Microwave-Mediated Extraction of Critical Metals from LED E-Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Microwave-Mediated Processing of LED E-Waste
2.2. Acid Treatment of the Recovered LED Chips
2.3. Characterizations
3. Results and Discussion
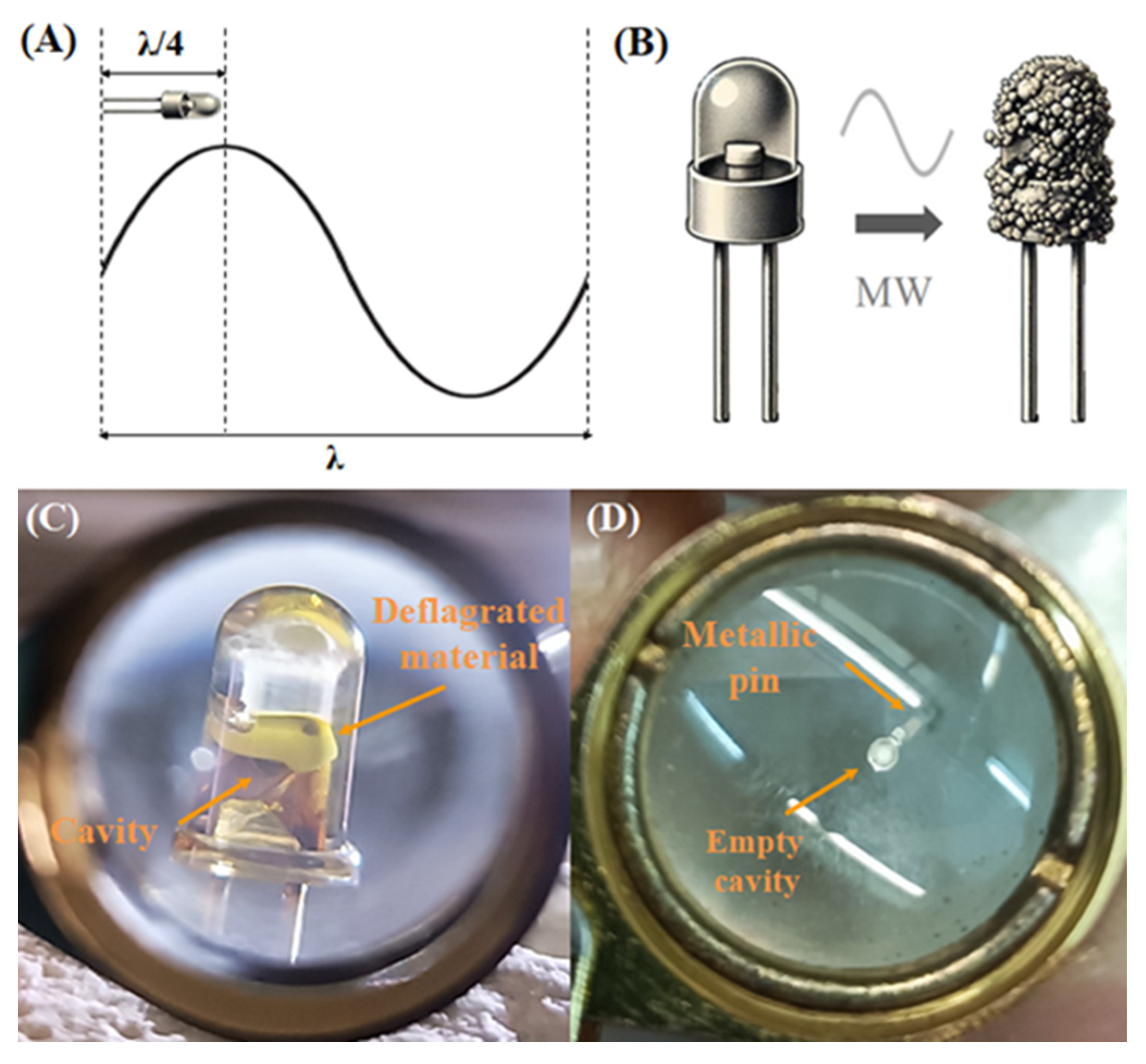
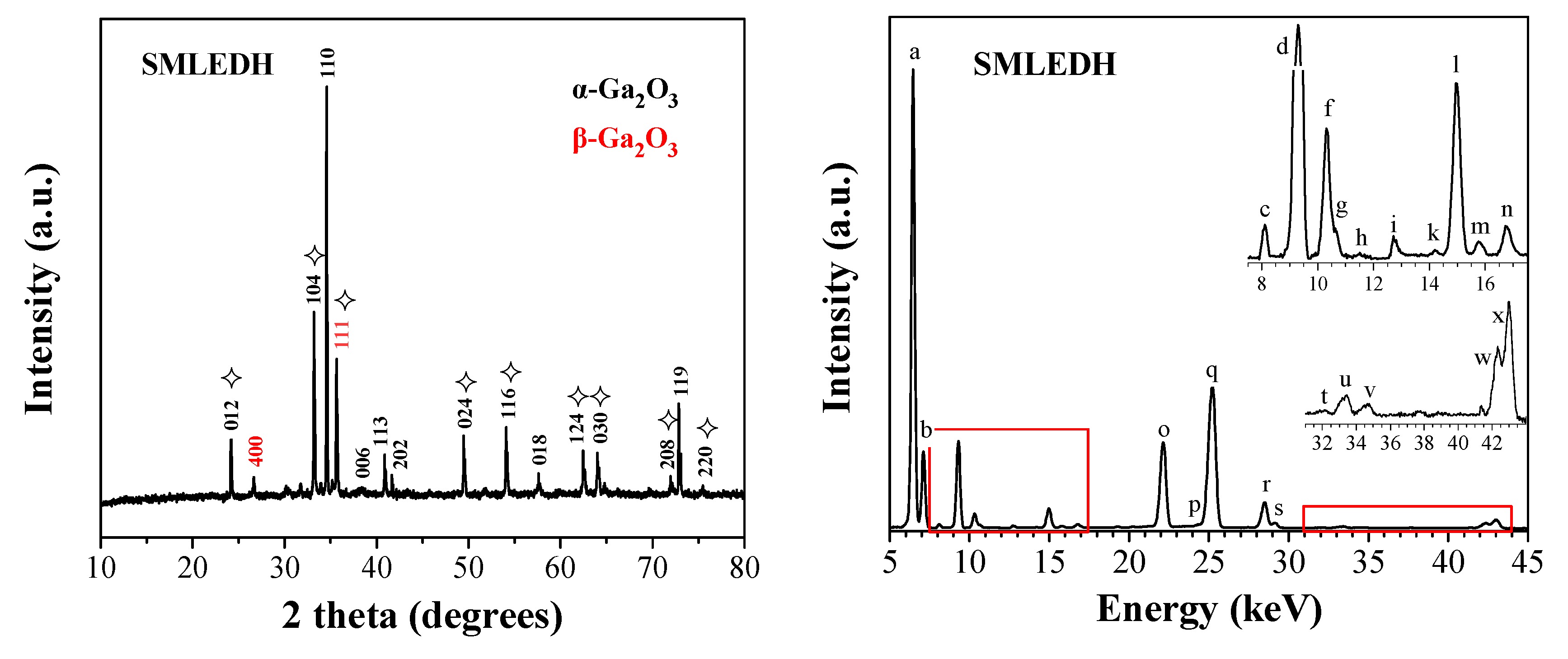
3.1. Microwave Separation Mechanism and Materials Characterization
3.2. Acid Leaching of Critical Metals from LED Chips
3.3. Microwave-Mediated Incineration vs. Direct Incineration
- (i)
- Thermal heating reduces the recovery efficiency within the reflective cavity, as some of the LED chip materials deform, adhere, or become trapped, making the separation and extraction of the chip more difficult. In particular, after conventional heat treatment, the LED chip was only partially recovered (with an efficiency of 45%, determined through mass measurements using a five-digit precision scale), with some material remaining trapped in the cavity (Figure 7). In the case of microwave treatment, the LED chip is 100% recoverable, with a mass remarkably close to the expected value of 0.1 mg per LED, leaving the reflective cavity completely empty (Figure 3D).
- (ii)
- Conventional heat treatment causes severe oxidation of the metallic pins into iron oxides, which compromises their structural integrity and renders them non-conductive (Figure 8). Silver can be detected in small amounts using XRF but not in the XRD in this case. When heated in air at 800 °C, the iron core oxidizes, forming a thick iron oxide layer. This layer, along with the fact that silver is present in small amounts, can completely obscure the detection of silver by XRD. Thus, direct heating is less favorable as it does not fully recover the LED chip material and causes irreversible damage to the metallic pins, ultimately reducing the recovery efficiency of the LED components.
3.4. Scalability, Limitations, and Challenges
4. Conclusions
- Microwave irradiation converts the plastic LED lens into a brittle char and weakens chip-to-metal pin bonds, enabling easy mechanical separation;
- A deflagration-like reaction initiated by the microwaves rapidly decomposes the plastic enclosure and enables the release of the LED chip;
- During microwave treatment, the LED chip becomes embedded in the charred lens residue, aiding its recovery;
- Calcination of the charred material produces a solid material containing critical elements such as Ga, As, In, Y, and Au;
- A two-step acid leaching process (aqua regia followed by hot HCl) efficiently extracts valuable metals;
- The method achieves an extraction efficiency of up to 96%, confirming its high effectiveness;
- The process is rapid and scalable, completing component separation in approximately one minute—making it well-suited for industrial recycling applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dillon, H.E.; Ross, C.; Dzombak, R. Environmental and energy improvements of LED lamps over time: A comparative life cycle assessment. LEUKOS 2019, 16, 229–237. [Google Scholar] [CrossRef]
- Wang, S.; Su, D.; Wu, Y.; Chai, Z. Application of life-cycle assessment to the eco-design of LED lighting products. Euro-Mediterr. J. Environ. Integr. 2020, 5, 41. [Google Scholar] [CrossRef]
- Lim, S.-R.; Kang, D.; Ogunseitan, O.A.; Schoenung, J.M. Potential environmental impacts from the metals in incandescent, compact fluorescent lamp (CFL), and light-emitting diode (LED) bulbs. Environ. Sci. Technol. 2013, 47, 1040–1047. [Google Scholar] [CrossRef]
- Rahman, S.M.M.; Pompidou, S.; Alix, T.; Laratte, B. A review of LED lamp recycling process from the 10 R strategy perspective. Sustain. Prod. Consum. 2021, 28, 1178–1191. [Google Scholar] [CrossRef]
- Wehbie, M.; Semetey, V. Characterization of end-of-life LED lamps: Evaluation of reuse, repair and recycling potential. Waste Manag. 2022, 141, 202–207. [Google Scholar] [CrossRef]
- Mandal, S.; Bakaruddin, B.R.B.; Jeon, S.; Lee, Y.; Kim, K.-W. Assessment of the recycling potential of valuable metals by mapping the elemental composition in discarded light-emitting diodes (LEDs). J. Environ. Manag. 2023, 328, 116900. [Google Scholar] [CrossRef]
- Illés, I.B.; Kékesi, T. The production of high-purity gallium from waste LEDs by combining sulfuric acid digestion, cation-exchange and electrowinning. J. Environ. Chem. Eng. 2023, 11, 110391. [Google Scholar] [CrossRef]
- Ruiz-Mercado, G.J.; Gonzalez, M.A.; Smith, R.L.; Meyer, D.E. A conceptual chemical process for the recycling of Ce, Eu, and Y from LED flat panel displays. Resour. Conserv. Recycl. 2017, 126, 42–49. [Google Scholar] [CrossRef]
- Cenci, M.P.; Dal Berto, F.C.; Castillo, B.W.; Veit, H.M. Precious and critical metals from wasted LED lamps: Characterization and evaluation. Environ. Technol. 2022, 43, 1870–1881. [Google Scholar] [CrossRef]
- Zhu, P.; Ma, Y.; Wang, Y.; Yang, Y.; Qian, G. Separation and recovery of materials from the waste light emitting diode (LED) modules by solvent method. J. Mater. Cycles Waste Manag. 2020, 22, 1184–1195. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, Z.; Zhang, Y.; Xu, Z. Recycling of metals (Ga, In, As and Ag) from waste light-emitting diodes in sub/supercritical ethanol. Resour. Conserv. Recycl. 2020, 155, 104695. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.-S.; Lee, C.G.; Hong, H.S. Recycling process for recovery of gallium from GaN an e-waste of LED industry through ball milling, annealing and leaching. Environ. Res. 2015, 138, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-S.; Hsu, L.-L.; Wang, L.-P. Recycling the GaN waste from LED industry by pressurized leaching method. Metals 2018, 8, 861. [Google Scholar] [CrossRef]
- Annoni, R.; Lange, L.C.; Amaral, M.C.S.; Silva, A.M.; Assunção, M.C.; Franco, M.B.; de Souza, W. Light emitting diode waste: Potential of metals concentration and acid reuse via the integration of leaching and membrane processes. J. Clean. Prod. 2020, 246, 119057. [Google Scholar] [CrossRef]
- Rebello, R.Z.; Lima, M.T.W.D.C.; Yamane, L.H.; Siman, R.R. Characterization of end-of-life LED lamps for the recovery of precious metals and rare earth elements. Resour. Conserv. Recycl. 2020, 153, 104557. [Google Scholar] [CrossRef]
- Zhan, L.; Xia, F.; Xia, Y.; Xie, B. Recycle gallium and arsenic from GaAs-based e-wastes via pyrolysis-vacuum metallurgy separation: Theory and feasibility. ACS Sustain. Chem. Eng. 2018, 6, 1336–1342. [Google Scholar] [CrossRef]
- Li, B.; Tao, R.; Wu, Y.; Zhang, W.; Liu, X.; Wang, W. Study on the pyrolysis characteristics and reaction mechanisms of WLED packaging materials. J. Anal. Appl. Pyrol. 2023, 170, 105935. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Liu, Z.; Ma, C. Recycling of waste printed circuit boards by microwave induced pyrolysis and featured mechanical processing. Ind. Eng. Chem. Res. 2011, 50, 11763–11769. [Google Scholar] [CrossRef]
- Anis, S.; Lestari, S.E. Production of liquid oil from thermolysis of electrical and electronic wastes (e-waste) under microwave irradiation. J. Phys. Sci. 2018, 29, 53–62. [Google Scholar] [CrossRef]
- Anis, S.; Prasetyo, E. Conversion of hand phone case waste into liquid fuels in a microwave reactor. IOP Conf. Ser. Earth Environ. Sci. 2019, 258, 012011. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Pan, M.-W.; Lo, S.-L. Hydrometallurgical metal recovery from waste printed circuit boards pretreated by microwave pyrolysis. Resour. Conserv. Recycl. 2020, 163, 105090. [Google Scholar] [CrossRef]
- Anis, S.; Qudus, N.; Kusumastuti, A. Treatment of electrical and electronic waste to reduce environmental issue. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 72, 148–156. [Google Scholar] [CrossRef]
- Risco, Á.; Sucunza, D.; González-Egido, S. Chemical recovery of waste electrical and electronic equipment by microwave-assisted pyrolysis: A review. J. Anal. Appl. Pyrol. 2021, 159, 105323. [Google Scholar] [CrossRef]
- Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Cheshmeh, Z.A. A comprehensive review on pyrolysis of e-waste and its sustainability. J. Clean. Prod. 2022, 333, 130191. [Google Scholar] [CrossRef]
- Horikoshi, S.; Hachisuga, N.; Serpone, N. Recycling of e-waste power cables using microwave-induced pyrolysis-process characteristics and facile recovery of copper metal. RSC Adv. 2024, 14, 29955–29964. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Spyrou, K.; Moschovas, D.; Avgeropoulos, A.; Salmas, C.; Karakassides, M.A. Fast microwave carbonization of aluminum-coated plastic wastes and perspectives: The examples of CDs and snack bags. Next Mater. 2025, 6, 100333. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Gioti, C.; Karakassides, M.A.; Gournis, D. Hypergolics in Carbon Nanomaterials Synthesis: New Paradigms and Perspectives. Molecules 2020, 25, 2207. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Chalmpes, N.; Giannelis, E.P.; Gournis, D.P.; Moschovas, D.; Avgeropoulos, A.; Salmas, C.; Karakassides, M.A. Total recycling of copper cable scrap and production of carbon using fast microwave technology. Eng 2025, 6, 74. [Google Scholar] [CrossRef]
- Chalmpes, N.; Tantis, I.; Alsmaeil, A.W.; Bourlinos, A.B.; Giannelis, E.P. Design, synthesis, and evaluation of noble metal nanoparticles and in situ-decorated carbon-supported nanoparticle electrocatalysts using hypergolic reactions. Chem. Mater. 2025, 36, 10616–10625. [Google Scholar] [CrossRef]
- Delgado, M.R.; Areán, C.O. Surface chemistry and pore structure of β-Ga2O3. Mater. Lett. 2003, 57, 2292–2297. [Google Scholar] [CrossRef]
- Shi, F.; Qiao, H. Influence of hydrothermal reaction time on crystal qualities and photoluminescence properties of β-Ga2O3 nanorods. J. Mater. Sci. Mater. Electron. 2020, 31, 20223–20231. [Google Scholar] [CrossRef]
- Meitei, S.R.; Devi, L.S.; Singh, N.K. Nanocrystalline β-Ga2O3 thin film prepared by electron beam evaporation for enhanced photodetection. J. Phys. D Appl. Phys. 2023, 56, 395104. [Google Scholar] [CrossRef]
- Bioud, Y.A.; Boucherif, A.; Belarouci, A.; Paradis, E.; Drouin, D.; Arès, R. Chemical composition of nanoporous layer formed by electrochemical etching of p-type GaAs. Nanoscale Res. Lett. 2016, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Verma, Y.; Rana, S.V.S. Hepatotoxic manifestations of arsenic trioxide loaded poly (lactide-coglycolide) nanoparticles in wistar rat. J. Anesth. Pain Med. 2024, 9, 1–9. [Google Scholar]
- El-Faramawy, N.; Gad, A.; Alazab, H.A.; Farouk, S. Thermoluminescence assessment of quartz veins emplaced in Talc-Schist Metamorphic Rocks, Egypt. J. Mater. Res. 2022, 37, 3784–3791. [Google Scholar] [CrossRef]
- Chen, W.-T.; Tsai, L.-C.; Tsai, F.-C.; Shu, C.-M. Recovery of gallium and arsenic from gallium arsenide waste in the electronics industry. Clean-Soil Air Water 2012, 40, 531–537. [Google Scholar] [CrossRef]
- de Oliveira, R.P.; Benvenuti, J.; Espinosa, D.C.R. A review of the current progress in recycling technologies for gallium and rare earth elements from light-emitting diodes. Renew. Sustain. Energy Rev. 2021, 145, 111090. [Google Scholar] [CrossRef]
- Vinhal, J.T.; de Oliveira, R.P.; Coleti, J.L.; Espinosa, D.C.R. Characterization of end-of-life LEDs: Mapping critical, valuable and hazardous elements in different devices. Waste Manag. 2022, 151, 113–122. [Google Scholar] [CrossRef]
- Balinski, A.; Recksiek, V.; Stoll, M.; Christesen, C.; Stelter, M. Liberation and separation of valuable components from LED modules: Presentation of two innovative approaches. Recycling 2022, 7, 26. [Google Scholar] [CrossRef]
- Illés, I.B.; Kékesi, T. A comprehensive aqueous processing of waste LED light bulbs to recover valuable metals and compounds. Sustain. Mater. Technol. 2023, 35, e00572. [Google Scholar] [CrossRef]
- Lavalley, J.C.; Daturi, M.; Montouillout, V.; Clet, G.; Areán, C.O.; Delgado, M.R.; Sahibed-dine, A. Unexpected similarities between the surface chemistry of cubic and hexagonal gallia polymorphs. Phys. Chem. Chem. Phys. 2003, 5, 1301–1305. [Google Scholar] [CrossRef]
- Chiang, J.-L.; Yadlapalli, B.K.; Chen, M.-I.; Wuu, D.-S. A review on gallium oxide materials from solution processes. Nanomaterials 2022, 12, 3601. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Saraswat, H.; Chaudhary, S.; Parkash, J.; Shin, H.-J.; Chae, K.-H.; Won, S.-O. Nano-structured phases of gallium oxide (GaOOH, α-Ga2O3, β-Ga2O3, γ-Ga2O3, δ-Ga2O3, and ε-Ga2O3): Fabrication, structural, and electronic structure investigations. Int. Nano Lett. 2020, 10, 71–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Li, Q.; Qu, C.; Zhang, L.; Xie, W. Optical and structural properties of Cu-doped β-Ga2O3 films. Mater. Sci. Eng. B 2011, 176, 846–849. [Google Scholar] [CrossRef]






| Element | Elemental Concentrations (% w/w) 1 | Leaching Efficiencies (%) 2 | ||||
|---|---|---|---|---|---|---|
| PNLED | SMLED | SMLEDH | SMLEDHH | SMLEDH | SMLEDHH | |
| Fe | 61.0 | 26.7 | 36.8 | 7 | 100 | |
| Ni | 37.0 | |||||
| Cu | 10.8 | 0.3 | 98 | 100 | ||
| Ga | 2.5 | 3.4 | 6.0 | 9 | 91 | |
| As | 1.3 | 327 * | 98 | 100 | ||
| Sr | 406 * | 92 * | 704 * | 85 | 93 | |
| Y | 0.24 | 0.32 | 10 | 100 | ||
| Zr | 202 * | 161 * | 0.1 | 46 | 81 | |
| Ru | 269 * | 138 * | 65 | 100 | ||
| Rh | 39 * | 100 | ||||
| Ag | 1.0 | 1.5 | 0.6 | 614 * | 73 | 96 |
| In | 570 * | 142 * | 83 | 100 | ||
| Sn | 2.0 | 1.3 | 0.5 | 59 | 99 | |
| Ba | 185 * | 40 * | 85 | 100 | ||
| La | 140 * | 158 * | 994 * | 24 | 73 | |
| Ce | 224 * | 265 * | 20 | 100 | ||
| Gd | 344 * | 567 * | 0 | 100 | ||
| Au | 1.0 | 1.5 | 100 | 100 | ||
| Pb | 426 * | 403 * | 443 * | 36 | 96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourlinos, A.B.; Papachristodoulou, C.; Markou, A.; Chalmpes, N.; Giannelis, E.P.; Gournis, D.P.; Salmas, C.E.; Karakassides, M.A. Microwave-Mediated Extraction of Critical Metals from LED E-Waste. ChemEngineering 2025, 9, 47. https://doi.org/10.3390/chemengineering9030047
Bourlinos AB, Papachristodoulou C, Markou A, Chalmpes N, Giannelis EP, Gournis DP, Salmas CE, Karakassides MA. Microwave-Mediated Extraction of Critical Metals from LED E-Waste. ChemEngineering. 2025; 9(3):47. https://doi.org/10.3390/chemengineering9030047
Chicago/Turabian StyleBourlinos, Athanasios B., Christina Papachristodoulou, Anastasios Markou, Nikolaos Chalmpes, Emmanuel P. Giannelis, Dimitrios P. Gournis, Constantinos E. Salmas, and Michael A. Karakassides. 2025. "Microwave-Mediated Extraction of Critical Metals from LED E-Waste" ChemEngineering 9, no. 3: 47. https://doi.org/10.3390/chemengineering9030047
APA StyleBourlinos, A. B., Papachristodoulou, C., Markou, A., Chalmpes, N., Giannelis, E. P., Gournis, D. P., Salmas, C. E., & Karakassides, M. A. (2025). Microwave-Mediated Extraction of Critical Metals from LED E-Waste. ChemEngineering, 9(3), 47. https://doi.org/10.3390/chemengineering9030047










