K. Long-Term Impact of Irrigation with Sewage Effluents on Heavy Metal Content in Soils, Crops and Groundwater—A Case Study. Agric. Ecosyst. Environ. 2005, 109, 310–322. [Google Scholar] [CrossRef]
- Nouri, J.; Mahvi, A.H.; Jahed, G.R.; Babaei, A.A. Regional Distribution Pattern of Groundwater Heavy Metals Resulting from Agricultural Activities. Environ. Geol. 2008, 55, 1337–1343. [Google Scholar] [CrossRef]
- Goel, P.K. Water Pollution Causes Effect and Control; New Age International Publishers: Delhi, India, 2006. [Google Scholar]
- Government of Canada Water Talk—Strontium in Drinking Water. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/water-talk-strontium.html (accessed on 7 July 2023).
- Négrel, P.; Petelet-Giraud, E.; Widory, D. Strontium Isotope Geochemistry of Alluvial Groundwater: A Tracer for Groundwater Resources Characterisation. Hydrol. Earth Syst. Sci. 2004, 8, 959–972. [Google Scholar] [CrossRef]
- Frankowski, M.; Zioła-Frankowska, A.; Siepak, J. Speciation of Aluminium Fluoride Complexes and Al3+ in Soils from the Vicinity of an Aluminium Smelter Plant by Hyphenated High Performance Ion Chromatography Flame Atomic Absorption Spectrometry Technique. Microchem. J. 2010, 95, 366–372. [Google Scholar] [CrossRef]
- Frankowski, M. Simultaneous Determination of Aluminium, Aluminium Fluoride Complexes and Iron in Groundwater Samples by New HPLC-UVVIS Method. Microchem. J. 2012, 101, 80–86. [Google Scholar] [CrossRef]
- Fernández-Luqueño, F.; López-Valdez, F.; Gamero-Melo, P.; Luna-Suárez, S.; Aguilera-González, E.; Martínez, A.; García-Guillermo, M.; Hernández-Martínez, G.; Herrera-Mendoza, R.; Álvarez-Garza, M.; et al. Heavy Metal Pollution in Drinking Water—A Global Risk for Human Health: A Review. Afr. J. Environ. Sci. Technol. 2013, 7, 567–584. [Google Scholar]
- Shakerkhatibi, M.; Mosaferi, M.; Pourakbar, M.; Ahmadnejad, M.; Safavi, N.; Banitorab, F. Comprehensive Investigation of Groundwater Quality in the North-West of Iran: Physicochemical and Heavy Metal Analysis. Groundw. Sustain. Dev. 2019, 8, 156–168. [Google Scholar] [CrossRef]
- Devic, G.; Djordjevic, D.; Sakan, S. Natural and Anthropogenic Factors Affecting the Groundwater Quality in Serbia. Sci. Total Environ. 2014, 468–469, 933–942. [Google Scholar] [CrossRef]
- L.W. Diamond, R.E.S. Sources of Manganese in the Environment. Environ. Geochem. Health 1989, 11, 17–27. [Google Scholar]
- Kumar, A.; Cabral-Pinto, M.; Kumar, A.; Kumar, M.; Dinis, P.A. Estimation of Risk to the Eco-Environment and Human Health of Using Heavy Metals in the Uttarakhand Himalaya, India. Appl. Sci. 2020, 10, 7078. [Google Scholar] [CrossRef]
- Struzyńska, L. A Glutamatergic Component of Lead Toxicity in Adult Brain: The Role of Astrocytic Glutamate Transporters. Neurochem. Int. 2009, 55, 151–156. [Google Scholar] [CrossRef]
- Butterworth, R.F. Metal Toxicity, Liver Disease and Neurodegeneration. Neurotox. Res. 2010, 18, 100–105. [Google Scholar] [CrossRef]
- Salonen, J.T.; Nyyssönen, K.; Korpela, H.; Tuomilehto, J.; Seppänen, R.; Salonen, R. High Stored Iron Levels Are Associated with Excess Risk of Myocardial Infarction in Eastern Finnish Men. Circulation 1992, 86, 803–811. [Google Scholar] [CrossRef]
- Tuomainen, T.P.; Punnonen, K.; Nyyssönen, K.; Salonen, J.T. Association between Body Iron Stores and the Risk of Acute Myocardial Infarction in Men. Circulation 1998, 97, 1461–1466. [Google Scholar] [CrossRef]
- Salonen, J.T.; Tuomainen, T.P.; Nyyssönen, K.; Lakka, H.M.; Punnonen, K. Relation between Iron Stores and Non-Insulin Dependent Diabetes in Men: Case-Control Study. Br. Med. J. 1998, 317, 727. [Google Scholar] [CrossRef]
- Tuomainen, T.; Nyyssônen, K.; Salonen, R.; Tervahauta, A.; Korpela, H.; Lakka, T.; Kaplan, G.; Salonen, J. Body Iron Stores Are Associated with Serum Insulin and Blood Glucose Concentrations. Diabetes Care 1997, 20, 426–428. [Google Scholar] [CrossRef]
- Rahman, M.F.; Ali, M.A.; Chowdhury, A.I.A.; Ravenscroft, P. Manganese in Groundwater in South Asia Needs Attention. ACS ES T Water 2023, 3, 1425–1428. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Gu, H.; Lu, L.; Li, L.; Pang, J.; Chen, F. The Genesis Mechanism and Health Risk Assessment of High Boron Water in the Zhaxikang Geothermal Area, South Tibet. Water 2022, 14, 3243. [Google Scholar] [CrossRef]
- McGregor, D.B.; Baan, R.A.; Partensky, C.; Rice, J.M.; Wilbourn, J.D. Evaluation of the Carcinogenic Risks to Humans Associated with Surgical Implants and Other Foreign Bodies—A Report of an IARC Monographs Programme Meeting. Eur. J. Cancer 2000, 36, 307–313. [Google Scholar] [CrossRef]
- Seilkop, S.K.; Oller, A.R. Respiratory Cancer Risks Associated with Low-Level Nickel Exposure: An Integrated Assessment Based on Animal, Epidemiological, and Mechanistic Data. Regul. Toxicol. Pharmacol. 2003, 37, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.S.; Ilyas, M.; Xu, R.Z.; Ahmad, W.; Wang, R. Concentrations of Lead in Groundwater and Human Blood in the Population of Palosai, a Rural Area in Pakistan: Human Exposure and Risk Assessment. Adsorpt. Sci. Technol. 2022, 2022, 8341279. [Google Scholar] [CrossRef]
- Khandare, A.L.; Validandi, V.; Rajendran, A.; Singh, T.G.; Thingnganing, L.; Kurella, S.; Nagaraju, R.; Dheeravath, S.; Vaddi, N.; Kommu, S.; et al. Health Risk Assessment of Heavy Metals and Strontium in Groundwater Used for Drinking and Cooking in 58 Villages of Prakasam District, Andhra Pradesh, India. Environ. Geochem. Health 2020, 42, 3675–3701. [Google Scholar] [CrossRef]
- Hart, K.A.; Kennedy, G.W.; Sterling, S.M. Distribution, Drivers, and Threats of Aluminum in Groundwater in Nova Scotia, Canada. Water 2021, 13, 1578. [Google Scholar] [CrossRef]


| Common Conditions | |
|---|---|
| Replicates | 3 |
| Pump speed | 15 rpm |
| Uptake time | 15 s |
| Rinse time | 30 s |
| Stabilization time | 10 s |
| Element | Wavelength (nm) | Read Time (s) | Nebulizer Pressure (kPa) |
|---|---|---|---|
| Al | 394.401 | 3 | 240 |
| B | 249.772 | 3 | 100 |
| Ba | 455.403 | 3 | 240 |
| Bi | 306.772 | 3 | 140 |
| Ca | 430.253 | 1 | 140 |
| Cd | 228.802 | 3 | 140 |
| Co | 340.512 | 3 | 240 |
| Cr | 425.433 | 3 | 240 |
| Cu | 324.754 | 1 | 240 |
| Fe | 371.993 | 3 | 120 |
| K | 776.897 | 1 | 200 |
| Li | 610.365 | 1 | 240 |
| Mg | 383.829 | 3 | 240 |
| Mn | 403.076 | 3 | 240 |
| Na | 589.592 | 1 | 80 |
| Ni | 352.454 | 3 | 240 |
| Pb | 405.781 | 5 | 240 |
| Sr | 407.771 | 1 | 200 |
| Zn | 213.857 | 5 | 140 |
| Variables | Mean | SD | CV | Min | Median | Max | WHO 2008 |
|---|---|---|---|---|---|---|---|
| T (°C) | 27.850 | 1.720 | 0.062 | 25.000 | 27.800 | 31.400 | - |
| pH | 7.307 | 0.515 | 0.070 | 6.780 | 7.110 | 8.570 | 6.5–8.5 |
| EC (µS/cm) | 4035.714 | 858.018 | 0.213 | 3100 | 3725 | 6200 | 1000 |
| Al (mg/L) | 0.309 | 0.083 | 0.269 | 0.220 | 0.290 | 0.520 | 0.2 |
| Fe (mg/L) | 0.214 | 0.092 | 0.429 | 0.110 | 0.185 | 0.400 | 0.3 |
| Mn (mg/L) | 0.440 | 0.111 | 0.253 | 0.300 | 0.400 | 0.710 | 0.5 |
| B (mg/L) | 0.626 | 0.426 | 0.681 | 0.192 | 0.454 | 1.408 | 0.5 |
| Ba (mg/L) | 0.015 | 0.009 | 0.628 | 0.004 | 0.011 | 0.034 | 0.7 |
| Bi (mg/L) | 0.144 | 0.108 | 0.753 | 0.000 | 0.146 | 0.282 | - |
| Cd (mg/L) | <Lod | <Lod | <Lod | <Lod | <Lod | <Lod | 0.003 |
| Co (mg/L) | <Lod | <Lod | <Lod | <Lod | <Lod | <Lod | - |
| Cr (mg/L) | 0.0081 | 0.010 | 1.231 | 0.000 | 0.000 | 0.023 | 0.05 |
| Cu (mg/L) | 0.004 | 0.007 | 1.612 | 0.000 | 0.000 | 0.020 | 1 |
| Li (mg/L) | <Lod | <Lod | <Lod | <Lod | <Lod | <Lod | - |
| Ni (mg/L) | 0.009 | 0.007 | 0.832 | 0.000 | 0.007 | 0.024 | 0.02 |
| Pb (mg/L) | 0.005 | 0.013 | 2.711 | 0.000 | 0.000 | 0.045 | 0.01 |
| Sr (mg/L) | 7.060 | 2.437 | 0.345 | 1.774 | 7.762 | 9.939 | - |
| Zn (mg/L) | 0.0075 | 0.012 | 1.613 | 0.000 | 0.000 | 0.037 | 3 |
| Parameters | Cross-Validation | K = 1 | K = 2 | K = 3 | Optimized Errors | Optimized K Value |
|---|---|---|---|---|---|---|
| Al | MPE | 0.00148 | 0.00184 | 0.0031 | 0.0029 | 2.86922 |
| RMSPE | 0.0835 | 0.08032 | 0.07948 | 0.07946 | ||
| Fe | MPE | 0.00106 | −0.00089 | −0.0016 | −0.00017 | 1.567 |
| RMSPE | 0.10417 | 0.10391 | 0.1048 | 0.10375 | ||
| Mn | MPE | 0.01766 | 0.02316 | 0.02614 | 0.01766 | 1 |
| RMSPE | 0.1278 | 0.1413 | 0.15272 | 0.1278 | ||
| B | MPE | 0.0356 | 0.0373 | 0.03102 | 0.0356 | 1 |
| RMSPE | 0.5338 | 0.5667 | 0.59156 | 0.5338 | ||
| Ba | MPE | −0.00016 | −0.00058 | −0.00091 | −0.00016 | 1 |
| RMSPE | 0.01152 | 0.01212 | 0.0127 | 0.01152 | ||
| Bi | MPE | −0.00032 | 0.00196 | 0.00393 | −0.00032 | 1 |
| RMSPE | 0.14673 | 0.15796 | 0.16672 | 0.14673 | ||
| Cr | MPE | 0.00104 | 0.00183 | 0.00234 | 0.00104 | 1 |
| RMSPE | 0.01179 | 0.01261 | 0.01337 | 0.01179 | ||
| Cu | MPE | 0.0006 | 0.00073 | 0.00072 | 0.0006 | 1 |
| RMSPE | 0.00975 | 0.01015 | 0.01044 | 0.00975 | ||
| Ni | MPE | 0.00109 | 0.00187 | 0.00235 | 0.00109 | 1 |
| RMSPE | 0.0079 | 0.0085 | 0.00905 | 0.0079 | ||
| Pb | MPE | −0.00027 | −0.00083 | −0.00122 | −0.00157 | 17.46576 |
| RMSPE | 0.01806 | 0.01802 | 0.018 | 0.018 | ||
| Sr | MPE | −0.09789 | −0.2642 | −0.39167 | −0.09789 | 1 |
| RMSPE | 2.75949 | 2.92814 | 3.09837 | 2.75949 | ||
| Zn | MPE | 0.00092 | 0.00181 | 0.00247 | 0.00165 | 1.79839 |
| RMSPE | 0.01226 | 0.01204 | 0.01227 | 0.01203 |
| Heavy Metals | Group 1 (n = 3) | Group 2 (n = 11) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | Mean | Median | Minimum | Maximum | |
| Al | 0.30 | 0.3 | 0.28 | 0.33 | 0.31 | 0.29 | 0.22 | 0.52 |
| Fe | 0.15 | 0.15 | 0.12 | 0.17 | 0.23 | 0.23 | 0.11 | 0.40 |
| Mn | 0.37 | 0.37 | 0.35 | 0.38 | 0.46 | 0.42 | 0.30 | 0.71 |
| B | 0.24 | 0.246 | 0.19 | 0.27 | 0.73 | 0.579 | 0.31 | 1.41 |
| Ba | 0.01 | 0.008 | 0.01 | 0.01 | 0.02 | 0.016 | 0.00 | 0.03 |
| Bi | 0.21 | 0.188 | 0.17 | 0.28 | 0.12 | 0.1 | 0.00 | 0.27 |
| Cr | 0.02 | 0.022 | 0.02 | 0.02 | 0.00 | 0 | 0.00 | 0.02 |
| Cu | 0.00 | 0 | 0.00 | 0.00 | 0.01 | 0.002 | 0.00 | 0.02 |
| Ni | 0.02 | 0.019 | 0.02 | 0.02 | 0.01 | 0.006 | 0.00 | 0.01 |
| Pb | 0.00 | 0 | 0.00 | 0.00 | 0.01 | 0 | 0.00 | 0.05 |
| Sr | 3.15 | 3.642 | 1.77 | 4.02 | 8.13 | 8.158 | 6.19 | 9.94 |
| Zn | 0.03 | 0.03 | 0.01 | 0.04 | 0.00 | 0.00 | 0.00 | 0.02 |
| Cases | Adults | Children | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chronic Daily Intake | Mean | Standard Deviation | Minimum | Median | Maximum | Mean | Standard Deviation | Minimum | Median | Maximum |
| Al | 0.00847 | 0.00228 | 0.00603 | 0.00795 | 0.01425 | 0.01977 | 0.00531 | 0.01406 | 0.01854 | 0.03324 |
| Fe | 0.00587 | 0.00252 | 0.00301 | 0.00507 | 0.01096 | 0.01370 | 0.00588 | 0.00703 | 0.01183 | 0.02557 |
| Mn | 0.01205 | 0.00305 | 0.00822 | 0.01096 | 0.01945 | 0.02813 | 0.00711 | 0.01918 | 0.02557 | 0.04539 |
| B | 0.01714 | 0.01168 | 0.00526 | 0.01242 | 0.03858 | 0.04000 | 0.02726 | 0.01227 | 0.02899 | 0.09001 |
| Ba | 0.00040 | 0.00025 | 0.00011 | 0.00029 | 0.00093 | 0.00093 | 0.00058 | 0.00026 | 0.00067 | 0.00217 |
| Cr | 0.00022 | 0.00027 | 0.00000 | 0.00000 | 0.00063 | 0.00052 | 0.00064 | 0.00000 | 0.00000 | 0.00147 |
| Cu | 0.00012 | 0.00019 | 0.00000 | 0.00000 | 0.00055 | 0.00028 | 0.00045 | 0.00000 | 0.00000 | 0.00128 |
| Ni | 0.00024 | 0.00020 | 0.00000 | 0.00018 | 0.00066 | 0.00057 | 0.00047 | 0.00000 | 0.00042 | 0.00153 |
| Pb | 0.00013 | 0.00036 | 0.00000 | 0.00000 | 0.00123 | 0.00031 | 0.00083 | 0.00000 | 0.00000 | 0.00288 |
| Sr | 0.19342 | 0.06676 | 0.04860 | 0.21266 | 0.27230 | 0.45131 | 0.15576 | 0.11341 | 0.49620 | 0.63537 |
| Zn | 0.00021 | 0.00033 | 0.00000 | 0.00000 | 0.00101 | 0.00048 | 0.00077 | 0.00000 | 0.00000 | 0.00237 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkat, A.; Bouaicha, F.; Ziad, S.; Mester, T.; Sajtos, Z.; Balla, D.; Makhloufi, I.; Szabó, G. The Integrated Use of Heavy-Metal Pollution Indices and the Assessment of Metallic Health Risks in the Phreatic Groundwater Aquifer—The Case of the Oued Souf Valley in Algeria. Hydrology 2023, 10, 201. https://doi.org/10.3390/hydrology10100201
Barkat A, Bouaicha F, Ziad S, Mester T, Sajtos Z, Balla D, Makhloufi I, Szabó G. The Integrated Use of Heavy-Metal Pollution Indices and the Assessment of Metallic Health Risks in the Phreatic Groundwater Aquifer—The Case of the Oued Souf Valley in Algeria. Hydrology. 2023; 10(10):201. https://doi.org/10.3390/hydrology10100201
Chicago/Turabian StyleBarkat, Ayoub, Foued Bouaicha, Sabrina Ziad, Tamás Mester, Zsófi Sajtos, Dániel Balla, Islam Makhloufi, and György Szabó. 2023. "The Integrated Use of Heavy-Metal Pollution Indices and the Assessment of Metallic Health Risks in the Phreatic Groundwater Aquifer—The Case of the Oued Souf Valley in Algeria" Hydrology 10, no. 10: 201. https://doi.org/10.3390/hydrology10100201
APA StyleBarkat, A., Bouaicha, F., Ziad, S., Mester, T., Sajtos, Z., Balla, D., Makhloufi, I., & Szabó, G. (2023). The Integrated Use of Heavy-Metal Pollution Indices and the Assessment of Metallic Health Risks in the Phreatic Groundwater Aquifer—The Case of the Oued Souf Valley in Algeria. Hydrology, 10(10), 201. https://doi.org/10.3390/hydrology10100201






