Abstract
In this study, we synthesized an activated biochar using Prosopis juliflora waste as a carbon source. Citric acid (CA) was used as the chemical agent for biochar activation. The removal of methylene blue (MB) using the fabricated biochar was investigated. A response surface methodology (RSM) experimental design was employed to evaluate the effects of synthesis parameters, including the temperature and the CA/biochar mass ratio, on the biochar’s MB removal efficiency. The impact of adsorption parameters, such as the biochar dosage, pH, MB concentration, and ionic strength, was also examined. Kinetic and isothermal adsorption studies were conducted to assess the efficacy of the activated biochar. The kinetic study revealed a maximum adsorption capacity (qe) of 37.6 mg/g and a rate constant of 0.0022 g mg−1 min−1, with the pseudo-second-order model providing the best fit. The isotherm study indicated that the Freundlich model best described the data, with KF = 37.8 mg/g and 1/nf = 0.498. Thermodynamic analysis showed that the MB adsorption onto the biochar was spontaneous (ΔG = −9.14 kJ/mol), endothermic (ΔH = 17.87 kJ/mol), and driven by an entropy increase (ΔS = 89.20 J/mol·K).
1. Introduction
Water pollution is a growing global environmental problem that affects the quality of life of millions of people and disrupts the balance of aquatic ecosystems [1,2]. The effective removal of these contaminants is essential in wastewater treatment processes. Among all common pollutants, dyes present one of the most significant challenges during the purification process. These dyes are commonly introduced into water sources through industrial, domestic, and pharmaceutical discharges [3,4]. Methylene blue (MB), a water-soluble organic cationic dye, is widely used for dyeing a wide range of products in industries such as textiles, lithography, paint, papermaking, leather, and cosmetics. Consequently, the rapid expansion of these industrial sectors contributes significantly to water pollution [5,6,7].
The presence of MB in water bodies leads to a series of negative impacts, which manifest in various forms. One of the most noticeable is visual pollution, which occurs even at low concentrations. Even at these lower concentrations, MB can absorb solar energy, negatively affecting the photosynthetic activity of aquatic organisms [8,9]. These impacts on photosynthetic organisms cause a decrease in dissolved oxygen levels, which may result in the death of ecosystems. The risks to human health from MB contamination include skin damage, eye and respiratory disorders, and digestive issues resulting from the consumption of contaminated water, with the consequent potential risk of cancer [10,11].
In the last decades, several treatment systems have been studied to mitigate these negative effects, although few have been successfully implemented on an industrial scale [12]. These include (i) photocatalytic degradation [13], (ii) ion-exchange membranes [14], (iii) combined adsorption/precipitation systems and chemical-biological degradation [15,16], and (iv) Fenton systems [17]. However, most of these methods have significant disadvantages, such as high costs, substantial by-product generation, the production of toxic secondary pollutants, high energy consumption, and the specific requirements for microbial growth conditions. Among various dye removal techniques, adsorption has emerged as a widely applicable, simple, and effective strategy for removing dyes from water [18,19].
Recently, several adsorbent materials have been used to remove dyes from water, including (i) biochar [20], (ii) activated carbon [21], (iii) carbon nanotubes [22], and (iv) clay-based materials [23]. Biochar is produced from raw materials and organic waste, materials that are often initially not directly usable, which leads to a substantial reduction in production costs compared to those of others sorbent materials [24]. In the last decades, biochar has been employed in different industries, and its specific use depends on the quality and characteristics of the biochar [25].
Biochar-based adsorption holds great potential for wastewater treatment in industries such as textiles, offering an economic and eco-friendly strategy. Its advantages include a lower cost compared to commercial adsorbents, a high surface area, an ecological profile, and a non-hazardous nature [26,27]. Different strategies have been employed to improve the chemical and physical properties of biochar, with the chemical modification of biochar being the most used technique for enhancing its properties, as it allows for increasing the porosity and surface area of biochar and introducing new functional groups [28]. When comparing chemical and physical activation treatments, chemical activation offers higher activity due to requiring lower temperatures and generating less burn-off char [29]. However, these modifications typically require strong acids or bases, and they can require aggressive methods that reduce biochar’s effectiveness and lead to environmental contamination during production [30]. Studies on the chemical activation of biochar using acidic treatment have focused on the application of inorganic acids (e.g., sulfuric acid, hydrochloric acid, and phosphoric acid). Organic acids are environmentally friendly alternatives to conventional chemical agents, and numerous studies have demonstrated their ability to effectively modify biochar at lower temperatures. Citric acid is eco-friendly and can enhance the accessibility of adsorption sites on the biochar surface. Citric acid, in particular, has been widely used to activate various carbonaceous materials for the removal of different pollutants [31,32,33]. Nazari et al. reported that the modification of biochar by citric acid increased the sorption capacity of Cd and Pb of biochar obtained from Chickpea straw [34].
Various types of natural waste can be used as a source for biochar production. This waste can be used as an alternative for energy recovery and valorization into value-added products. In rural places, these materials are abundant and not expensive [35]. However, despite its potential, rural biomass is not used in practical applications and is discarded. Prosopis juliflora is a species that can be found in the Caribbean region, specifically in the Departamento del Atlántico (Colombia), and that produces seeds which are typically discarded or burned as solid waste. This material has demonstrated potential as a source of biochar for dye removal from water. For instance, Vasiraja et al. successfully used activated carbon derived from P. juliflora stems to remove methylene blue (MB) dye from water [36]. The same research group also applied this material to eliminate heavy metals from textile industry effluent [37]. More recently, Díaz et al. produced biochar using P. juliflora as a raw material [38]. While chemical activation significantly improves the adsorption capacity of carbon-based materials, there is limited research on chemically activated biochar made from agricultural waste. Therefore, further research into the chemical activation of biochar derived from P. juliflora biomass is necessary. In this work, we investigated the removal of methylene blue (MB) using chemically activated biochar synthesized from P. juliflora waste.
2. Materials and Methods
2.1. Synthesis and Characterization of Chemically Active Biochar
The collected Prosopis juliflora waste seeds were washed with water and then dried in an oven at 105 °C for 24 h. The dried biomass was pulverized and subjected to pyrolysis in a batch reactor, with a heating rate of 5 °C/min being maintained under an inert atmosphere (using N2(g) at 99.9%) until 500 °C was reached. At this temperature, the pyrolysis process was carried out for 2 h [33]. Following pyrolysis, the resulting biochar was washed with water to eliminate residual ash and impurities. For the chemical modification process, 5 g of biochar were added to 150 mL of citric acid (CA) solution at varying reagent ratios, as specified in Table 1. The mixture was stirred at 300 rpm for 2 h. Subsequently, the citric acid-impregnated biochar was heated at 70 °C for 12 h to complete the modification process. Once the biochar was dehydrated, it was thermally activated at three different temperatures (see Table 1) for 2 h, then allowed to cool to room temperature. The resulting modified AC biochar (AC-WB) was washed several times with distilled water to remove any residual activating agent, and was finally dried at 60 °C for 14 h. A response surface methodology (RSM) study combined with an ANOVA assay was conducted to identify the most significant factors that influence biochar functionalization. Table 1 presents the experimental parameters investigated.

Table 1.
Experimental parameters used in the synthesis design.
The biochar was characterized by scanning electron microscopy (SEM) to examine its morphology. Additionally, Fourier-transform infrared (FTIR) spectroscopy was carried out to identify its chemical functional groups.
2.2. Study of Adsorption Process Parameters
The ionic strength was adjusted using the salt addition method by introducing NaCl at different concentrations (0.0–0.5 M). After stirring for 2 h, the final concentration was measured by spectrophotometry at 665 nm. The pH (3, 9), biochar load (1, 3 g L−1), and methylene blue (MB) concentration (10, 40 mg L−1) values were studied to determine their effect on removal efficacy. In the kinetic isothermal study, the biochar was added to MB solution, then the system was stirred. The adsorption process was monitored using UV-vis spectrophotometry (at λ = 665 nm) every 5 min until the adsorption/desorption equilibrium was reached. The adsorption capacity of MB on the biochar surface was determined using the following equation:
where qe is the amount (mg) of MB adsorbed per gram of biochar (mg/g) at equilibrium; Co is the initial MB concentration (mg L−1); Ce is the MB concentration at equilibrium; V (L) is the volume of the system; and m (g) is the amount of biochar.
In order to determine the point of zero charge (PZC) of biochar, a salt addition method was employed. After salt addition, the pH was adjusted within the range of 2–12. The solution was then shaken for 24 h, and the final pH values were recorded. The samples were subsequently filtered, and the final pH was measured to determine the change in pH (ΔpH) for each solution [39].
2.3. Kinetic and Thermodynamic Study of the Adsorption Process
In the kinetic study, 100 mg of biochar was added to an Erlenmeyer flask with 100 mL of an MB solution (40 mg L−1). The suspension was stirred at 400 rpm at 298 K, and the pH was adjusted to 9 over 120 min. For the adsorption isotherm study, the following procedure was followed: 75 mg of biochar (B9) was added to containers with 75 mL of an MB solution at different concentrations (10–160 mg L−1). The system was stirred at 400 rpm at pH = 9 for 100 min. The temperature effect on the MB remotion on biochar B9 was studied. The physical chemical parameters were calculated using the Arrhenius equation:
where K is the equilibrium constant, T is temperature (K) and, R = 8.314 J mol−1 K−1 [40].
3. Results and Discussion
3.1. Spectroscopic Characterization
The FTIR absorbance spectrum acts as a structural fingerprint, revealing the optical responses of surface functional groups and offering valuable insights into the chemical characteristics of materials. Furthermore, the positive or negative charge of the biochar surface was determined by the randomly oriented polyaromatic clusters and heteroatomic functional groups that were observed on its surface [41]. Figure 1 shows the FTIR spectra of the biochar synthesized in this study. The band observed at 3280 cm−1 corresponds to hydroxyl groups (–OH). Notably, the intensity of this band is higher for the unmodified biochar (B, see Figure 1) compared to the modified biochar. The bands between 2920 and 2850 cm−1 are attributed to the stretching vibrations of C–H bonds in both sp2 and sp3 configurations. Bands in the range of 2400–2300 cm−1 represent characteristic overtones of unsaturated conjugated or disubstituted carbon bonds, which are typical of biochar materials [42,43]. When comparing the FTIR spectrum of the modified biochar to that of the unmodified biochar, a shift is observed in the band around 1200 cm−1 for the unmodified biochar, which moves to 970 cm−1 in the modified biochar. This shift is likely due to the introduction of oxygenated functional groups, such as carboxyl (–COOH), on the biochar surface. Additionally, the band at 1150 cm−1 shows increased intensity, which can be attributed to the formation of ester (C–O–C) bonds [44].
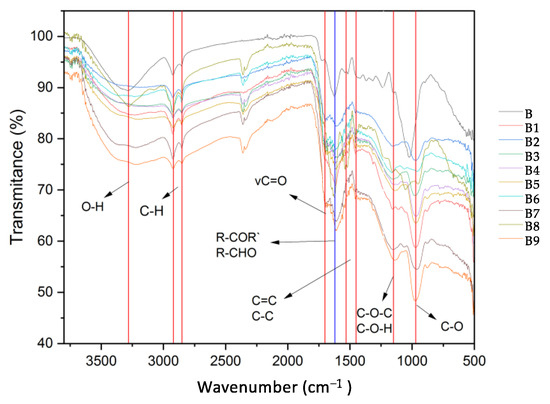
Figure 1.
FTIR spectra of biochar synthesized in this work.
3.2. Morphological Characterization
The changes in the morphology of the activated biochar are shown in Figure 2. The images illustrate the surface morphology of the biochar samples, highlighting differences in porosity and texture at different scales of magnification. Figure 2 highlights the effect of the CA/biochar ratio at 403 K, the highest activation temperature. The results indicate that all samples exhibited heterogeneous structures. SEM images revealed macroporous and rough surface textures across all samples, which were influenced by both the temperature and the chemical activation process. The biochar porosity increases with higher CA/biochar ratios, as shown in Figure 2. Citric acid acts as a dehydrating agent, which affects the pyrolytic decomposition and helps prevent the formation of bitumen, and thereby promotes the content of activated carbon [45]. A comparison of biochar B1 and B3 shows a noticeable change in the microscopic morphology of the biochar after the activation temperature was increased. The pores became more prominent due to the removal of volatile matter during activation [46].
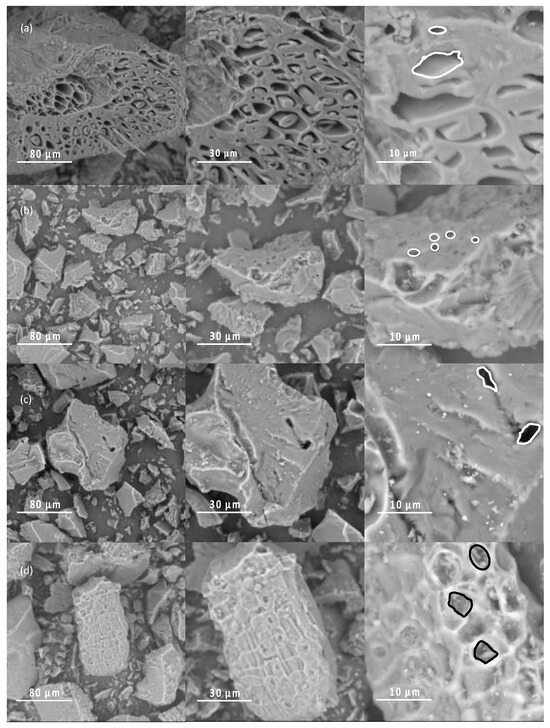
Figure 2.
SEM images at various magnifications of biochar synthesized in this study: (a) B1, (b) B3, (c) B6, and (d) B9. (Inside figure pore differences between samples is highlighted).
3.3. Study Experimental Factors Effect on MB Removal
To determine the influence of synthesis parameters (Table 1), a response surface methodology (RSM) design was applied in this study. Figure 3 presents the results, showing the response surface plots of the Box–Behnken design for MB removal using different biochar samples synthesized according to the information listed in Table 1. The MB removal was more efficient in materials with a higher CA/biochar ratio and higher activation temperature. Table S1 provides the statistical results from an ANOVA assay. These results confirm that both the CA/biochar ratio and the activation temperature are statistically significant factors in biochar’s removal efficacy. This behavior can be attributed to the new functional groups generated during chemical activation. Furthermore, at higher temperatures, the degree of aromatization is enhanced, which aids in the adsorption of MB onto active biochar [47,48]. Based on these finding, biochar B9 was selected as the best material for studying the MB removal process. Table 2 lists the effects of experimental parameters on MB remotion using B9, and Table S2 shows the statistical results from ANOVA analysis. Among these parameters, pH is an important parameter due to it affects the charge of both the B9 surface and the MB molecules. The results show that the removal efficacy increases with the pH (see Table 2). This is due to the deprotonation of functional groups on the biochar surface at higher pH values, which leads to a greater electron density. As a result, interactions between the B9 biochar’s surface and the MB are enhanced, which facilitates the adsorption process.
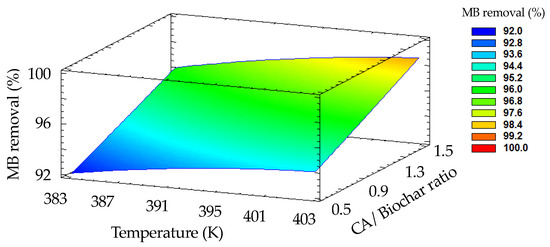
Figure 3.
Effect of activation temperature and CA/biochar ratio parameters on removal efficiency of MB. Data are plotted according to information in Table 1 (MB concentration = 10 mg L−1; biochar dosage = 0.100 g; stirring rate = 400 rpm; pH = 9).

Table 2.
Effect of experimental parameters on MB removal efficiency using modified biochar.
Additionally, the amount of biochar used significantly affects the MB removal efficiency (Table 2). A higher biochar dosage increases the number of active sites, and thereby enhances the overall removal efficiency. Similarly, Table 2 suggests that, at higher MB concentrations, the removal efficiency decreases. This reduction occurs because a higher concentration of MB in the system leads to faster saturation of the biochar surface. Furthermore, electrostatic repulsion between MB cations may hinder further adsorption, reducing the efficiency of the removal process.
The surface charge of biochar, particularly its point zero charge (PZC), is a crucial parameter in determining its adsorption performance. Figure 4a shows the determination of the PZC for biochar B9, showing that, at pH levels less than 2.66, the B9 surface carries a positive charge, which reduces its MB removal efficiency. In contrast, at pH levels above 2.66, the biochar surface achieves a negative density, which assists in the adsorption of MB, a cationic dye. These findings are consistent with the trends observed in Figure 3.
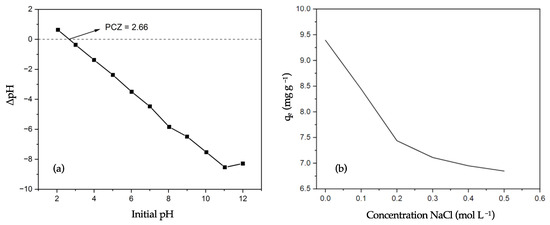
Figure 4.
(a) PZC estimation for biochar B9 (1.0 g of biochar in 50 mL of 0.01 M NaCl solution at 298 K). (b) IS effect on the qe value for MB removal using biochar B9 (MB concentration = 10 mg L−1; biochar dosage = 0.100 g; stirring rate = 400 rpm; pH = 9; temperature = 298 K).
Figure 4b illustrates the effect of the ionic strength (IS) on the MB adsorption. As the IS increases, the adsorption capacity (qe) decreases due to electrostatic screening. This phenomenon occurs because the presence of additional ions in the solution disrupts electrostatic interactions between the MB, biochar, and water molecules. Electrostatic screening can result from ionic binding, which reduces the effective surface charge density of biochar and weakens its interaction with MB, ultimately lowering the adsorption efficiency [49,50].
3.4. Kinetic Study
Figure 5 shows the fitting results for the kinetic data of MB adsorption on B9. The pseudo-first-order model (SFO), the pseudo-second-order model (SSO), and the intraparticle diffusion model were applied to fit the experimental data, as shown in Equations (3)–(5), respectively [51,52]:
where qt represents the amount of MB adsorbed by the biochar (mg g−1) at time t, and qe is the adsorption capacity (mg g−1) at equilibrium. k1 (min−1) is the rate constant for the SFO model, whereas k2 (g mg−1 min−1) corresponds to the rate constant for the SSO model. Additionally, kid (mg/g−1 min1/2) represents the intraparticle diffusion constant. Table 3 lists the corresponding kinetic parameters obtained from the fitting process.
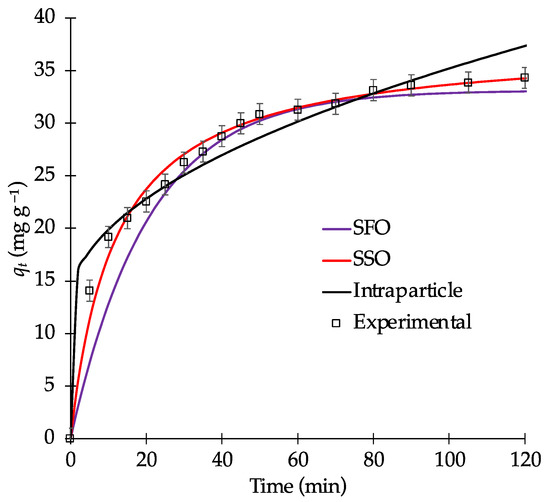
Figure 5.
Kinetic fitting for MB adsorption on biochar B9 (biochar loading = 0.100 g; [MB] = 40 mg L−1; pH = 9, temperature = 298 K; stirring rate = 400 rpm).

Table 3.
Results of kinetic modeling for MB adsorption onto biochar B9.
To determine the best-fitting model, the fitting correlation coefficient (R2) and the average relative error (ARE) were employed. Figure 5 shows that the surface uptake reaches equilibrium after 100 min, which indicates that this time is required to achieve the maximum MB removal from water. Among the three models, the intraparticle diffusion model exhibited the lowest R2 value, which suggests that it does not adequately describe the MB adsorption. Figure S1 shows the linear fit of the experimental data using the intraparticle model. Although the total data yielded an R2 of 0.933, two distinct regions are observed in Figure S1, suggesting that MB adsorption on biochar may be influenced by multiple mechanisms (e.g., external diffusion and adsorption onto active sites) [53]. If the fitting line (red line in Figure S1) passes through the origin, the adsorption process is dominated by intraparticle diffusion. If it does not, a multiple adsorption process is occurring [51]. In this case, the red line does not pass exactly through the origin, which indicates that intraparticle diffusion is not the only rate-limiting step in the process.
Furthermore, as shown in Table 3, the SSO model provided the best fitting results. The calculated qe values obtained from the SSO model closely matched the experimental data, which indicates its suitability for describing the adsorption process. This model suggests that the adsorption process can occur mainly through chemisorption. However, electrostatic interactions on the biochar surface significantly affect its interaction with MB molecules. As a cationic dye, MB can readily interact with negatively charged functional groups on the biochar surface, such as hydroxyl and carboxyl groups, through hydrogen bonding. Additionally, other interactions, such as π–π stacking and cation–π interaction [54], may also contribute to the adsorption mechanism.
The SSO model has been widely reported to describe the kinetic behavior of MB adsorption on modified biochar. Nizam et al. [55] applied the SSO model to fit experimental data for MB adsorption onto cornstalk biochar. Similarly, Ying et al. [56] found that the SSO model was suitable to describe the MB adsorption on soybean dreg biochar. In a previous study, our group reported an adsorption capacity of 2.94 mg g−1 for biochar obtained from Prosopis juliflora seed waste [38]. In the present work, after chemical activation with citric acid using the same raw material, the remotion efficiency of the biochar significantly increased to 37.6 1.9 mg g−1. Furthermore, this remotion efficiency value is comparable to values reported in other studies [48]. Baloo et al. [57] reported a qe value of 24.0 mg g−1 for MB adsorption onto oil palm waste-derived biochar. Similarly, Chaukura et al. [58] reported a qe value of 23.7 mg g−1 for methyl orange (MO) adsorption onto Fe2O3-biochar nanocomposites. Oliveira et al. [59] achieved a higher qe value of 75 mg g−1 for MB adsorption using FeCl3-activated carbon. Rashid et al. reported a similar trend in the removal of fluorine from water using active carbon modified with citric acid. They reported that the removal efficiency reached a qe value of 1.65 mg g−1, which was more than twice the value obtained with unmodified biochar [60]. Sun et al. produced biochar from eucalyptus sawdust and studied the effect of chemical activation on MB removal from water. They reported that citric acid-activated biochar exhibited higher MB removal efficiency than bare biochar, with a qe value of 9.5 mg g−1, [61].
3.5. Isothermal and Thermodynamic Study
To analyze the isothermal results, three adsorption models were employed: (i) the Langmuir isotherm, (ii) the Freundlich isotherm, and (iii) the Temkin isotherms models, according to [62,63]:
where qe represent the amount of MB adsorbed on the biochar (mg g−1) and Ce is the equilibrium concentration of MB (mg L−1). In Equation (6), KL is the Langmuir constant (L mg−1). In Equation (7), KF and nF are the Freundlich constants. In Equation (8), B is the Temkin constant and AT is the equilibrium binding constant (L mg−1). Figure 6 shows the isothermal fitting results, and Table 4 lists the physicochemical parameters.
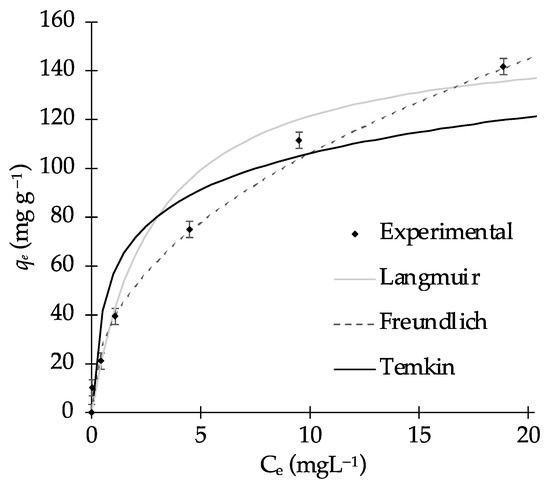
Figure 6.
Isothermal fitting for MB adsorption on biochar B9 (biochar loading = 0.075 g; pH = 9, temperature = 298 K, time contact = 100 min, stirring rate = 400 rpm).

Table 4.
Isotherm modeling results for MB adsorption on biochar.
Among the three models that we applied, the Temkin model exhibited the lowest R2 value and the highest ARE, which suggests that it does not accurately describe MB adsorption. This model assumes that dye adsorption follows a multilayer process and disregards extremely high and low adsorbate concentrations in liquid phase [63].
Conversely, the Langmuir model showed suitable R2 and ARE values (see Figure 6), which indicates that the adsorption occurs via a monolayer mechanism. However, the best fitting results were obtained using the Freundlich model, which describes dye adsorption on the catalyst as a multilayer process on a heterogeneous surface. The Freundlich model is employed to describe adsorption processes on heterogeneous surfaces. Additionally, a 1/nF value smaller than 1 suggests that the adsorption of MB on biochar is favorable [64]. Previous studies have proposed that the 1/nF value can provide information on surface heterogeneity, with values of 1/nF < 1 indicating high surface heterogeneity [65]. This behavior is consistent with our morphological analysis (see Section 3.2).
A comparison of the previously reported KF value for Prosopis juliflora seed waste biochar (1.45) with the KF obtained in this work (37.78 2.8, see Table 4) clearly demonstrates that activation with citric acid (CA) significantly enhances the chemical properties of the biochar surface. The increase in active carbon content and changes in surface chemistry contribute to an improved porous structure. Modification with CA can introduce carboxylic functional groups on the biochar surface. Furthermore, CA can penetrate the carbon structure, promoting the formation of small pores in the activated carbon and enhancing its morphological properties [45,66].
This thermodynamic study provides insights into the spontaneity of MB adsorption onto biochar. Figure S2 shows the fitting plot for Equation (1), based on evaluations conducted at four different temperatures (see Table 5). The results indicate that MB adsorption on biochar is a thermodynamically spontaneous process (ΔG < 0). Moreover, the reduction in ΔG values with an increasing temperature suggests that adsorption is favored at higher temperatures. This is consistent with the enthalpy value (ΔH > 0), which confirms that the process is endothermic. Additionally, MB adsorption on biochar is entropically assisted (ΔS > 0), which can be attributed to the degree of hydration of MB molecules. At the molecular level, MB adsorption on biochar surfaces displaces hydration water from both the MB and biochar surface, which leads to an increase in the molecular conformational states.

Table 5.
Thermodynamic adsorption values for dye adsorption onto modified biochar.
The thermodynamic values obtained in this study are comparable to those reported for other biochars. Table 5 summarizes the thermodynamic studies on the removal of MB using modified biochar. The thermodynamic parameters were obtained from the linear fitting of the Arrhenius equation (see Figure S1).
4. Conclusions
In this work, MB was removed from an aqueous solution using chemically activated biochar. The biochar was synthesized from Prosopis juliflora waste seeds using pyrolysis, with citric acid (CA) serving as the chemical activation agent. The FTIR spectra reveal the characteristic functional groups present in the biochar samples, with vibrational bands corresponding to hydroxyl groups (–OH), C–H bond stretches, and overtones of unsaturated conjugated carbon bonds. Shifts in the bands were observed between the unmodified and modified biochar, which indicates the introduction of oxygenated groups and ester bonds on the surface of the material. The optimal conditions for synthesizing active biochar were a temperature of 403 K and a CA/biochar ratio of 1.5, which achieved 98% MB removal. The optimal values of the pH and adsorption time were pH = 9 and 100 min, respectively, which resulted in a qe value of 37.6 mg g−1 for MB removal. The pseudo-second-order and Freundlich isotherm models were found to be suitable to describe the kinetic and isothermal behavior of the biochar. Additionally, the MB adsorption on the active biochar was thermodynamically spontaneous, with the adsorption process being enhanced at higher temperatures. Overall, the results suggest that the activation process increases the removal capacity of the biochar, making it a promising material for environmental applications, particularly for the removal of dyes from water.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemengineering9030064/s1, Table S1: ANOVA results to activation biochar synthesis; Table S2: ANOVA results to MB adsorption on biochar B9; Figure S1. Intra-particle diffusion fitting for experimental data of MB adsorption on biochar; Figure S2: Linear fitting of Arrhenius equation of experimental data of MB adsorption on biochar B9.
Author Contributions
Conceptualization, C.D.-U. and W.V.; methodology, A.A., C.D.-U., W.V., F.D. and E.M.-V.; validation, A.A. and F.D.; formal analysis, A.A., C.D.-U. and W.V.; investigation, A.A., C.D.-U., W.V., F.D. and E.M.-V.; resources, C.D.-U., W.V. and E.M.-V.; data curation, A.A. and F.D.; writing—original draft preparation, A.A., C.D.-U., W.V., F.D. and E.M.-V.; writing—review and editing, A.A., C.D.-U., W.V., F.D. and E.M.-V.; supervision, C.D.-U. and W.V.; project administration, C.D.-U., W.V. and E.M.-V.; funding acquisition, C.D.-U., W.V. and E.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad del Atlántico.
Data Availability Statement
Data are contained within the article.
Acknowledgments
C.D.-U.: W.V., would like to thank Universidad del Atlántico. E.M.-V., thanks Engineer J. Betancourt for the SEM measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Somma, R.; Kumar, V.; Barco, J. Surface water, groundwater, and soil pollution: Sustainable water and soils resources management and human health risk assessment and ecology. Chemosphere 2023, 337, 139295. [Google Scholar] [CrossRef]
- Weng, C.H. Water environment and recent advances in pollution control technologies. Environ. Sci. Pollut. Res. 2022, 29, 12462–12464. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, Toxicity and Carcinogenicity of Organic Dyes and their Catalytic Bio-Remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef]
- Sudarshan, S.; Harikrishnan, S.; RathiBhuvaneswari, G.; Alamelu, V.; Aanand, S.; Rajasekar, A.; Govarthanan, M. Impact of textile dyes on human health and bioremediation of textile industry effluent using microorganisms: Current status and future prospects. J. Appl. Microbiol. 2023, 134, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Cwalinski, T.; Polom, W.; Marano, L.; Roviello, G.; D’angelo, A.; Cwalina, N.; Matuszewski, M.; Roviello, F.; Jaskiewicz, J.; Polom, K. Methylene Blue—Current Knowledge, Fluorescent Properties, and Its Future Use. J. Clin. Med. 2020, 9, 3538. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- da Costa, S.R.; da Costa Monteiro, M.; da Silva Júnior, F.M.R.; Sandrini, J.Z. Methylene blue toxicity in zebrafish cell line is dependent on light exposure. Cell Biol. Int. 2016, 40, 895–905. [Google Scholar] [CrossRef]
- Krishna Moorthy, A.; Govindarajan Rathi, B.; Shukla, S.P.; Kumar, K.; Shree Bharti, V. Acute toxicity of textile dye Methylene blue on growth and metabolism of selected freshwater microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef]
- Xiao, J.; Shen, Y.; Yang, X.; Wei, M.; Meng, W.; Wang, Z. Methylene blue can increase the number of lymph nodes harvested in colorectal cancer: A meta-analysis. Int. J. Colorectal Dis. 2023, 38, 50. [Google Scholar] [CrossRef]
- Malik, S.; Andleeb, F.; Ullah, H. Multimodal imaging of skin lesions by using methylene blue as cancer biomarker. Microsc. Res. Tech. 2020, 83, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Uribe, C.; Florez, J.; Vallejo, W.; Duran, F.; Puello, E.; Roa, V.; Schott, E.; Zarate, X. Removal and photocatalytic degradation of methylene blue on ZrO2 thin films modified with Anderson-Polioxometalates (Cr3+, Co3+, Cu2+): An experimental and theoretical study. J. Photochem. Photobiol. A Chem. 2024, 454, 115689. [Google Scholar] [CrossRef]
- Ali Dheyab, M.; Abdul Aziz, A.; Jameel, M.S.; Moradi Khaniabadi, P.; Mehrdel, B. Sonochemical-assisted synthesis of highly stable gold nanoparticles catalyst for decoloration of methylene blue dye. Inorg. Chem. Commun. 2021, 127, 108551. [Google Scholar] [CrossRef]
- Chen, W.; Ma, H.; Xing, B. Electrospinning of multifunctional cellulose acetate membrane and its adsorption properties for ionic dyes. Int. J. Biol. Macromol. 2020, 158, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, C.; Posada, M.; Hormiga, A.; Peña, J.; Diaz-Uribe, C.; Vallejo, W.; Muñoz-Acevedo, A.; Roa, V.; Schott, E.; Zarate, X. Antimicrobial Activity against Fusarium oxysporum f. sp. dianthi of TiO2/ZnO Thin Films under UV Irradiation: Experimental and Theoretical Study. ACS Omega 2024, 9, 31546–31555. [Google Scholar] [CrossRef]
- Duran, F.; Díaz-Uribe, C.; Vallejo, W.; Vargas, X.; Romero Bohorquez, A.R.; Schott, E.; Zarate, X. Thermodynamic Study of Mehtylene Blue Adsorption and Photocatalytic Degradation on The N-Doped TiO2 Thin Films: A DFT and Experimental Study. ChemistrySelect 2024, 9, e202401559. [Google Scholar] [CrossRef]
- Shi, G.; Zeng, S.; Liu, Y.; Xiang, J.; Deng, D.; Wu, C.; Teng, Q.; Yang, H. Efficient heterogeneous Fenton-like degradation of methylene blue using green synthesized yeast supported iron nanoparticles. Ecotoxicol. Environ. Saf. 2023, 263, 115240. [Google Scholar] [CrossRef]
- Mustapha, O.R.; Osobamiro, T.M.; Sanyaolu, N.O.; Alabi, O.M. Adsorption study of Methylene blue dye: An effluents from local textile industry using Pennisteum pupureum (elephant grass). Int. J. Phytoremediation 2023, 25, 1348–1358. [Google Scholar] [CrossRef]
- Mussa, Z.H.; Al-Ameer, L.R.; Al-Qaim, F.F.; Deyab, I.F.; Kamyab, H.; Chelliapan, S. A comprehensive review on adsorption of methylene blue dye using leaf waste as a bio-sorbent: Isotherm adsorption, kinetics, and thermodynamics studies. Environ. Monit. Assess. 2023, 195, 940. [Google Scholar] [CrossRef]
- Hou, M.; He, Y.; Yang, X.; Yang, Y.; Lin, X.; Feng, Y.; Kan, H.; Hu, H.; He, X.; Liu, C. Preparation of Biomass Biochar with Components of Similar Proportions and Its Methylene Blue Adsorption. Molecules 2023, 28, 6261. [Google Scholar] [CrossRef]
- Fito, J.; Abewaa, M.; Mengistu, A.; Angassa, K.; Ambaye, A.D.; Moyo, W.; Nkambule, T. Adsorption of methylene blue from textile industrial wastewater using activated carbon developed from Rumex abyssinicus plant. Sci. Rep. 2023, 13, 5427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Wang, M.; Chen, B.; Sun, Y.; Chen, K.; Du, Q.; Pi, X.; Wang, Y. Adsorption of Methylene Blue from Aqueous Solution Using Gelatin-Based Carboxylic Acid-Functionalized Carbon Nanotubes@Metal–Organic Framework Composite Beads. Nanomaterials 2022, 12, 2533. [Google Scholar] [CrossRef] [PubMed]
- Sellak, S.; Bensalah, J.; Ouaddari, H.; Safi, Z.; Berisha, A.; Draoui, K.; Barrak, I.; Guedira, T.; Bourhia, M.; Ibenmoussa, S.; et al. Adsorption of Methylene Blue Dye and Analysis of Two Clays: A Study of Kinetics, Thermodynamics, and Modeling with DFT, MD, and MC Simulations. ACS Omega 2024, 9, 15175–15190. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, D.; Zhylina, M.; Shmeltser, K. Modern Use of Biochar in Various Technologies and Industries: A Review. Chem. Chem. Technol. 2024, 18, 232–243. [Google Scholar] [CrossRef]
- Kocsis, T.; Ringer, M.; Biró, B. Characteristics and Applications of Biochar in Soil–Plant Systems: A Short Review of Benefits and Potential Drawbacks. Appl. Sci. 2022, 12, 4051. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Gale, M.; Nguyen, T.; Moreno, M.; Gilliard-Abdulaziz, K.L. Physiochemical Properties of Biochar and Activated Carbon from Biomass Residue: Influence of Process Conditions to Adsorbent Properties. ACS Omega 2021, 6, 10224–10233. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2019, 35, 777–815. [Google Scholar] [CrossRef]
- Angin, D.; Altintig, E.; Köse, T.E. Influence of process parameters on the surface and chemical properties of activated carbon obtained from biochar by chemical activation. Bioresour. Technol. 2013, 148, 542–549. [Google Scholar] [CrossRef]
- Lonappan, L.; Liu, Y.; Rouissi, T.; Brar, S.K.; Surampalli, R.Y. Development of biochar-based green functional materials using organic acids for environmental applications. J. Clean. Prod. 2020, 244, 118841. [Google Scholar] [CrossRef]
- Mihoub, A.; Amin, A.E.-E.A.Z.; Motaghian, H.R.; Saeed, M.F.; Naeem, A. Citric Acid (CA)–Modified Biochar Improved Available Phosphorus Concentration and Its Half-Life in a P-Fertilized Calcareous Sandy Soil. J. Soil Sci. Plant Nutr. 2022, 22, 465–474. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Liu, S.; Tan, X.; Zeng, G.; Zeng, W.; Ding, Y.; Cao, W.; Zheng, B. Enhanced adsorption of methylene blue by citric acid modification of biochar derived from water hyacinth (Eichornia crassipes). Environ. Sci. Pollut. Res. 2016, 23, 23606–23618. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Rahimi, G.; Khademi Jolgeh Nezhad, A. Effectiveness of native and citric acid-enriched biochar of Chickpea straw in Cd and Pb sorption in an acidic soil. J. Environ. Chem. Eng. 2019, 7, 103064. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Wan Mahari, W.A.; Lee, X.Y.; Han, C.S.; Vo, D.V.N.; Van Le, Q.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Vasiraja, N.; Saravana, R.; Joshua, A.; Kennedy, S.; Jeen, J. Sustainable Methylene Blue dye removal with activated carbon from Prosopis juliflora stem. Int. J. Phytoremediation 2024, 4, 472–480. [Google Scholar]
- Vasiraja, N.; Saravana Sathiya Prabhahar, R.; Joshua, A. Preparation and Physio–Chemical characterisation of activated carbon derived from prosopis juliflora stem for the removal of methylene blue dye and heavy metal containing textile industry effluent. J. Clean. Prod. 2023, 397, 136579. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Walteros, L.; Duran, F.; Vallejo, W.; Romero Bohórquez, A.R. Prosopis juliflora Seed Waste as Biochar for the Removal of Blue Methylene: A Thermodynamic and Kinetic Study. ACS Omega 2022, 7, 42916–42925. [Google Scholar] [CrossRef]
- Cardenas-Peña, A.M.; Ibanez, J.G.; Vasquez-Medrano, R. Determination of the Point of Zero Charge for Electrocoagulation Precipitates from an Iron Anode. Int. J. Electrochem. Sci. 2012, 7, 6142–6153. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Ortiz, J.; Duran, F.; Vallejo, W.; Fals, J. Methyl Orange Adsorption on Biochar Obtained from Prosopis juliflora Waste: Thermodynamic and Kinetic Study. ChemEngineering 2023, 7, 114. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Guo, C.; Zou, J.; Yang, J.; Wang, K.; Song, S. Surface characterization of maize-straw-derived biochar and their sorption mechanism for Pb2+ and methylene blue. PLoS ONE 2020, 15, e0238105. [Google Scholar]
- Singh, B.; Fang, Y.; Johnston, C.T. A Fourier-Transform Infrared Study of Biochar Aging in Soils. Soil Sci. Soc. Am. J. 2016, 80, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Sarkar, D.; Panicker, T.F.; Kumar Mishra, R.; Srinivas Kini, M. A comprehensive review of production and characterization of biochar for removal of organic pollutants from water and wastewater. Water-Energy Nexus 2024, 7, 243–265. [Google Scholar] [CrossRef]
- Yu, O.Y.; Raichle, B.; Sink, S. Impact of biochar on the water holding capacity of loamy sand soil. Int. J. Energy Environ. Eng. 2013, 4, 44. [Google Scholar] [CrossRef]
- Dwivedi, S.; Dey, S. Review on biochar as an adsorbent material for removal of dyes from waterbodies. Int. J. Environ. Sci. Technol. 2023, 20, 9335–9350. [Google Scholar] [CrossRef]
- Newcombe, G.; Drikas, M. Adsorption of NOM onto activated carbon: Electrostatic and non-electrostatic effects. Carbon 1997, 35, 1239–1250. [Google Scholar] [CrossRef]
- Collins, K.D.; Neilson, G.W.; Enderby, J.E. Ions in water: Characterizing the forces that control chemical processes and biological structure. Biophys. Chem. 2007, 128, 95–104. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Behera, A.K.; Shadangi, K.P.; Sarangi, P.K. Efficient removal of Rhodamine B dye using biochar as an adsorbent: Study the performance, kinetics, thermodynamics, adsorption isotherms and its reusability. Chemosphere 2024, 354, 141702. [Google Scholar] [CrossRef]
- Doǧan, M.; Abak, H.; Alkan, M. Adsorption of methylene blue onto hazelnut shell: Kinetics, mechanism and activation parameters. J. Hazard. Mater. 2009, 164, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Sui, X. Synthesis of fibrous LaFeO3 perovskite oxide for adsorption of Rhodamine B. Ecotoxicol. Environ. Saf. 2019, 168, 35–44. [Google Scholar] [CrossRef]
- Nizam, N.U.M.; Hanafiah, M.M.; Mahmoudi, E.; Halim, A.A.; Mohammad, A.W. The removal of anionic and cationic dyes from an aqueous solution using biomass-based activated carbon. Sci. Rep. 2021, 11, 8623. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Chen, X.; Li, H.; Liu, X.; Zhang, C.; Zhang, J.; Yi, G. Efficient Adsorption of Methylene Blue by Porous Biochar Derived from Soybean Dreg Using a One-Pot Synthesis Method. Molecules 2021, 26, 661. [Google Scholar] [CrossRef]
- Baloo, L.; Isa, M.H.; Sapari, N.B.; Jagaba, A.H.; Wei, L.J.; Yavari, S.; Razali, R.; Vasu, R. Adsorptive removal of methylene blue and acid orange 10 dyes from aqueous solutions using oil palm wastes-derived activated carbons. Alexandria Eng. J. 2021, 60, 5611–5629. [Google Scholar] [CrossRef]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Synthesis, characterisation and methyl orange adsorption capacity of ferric oxide–biochar nano-composites derived from pulp and paper sludge. Appl. Water Sci. 2017, 7, 2175–2186. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Pereira, E.; Guimaraes, I.R.; Vallone, A.; Pereira, M.; Mesquita, J.P.; Sapag, K. Preparation of activated carbons from coffee husks utilizing FeCl3 and ZnCl2 as activating agents. J. Hazard. Mater. 2009, 165, 87–94. [Google Scholar] [CrossRef]
- Rashid, U.S.; Bezbaruah, A.N. Citric acid modified granular activated carbon for enhanced defluoridation. Chemosphere 2020, 252, 126639. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Húmpola, P.D.; Odetti, H.S.; Fertitta, A.E.; Vicente, J.L. Thermodynamic analysis of adsorption models of phenol in liquid phase on different activated carbons. J. Chil. Chem. Soc. 2013, 58, 1541–1544. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Huang, X.; Zhang, Q.; Wang, T.; Guo, X. Guideline for modeling solid-liquid adsorption: Kinetics, isotherm, fixed bed, and thermodynamics. Chemosphere 2024, 349, 140736. [Google Scholar] [CrossRef]
- Gratuito, M.K.B.; Panyathanmaporn, T.; Chumnanklang, R.A.; Sirinuntawittaya, N.; Dutta, A. Production of activated carbon from coconut shell: Optimization using response surface methodology. Bioresour. Technol. 2008, 99, 4887–4895. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of methylene blue on chemically modified lychee seed biochar: Dynamic, equilibrium, and thermodynamic study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, F.; Ye, L.; Yang, X.; Song, Y.; Wang, H. Methylene blue and acid red adsorption on biochar made from modified sugarcane bagasse: A dynamic, equilibrium, and thermodynamic investigation. Adsorpt. Sci. Technol. 2024, 42, 1–22. [Google Scholar] [CrossRef]
- Plentz Gomes Vasconcelos, L.; Almeida Albuquerque, A.; Roberta Cabral Ribeiro, K.; Beatriz Oliveira Palmeira, M.; Thalis Vaz da Costa Capistrano, R.; Inácio Soletti, J.; Helena Vieira Carvalho, S.; Daltro Bispo, M. Comparison of adsorption potential of methylene blue and 17β-stradiol on biochar, activated biochar and catalytic biochar from lignocellulosic waste. J. Ind. Eng. Chem. 2024, 144, 585–595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).