Biodiesel Carbonaceous Nanoparticle-Supported Potassium Carbonate as a Catalyst for Biodiesel Production via Transesterification
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Characterizations
2.3. Response Surface Methodology (RSM)
3. Results and Discussion
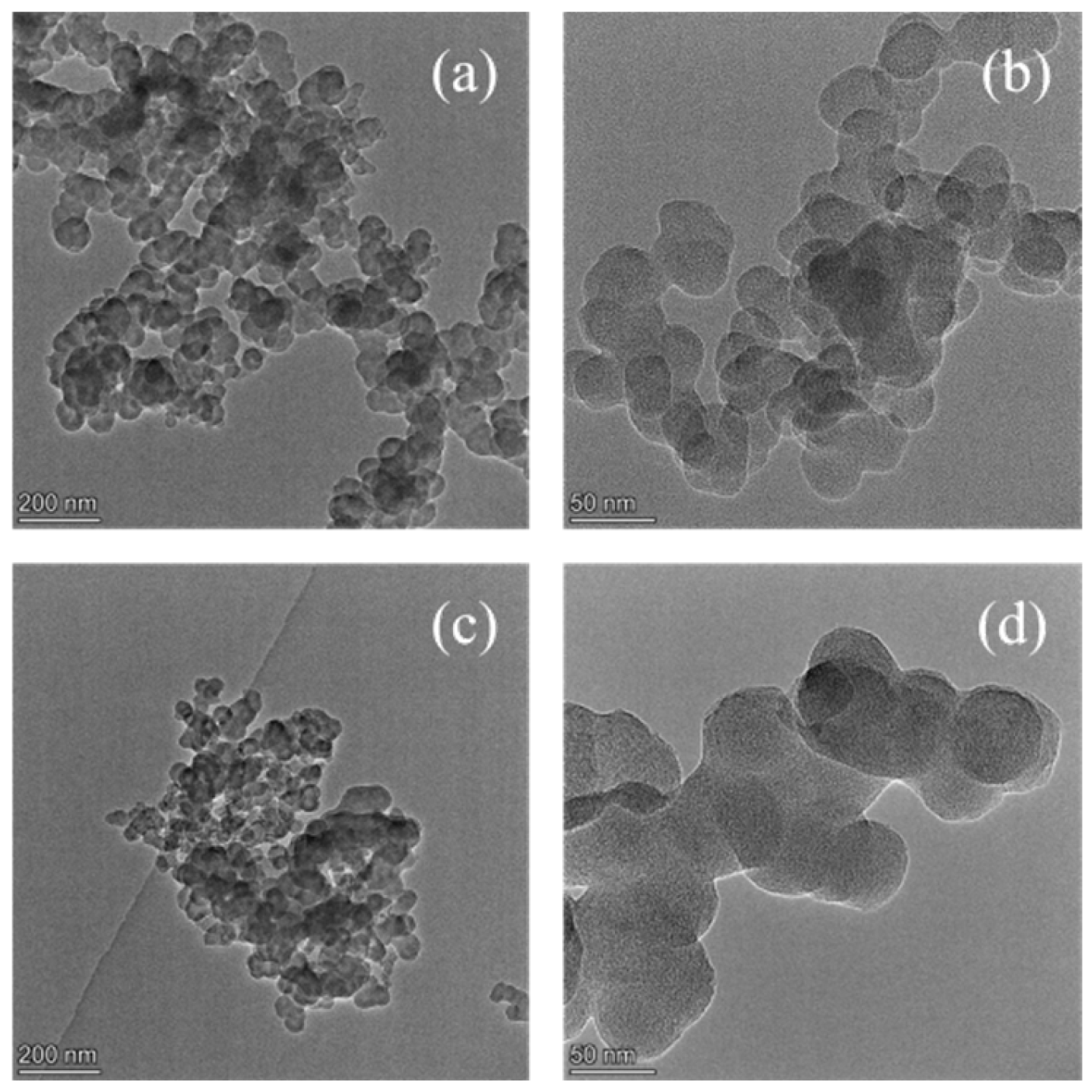
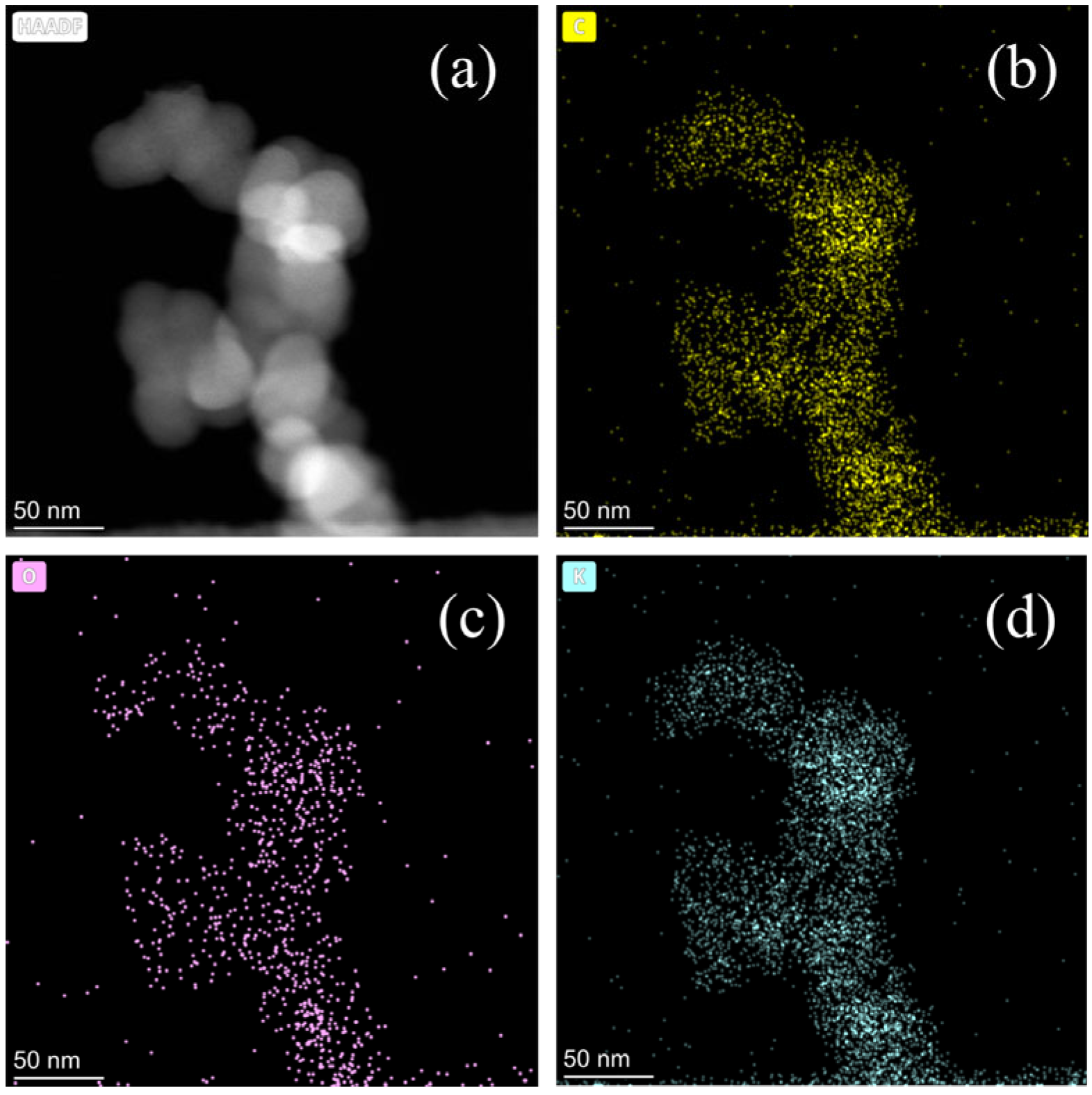
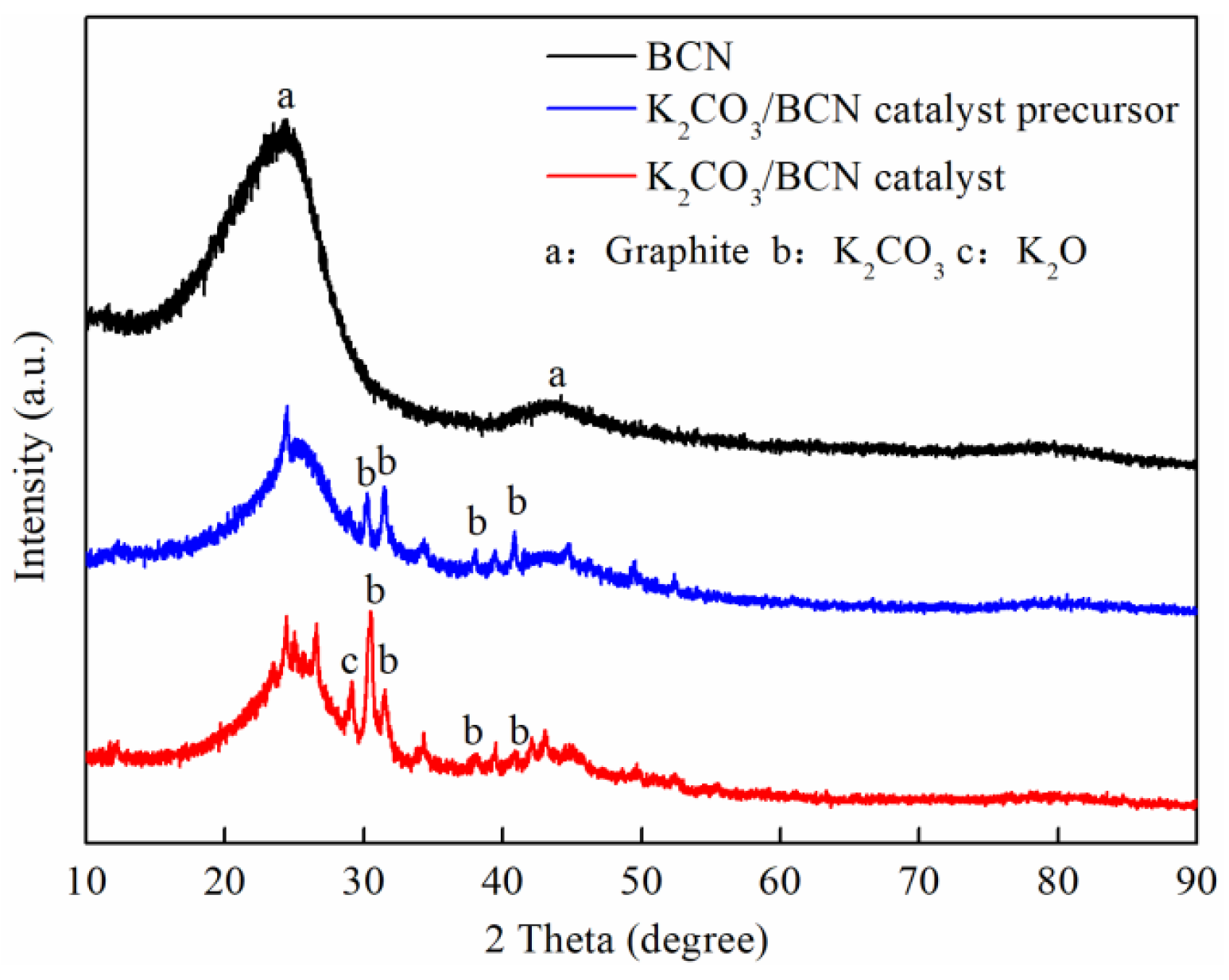
3.1. Catalysts Characterization
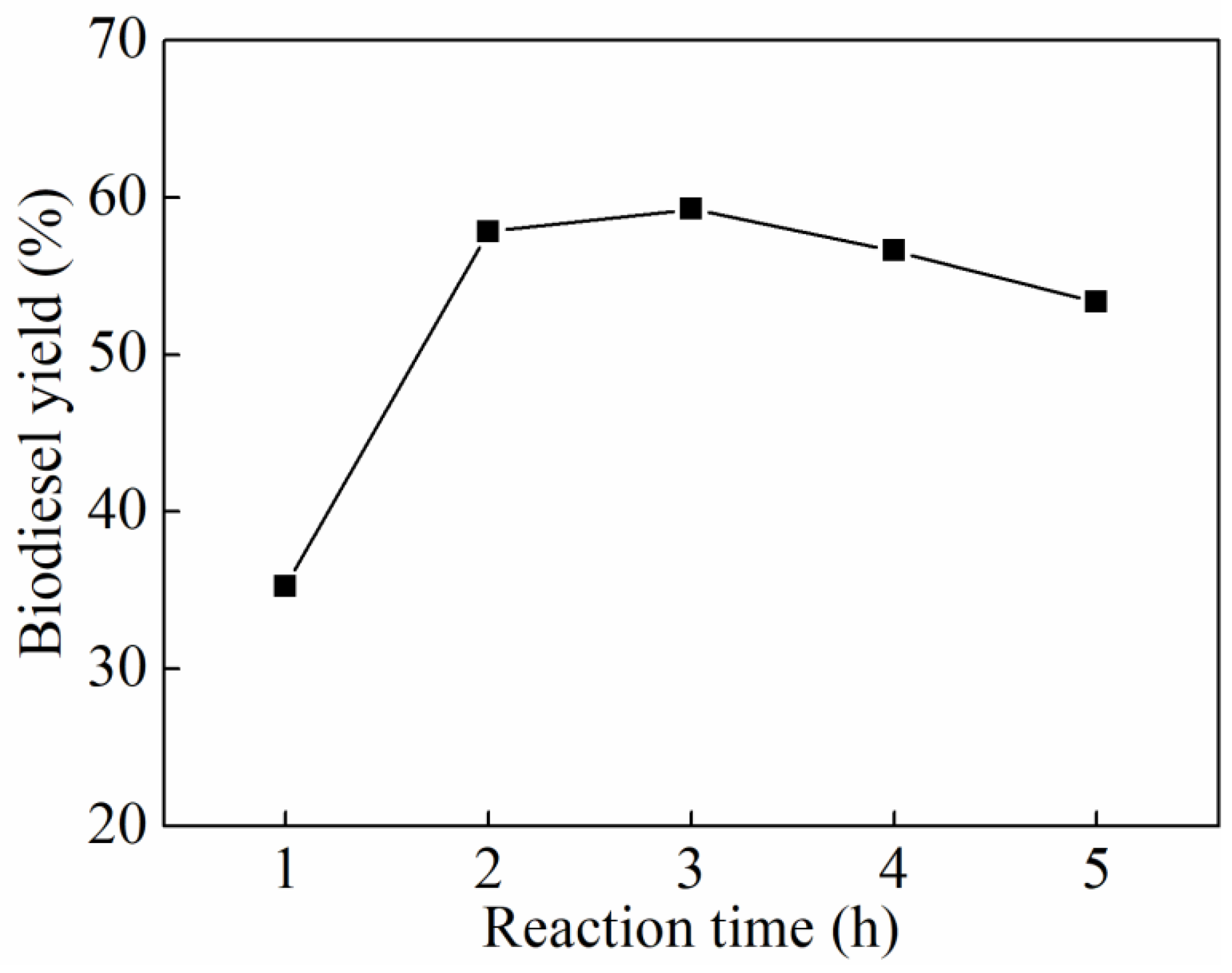
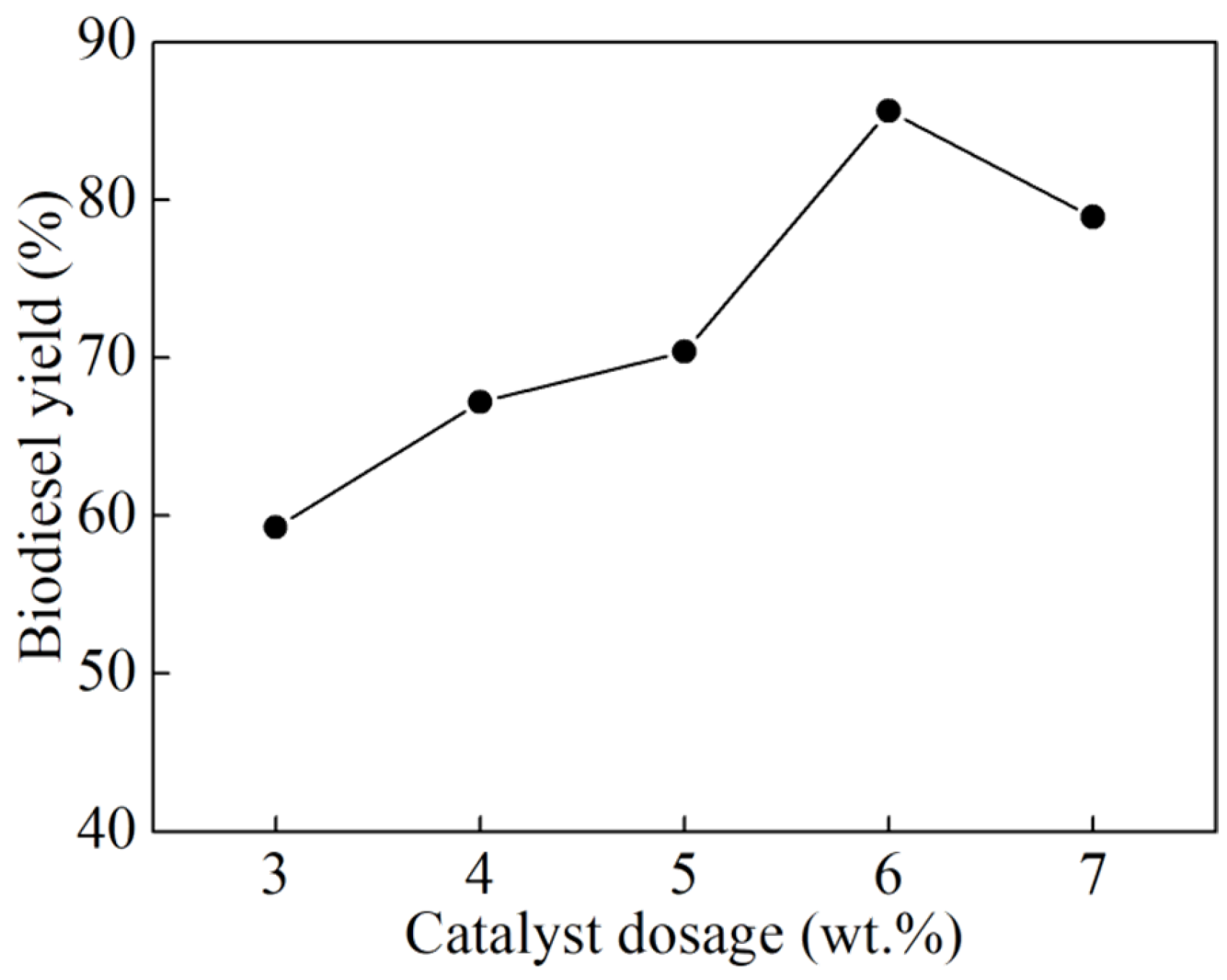
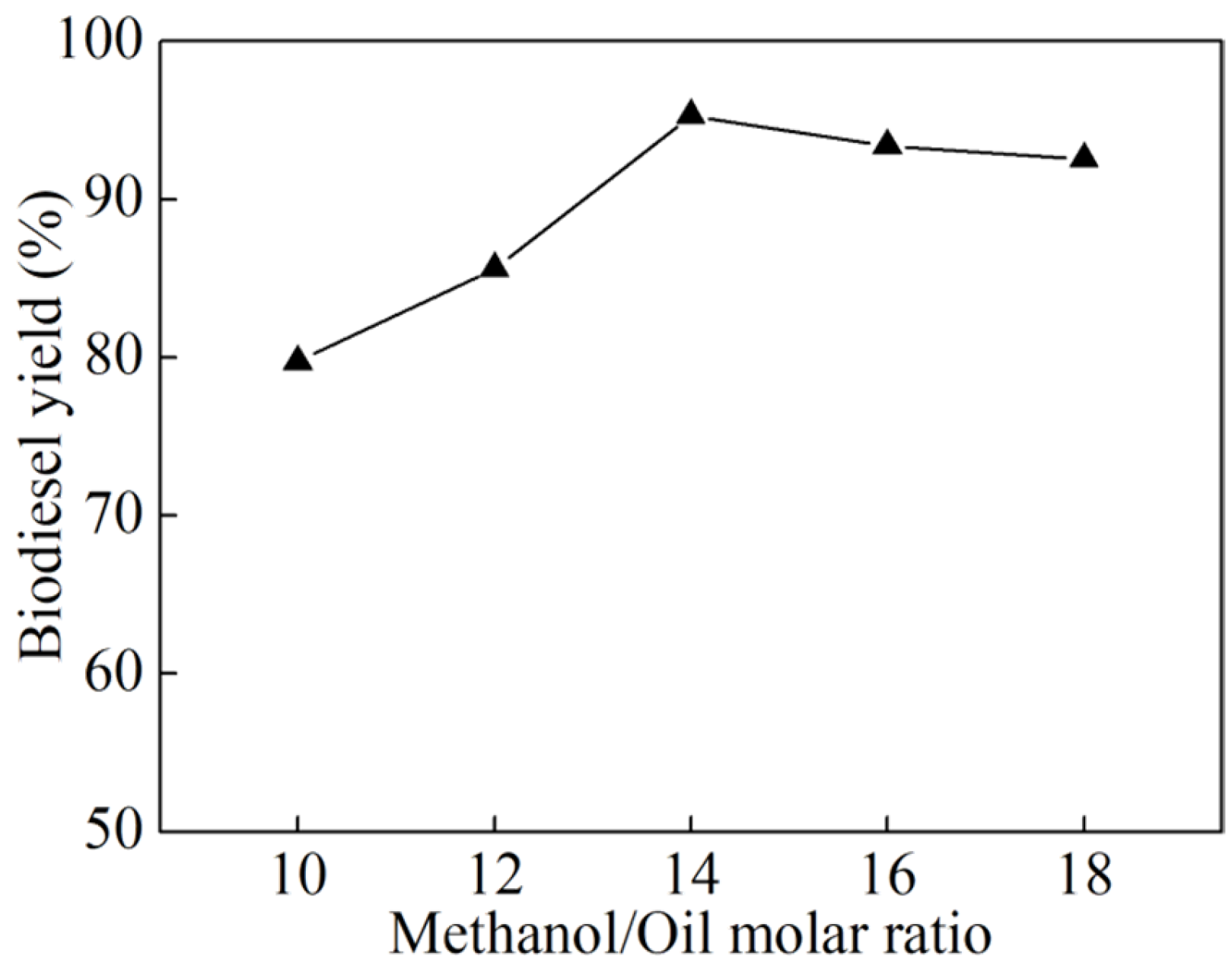
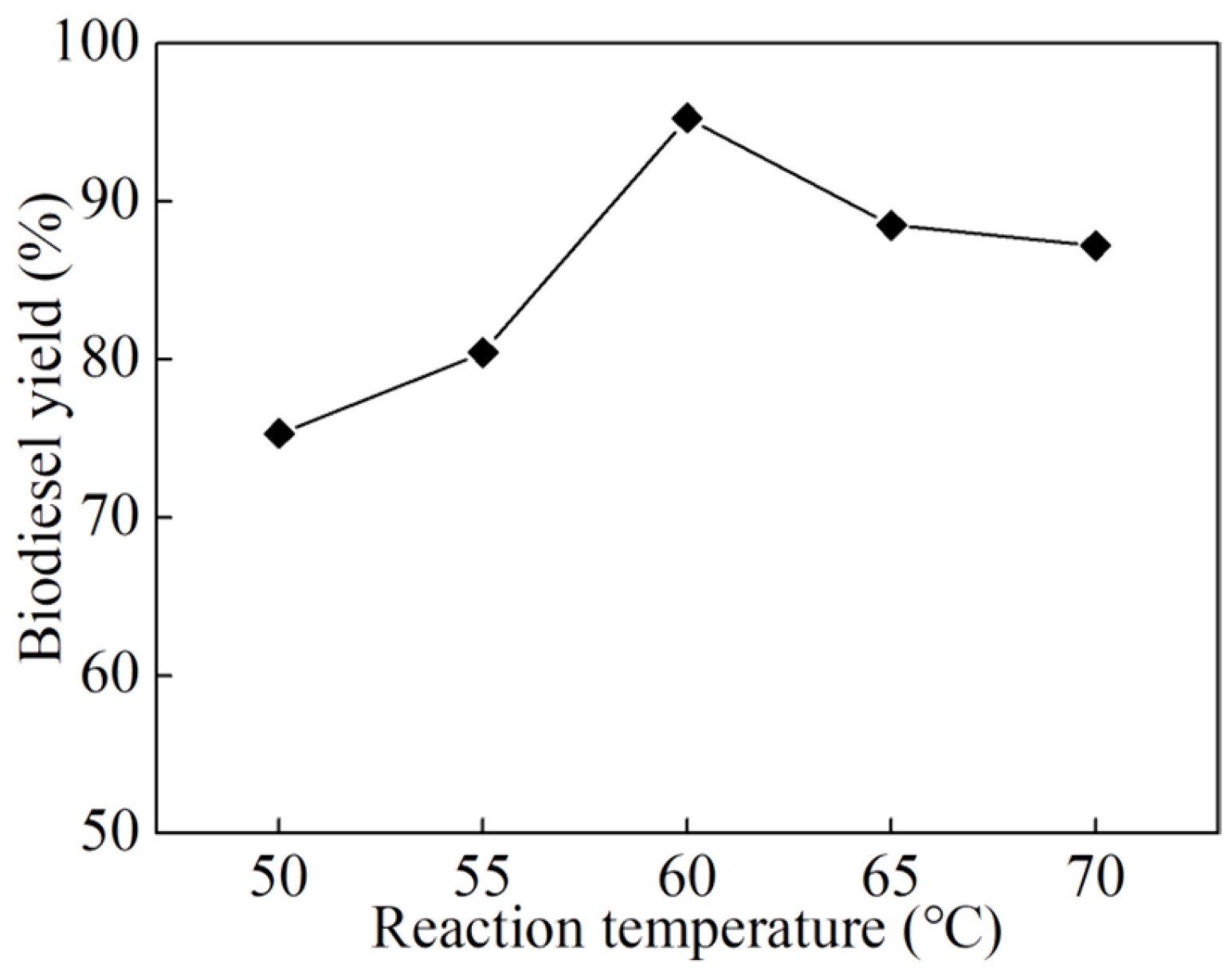
3.2. Single-Factor Experiments
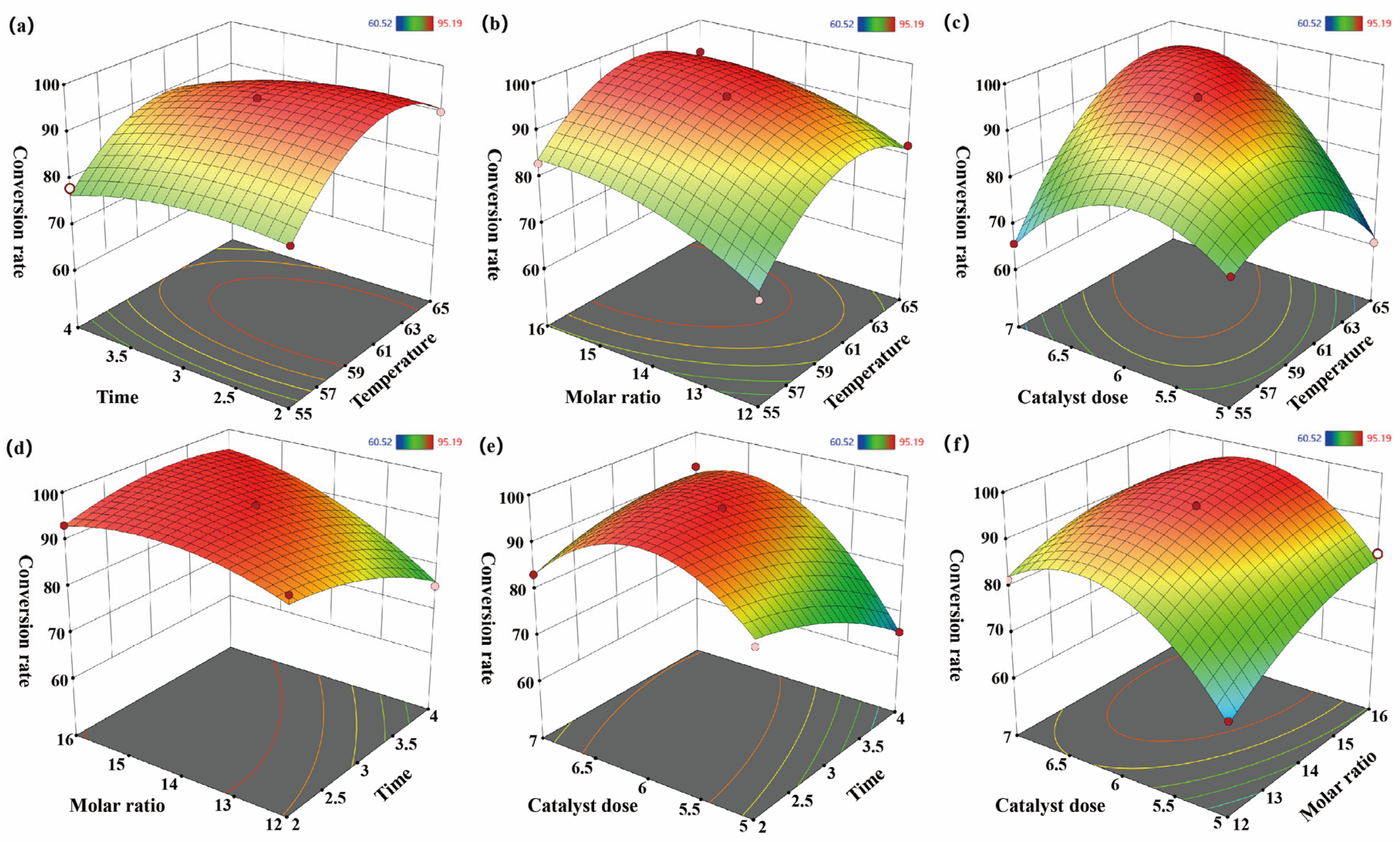
3.3. Response Surface Optimization Experiment
3.3.1. Box–Behnken Design (BBD) of Experiments
3.3.2. Quadratic Model Analysis
3.4. Characterization Analysis of Response Surface Methodology
4. Conclusions
- (1)
- The K2CO3/BCN catalyst exhibits a spherical particle structure with chain-like aggregation attributed to van der Waals interactions. The presence of active components, K2CO3 and K2O, confirms its classification as a typical supported solid alkali catalyst with excellent physicochemical stability.
- (2)
- Systematic single-factor optimization experiments identified the optimal conditions for biodiesel synthesis as follows: a catalyst dosage of 6 wt% (relative to the mass of raw oil), an alcohol-to-oil molar ratio of 14:1, a reaction temperature of 60 °C, and a reaction time of 3 h. Under these conditions, a high biodiesel yield of 95.29% was obtained, underscoring the superior catalytic efficiency of the K2CO3/BCN catalyst in the transesterification process.
- (3)
- An RSM optimization model was constructed using a four-factor, three-level BBD, accompanied by a quadratic polynomial regression equation established based on 29 experimental runs, systematically revealing the interaction effects among reaction temperature, reaction time, alcohol-to-oil molar ratio, and catalyst dosage. The optimized conditions identified by the model—temperature of 61.1 °C, reaction time of 3.3 h, alcohol-to-oil molar ratio of 14.2:1, and catalyst dosage of 6.1%—yielded a biodiesel conversion rate of 95.37% in validation experiments, confirming the model’s reliability and applicability for process optimization.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2017, 9, 273–289. [Google Scholar] [CrossRef]
- Betha, R.; Balasubramanian, R. Particulate emissions from a stationary engine fueled with ultra-low-sulfur diesel and waste-cooking-oil-derived biodiesel. J. Air Waste Manag. Assoc. 2011, 61, 1063–1069. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Wang, W.; Ni, Z.; Ding, B.; Zhang, H. Experimental study on lubrication performance of main components of biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 47, 1–15. [Google Scholar] [CrossRef]
- Suhara, A.; Karyadi; Herawan, S.G.; Tirta, A.; Idris, M.; Roslan, M.F.; Putra, N.R.; Hananto, A.L.; Veza, I. Biodiesel sustainability: Review of progress and challenges of biodiesel as sustainable biofuel. Clean Technol. 2024, 6, 886–906. [Google Scholar] [CrossRef]
- Pydimalla, M.; Husaini, S.; Kadire, A.; Kumar Verma, R. Sustainable biodiesel: A comprehensive review on feedstock, production methods, applications, challenges and opportunities. Mater. Today Proc. 2023, 92, 458–464. [Google Scholar] [CrossRef]
- Idoine, N.E.; Raycraft, E.R.; Hobbs, S.F.; Everett, P.; Evans, E.J.; Mills, A.J.; Currie, D.; Horn, S.; Shaw, R.A.; MacKenzie, A.C. World Mineral Production 2018–2022; BGS Press: Nottingham, UK, 2024; Available online: https://nora.nerc.ac.uk/id/eprint/537241/1/World%20Mineral%20Production%202018%20to%202022.pdf (accessed on 30 September 2025).
- European Union (EU). Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC. Available online: https://eur-lex.europa.eu/eli/dir/2009/28/oj/eng (accessed on 31 July 2025).
- Chanakaewsomboon, I.; Phoungthong, K.; Palamanit, A.; Seechamnanturakit, V.; Cheng, C.K. Biodiesel produced using potassium methoxide homogeneous alkaline catalyst: Effects of various factors on soap formation. Biomass Convers. Biorefin. 2021, 13, 9237–9247. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Niakousari, M.; Peykar, A.; Safaeipour, T. Microwave-assisted transesterification of sour cherry kernel oil for biodiesel production: Comparison with ultrasonic bath-, ultrasonic probe-, and ohmic-assisted transesterification methods. Grasas Aceites 2024, 75, e545. [Google Scholar] [CrossRef]
- Olagunju, O.A.; Musonge, P.; Kiambi, S.L. Production and optimization of biodiesel in a membrane reactor, using a solid base catalyst. Membranes 2022, 12, 674. [Google Scholar] [CrossRef]
- Cong, W.-J.; Wang, Y.-T.; Li, H.; Fang, Z.; Sun, J.; Liu, H.-T.; Liu, J.-T.; Tang, S.; Xu, L. Direct production of biodiesel from waste oils with a strong solid base from alkalized industrial clay ash. Appl. Energy 2020, 264, 114735. [Google Scholar] [CrossRef]
- Raising Rathod, D.; Suresh Karkal, S.; Jamadar, A.S.; Hashem, A.M.A.; Suresh, P.V.; Mamatha, S.S.; Kudre, T.G. Prospects of novel heterogeneous base catalysts and nanocatalysts in achieving sustainable biodiesel production. Int. J. Green Energy 2023, 21, 1017–1042. [Google Scholar] [CrossRef]
- Kingkam, W.; Maisomboon, J.; Khamenkit, K.; Nuchdang, S.; Nilgumhang, K.; Issarapanacheewin, S.; Rattanaphra, D. Preparation of CaO@CeO2 solid base catalysts used for biodiesel production. Catalysts 2024, 14, 240. [Google Scholar] [CrossRef]
- Singh, S.; Bairagi, P.K.; Verma, N. Candle soot-derived carbon nanoparticles: An inexpensive and efficient electrode for microbial fuel cells. Electrochim. Acta 2018, 264, 119–127. [Google Scholar] [CrossRef]
- Xiang, C.; Liu, S.Y.; Fu, Y.; Chang, J. A quick method for producing biodiesel from soy sauce residue under supercritical carbon dioxide. Renew. Energy 2019, 134, 739–744. [Google Scholar] [CrossRef]
- Baig, A.; Ng, F.T.T. A single-step solid acid-catalyzed process for the production of biodiesel from high free fatty acid feedstocks. Energy Fuels 2010, 24, 4712–4720. [Google Scholar] [CrossRef]
- Chen, X.; Qian, W.-W.; Lu, X.-P.; Han, P.-F. Preparation of biodiesel catalysed by KF/CaO with ultrasound. Nat. Prod. Res. 2012, 26, 1249–1256. [Google Scholar] [CrossRef]
- Weremfo, A.; Abassah-Oppong, S.; Adulley, F.; Dabie, K.; Seidu-Larry, S. Response surface methodology as a tool to optimize the extraction of bioactive compounds from plant sources. J. Sci. Food Agric. 2023, 103, 26–36. [Google Scholar] [CrossRef]
- Breig, S.J.M.; Luti, K.J.K. Response surface methodology: A review on its applications and challenges in microbial cultures. Mater. Today Proc. 2021, 42, 2277–2284. [Google Scholar] [CrossRef]
- Mäkelä, M. Experimental design and response surface methodology in energy applications: A tutorial review. Energy Convers. Manag. 2017, 151, 630–640. [Google Scholar] [CrossRef]
- Abu-Ghazala, A.H.; Abdelhady, H.H.; Mazhar, A.A.; El-Deab, M.S. Exceptional room temperature catalytic transesterification of waste cooking oil to biodiesel using environmentally-benign K2CO3/γ-Al2O3 nano-catalyst. Chem. Eng. J. 2023, 474, 145784. [Google Scholar] [CrossRef]
- Li, C.; Wei, D.; Zhuang, Y.; Song, R.; Hu, X. Effect of biodiesel soot on tribological behavior of liquid paraffin. China Pet. Process. Petrochem. Technol. 2018, 20, 106–113. [Google Scholar]
- Deng, X.; Mammen, L.; Butt, H.J.; Vollmer, D. Candle soot as a template for a transparent robust super-amphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef]
- Li, C.; Hu, X.; Feng, W.; Wu, B.; Wu, K. A supported solid base catalyst synthesized from green biomass ash for biodiesel production. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 40, 142–147. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, E.Z.; Liu, T.X.; Hu, X.G. Characterization of morphology, structure and composition of soot particles from biomass fuel. CIESC J. 2015, 66, 441–448. [Google Scholar] [CrossRef]
- Sulaiman, N.F.; Wan Abu Bakar, W.A.; Ali, R. Response surface methodology for the optimum production of biodiesel over Cr/Ca/γ-Al2O3 catalyst: Catalytic performance and physicochemical studies. Renew. Energy 2017, 113, 697–705. [Google Scholar] [CrossRef]
- Li, F.-J.; Li, H.-Q.; Wang, L.-G.; Cao, Y. Waste carbide slag as a solid base catalyst for effective synthesis of biodiesel via transesterification of soybean oil with methanol. Fuel Process. Technol. 2015, 131, 421–429. [Google Scholar] [CrossRef]
- Ergan, B.T.; Yılmazer, G.; Bayramoğlu, M. Fast, High Quality and Low-Cost Biodiesel Production using Dolomite Catalyst in an Enhanced Microwave System with Simultaneous Cooling. Clean. Chem. Eng. 2022, 3, 100051. [Google Scholar] [CrossRef]
- Oza, S.; Kodgire, P.; Kachhwaha, S.S. Analysis of RSM based BBD and CCD techniques applied for biodiesel production from waste cotton-seed cooking oil via ultrasound method. Anal. Chem. Lett. 2022, 12, 86–101. [Google Scholar] [CrossRef]
- Roslan, N.A.; Zainal Abidin, S.; Abdullah, N.; Osazuwa, O.U.; Abdul Rasid, R.; Yunus, N.M. Esterification reaction of free fatty acid in used cooking oil using sulfonated hypercrosslinked exchange resin as catalyst. Chem. Eng. Res. Des. 2022, 180, 414–424. [Google Scholar] [CrossRef]
- Kefale Mangesha, Y.; Nallamothu, R.B.; Ancha, V.R.; Tesfaye Tefera, N. Optimization, Production, and characterization of cottonseed methyl ester based on Box-Behnken in response surface design and gas Chromatography-Mass spectrum analysis. Energy Convers. Manag. X 2024, 23, 100619. [Google Scholar] [CrossRef]
- Nirmala, N.; Dawn, S.S. Optimization of Chlorella variabilis. MK039712.1 lipid transesterification using Response Surface Methodology and analytical characterization of biodiesel. Renew. Energy 2021, 179, 1663–1673. [Google Scholar] [CrossRef]

| Run | A (°C) | B (h) | C | D (wt%) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 55 | 3 | 14 | 7 | 65.81 |
| 2 | 60 | 4 | 12 | 6 | 75.42 |
| 3 | 60 | 3 | 14 | 6 | 95.12 |
| 4 | 65 | 2 | 14 | 6 | 90.08 |
| 5 | 60 | 3 | 14 | 6 | 95.19 |
| 6 | 60 | 2 | 14 | 7 | 83.35 |
| 7 | 55 | 2 | 14 | 6 | 80.45 |
| 8 | 55 | 3 | 12 | 6 | 69.16 |
| 9 | 60 | 4 | 14 | 5 | 65.53 |
| 10 | 60 | 3 | 14 | 6 | 95.13 |
| 11 | 65 | 3 | 14 | 5 | 60.52 |
| 12 | 55 | 3 | 14 | 5 | 74.16 |
| 13 | 60 | 3 | 14 | 6 | 95.03 |
| 14 | 60 | 2 | 14 | 5 | 82.3 |
| 15 | 60 | 4 | 16 | 6 | 92.61 |
| 16 | 60 | 3 | 12 | 7 | 81.63 |
| 17 | 60 | 3 | 16 | 7 | 89.3 |
| 18 | 60 | 4 | 14 | 7 | 92.01 |
| 19 | 60 | 2 | 12 | 6 | 91.78 |
| 20 | 65 | 3 | 16 | 6 | 92.64 |
| 21 | 65 | 4 | 14 | 6 | 84.05 |
| 22 | 60 | 3 | 12 | 5 | 66.79 |
| 23 | 60 | 3 | 14 | 6 | 94.89 |
| 24 | 60 | 2 | 16 | 6 | 93.15 |
| 25 | 65 | 3 | 12 | 6 | 82.39 |
| 26 | 65 | 3 | 14 | 7 | 92.55 |
| 27 | 60 | 3 | 16 | 5 | 82.65 |
| 28 | 55 | 4 | 14 | 6 | 78.16 |
| 29 | 55 | 3 | 16 | 6 | 83.12 |
| Source | Sum of Squares | Degree of Freedom (Dom) | Mean Squares | F-Value | p-Value × Prob > F |
|---|---|---|---|---|---|
| Model | 3037.26 | 14 | 216.95 | 80.13 | <0.0001 |
| A—Temperature | 219.91 | 1 | 219.91 | 81.23 | <0.0001 |
| B—Time | 92.57 | 1 | 92.57 | 34.19 | <0.0001 |
| C—Molar ratio | 366.31 | 1 | 366.31 | 135.30 | <0.0001 |
| D—Catalyst addition | 440.44 | 1 | 440.44 | 162.69 | <0.0001 |
| AB | 3.50 | 1 | 3.50 | 1.29 | 0.2748 |
| AC | 3.44 | 1 | 3.44 | 1.27 | 0.2785 |
| AD | 407.64 | 1 | 407.64 | 150.57 | <0.0001 |
| BC | 62.57 | 1 | 62.57 | 23.11 | 0.0003 |
| BD | 161.67 | 1 | 161.67 | 59.72 | <0.0001 |
| CD | 16.77 | 1 | 16.77 | 6.19 | 0.0260 |
| A2 | 601.97 | 1 | 601.97 | 222.35 | <0.0001 |
| B2 | 45.84 | 1 | 45.84 | 16.93 | 0.0011 |
| C2 | 88.31 | 1 | 88.31 | 32.62 | <0.0001 |
| D2 | 887.14 | 1 | 887.14 | 327.69 | <0.0001 |
| Residual | 37.90 | 14 | 2.71 | ||
| Lack of fit | 37.85 | 10 | 3.78 | 277.88 | <0.0001 |
| Pure error | 0.0545 | 4 | 0.0136 | ||
| Corr. total | 3075.16 | 28 | |||
| R2 = 0.9877 | |||||
| R2 Adj. = 0.9753 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Shi, T.; Chen, Y.; Zhang, L.; Yang, Z.; Xu, L.; Luo, Y.; Xu, X. Biodiesel Carbonaceous Nanoparticle-Supported Potassium Carbonate as a Catalyst for Biodiesel Production via Transesterification. ChemEngineering 2025, 9, 116. https://doi.org/10.3390/chemengineering9060116
Li C, Shi T, Chen Y, Zhang L, Yang Z, Xu L, Luo Y, Xu X. Biodiesel Carbonaceous Nanoparticle-Supported Potassium Carbonate as a Catalyst for Biodiesel Production via Transesterification. ChemEngineering. 2025; 9(6):116. https://doi.org/10.3390/chemengineering9060116
Chicago/Turabian StyleLi, Chuan, Tianyu Shi, Yijun Chen, Li Zhang, Zhiquan Yang, Lin Xu, Yong Luo, and Xiaoyong Xu. 2025. "Biodiesel Carbonaceous Nanoparticle-Supported Potassium Carbonate as a Catalyst for Biodiesel Production via Transesterification" ChemEngineering 9, no. 6: 116. https://doi.org/10.3390/chemengineering9060116
APA StyleLi, C., Shi, T., Chen, Y., Zhang, L., Yang, Z., Xu, L., Luo, Y., & Xu, X. (2025). Biodiesel Carbonaceous Nanoparticle-Supported Potassium Carbonate as a Catalyst for Biodiesel Production via Transesterification. ChemEngineering, 9(6), 116. https://doi.org/10.3390/chemengineering9060116







