Theoretical Design of Acridone-Core Energetic Materials: Assessment of Detonation Properties and Potential as Insensitive, Thermally Stable High-Energy Materials
Abstract
1. Introduction
2. Methods
- Politzer’s equations [35], which incorporate the 0.001 e/bohr3 isosurface and surface potential balance;
- Calculations from molecular weight by B3LYP/cc-pVTZ and molar volume;
- ACD/ChemSketch [36], where density is estimated using a machine learning algorithm trained on compounds with known densities, expressed as a function of molecular volume, electronic properties, and intermolecular interaction patterns. The obtained densities were compared.
3. Results and Discussion
3.1. Stability
3.1.1. Thermal Stability
3.1.2. Chemical Stability
3.2. Sensitivity
3.3. Detonation Properties
3.3.1. Density
3.3.2. Detonation Velocity and Pressure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDE | Cohesive energy per atom | |
| HOMO-LUMO gap | Difference between the highest occupied and the lowest unoccupied orbitals | |
| OB | Oxygen Balance (calculated to CO2) | |
| PYX | 3,5-Dinitro-N,N’-bis(2,4,6-trinitrophenyl)-2,6-pyridinediamine | |
| Acri1 | C13H5N5O9 | 2,4,5,7-Tetranitroacridin-9(10 H)-one |
| Acri2 | C13H5N5O11 | 3,6-Dihydroxy-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri3 | C13H3Cl2N5O7 | 3,6-Dichloro-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri4 | C13H3F2N5O7 | 3,6-Difluoro-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri5 | C13H7N7O9 | 3,6-Diamino-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri6 | C15H9N9O13 | 3,6-Bis[methyl(nitro)amino]-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri7 | C13H5N5O13 | 1,3,6,8-Tetrahydroxy-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri8 | C13HCl4N5O9 | 1,3,6,8-Tetrachloro-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri9 | C13HF4N5O9 | 1,3,6,8-Tetrafluoro-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri10 | C13H9N9O9 | 1,3,6,8-Tetraamino-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri11 | C17H13N13O17 | 1,3,6,8-Tetrakis[methyl(nitro)amino]-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri12 | C19H6N8O15 | 10-Picryl-2,4,5,7-tetranitroacridin-9(10 H)-one |
| Acri13 | C19H8N10O15 | 10-Picryl-3,6-Diamino-2,4,5,7-tetranitroacridin -9(10 H)-one |
| Acri14 | C19H10N12O15 | 10-Picryl-1,3,6,8-tetraamino-2,4,5,7-tetranitroacridin -9(10 H)-one |
| Acri15 | C19H12N14O15 | 10-(3’,5’-Diamino-picryl)-1,3,6,8-tetraamino-2,4,5,7-tetranitroacridin -9(10 H)-one |
Appendix A
| Assignation | Structure | Molecular Formula | MW | Calculated Elemental Composition Data | ||||
|---|---|---|---|---|---|---|---|---|
| C, % | H, % | F or Cl, % | N, % | O, % | ||||
| Acri1 | 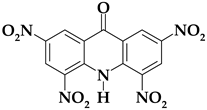 | C13H5N5O9 | 375.21 | 41.62 | 1.34 | 0 | 18.67 | 38.36 |
| Acri2 |  | C13H5N5O11 | 407.21 | 38.34 | 1.24 | 0 | 17.20 | 43.22 |
| Acri3 | 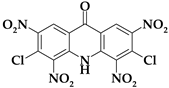 | C13H3Cl2N5O9 | 444.10 | 35.16 | 0.68 | 15.97 (Cl) | 15.77 | 32.42 |
| Acri4 | 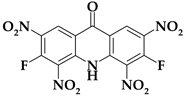 | C13H3F2N5O9 | 411.19 | 37.97 | 0.74 | 9.24 (F) | 17.03 | 35.02 |
| Acri5 | 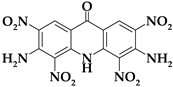 | C13H7N7O9 | 405.24 | 38.53 | 1.74 | 0 | 24.19 | 35.53 |
| Acri6 |  | C15H9N9O13 | 523.29 | 34.43 | 1.73 | 0 | 24.09 | 39.75 |
| Acri7 | 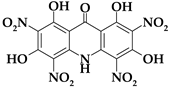 | C13H5N5O11 | 407.21 | 38.34 | 1.24 | 0 | 17.20 | 43.22 |
| Acri8 |  | C13HCl4N5O9 | 512.99 | 30.44 | 0.20 | 27.64 (Cl) | 13.65 | 28.07 |
| Acri9 | 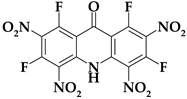 | C13HF4N5O9 | 447.17 | 34.92 | 0.23 | 16.99 (F) | 15.66 | 32.20 |
| Acri10 | 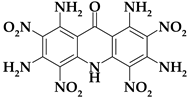 | C13H9N9O9 | 435.27 | 35.87 | 2.08 | 0 | 28.96 | 33.08 |
| Acri11 | 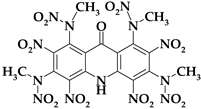 | C17H13N13O17 | 671.37 | 30.41 | 1.95 | 0 | 27.12 | 40.51 |
| Acri12 |  | C19H6N8O15 | 586.30 | 38.92 | 1.03 | 0 | 19.11 | 40.93 |
| Acri13 |  | C19H8N10O15 | 616.33 | 37.03 | 1.31 | 0 | 22.73 | 38.94 |
| Acri14 |  | C19H10N12O15 | 646.36 | 35.31 | 1.56 | 0 | 26.00 | 37.13 |
| Acri15 |  | C19H12N14O15 | 676.39 | 33.74 | 1.79 | 0 | 28.99 | 35.48 |
References
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Baguley, B.C.; Wakelin, L.P.G.; Jacintho, J.D.; Kovacic, P. Mechanisms of action of DNA intercalating acridine-based drugs: How important are contributions from electron transfer and oxidative stress? Curr. Med. Chem. 2003, 10, 2643–2649. [Google Scholar] [CrossRef]
- Şen, N.; Pons, J.-F.; Kurtay, G.; Yüksel, B.; Nazir, H.; Khumsri, A.; Atakol, O. New explosive materials through covalent and non-covalent synthesis: Investigation of explosive properties of trinitrotoluene: Acridine and picric acid: Acridine. J. Mol. Struct. 2023, 1292, 136080. [Google Scholar] [CrossRef]
- Şen, N.; Pons, J.-F.; Zorlu, Y.; Dossi, E.; Persico, F.; Temple, T.; Aslan, N.; Khumsri, A. Synthesis, structure characterization, Hirshfeld surface analysis, and computational studies of 3-nitro-1,2,4-triazol-5-one (NTO): Acridine. Struct. Chem. 2024, 35, 1865–1879. [Google Scholar] [CrossRef]
- Drake, G.W.; Hawkins, T.; Brand, A.; Hall, L.; Mckay, M.; Vij, A.; Ismail, I. Energetic, low-melting salts of simple heterocycles. Propellants Explos. Pyrotech. 2003, 28, 174–180. [Google Scholar] [CrossRef]
- Darwich, C.; Klapötke, T.M.; Sabaté, C.M. 1,2,4-Triazolium-cation-based energetic salts. Chem. Eur. J. 2008, 14, 5756–5771. [Google Scholar] [CrossRef]
- Tao, G.H.; Tang, M.; He, L.; Ji, S.P.; Nie, F.D.; Huang, M. Synthesis, structure and property of 5-aminotetrazolate room-temperature ionic liquids. Eur. J. Inorg. Chem. 2012, 2012, 3070–3078. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, M.; He, J.; Yu, P.; Guo, X.; Liu, Y.; Gao, H.; Yin, P. Exchanging of NH2/NHNH2/NHOH groups: An effective strategy for balancing the energy and safety of fused-ring energetic materials. Chem. Eng. J. 2023, 466, 143333. [Google Scholar] [CrossRef]
- Man, T.; Wang, K.; Zhang, J.; Niu, X.; Zhang, T. Theoretical Studies of High-nitrogen-containing Energetic Compounds Based on the s-Tetrazine Unit. Cent. Eur. J. Energ. Mater. 2013, 10, 171–189. [Google Scholar]
- Zhang, Y.; Parrish, D.; Shreeve, J. Derivatives of 5-nitro-1,2,3-2H-triazole—High performance energetic materials. J. Mater. Chem. A 2013, 1, 585–593. [Google Scholar] [CrossRef]
- Cao, W.; Qin, J.; Zhang, J.; Sinditskii, V. 4,5-Dicyano-1,2,3-Triazole—A Promising Precursor for a New Family of Energetic Compounds and Its Nitrogen-Rich Derivatives: Synthesis and Crystal Structures. Molecules 2021, 26, 6735. [Google Scholar] [CrossRef]
- Gu, H.; Xiong, H.; Yang, H.; Cheng, G. Tricyclic nitrogen-rich cation salts based on 1,2,3-triazole: Chemically stable and insensitive candidates for novel gas generant. Chem. Eng. J. 2021, 408, 128021. [Google Scholar] [CrossRef]
- Feng, S.; Yin, P.; He, C.; Pang, S.; Shreeve, J. Tunable Dimroth rearrangement of versatile 1,2,3-triazoles towards high performance energetic materials. J. Mater. Chem. A 2021, 9, 12291–12298. [Google Scholar] [CrossRef]
- Lai, Q.; Fei, T.; Yin, P.; Shreeve, J. 1,2,3-Triazole with linear and branched catenated nitrogen chains—The role of regiochemistry in Energetic Materials. Chem. Eng. J. 2020, 410, 128148. [Google Scholar] [CrossRef]
- Yao, W.; Xue, Y.; Qian, L.; Yang, H.; Cheng, G. Combination of 1,2,3-triazole and 1,2,4-triazole frameworks for new high-energy and low-sensitivity compounds. Energ. Mater. Front. 2021, 2, 131–138. [Google Scholar] [CrossRef]
- Yadav, A.K.; Kukreja, S.; Dharavath, S. Highly Promising Primary Explosive: A Metal-Free, Fluoro-Substituted Azo-Triazole with Unmatched Safety and Performance. JACS Au 2025, 5, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lease, N.; Spielvogel, K.D.; Davis, J.V.; Tisdale, J.T.; Klamborowski, L.M.; Cawkwell, M.J.; Manner, V.W. Halogenated PETN derivatives: Interplay between physical and chemical factors in explosive sensitivity. Chem. Sci. 2023, 14, 7044–7056. [Google Scholar] [CrossRef]
- Nie, X.; Lei, C.; Xiong, H.; Cheng, G.; Yang, H. Methylation of a triazole-fused framework to create novel insensitive energetic materials. Energ. Mater. Front. 2020, 1, 165–171. [Google Scholar] [CrossRef]
- Chen, F.; Song, S.; Zhang, Q.; Wang, Y. Modification of an N-methyl group toward a new energetic melt-castable material with a good energy–stability balance. Synlett 2024, 35, 2015–2021. [Google Scholar] [CrossRef]
- Guo, D.; Wei, Y.; Zybin, S.V.; Liu, Y.; Huang, F.; Goddard, W.A. III Detonation performance of insensitive nitrogen-rich nitroenamine energetic materials predicted from first-principles reactive molecular dynamics simulations. JACS Au 2024, 4, 1605–1614. [Google Scholar] [CrossRef]
- Lease, N.; Spielvogel, K.D.; Cawkwell, M.J.; Manner, V.W. Synthesis and investigation into explosive sensitivity for a series of new picramide explosives. J. Energ. Mater. 2024, 1–21. [Google Scholar] [CrossRef]
- Becke, A. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations, I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.2; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Cardia, R.; Malloci, G.; Mattoni, A.; Cappellini, G. Effects of TIPS-Functionalization and Perhalogenation on the Electronic, Optical, and Transport Properties of Angular and Compact Dibenzochrysene. J. Phys. Chem. A 2014, 118, 5170–5177. [Google Scholar] [CrossRef]
- Cardia, R.; Malloci, G.; Rignanese, G.; Blasé, X.; Molteni, E.; Cappellini, G. Electronic and optical properties of hexathiapentacene in the gas and crystal phases. Phys. Rev. B 2016, 93, 235132. [Google Scholar] [CrossRef]
- Dardenne, N.; Cardia, R.; Li, J.; Malloci, G.; Cappellini, G.; Blasé, X.; Charlier, J.; Rignanese, G. Tuning Optical Properties of Dibenzochrysenes by Functionalization: A Many-Body Perturbation Theory Study. J. Phys. Chem. C 2017, 121, 24480–24488. [Google Scholar] [CrossRef]
- Antidormi, A.; Aprile, G.; Cappellini, G.; Cara, E.; Cardia, R.; Colombo, L.; Farris, R.; d’Ischia, M.; Mehrabanian, M.; Melis, C.; et al. Physical and Chemical Control of Interface Stability in Porous Si–Eumelanin Hybrids. J. Phys. Chem. C 2018, 122, 28405–28415. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Inclusions of Si-atoms in Graphene nanostructures: A computational study on the ground-state electronic properties of Coronene and Ovalene. J. Phys. Conf. Ser. 2018, 956, 012020. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Si-atoms substitutions effects on the electronic and optical properties of coronene and ovalene. New J. Phys. 2018, 20, 113008. [Google Scholar] [CrossRef]
- Kumar, A.; Cardia, R.; Cappellini, G. Electronic and optical properties of chromophores from bacterial cellulose. Cellulose 2018, 25, 2191–2203. [Google Scholar] [CrossRef]
- Szafran, M.; Koput, J. Ab initio and DFT calculations of structure and vibrational spectra of pyridine and its isotopomers. J. Mol. Struct. 2001, 565–566, 439–448. [Google Scholar] [CrossRef]
- Begue, D.; Carbonniere, P.; Pouchan, C. Calculations of Vibrational Energy Levels by Using a Hybrid ab Initio and DFT Quartic Force Field: Application to Acetonitrile. J. Phys. Chem. A 2005, 109, 4611–4616. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Kaya, C. A New equation based on ionization energies and electron affinities of atoms for calculating of group electronegativity. Comput. Theor. Chem. 2015, 1052, 42–46. [Google Scholar] [CrossRef]
- Politzer, P.; Martinez, J.; Murray, J.; Concha, M.; Toro-Labbé, A. An electrostatic interaction correction for improved crystal density prediction. Mol. Phys. 2009, 107, 2095–2101. [Google Scholar] [CrossRef]
- Spessard, G.O. ACD Labs/LogP dB 3.5 and ChemSketch 3.5. J. Chem. Inf. Comput. Sci. 1998, 38, 1250–1253. [Google Scholar] [CrossRef]
- Keshavarz, M.; Zamani, A. A simple and reliable method for predicting the detonation velocity of CHNOFCl and aluminized explosives. Cent. Eur. Energ. Mater. 2015, 12, 13–33. [Google Scholar]
- Keshavarz, M.; Pouretedal, H. An empirical method for predicting detonation pressure of CHNOFCl explosives. Thermochim. Acta 2004, 414, 203–208. [Google Scholar] [CrossRef]
- Zahari, N.; Montazeri, M.; Hoseini, S. Estimation of the Detonation Pressure of Co-crystal Explosives through a Novel, Simple and Reliable Model. Cent. Eur. J. Energ. Mater. 2020, 17, 492–505. [Google Scholar] [CrossRef]
- Meyer, R.; Köhler, J.; Homburg, A. Explosives, 6th ed.; Wiley-VCH: Weinheim, Germany, 2007; ISBN 978-3-527-31656-4. [Google Scholar]
- Rajakumar, B.; Arathala, P.; Muthiah, B. Thermal Decomposition of 2-Methyltetrahydrofuran behind Reflected Shock Waves over the Temperature Range of 1179–1361 K. J. Phys. Chem. A 2021, 125, 5406–5423. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Witkowski, T.G. 5, 5′-Bis (2, 4, 6-trinitrophenyl)-2, 2′-bi (1, 3, 4-oxadiazole)(TKX-55): Thermally Stable Explosive with Outstanding Properties. ChemPlusChem 2016, 81, 357–360. [Google Scholar] [CrossRef]
- Pagoria, P. A comparison of the structure, synthesis, and properties of insensitive energetic compounds. Propellants Explos. Pyrotech. 2016, 41, 452–469. [Google Scholar] [CrossRef]
- Gutowski, Ł.; Cudziło, S. Synthesis and properties of novel nitro-based thermally stable energetic compounds. Def. Technol. 2021, 17, 775–784. [Google Scholar] [CrossRef]
- Agrawal, J.P.; Hodgson, R. Organic Chemistry of Explosives; John Wiley & Sons Ltd.: Chichester, UK, 2007; pp. 163–167. ISBN 0-470-02967-6. [Google Scholar]
- Ali, H.M.; Ali, I.H. Thermal and thermo-oxidative stability of a series of palladium and platinum ferrocenylamine sulfides and selenides. J. Chem. Res. 2022, 46, 17475198221141979. [Google Scholar] [CrossRef]
- Green, S.P.; Wheelhouse, K.M.; Payne, A.D.; Hallett, J.P.; Miller, P.W.; Bull, J.A. Thermal stability and explosive hazard assessment of diazo compounds and diazo transfer reagents. Org. Process Res. Dev. 2020, 24, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Khunnawutmanotham, N.; Jumpathong, W.; Eurtivong, C.; Chimnoi, N.; Techasakul, S. Synthesis, cytotoxicity evaluation, and molecular modeling studies of 2,N10-substituted acridones as DNA-intercalating agents. J. Chem. Res. 2020, 44, 410–425. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Ye, H.; McKenney, R.L.; Bolduc, P.R. Thermal stability studies on a homologous series of nitroarenes. J. Phys. Chem. 1995, 99, 9593–9602. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Talawar, M.B.; Sivabalan, R.; Dhavale, D.D.; Asthana, S.N.; Krishnamurthy, V.N. Synthesis, characterization and thermolysis studies on new derivatives of 2,4,5-trinitroimidazoles: Potential insensitive high energy materials. J. Hazard. Mater. 2007, 143, 192–197. [Google Scholar] [CrossRef]
- Yang, W.; Lu, H.; Liao, L.; Fan, G.; Ma, Q.; Huang, J. Synthesis and single crystal structure of fully substituted polynitrobenzene derivatives for high energy materials. RSC Adv. 2018, 8, 2203–2208. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Vansweevelt, H.; Vanquickenborne, L.G. Remarkable effect of fluorine and chlorine atoms on the stability of 1H-phosphirene. Chem. Berichte 1992, 125, 923–927. [Google Scholar] [CrossRef]
- Rice, B.M.; Byrd, E.F.C. Relationships with oxygen balance and bond dissociation energies. Comput. Theor. Chem. 2022, 22, 67–75. [Google Scholar] [CrossRef]
- Zeman, S.; Jungova, M. Sensitivity and Performance of Energetic Materials. Propellants Explos. Pyrotech. 2016, 41, 426–451. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C. Review of the molecular and crystal correlations on sensitivities of energetic materials. J. Hazard. Mater. 2020, 398, 122910. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. High Performance, Low Sensitivity: Conflicting or Compatible? Propellants Explos. Pyrotech. 2016, 41, 414–425. [Google Scholar] [CrossRef]
- Džingalašević, V.; Antić, G.; Mlađenović, D. Ratio of detonation pressure and critical pressure of high explosives with different compounds. Sci. Tech. Rev. 2004, 5, 72–76. [Google Scholar]
- Lal, S.; Gao, H.; Shreeve, J.M. New approach for predicting crystal densities of energetic materials. New J. Chem. 2024, 48, 17947–17952. [Google Scholar] [CrossRef]
- Wen, L.; Wang, B.; Yu, T.; Lai, W.; Shi, J.; Liu, M.; Liu, Y. Accelerating the search of CHONF-containing highly energetic materials by combinatorial library design and high-throughput screening. Fuel 2022, 310, 122241. [Google Scholar] [CrossRef]
- Tamuliene, J.; Sarlauskas, J. Polynitrobenzene Derivatives, Containing -CF3, -OCF3, and -O(CF2)nO- Functional Groups, as Candidates for Perspective Fluorinated High-Energy Materials: Theoretical Study. Energies 2024, 17, 6126. [Google Scholar] [CrossRef]
- Tselinskii, I.V. Applications of energetic materials in engineering, technology and national economy. Soros Educat. J. 1997, 11, 46–52. [Google Scholar]
- Fallas, J.A.; González, L.; Corral, I. Density functional theory rationalization of the substituent effects in trifluoromethyl-pyridinol derivatives. Tetrahedron 2009, 65, 232–239. [Google Scholar] [CrossRef]
- Kumar, P.; Ghule, V.D.; Dharavath, S. Advancing energetic chemistry: The first synthesis of sulfur-based C–C bonded thiadiazole-pyrazine compounds with a nitrimino moiety. Dalton Trans. 2024, 53, 19112–19115. [Google Scholar] [CrossRef]
- Chandler, J.; Ferguson, R.E.; Forbes, J.; Kuhl, A.L.; Oppenheim, A.K.; Spektor, R. Confined combustion of TNT explosion products in air. In Proceedings of the 8th International Colloquium on Dust Explosions, Schaumburg, IL, USA, 21–25 September 1998; Available online: https://www.osti.gov/biblio/3648 (accessed on 8 February 2025).
- Mahajan, T.; Bhargava, G.; Sharma, H. Theoretical Exploration on Polynitrobenzo/Heteroaryl Pyrazines as Useful High-Energy-Density Materials. ChemistrySelect 2025, 10, e0227. [Google Scholar] [CrossRef]
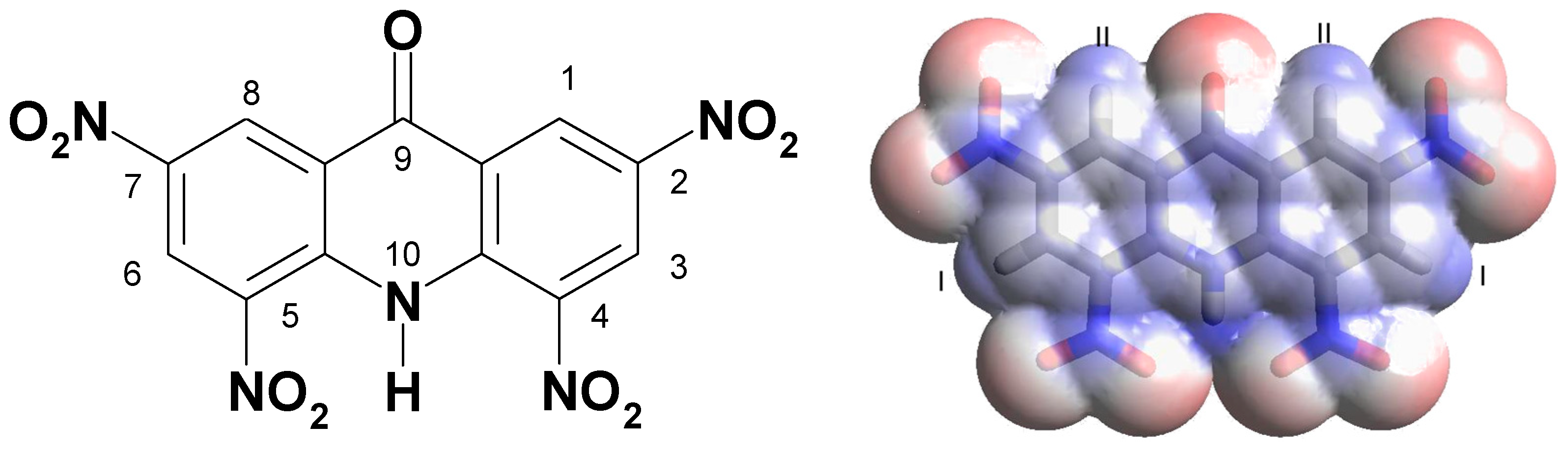
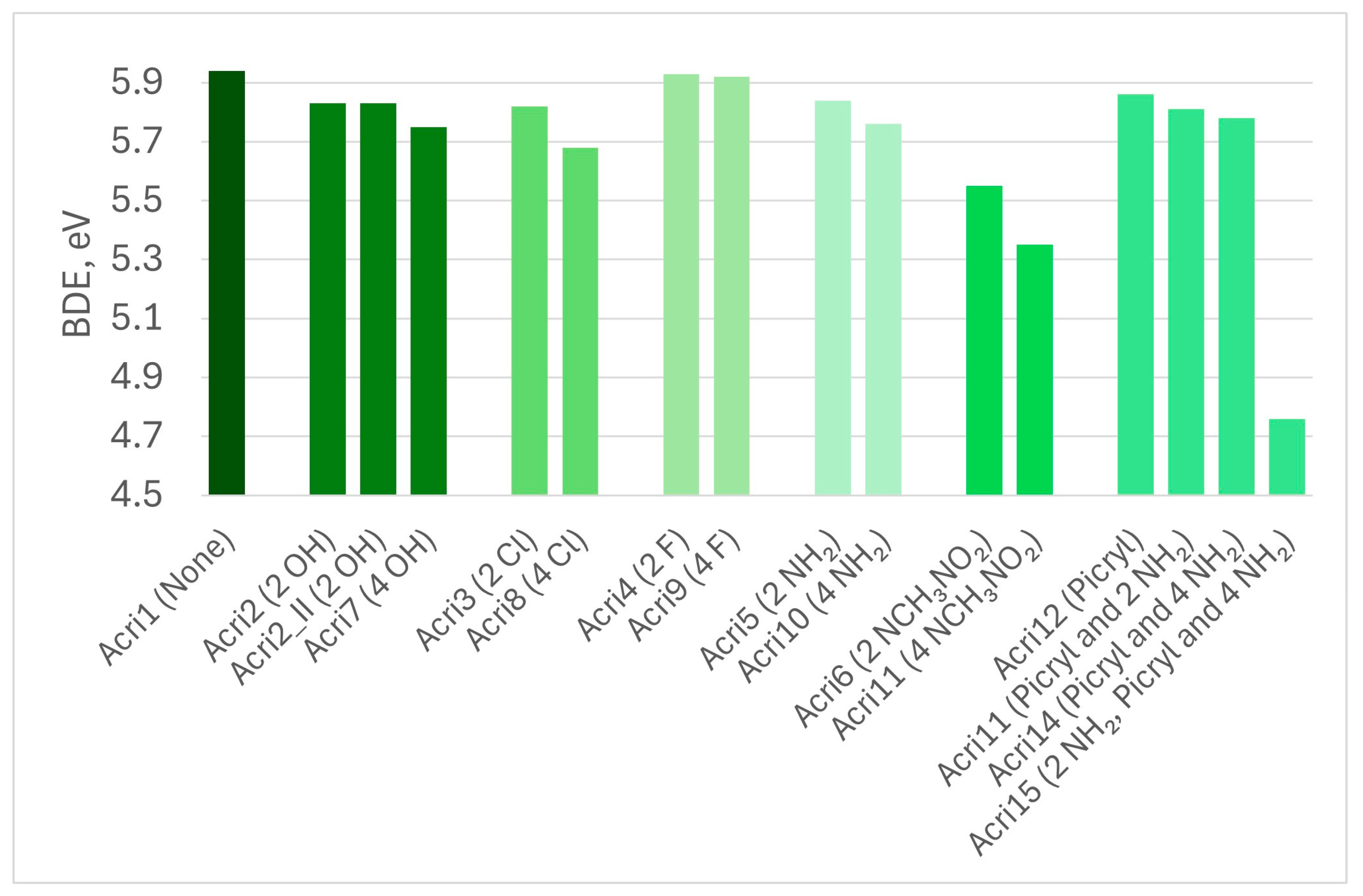
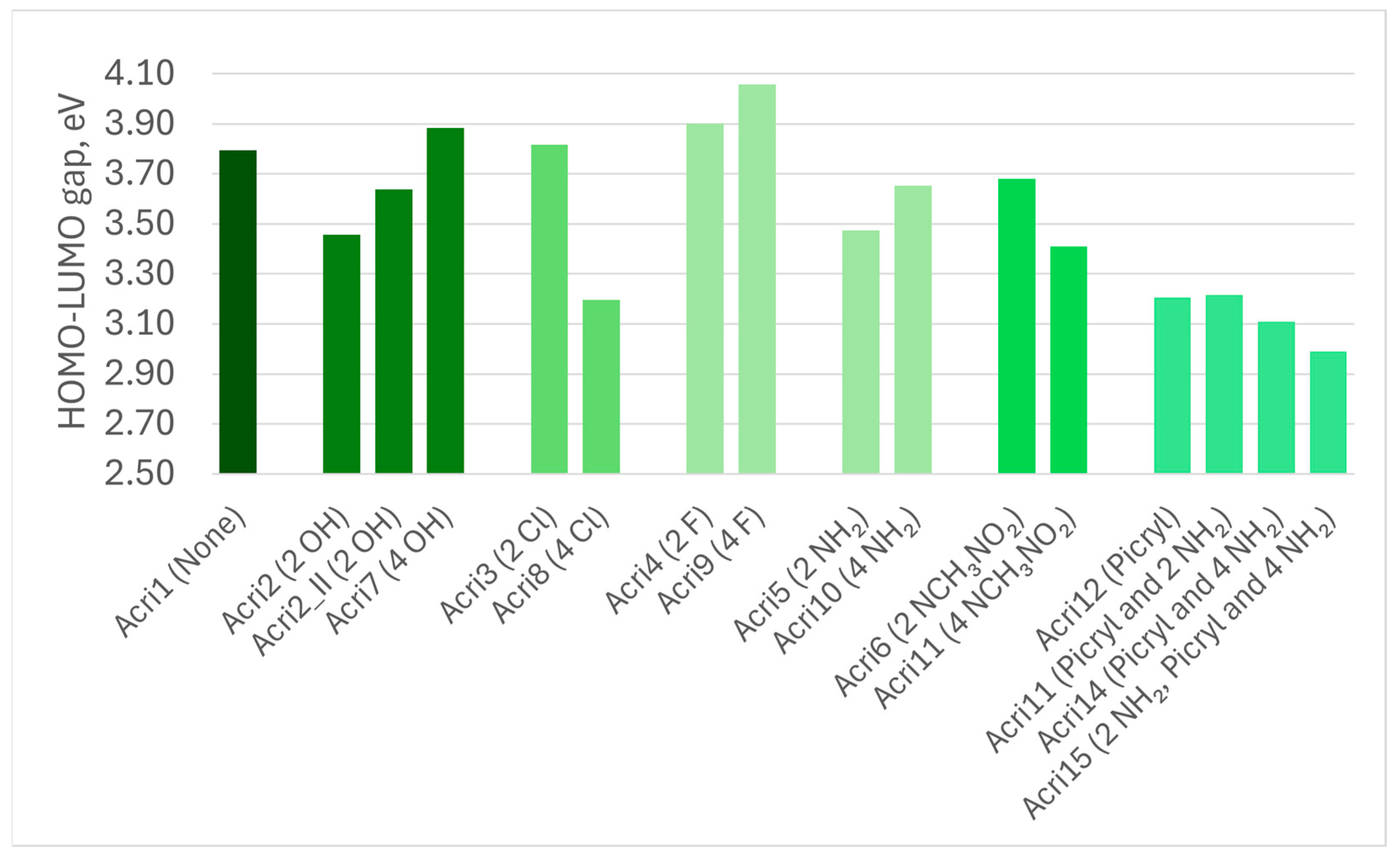
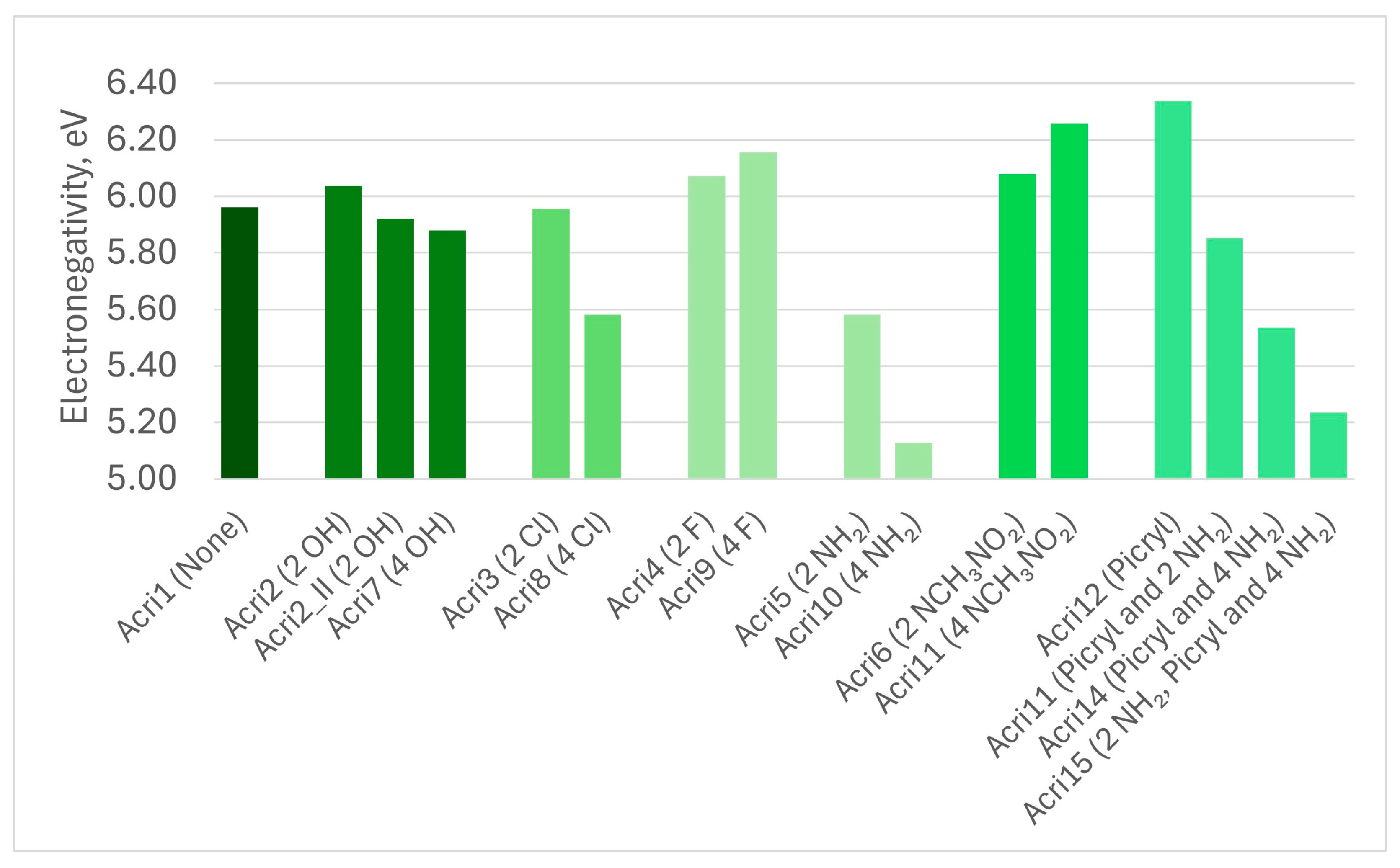
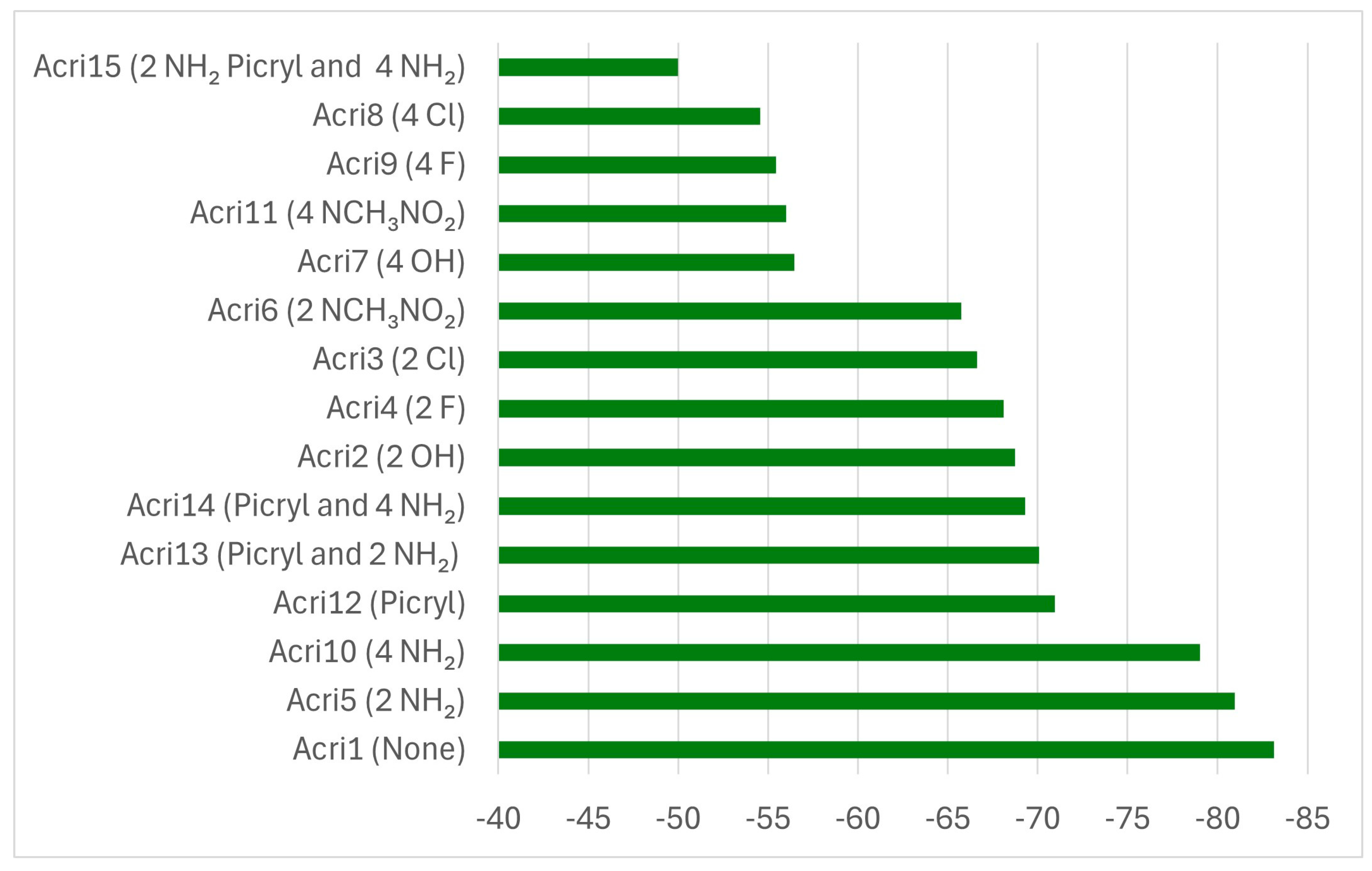
| Abbreviation | Substitutions | Gap, eV | Hard, eV | Elec, eV | Ind | BDE |
|---|---|---|---|---|---|---|
| Acri1 | None | 3.79 | 1.90 | 5.96 | 0.86 | 5.94 |
| Acri2 | 2 OH | 3.46 | 1.73 | 6.04 | 0.83 | 5.83 |
| Acri2_II | 2 OH | 3.64 | 1.82 | 5.92 | 0.85 | 5.83 |
| Acri7 | 4 HO | 3.88 | 1.94 | 5.88 | 0.87 | 5.75 |
| Acri3 | 2 Cl | 3.82 | 1.91 | 5.96 | 0.86 | 5.82 |
| Acri8 | 4 Cl | 3.20 | 1.60 | 5.58 | 0.80 | 5.68 |
| Acri4 | 2 F | 3.90 | 1.95 | 6.07 | 0.87 | 5.93 |
| Acri9 | 4 F | 4.06 | 2.03 | 6.16 | 0.88 | 5.92 |
| Acri5 | 2 NH2 | 3.47 | 1.74 | 5.58 | 0.83 | 5.84 |
| Acri10 | 4 NH2 | 3.65 | 1.83 | 5.13 | 0.85 | 5.76 |
| Acri6 | 2 NCH3NO2 | 3.68 | 1.84 | 6.08 | 0.85 | 5.55 |
| Acri11 | 4 NCH3NO2 | 3.41 | 1.71 | 6.26 | 0.83 | 5.35 |
| Acri12 | Picryl | 3.21 | 1.60 | 6.34 | 0.81 | 5.86 |
| Acri13 | Picryl and 2 NH2 | 3.22 | 1.61 | 5.85 | 0.81 | 5.81 |
| Acri14 | Picryl and 4 NH2 | 3.11 | 1.55 | 5.54 | 0.79 | 5.78 |
| Acri15 | 2 NH2 in Picryl and 4 NH2 | 2.99 | 1.50 | 5.24 | 0.78 | 4.76 |
| Abbreviation | Substitutions | ρgaus, g/cm3 | ρKev, g/cm3 | ρacd, g/cm3 |
|---|---|---|---|---|
| Acri1 | None | 1.70 | 1.60 | 1.82 |
| Acri2 | 2 OH | 1.98 | 1.89 | 2.01 |
| Acri2_II | 2 OH | 1.95 | 1.86 | 2.01 |
| Acri7 | 4 HO | 1.91 | 1.81 | 2.20 |
| Acri3 | 2 Cl | 1.81 | 1.72 | 1.93 |
| Acri8 | 4 Cl | 2.10 | 2.02 | 2.02 |
| Acri4 | 2 F | 1.84 | 1.75 | 1.92 |
| Acri9 | 4 F | 1.83 | 1.73 | 2.01 |
| Acri5 | 2 NH2 | 1.88 | 1.79 | 1.92 |
| Acri10 | 4 NH2 | 1.66 | 1.56 | 2.02 |
| Acri6 | 2 NCH3NO2 | 1.85 | 1.75 | 1.91 |
| Acri11 | 4 NCH3NO2 | 1.71 | 1.61 | 1.96 |
| Acri12 | Picryl | 1.75 | 1.65 | 1.95 |
| Acri13 | Picryl and 2 NH2 | 1.94 | 1.85 | 2.02 |
| Acri14 | Picryl and 4 NH2 | 2.14 | 2.06 | 2.09 |
| Acri15 | 2 NH2 in Picryl and 4 NH2 | 1.96 | 1.87 | 2.15 |
| Abbreviation | Substitutions | Pgaus, GPa | PKev, GPa | Pacd, GPa |
|---|---|---|---|---|
| Acri1 | None | 9.76 | 8.49 | 11.43 |
| Acri2 | 2 OH | 14.45 | 13.06 | 14.91 |
| Acri2_II | 2 OH | 13.98 | 12.58 | 14.91 |
| Acri7 | 4 HO | 14.03 | 12.56 | 19.05 |
| Acri3 | 2 Cl | 11.53 | 10.33 | 13.28 |
| Acri8 | 4 Cl | 15.93 | 14.67 | 14.70 |
| Acri4 | 2 F | 12.22 | 10.86 | 13.35 |
| Acri9 | 4 F | 12.56 | 11.09 | 15.31 |
| Acri5 | 2 NH2 | 14.29 | 12.81 | 15.07 |
| Acri10 | 4 NH2 | 12.40 | 10.79 | 19.03 |
| Acri6 | 2 NCH3NO2 | 16.15 | 14.44 | 17.26 |
| Acri11 | 4 NCH3NO2 | 16.64 | 14.62 | 22.19 |
| Acri12 | Picryl | 12.92 | 11.33 | 16.33 |
| Acri13 | Picryl and 2 NH2 | 11.53 | 10.33 | 13.28 |
| Acri14 | Picryl and 4 NH2 | 24.36 | 22.49 | 23.02 |
| Acri15 | 2 NH2 in Picryl and 4 NH2 | 18.91 | 17.12 | 23.06 |
| Abbreviation | Substitutions | Dgaus, km/s | DKev, km/s | Dacd, km/s |
|---|---|---|---|---|
| Acri1 | None | 5.70 | 5.48 | 5.98 |
| Acri2 | 2 OH | 6.43 | 6.23 | 6.49 |
| Acri2_II | 2 OH | 6.36 | 6.16 | 6.49 |
| Acri7 | 4 HO | 6.37 | 6.15 | 7.04 |
| Acri3 | 2 Cl | 5.99 | 5.80 | 6.26 |
| Acri8 | 4 Cl | 6.63 | 6.46 | 6.46 |
| Acri4 | 2 F | 6.10 | 5.89 | 6.27 |
| Acri9 | 4 F | 6.15 | 5.92 | 6.55 |
| Acri5 | 2 NH2 | 6.41 | 6.19 | 6.52 |
| Acri10 | 4 NH2 | 6.13 | 5.88 | 7.04 |
| Acri6 | 2 NCH3NO2 | 6.66 | 6.43 | 6.81 |
| Acri11 | 4 NCH3NO2 | 6.73 | 6.45 | 7.42 |
| Acri12 | Picryl | 6.21 | 5.96 | 6.69 |
| Acri13 | Picryl and 2 NH2 | 5.99 | 5.80 | 6.26 |
| Acri14 | Picryl and 4 NH2 | 7.66 | 7.45 | 7.51 |
| Acri15 | 2 NH2 in Picryl and 4 NH2 | 7.02 | 6.79 | 7.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamuliene, J.; Sarlauskas, J. Theoretical Design of Acridone-Core Energetic Materials: Assessment of Detonation Properties and Potential as Insensitive, Thermally Stable High-Energy Materials. ChemEngineering 2025, 9, 130. https://doi.org/10.3390/chemengineering9060130
Tamuliene J, Sarlauskas J. Theoretical Design of Acridone-Core Energetic Materials: Assessment of Detonation Properties and Potential as Insensitive, Thermally Stable High-Energy Materials. ChemEngineering. 2025; 9(6):130. https://doi.org/10.3390/chemengineering9060130
Chicago/Turabian StyleTamuliene, Jelena, and Jonas Sarlauskas. 2025. "Theoretical Design of Acridone-Core Energetic Materials: Assessment of Detonation Properties and Potential as Insensitive, Thermally Stable High-Energy Materials" ChemEngineering 9, no. 6: 130. https://doi.org/10.3390/chemengineering9060130
APA StyleTamuliene, J., & Sarlauskas, J. (2025). Theoretical Design of Acridone-Core Energetic Materials: Assessment of Detonation Properties and Potential as Insensitive, Thermally Stable High-Energy Materials. ChemEngineering, 9(6), 130. https://doi.org/10.3390/chemengineering9060130








