Dissolution Behavior and Kinetics of Copper Sulfide Concentrate in Choline Chloride DES
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Deep Eutectic Solvents
2.3. Characterization
2.3.1. Chemical Composition Analysis (XRF)
2.3.2. Mineralogical and Phase Analysis (XRD)
2.3.3. Elemental Analysis of Leachates (ICP-OES)
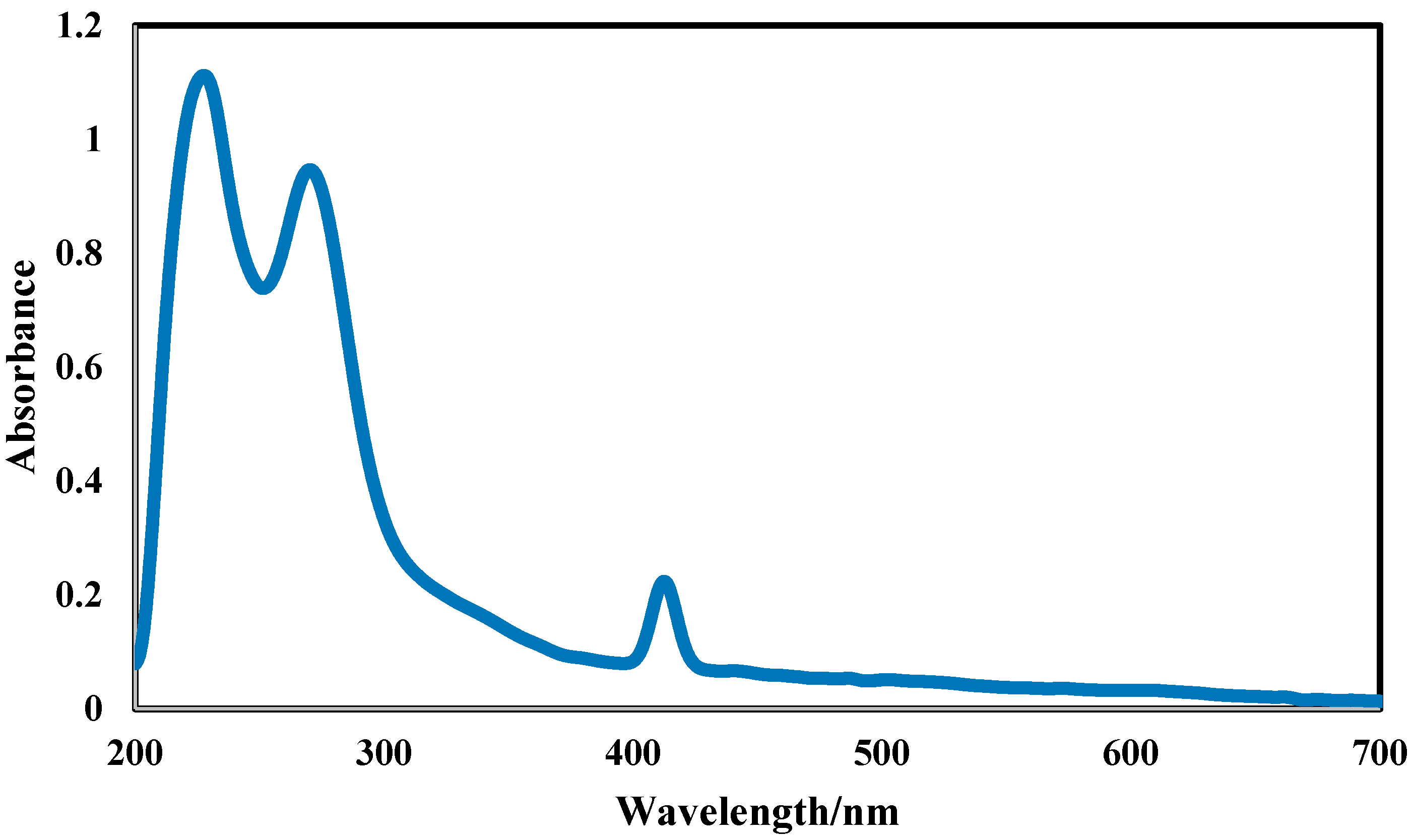
2.3.4. Optical Properties and Complexation (UV-Vis Spectroscopy)
2.3.5. Thermal Stability Analysis (TGA/DTG)
2.3.6. Calculation of Leaching Efficiency
3. Results
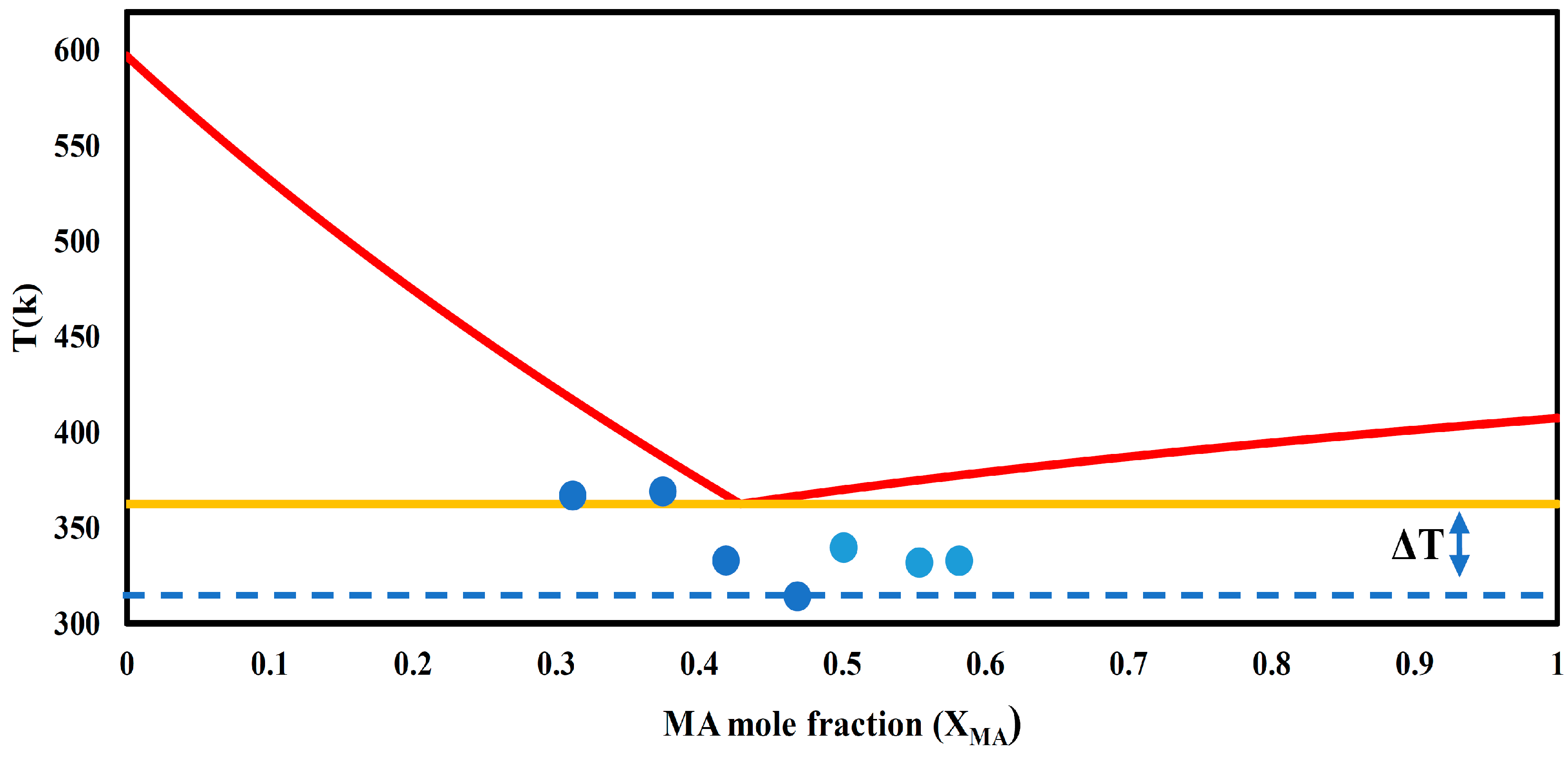
3.1. Determination of Eutectic Temperature
- -
- Choline chloride (ChCl): Enthalpy of fusion (ΔHm,ChCl) = 4300 kJ/mol, melting point (Tm) = 324 °C.
- -
- Malonic acid (Ma): Enthalpy of fusion (ΔHm,Ma)= 23,100 kJ/mol, melting point (Tm) = 134 °C.
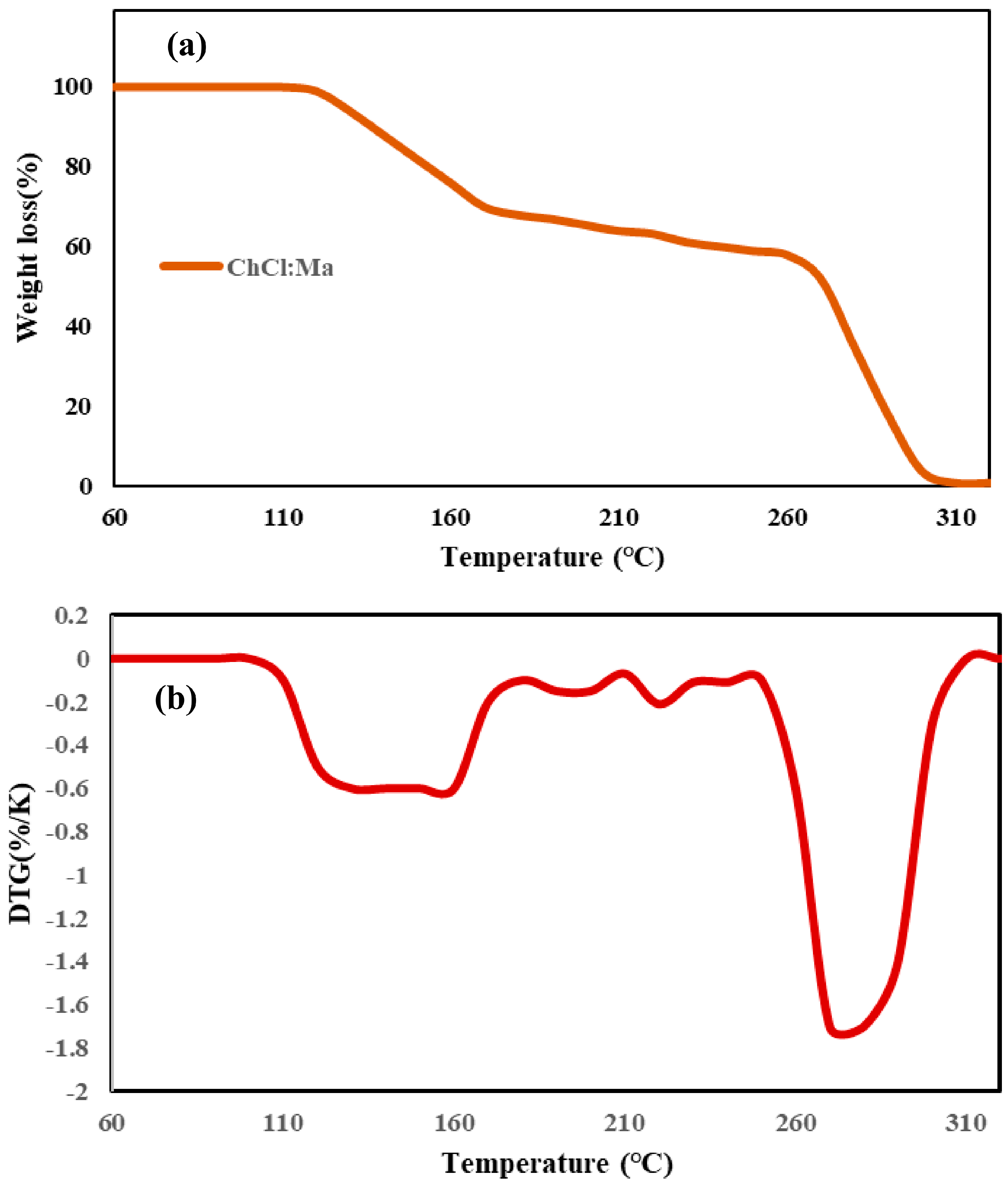
3.2. The Thermal Stability of DESs
- A peak at 130–160 °C (malonic acid decomposition)
- A peak at 270–300 °C (choline chloride degradation)
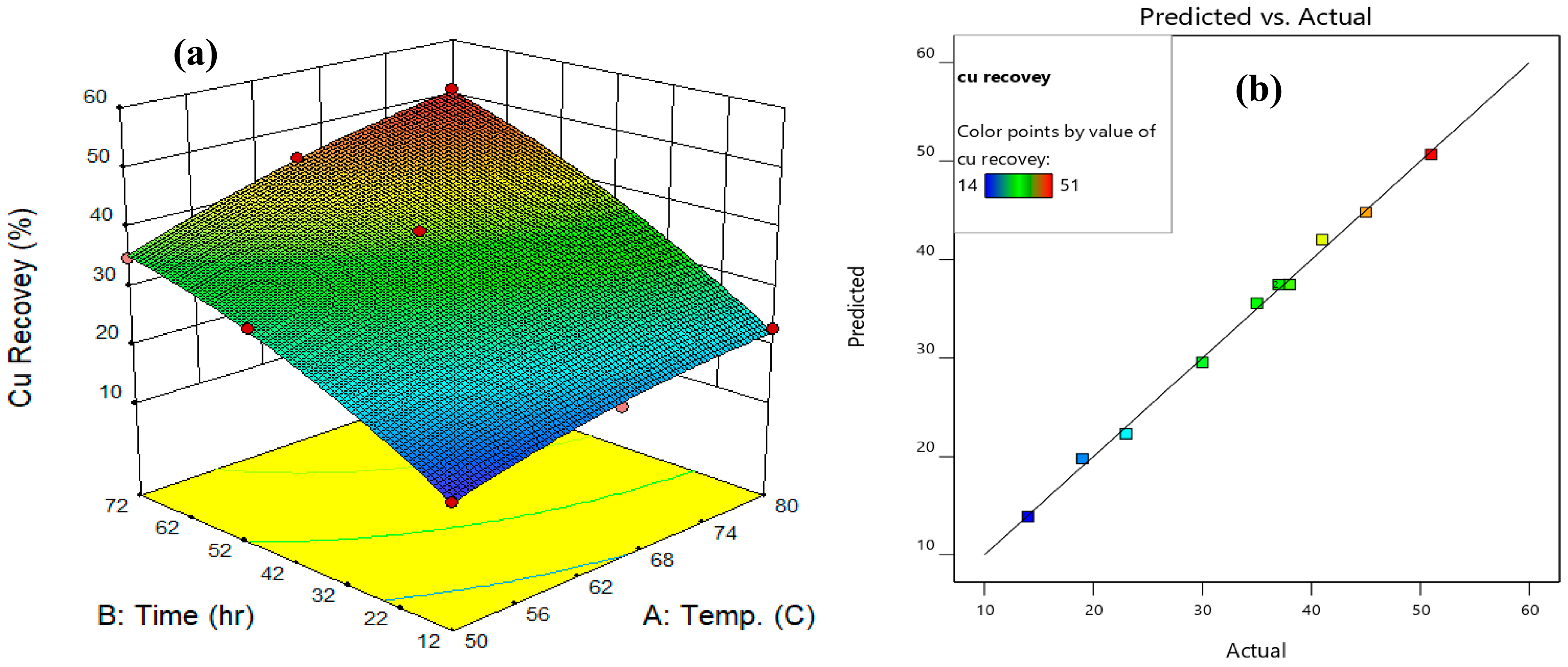
3.3. Response Surface Methodology Studies
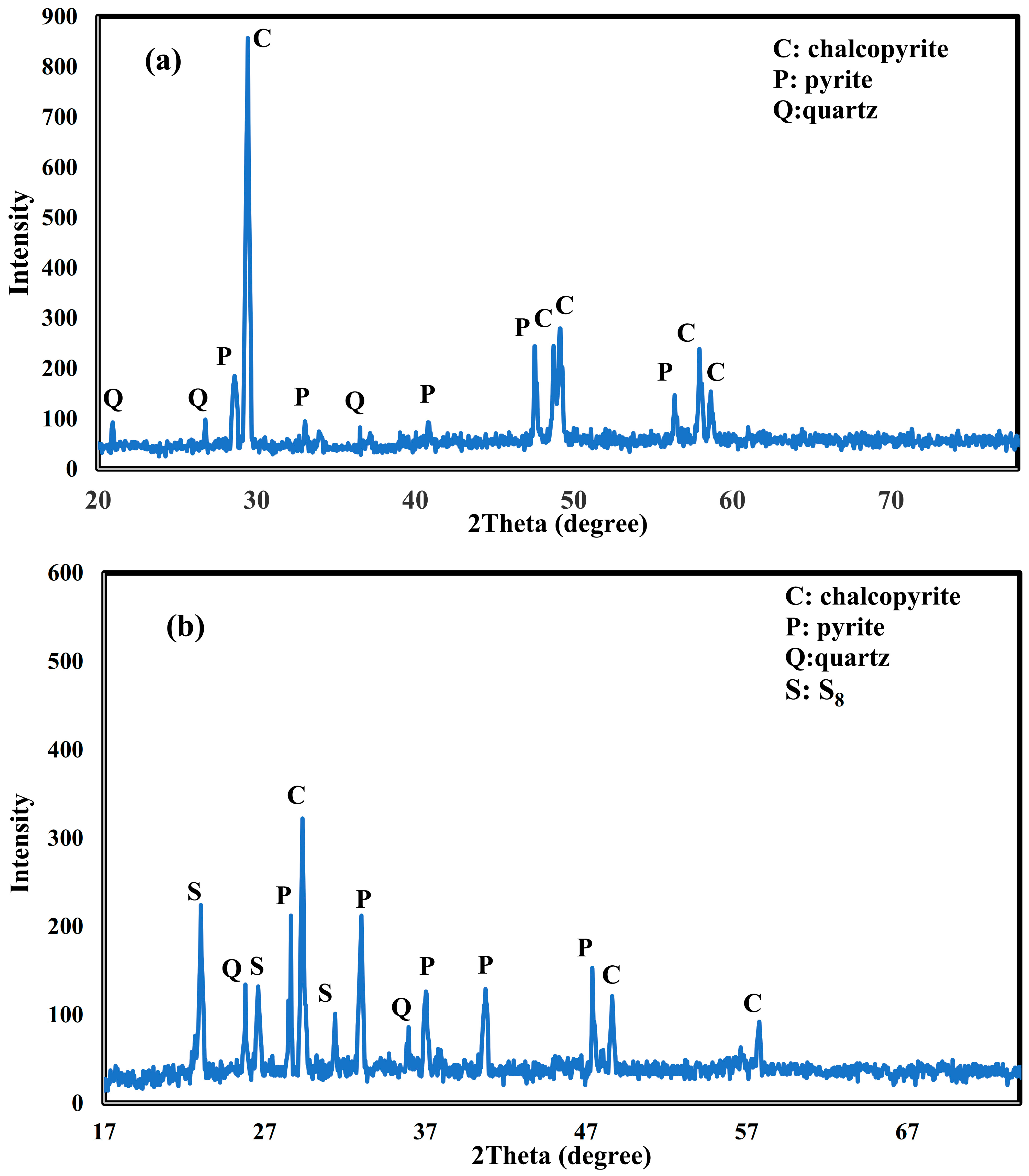
3.4. Mineralogical Studies on Chalcopyrite
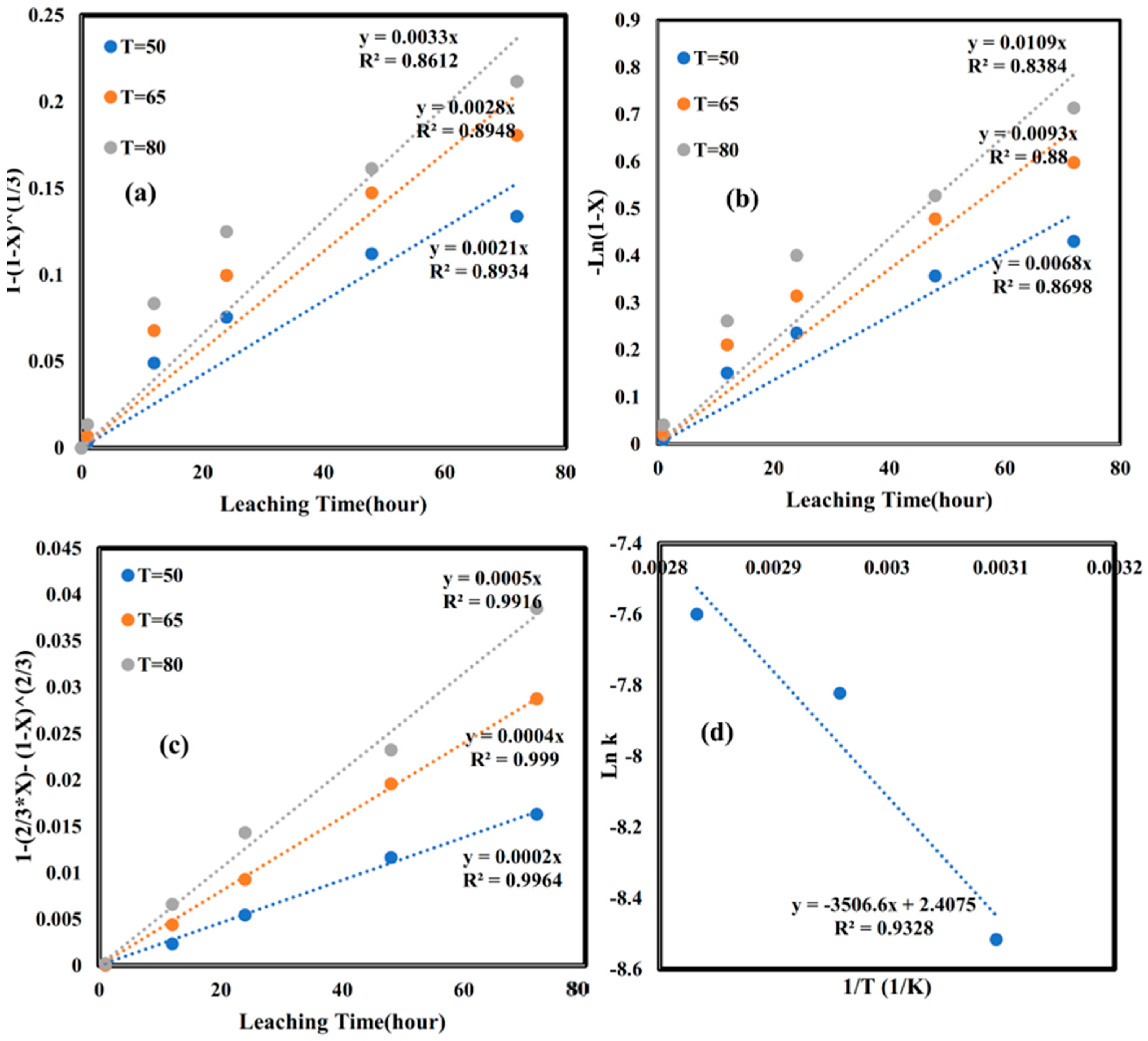
3.5. Kinetics of Dissolution for Cu and Fe
- (1)
- Chemical reaction control (Equation (4)), representing surface-controlled kinetics.
- (2)
- Diffusion control (Equation (5)), governing ash-layer mass transfer limitations.
- (3)
- Mixed control (Equation (6)), accounting for combined surface reaction and diffusion effects.
- Chemical control model (Equation (4), Figure 5a): The results showed poor correlation with experimental data, indicating that surface chemical reactions were not rate-limiting.
- Mixed control model (Equation (6), Figure 5b): While this model showed better agreement than the chemical control model, it still could not fully describe the experimental data.
- Diffusion control model (Equation (5), Figure 5c): This model provided the best fit to the experimental data (with acceptable R2 values), suggesting that the reaction was controlled by ash layer diffusion.
- Chemical reaction control model (Figure 6a): The experimental data showed poor correlation (R2 = 0.93), indicating that surface chemical reactions were not rate-limiting.
- Mixed control model (Figure 6b): While showing slightly better fit (R2 = 0.95), this model still failed to adequately describe the dissolution behavior.
- Diffusion control model (Figure 6c): Demonstrated excellent agreement with the experimental data (R2 = 0.99), confirming ash layer diffusion as the rate-controlling mechanism through Equation (5).
3.6. Proposed Dissolution Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, T.F.; Siow, K.S. Comparing the mechanical and thermal-electrical properties of sintered copper (Cu) and sintered silver (Ag) joints. J. Alloys Compd. 2021, 866, 158783. [Google Scholar] [CrossRef]
- Jena, S.S.; Tripathy, S.K.; Mandre, N.; Venugopal, R.; Farrokhpay, S. Sustainable use of copper resources: Beneficiation of low-grade copper ores. Minerals 2022, 12, 545. [Google Scholar] [CrossRef]
- Yang, W.; Tang, Y.; Huang, B.; Han, G.; Feng, Q. Experimental and molecular dynamics simulation insights into enhanced flotation of sulfidized smithsonite in a Cu–Pb dual activation system. Green Smart Min. Eng. 2025, 2, 8–17. [Google Scholar] [CrossRef]
- Atesoglu, G.; Atilgan, İ. Effect of roasting temperature on the leaching of chalcopyrite concentrate in sulphuric acid. Min. Metall. Explor. 2022, 39, 2199–2208. [Google Scholar] [CrossRef]
- Estay, H.; Barros, L.; Troncoso, E. Metal sulfide precipitation: Recent breakthroughs and future outlooks. Minerals 2021, 11, 1385. [Google Scholar] [CrossRef]
- Fan, Y.; Kong, Y.; Jiang, P.; Zhang, G.; Cong, J.; Shi, X.; Liu, Y.; Zhang, P.; Zhang, R.; Huang, Y. Development and challenges of deep eutectic solvents for cathode recycling of end-of-life lithium-ion batteries. Chem. Eng. J. 2023, 463, 142278. [Google Scholar] [CrossRef]
- Barlow, B. Leaching Kinetics of Carbonatite and Silicate Based Chalcopyrite Minerals. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2020. [Google Scholar]
- Fang, C.; Yu, S.; Wang, X.; Zhao, H.; Qin, W.; Qiu, G.; Wang, J. Synchrotron radiation XRD investigation of the fine phase transformation during synthetic chalcocite acidic ferric sulfate leaching. Minerals 2018, 8, 461. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Wang, G.; Yang, H. Copper leaching from complex chalcopyrite-rich ores: Utilizing mechanical activation and wastewater-based sulfuric acid system. Sep. Purif. Technol. 2025, 354, 128631. [Google Scholar] [CrossRef]
- Mohanraj, G.; Rahman, M.; Arya, S.; Barman, R.; Krishnendu, P.; Meena, S.S. Characterization study and recovery of copper from low grade copper ore through hydrometallurgical route. Adv. Powder Technol. 2022, 33, 103382. [Google Scholar] [CrossRef]
- Moradi, M.; Karimi, S.; Behnajady, B. The Effect of the Third Component on the Dissolution of Chalcopyrite in Deep Eutectic Solvents Based on Choline Chloride, and P-Toluenesulfonic Acid. In Proceedings of the 3rd International Conference & 7th National Conference on Materials, Metallurgy, Mining, Ahvaz, Iran, 7 February 2024. [Google Scholar]
- Nagar, N.; Garg, H.; Dhaka, M.; Gahan, C.S. Bioleaching of zinc sulfide concentrate in redox-controlled fed-batch process compared to redox non-controlled batch process. J. Sustain. Metall. 2022, 8, 333–342. [Google Scholar] [CrossRef]
- Gantait, A.; Debnath, S.; Anand, A.; Jakhar, S. Fluid evolution, metal source, and ore genesis of sulfide mineralization, Nim Ka Thana Copper Belt, Rajasthan, India: Evidence from mineral chemistry, fluid inclusions and sulfur isotope geochemistry. Arab. J. Geosci. 2023, 16, 167. [Google Scholar] [CrossRef]
- Chen, W.-H.; Uribe, M.C.; Kwon, E.E.; Lin, K.-Y.A.; Park, Y.-K.; Ding, L.; Saw, L.H. A comprehensive review of thermoelectric generation optimization by statistical approach: Taguchi method, analysis of variance (ANOVA), and response surface methodology (RSM). Renew. Sustain. Energy Rev. 2022, 169, 112917. [Google Scholar] [CrossRef]
- Kalinina, E.; Pikalova, E. Opportunities, challenges and prospects for electrodeposition of thin-film functional layers in solid oxide fuel cell technology. Materials 2021, 14, 5584. [Google Scholar] [CrossRef]
- Bilal, M.; Park, I.; Hornn, V.; Ito, M.; Hassan, F.U.; Jeon, S.; Hiroyoshi, N. The challenges and prospects of recovering fine copper sulfides from tailings using different flotation techniques: A review. Minerals 2022, 12, 586. [Google Scholar] [CrossRef]
- Feng, Q.; Lu, W.; Wang, H.; Zhang, Q. Mechanistic insights into stepwise activation of malachite for enhancing surface reactivity and flotation performance. Int. J. Miner. Metall. Mater. 2024, 31, 2159–2172. [Google Scholar] [CrossRef]
- Karimi, S.; Mohammadpour, P.; Esmailzadeh, M.; Izadi, M. Sustainable synthesis and application of green deep eutectic solvent in chalcopyrite leaching: A combined experimental and molecular dynamic simulation approach. Nano-Struct. Nano-Objects 2025, 42, 101481. [Google Scholar] [CrossRef]
- Zeng, C.; Feng, B.; Wang, Z.; Xing, Y. Research progress of chalcopyrite and galena flotation separation reagents: A review. J. Ind. Eng. Chem. 2025, 146, 24–34. [Google Scholar] [CrossRef]
- Rios, L.A.; Barraza, M.J.; Robles, P.A.; Quezada, G.R. Chalcopyrite Flotation, Molecular Design and Smart Industry: A Review. Int. J. Mol. Sci. 2025, 26, 3613. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Solvometallurgy: An emerging branch of extractive metallurgy. J. Sustain. Metall. 2017, 3, 570–600. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Holyoak, F.; Frisch, G.; Hartley, J.; Jenkin, G.R. Electrocatalytic recovery of elements from complex mixtures using deep eutectic solvents. Green Chem. 2015, 17, 2172–2179. [Google Scholar] [CrossRef]
- Khan, A.S.; Ibrahim, T.H.; Jabbar, N.A.; Khamis, M.I.; Nancarrow, P.; Mjalli, F.S. Ionic liquids and deep eutectic solvents for the recovery of phenolic compounds: Effect of ionic liquids structure and process parameters. RSC Adv. 2021, 11, 12398–12422. [Google Scholar] [CrossRef]
- Anggara, S.; Bevan, F.; Harris, R.C.; Hartley, J.M.; Frisch, G.; Jenkin, G.R.; Abbott, A.P. Direct extraction of copper from copper sulfide minerals using deep eutectic solvents. Green Chem. 2019, 21, 6502–6512. [Google Scholar] [CrossRef]
- Huntington, V.E.; Coulon, F.; Wagland, S.T. Innovative resource recovery from industrial sites: A critical review. Sustainability 2022, 15, 489. [Google Scholar] [CrossRef]
- Bevan, F.R. Electrochemical Processing of Metal Chalcogenides in Deep Eutectic Solvents. Ph.D. Thesis, University of Leicester, Leicester, UK, 2019. [Google Scholar]
- Van den Bossche, A.; Binnemans, K.; Dehaen, W. Recovery of valuable metals from urban waste via oxidative dissolution in polyhalide ionic liquids. In Proceedings of the International Symposium on Grids & Clouds (ISGC), Taipei, Taiwan, 9–13 March 2020. [Google Scholar]
- Araújo, C.F.; Abranches, D.O.; Coutinho, J.A.; Vaz, P.D.; Ribeiro-Claro, P.; Nolasco, M.M. Good vibrations: Understanding deep eutectic solvents through the lens of vibrational spectroscopy. Appl. Spectrosc. Rev. 2025, 60, 137–192. [Google Scholar] [CrossRef]
- Behnajady, B.; Najafi, M.; Karimi, S. A new approach to direct chemical leaching of Sungun chalcopyrite concentrate via green deep eutectic solvent choline chloride-ρ-toluenesulfonic acid and MD simulation. J. Taiwan Inst. Chem. Eng. 2025, 172, 106118. [Google Scholar] [CrossRef]
- Moradi, M.; Karimi, S.; Behnajady, B.; Esmailzadeh, M. Green Solvent-Driven Chalcopyrite Dissolution: Ternary DES (ChCl/MOA/PTSA) for High-Efficiency Copper Extraction via RSM Optimization, Kinetics, and Molecular Dynamics Insights. Miner. Eng. 2025, 233, 109606. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Carlesi, C.; Harris, R.C.; Abbott, A.P.; Jenkin, G.R. Chemical dissolution of chalcopyrite concentrate in choline chloride ethylene glycol deep eutectic solvent. Minerals 2022, 12, 65. [Google Scholar] [CrossRef]
- Valverde, P.; Green, T.; Roy, S. Effect of water on the electrodeposition of copper from a deep eutectic solvent. J. Appl. Electrochem. 2020, 50, 699–712. [Google Scholar] [CrossRef]
- Stando, G.; Hannula, P.-M.; Kumanek, B.; Lundström, M.; Janas, D. Copper recovery from industrial wastewater-Synergistic electrodeposition onto nanocarbon materials. Water Resour. Ind. 2021, 26, 100156. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, A.; Kashyap, H.K. How hydration affects the microscopic structural morphology in a deep eutectic solvent. J. Phys. Chem. B 2020, 124, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Behmadi, R.; Mirzaei, M.; Afshar, M.R.; Najafi, H. Investigation of chalcopyrite removal from low-grade molybdenite using response surface methodology and its effect on molybdenum trioxide morphology by roasting. RSC Adv. 2023, 13, 14899–14913. [Google Scholar] [CrossRef]
- Wang, L.; Song, S.; Gao, H.; Wang, L.; Yang, S.; Liu, C. The optimization and characterization of the recycling utilization of raffinate in the copper leaching process. J. Mater. Res. Technol. 2020, 9, 2214–2222. [Google Scholar] [CrossRef]
- Aragón-Tobar, C.F.; Endara, D.; de la Torre, E. Dissolution of metals (Cu, Fe, Pb, and Zn) from different metal-bearing species (sulfides, oxides, and sulfates) using three deep eutectic solvents based on choline chloride. Molecules 2024, 29, 290. [Google Scholar] [CrossRef]
- Prabhune, A.; Dey, R. Green and sustainable solvents of the future: Deep eutectic solvents. J. Mol. Liq. 2023, 379, 121676. [Google Scholar] [CrossRef]
- Mannu, A.; Blangetti, M.; Baldino, S.; Prandi, C. Promising technological and industrial applications of deep eutectic systems. Materials 2021, 14, 2494. [Google Scholar] [CrossRef] [PubMed]
- Röbbert, Y. Mobilization and Isotope Fractionation of Uranium, Copper and Iron in the Environment-Implications for (Bio) Remediation of Contaminated Sites and Mine Tailings. Ph.D. Thesis, Leibniz Universität Hannover, Hannover, Germany, 2021. [Google Scholar]
- Li, X.; Monnens, W.; Li, Z.; Fransaer, J.; Binnemans, K. Solvometallurgical process for extraction of copper from chalcopyrite and other sulfidic ore minerals. Green Chem. 2020, 22, 417–426. [Google Scholar] [CrossRef]
- Teimouri, S.; Potgieter, J.H.; Billing, C.; Conradie, J. The feasibility of pyrite dissolution in the deep eutectic solvent ethaline: Experimental and theoretical study. J. Mol. Liq. 2023, 392, 123468. [Google Scholar] [CrossRef]
- Lu, B.; Du, R.; Wang, G.; Wang, Y.; Dong, S.; Zhou, D.; Wang, S.; Li, C. High-efficiency leaching of valuable metals from waste Li-ion batteries using deep eutectic solvents. Environ. Res. 2022, 212, 113286. [Google Scholar] [CrossRef]
- Gaweł, B.A.; Ulvensøen, A.; Łukaszuk, K.; Arstad, B.; Muggerud, A.M.F.; Erbe, A. Structural evolution of water and hydroxyl groups during thermal, mechanical and chemical treatment of high purity natural quartz. RSC Adv. 2020, 10, 29018–29030. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Sanaeepur, H.; Amooghin, A.E.; Shirazi, M.M.A. Recovery of precious metals from industrial wastewater towards resource recovery and environmental sustainability: A critical review. Desalination 2022, 527, 115510. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Tian, G.; Liu, H. Review on the mineral processing in ionic liquids and deep eutectic solvents. Miner. Process. Extr. Metall. Rev. 2024, 45, 130–153. [Google Scholar] [CrossRef]
- Kollau, L.J.; Vis, M.; Van Den Bruinhorst, A.; de With, G.; Tuinier, R. Activity modelling of the solid–liquid equilibrium of deep eutectic solvents. Pure Appl. Chem. 2019, 91, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- González-Rivera, J.; Husanu, E.; Mero, A.; Ferrari, C.; Duce, C.; Tine, M.R.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Insights into microwave heating response and thermal decomposition behavior of deep eutectic solvents. J. Mol. Liq. 2020, 300, 112357. [Google Scholar] [CrossRef]
- Ghadamgahi, S.M.; Babakhani, A.; Barati Darband, G.; Shalchian, H.; Behmadi, R. Solvometallurgical properties of choline chloride-based deep eutectic solvents for copper extraction from chalcopyrite: Optimization and analysis. Mining 2025, 5, 8. [Google Scholar] [CrossRef]
- Ghodrati, S.; Nakhaei, F.; VandGhorbany, O.; Hekmati, M. Modeling and optimization of chemical reagents to improve copper flotation performance using response surface methodology. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 1633–1648. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Zhu, L.; Liu, B.; Zhou, K.; Xu, J.; Guo, C. Eco-friendly oxidation leaching from chalcopyrite powder and kinetics assisted by sodium chloride in organic acid media. Adv. Powder Technol. 2022, 33, 103547. [Google Scholar] [CrossRef]
- Nadimi, H.; Sarpoolaky, H.; Soltanieh, M. Formation reaction kinetics of nanocrystalline TiC via molten LiCl–KCl applying shrinking core model. Ceram. Int. 2021, 47, 12859–12869. [Google Scholar] [CrossRef]
- Wei, L.; Pu, X.; Cheng, D.; Wang, Y. Measurement of the concentration-and temperature-dependent diffusion coefficient and activation energy via diffusion image analysis. AIP Adv. 2022, 12, 105013. [Google Scholar] [CrossRef]
- Arnaut, L.; Formosinho, S.; Burrows, H. Elementary Reactions in Solution; Chemical Kinetics; Elsevier: Amsterdam, The Netherland, 2007; pp. 223–250. [Google Scholar]
- Tombal, T.D.; Kursun, I.; Terzi, M.; Gungoren, C. Optimization and Kinetics of Chalcopyrite Leaching in the Presence of Hydrochloric and Nitric Acids, along with a Particle Shape-Based Evaluation. JOM 2025, 77, 3589–3599. [Google Scholar] [CrossRef]

| Fe | S | Cu | SiO2 | Al2O3 | CaO | TiO2 | MgO |
|---|---|---|---|---|---|---|---|
| 32.52 | 36.4 | 22.46 | 4.74 | 1.17 | 0.49 | 0.41 | 0.48 |
| Variables | Levels | ||||
|---|---|---|---|---|---|
| Low Actual | High Actual | Low Coded | High Coded | Mean | |
| Temperature (A) | 50 °C | 80 °C | −1 | 1 | 65 °C |
| Time (B) | 24 h | 72 h | −1 | 1 | 48 h |
| Run | Factor 1 | Factor 2 | Response | Response |
|---|---|---|---|---|
| A: Temperature (°C) | B: Time (h) | Efficiency of Cu Recovery (%) | Efficiency of Fe Recovery (%) | |
| A: Choline Chloride–Malonic Acid | ||||
| 1 | 80 | 24 | 33.1 | 31.9 |
| 2 | 50 | 24 | 21.3 | 12 |
| 3 | 65 | 48 | 37.9 | 36.2 |
| 4 | 65 | 24 | 27.4 | 24.7 |
| 5 | 65 | 72 | 45.2 | 43.7 |
| 6 | 65 | 48 | 36.3 | 36.8 |
| 7 | 50 | 72 | 35.2 | 23.9 |
| 8 | 65 | 48 | 38.1 | 35.1 |
| 9 | 80 | 72 | 51.4 | 54.3 |
| 10 | 50 | 48 | 30.1 | 19.8 |
| 11 | 80 | 48 | 41.6 | 46.5 |
| A: Choline Chloride–Malonic Acid | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
| Model | 1561.05 | 5 | 312.21 | 278.34 | <0.0001 | significant |
| A—temp. | 596.34 | 1 | 596.34 | 486.46 | <0.0001 | |
| B—time | 834.12 | 1 | 834.12 | 678.57 | <0.0001 | |
| AB | 0.0361 | 1 | 0.0361 | 0.0286 | 0.8724 | |
| A2 | 1.05 | 1 | 1.05 | 0.875 | 0.3925 | |
| B2 | 46.12 | 1 | 46.12 | 37.59 | 0.0017 | |
| Residual | 6.1 | 5 | 1.22 | |||
| Lack of Fit | 4.1533 | 3 | 1.3844 | 1.422 | 0.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghadamgahi, M.; Babakhani, A.; Shalchian, H.; Barati Darband, G.; Shiri, H.R. Dissolution Behavior and Kinetics of Copper Sulfide Concentrate in Choline Chloride DES. ChemEngineering 2025, 9, 132. https://doi.org/10.3390/chemengineering9060132
Ghadamgahi M, Babakhani A, Shalchian H, Barati Darband G, Shiri HR. Dissolution Behavior and Kinetics of Copper Sulfide Concentrate in Choline Chloride DES. ChemEngineering. 2025; 9(6):132. https://doi.org/10.3390/chemengineering9060132
Chicago/Turabian StyleGhadamgahi, Mojtaba, Abolfazl Babakhani, Hossein Shalchian, Ghasem Barati Darband, and Hamid Reza Shiri. 2025. "Dissolution Behavior and Kinetics of Copper Sulfide Concentrate in Choline Chloride DES" ChemEngineering 9, no. 6: 132. https://doi.org/10.3390/chemengineering9060132
APA StyleGhadamgahi, M., Babakhani, A., Shalchian, H., Barati Darband, G., & Shiri, H. R. (2025). Dissolution Behavior and Kinetics of Copper Sulfide Concentrate in Choline Chloride DES. ChemEngineering, 9(6), 132. https://doi.org/10.3390/chemengineering9060132







