Solving a Challenge in the Tequila Industry: A New Continuous Rectification Process for Reducing Higher Alcohols and Obtaining Products Within the Official Tequila Standard
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Distillation of Ordinario at Pilot Level
2.2.1. Horizontal Continuous Distillation Process
- Residence Time: 4 h (Feed = 250 mL/min) and 2 h (Feed = 500 mL/min).
- Distilled/Feed (D/F) = 0.2.
- During the continuous process, fractions (f1, f2, f3, f4, and f5) were collected once the processes reached a stationary state.
2.2.2. Batch Distillation
2.3. Alcoholic Content
2.4. Volatile Composition Determination
2.5. Statistical Analysis
3. Results and Discussion
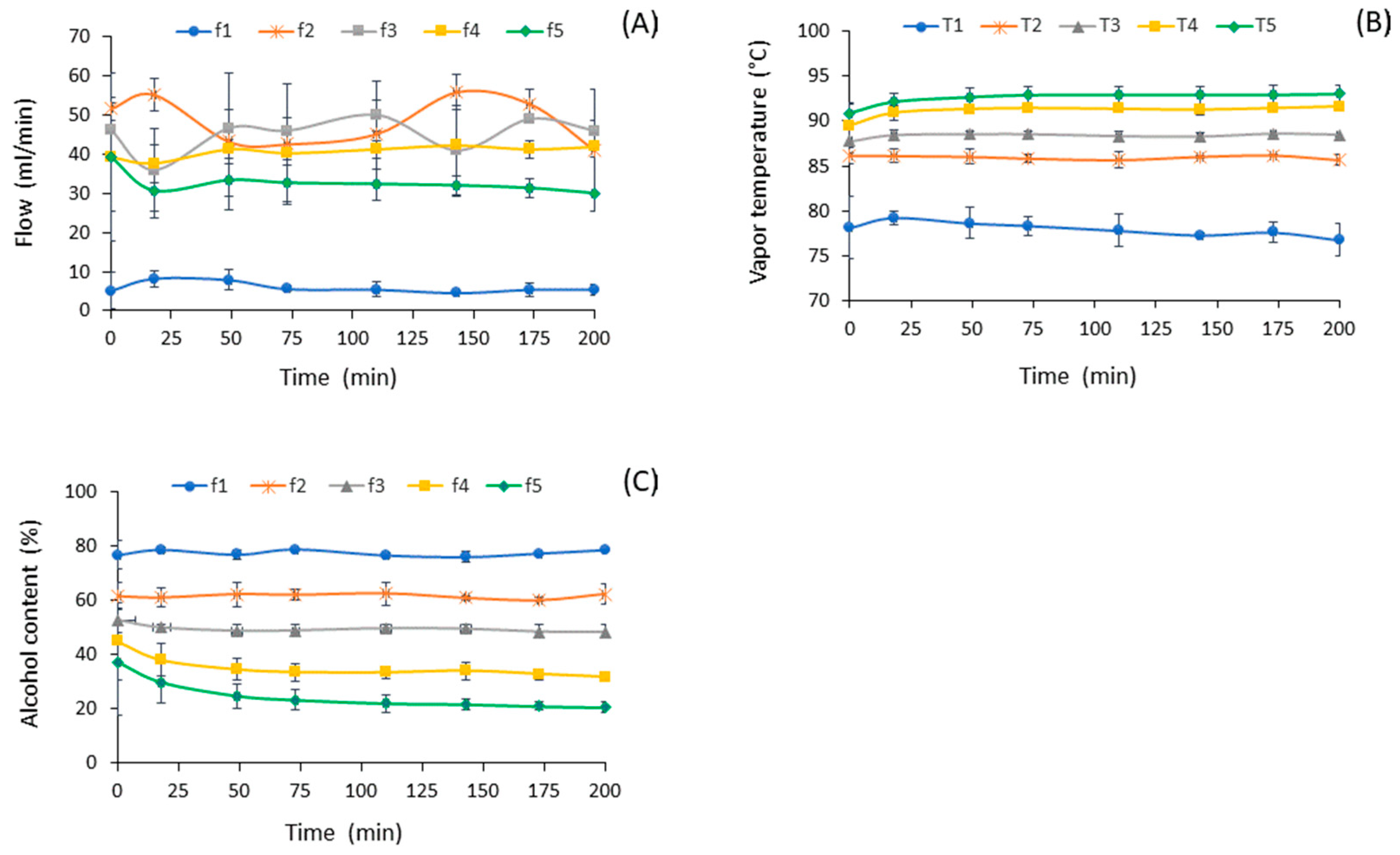
3.1. Stationary State
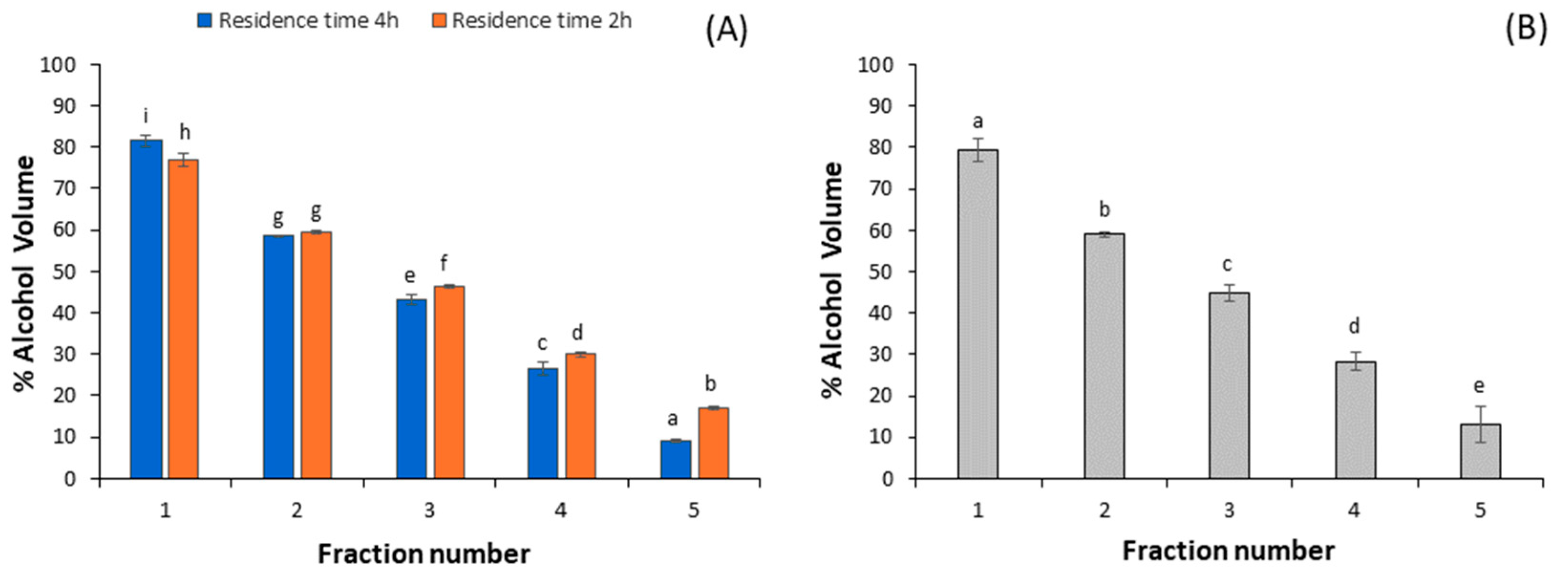
3.2. Alcoholic Concentration of Distilled Fractions
3.3. Volatile Composition of Fractions
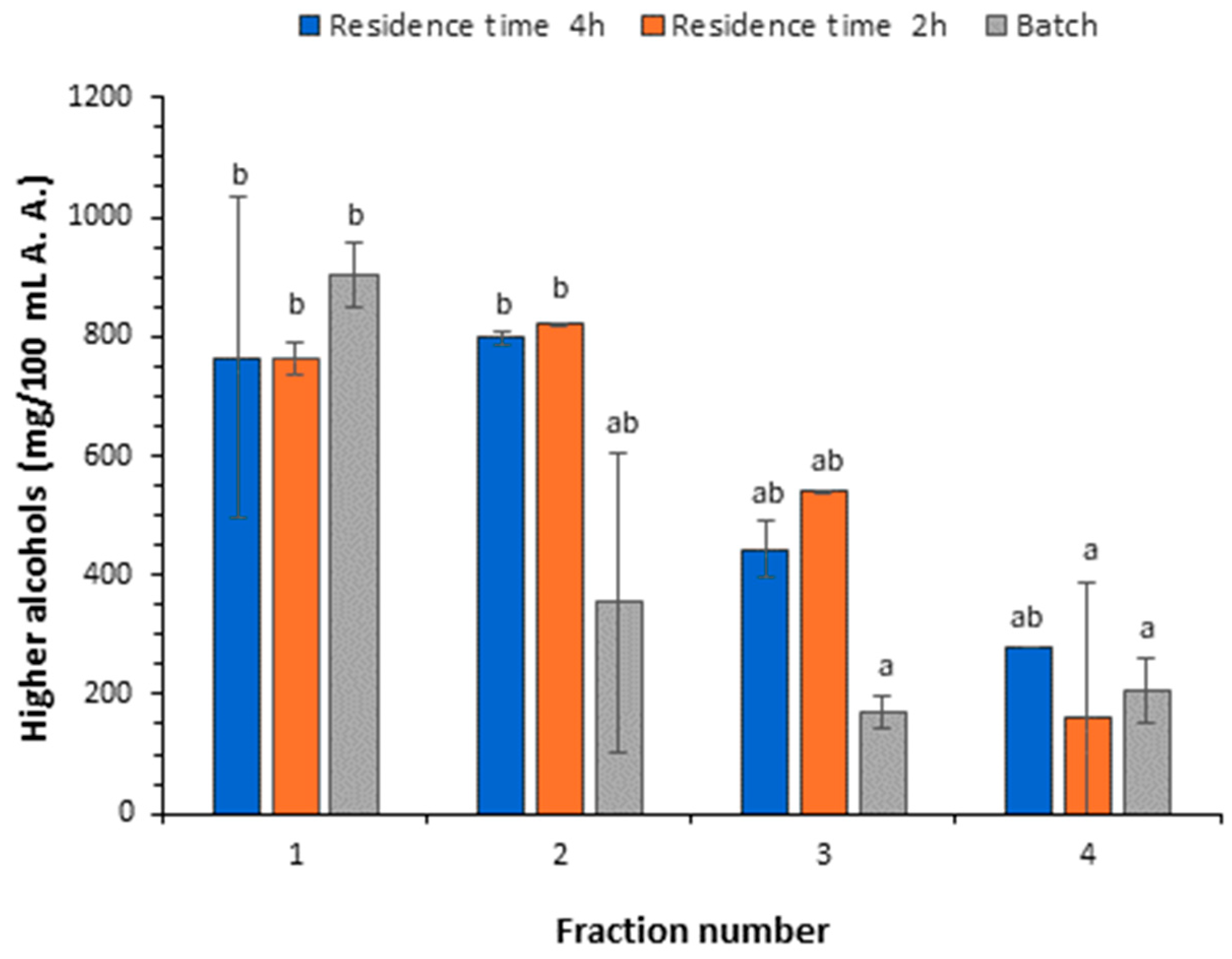
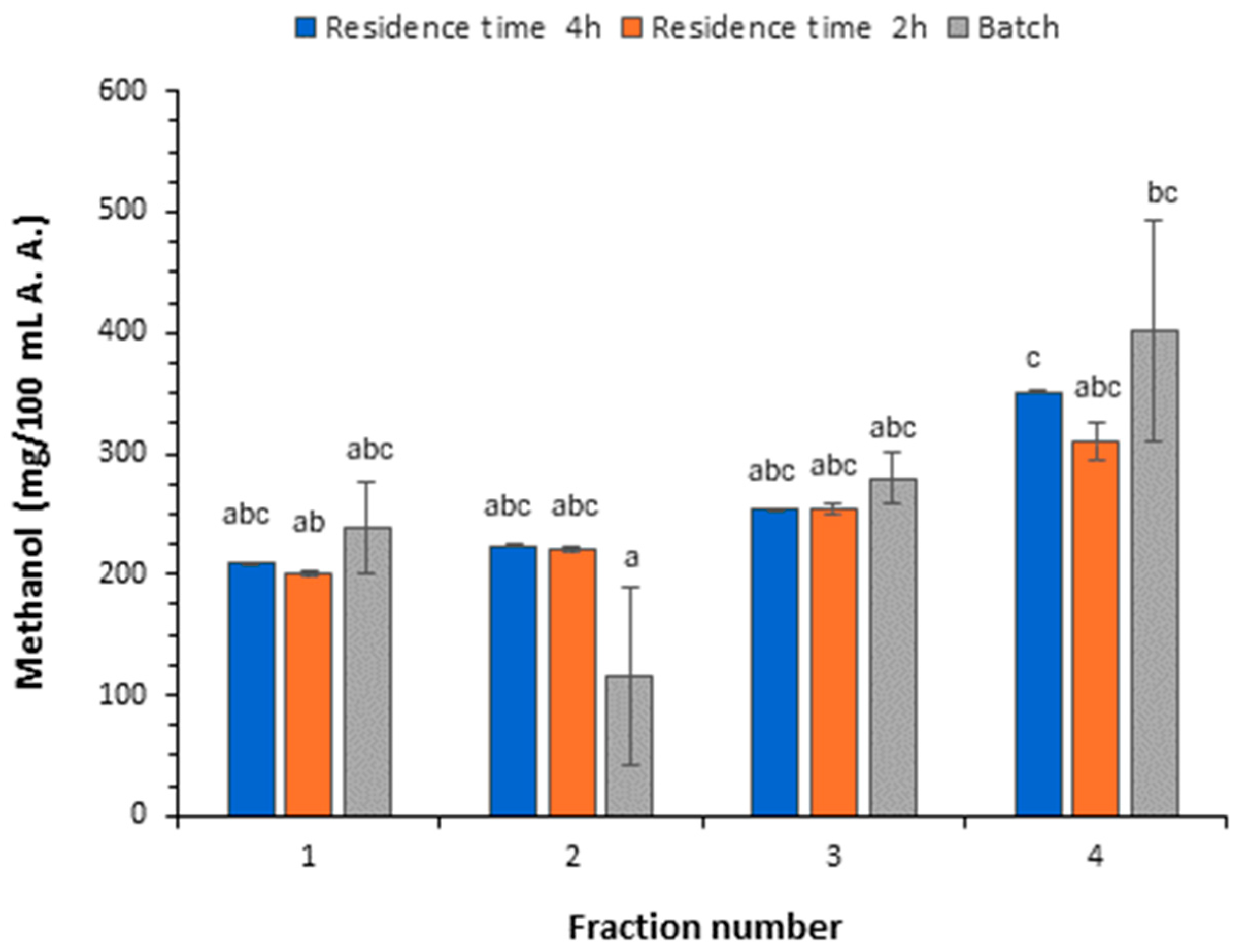
3.3.1. Content of Higher Alcohols and Methanol
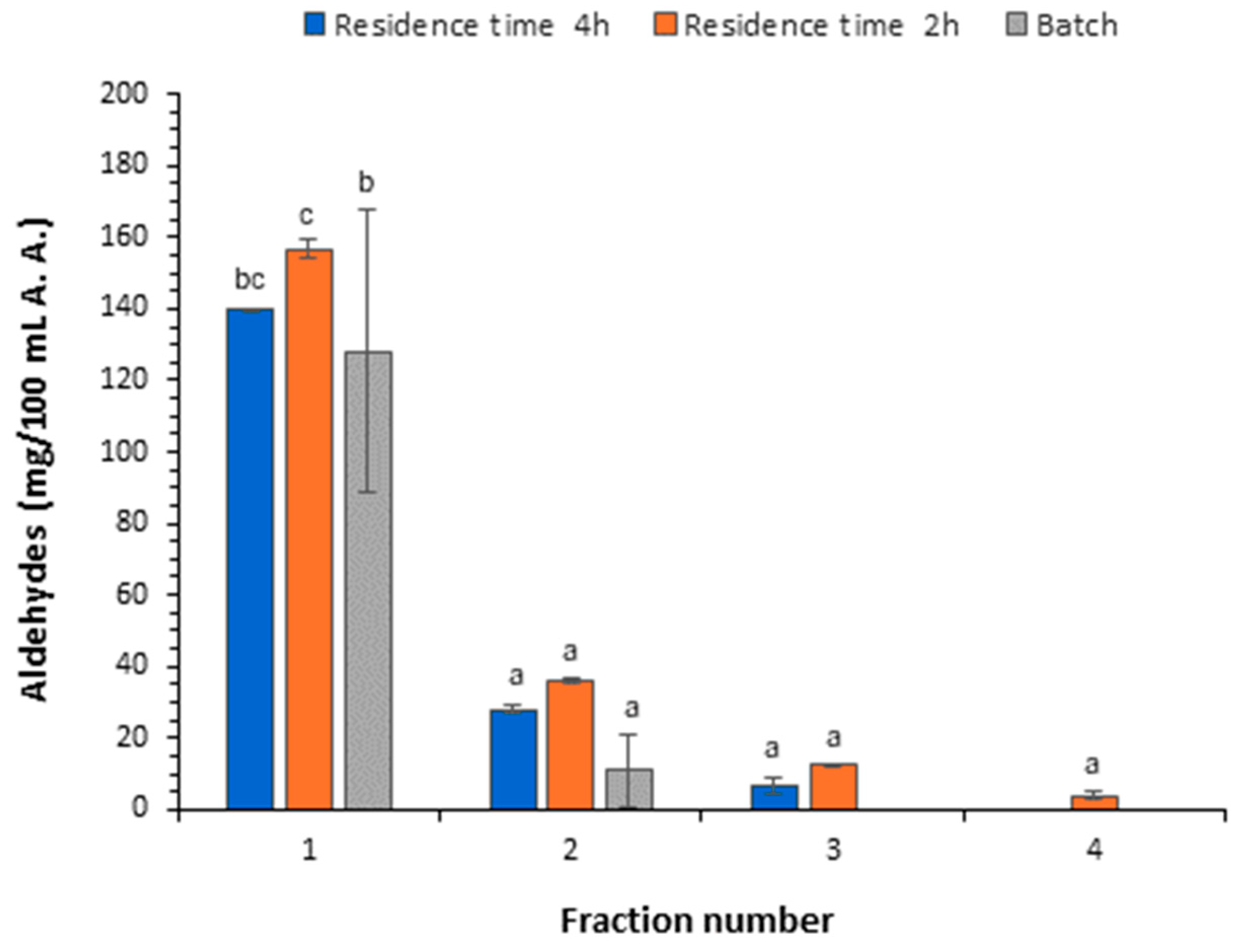
3.3.2. Content of Aldehydes, Esters, and Furfural
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fonseca-Aguiñaga, R.; Gómez-Ruiz, H.; Miguel-Cruz, F.; Romero-Cano, L.A. Analytical Characterization of Tequila (Silver Class) Using Stable Isotope Analyses of C, O and Atomic Absorption as Additional Criteria to Determine Authenticity of Beverage. Food Control 2020, 112, 107161. [Google Scholar] [CrossRef]
- Cedeño, M.C. Tequila Production. Crit. Rev. Biotechnol. 1995, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- NOM-006-SCFI-2012; Bebidas Alcohólicas-Tequila-Especificaciones. Secretaría de Comercio y Fomento Industrial. Diario Oficial de la Federación: Mexico City, México, 2012.
- Villanueva-Rodríguez, S.J.; Rodríguez-Garay, B.; Prado-Ramírez, R.; Gschaedler, A. Tequila: Raw Material, Classification, Process, and Quality Parameters. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 283–289. ISBN 978-0-12-384953-3. [Google Scholar]
- Prado-Jaramillo, N.; Estarrón-Espinosa, M.; Escalona-Buendía, H.; Cosío-Ramírez, R.; Martín-del-Campo, S.T. Volatile Compounds Generation during Different Stages of the Tequila Production Process. A Preliminary Study. LWT Food Sci. Technol. 2015, 61, 471–483. [Google Scholar] [CrossRef]
- Martín-del-Campo, S.T.; López-Ramírez, J.E.; Estarrón-Espinosa, M. Evolution of Volatile Compounds during the Maturation Process of Silver Tequila in New French Oak Barrels. LWT 2019, 115, 108386. [Google Scholar] [CrossRef]
- Peña-Alvarez, A.; Capella, S.; Juárez, R.; Labastida, C. Determination of Terpenes in Tequila by Solid Phase Microextraction-Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2006, 1134, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Zanghelini, G.; Giampaoli, P.; Athès, V.; Vitu, S.; Wilhelm, V.; Esteban-Decloux, M. Charentaise Distillation of Cognac. Part I: Behavior of Aroma Compounds. Food Res. Int. 2024, 178, 113977. [Google Scholar] [CrossRef] [PubMed]
- Sacher, J.; García-Llobodanin, L.; López, F.; Segura, H.; Pérez-Correa, J.R. Dynamic Modeling and Simulation of an Alembic Pear Wine Distillation. Food Bioprod. Process. 2013, 91, 447–456. [Google Scholar] [CrossRef]
- Alemán-Nava, G.S.; Gatti, I.A.; Parra-Saldivar, R.; Dallemand, J.-F.; Rittmann, B.E.; Iqbal, H.M.N. Biotechnological Revalorization of Tequila Waste and By-Product Streams for Cleaner Production—A Review from Bio-Refinery Perspective. J. Clean Prod. 2018, 172, 3713–3720. [Google Scholar] [CrossRef]
- Sahraoui, N.; Vian, M.A.; Bornard, I.; Boutekedjiret, C.; Chemat, F. Improved Microwave Steam Distillation Apparatus for Isolation of Essential Oils: Comparison with Conventional Steam Distillation. J. Chromatogr. A 2008, 1210, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Prado-Ramírez, R. Destilacion. In Ciencia y Tecnología del Tequila: Avances y Perspectivas; CIATEJ: Guadalajara, Mexico, 2015; p. 18230. [Google Scholar]
- Romero-Cano, L.A.; Zárate-Guzmán, A.I.; Nájar-Guzmán, R.; Warren-Vega, W.M.; Campos-Rodríguez, A. Simulation of a Steam Generation Plant Useful in the Tequila Production Process Employing Different Fuels as a Novel Strategy for Environmental Impact Assessment. J. Clean Prod. 2024, 440, 140983. [Google Scholar] [CrossRef]
- Padilla, J.D.; Vega, H.; Alba, A.; Rodríguez, E. Sistema Multifuncional de Destilación, Evaporación y Extracción de Moléculas Orgánicas Derivadas de Productos Naturales; CIATEJ: Guadalajara, Mexico, 2015. [Google Scholar]
- Mirna, E.-E.; Mariela, R.-P.; Daniel, P.l.R.J.; Rogelio, P.-R. Innovation in Continuous Rectification for Tequila Production. Processes 2019, 7, 283. [Google Scholar] [CrossRef]
- NMX-V-013-NORMEX-2019; Bebidas Alcohólicas-Determinación del Contenido Alcohólico (por Ciento de Alcohol en Volumen a 20 °C) (% Alc. Vol.)-Métodos de Ensayo (Prueba). Diario Oficial de la Federación: Mexico City, México, 2020.
- NMX-V-004-NORMEX-2018; Bebidas Alcohólicas-Determinación de Furfural-Métodos de Ensayo (Prueba). Diario Oficial de la Federación: Mexico City, México, 2020.
- NMX-V-005-NORMEX-2018; Bebidas Alcohólicas-Determinación de Aldehídos, Ésteres, Metanol y Alcoholes Superiores-Métodos de Ensayo (Prueba). Diario Oficial de la Federación: Mexico City, México, 2020.
- Nolasco-Cancino, H.; Jarquín Martinez, D.; Ruíz Teran, F.; Santiago-Urbina, J.A. Behavior of volatile compounds regulated by the Mexican c during the distillation of artisanal Mezcal. Rev. De Cienc. Biológicas Y De La Salud 2022, 24. [Google Scholar]
- Einfalt, D.; Rieke-Zapp, J.; Quintanilla Bellucci, A.; Sommerfeld, K.; Schwarz, S.; Lachenmeier, D.W. Methanol Mitigation during Manufacturing of Fruit Spirits with Special Consideration of Novel Coffee Cherry Spirits. Molecules 2021, 26, 2585. [Google Scholar] [CrossRef]
- Resa, J.M.; Goenaga, J.M. Measurement and Modeling of Phase Equilibria for Ethanol + Water + Methanol at Isobaric Condition. J. Chem. Eng. Data 2006, 51, 2114–2120. [Google Scholar] [CrossRef]
- Rodriguez-Donis, I.; Gerbaud, V.; Joulia, X. Thermodynamic Insights on the Feasibility of Homogeneous Batch Extractive Distillation, 1. Azeotropic Mixtures with a Heavy Entrainer. Ind. Eng. Chem. Res. 2009, 48, 3544–3559. [Google Scholar] [CrossRef]




| Compound | Final Distillate, P1 a | Final Distillate, P2 b | NOM-006-SCFI-2012 [3] c Specifications | |
|---|---|---|---|---|
| mg/100 mL of Anhydrous Alcohol | Minimum | Maximum | ||
| Aldehydes | 16.18 | 14.65 | 0 | 40 |
| Methanol | 264.91 | 268.30 | 30 | 300 |
| Esters | 96.22 | 88.46 | 2 | 200 |
| Higher alcohols | 461.76 | 444.82 | 20 | 500 |
| Furfural | 1.76 | 1.80 | 0 | 4 |
| Compound | Fraction 1 | Fraction 2 | Fraction 3 | Fraction 4 |
|---|---|---|---|---|
| Concentration in mg/100 mL of Anhydrous Alcohol | ||||
| Acetaldehyde | 156.57 ± 2.58 c | 36.12 ± 0.78 b | 12.50 ± 0.33 ab | 3.91 ± 1.26 a |
| Ethyl acetate | 785.79 ± 28.25 b | 183.98 ± 5.42 a | 36.84 ± 1.22 a | 12.24 ± 0.52 a |
| Methanol | 201.01 ± 2.49 a | 220.74 ± 1.22 a | 254.17 ± 5.11 b | 310.98 ± 15.44 c |
| 2-Butanol | 39.06 ± 0.45 a | 26.64 ± 0.54 a | 15.65 ± 0.07 a | 8.40 ± 0.19 a |
| 1-Propanol | 36.32 ± 45.52 a | 198.66 ± 0.97 a | 168.72 ± 0.66 a | 136.68 ± 3.30 a |
| 1-Butanol | 1.14 ± 0.01 b | 1.26 ± 0.09 b | 1.03 ± 0.01 ab | 0.48 ± 0.014 a |
| Isoamyl alcohol | 499.66 ± 14.06 b | 455.05 ± 1.98 b | 273.40 ± 0.44 a | 141.29 ± 6.92 a |
| 1-Pentanol | 0.66 ± 0.04 ab | 0.34 ± 0.26 a | 0.68 ± 0.01 b | 1.06 ± 0.11 c |
| Ethyl lactate | 3.59 ± 0.08 a | 13.08 ± 0.01 a | 22.40 ± 0.74 b | 40.28 ± 4.02 c |
| Furfural | 0.74 ± 0.08 a | 1.20 ± 0.01 a | 1.55 ± 0.03 b | 2.41 ± 0.05 c |
| ∑Higher alcohols | 576.74 ± 60.08 b | 681.94 ± 3.84 b | 459.47 ± 1.18 a | 287.90 ± 10.54 a |
| ∑Esters | 789.34 ± 28.33 b | 197.06 ± 5.44 a | 59.24 ± 1.95 a | 52.53 ± 4.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Martínez, H.; Tejeda-Arandas, I.G.; Estarrón-Espinosa, M.; Padilla-de la Rosa, J.D. Solving a Challenge in the Tequila Industry: A New Continuous Rectification Process for Reducing Higher Alcohols and Obtaining Products Within the Official Tequila Standard. ChemEngineering 2025, 9, 59. https://doi.org/10.3390/chemengineering9030059
Flores-Martínez H, Tejeda-Arandas IG, Estarrón-Espinosa M, Padilla-de la Rosa JD. Solving a Challenge in the Tequila Industry: A New Continuous Rectification Process for Reducing Higher Alcohols and Obtaining Products Within the Official Tequila Standard. ChemEngineering. 2025; 9(3):59. https://doi.org/10.3390/chemengineering9030059
Chicago/Turabian StyleFlores-Martínez, Héctor, Isaac Guadalupe Tejeda-Arandas, Mirna Estarrón-Espinosa, and José Daniel Padilla-de la Rosa. 2025. "Solving a Challenge in the Tequila Industry: A New Continuous Rectification Process for Reducing Higher Alcohols and Obtaining Products Within the Official Tequila Standard" ChemEngineering 9, no. 3: 59. https://doi.org/10.3390/chemengineering9030059
APA StyleFlores-Martínez, H., Tejeda-Arandas, I. G., Estarrón-Espinosa, M., & Padilla-de la Rosa, J. D. (2025). Solving a Challenge in the Tequila Industry: A New Continuous Rectification Process for Reducing Higher Alcohols and Obtaining Products Within the Official Tequila Standard. ChemEngineering, 9(3), 59. https://doi.org/10.3390/chemengineering9030059







