Research of the Process of Obtaining Monocalcium Phosphate from Unconditional Phosphate Raw Materials
Abstract
1. Introduction
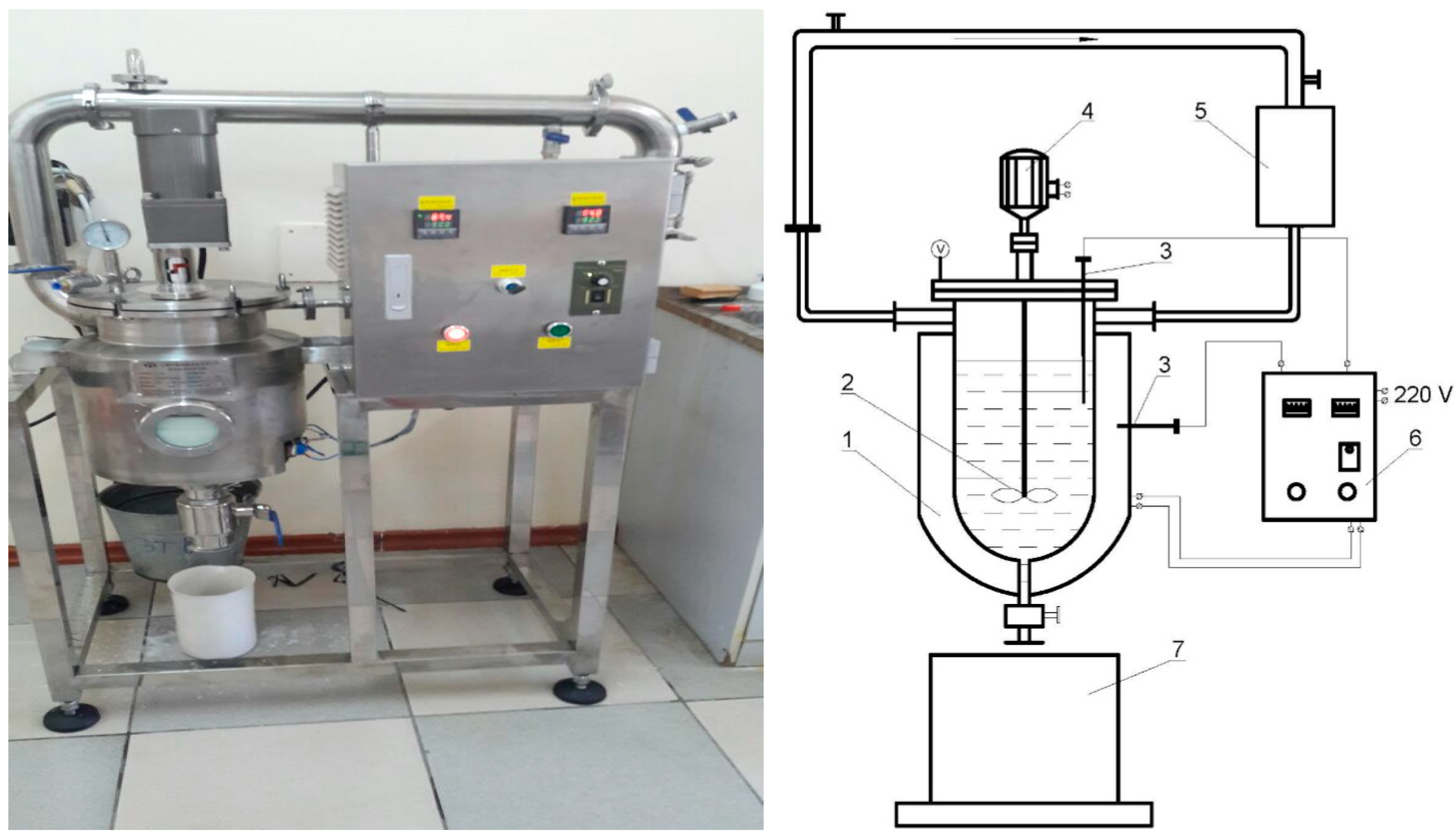
2. Research Methodology
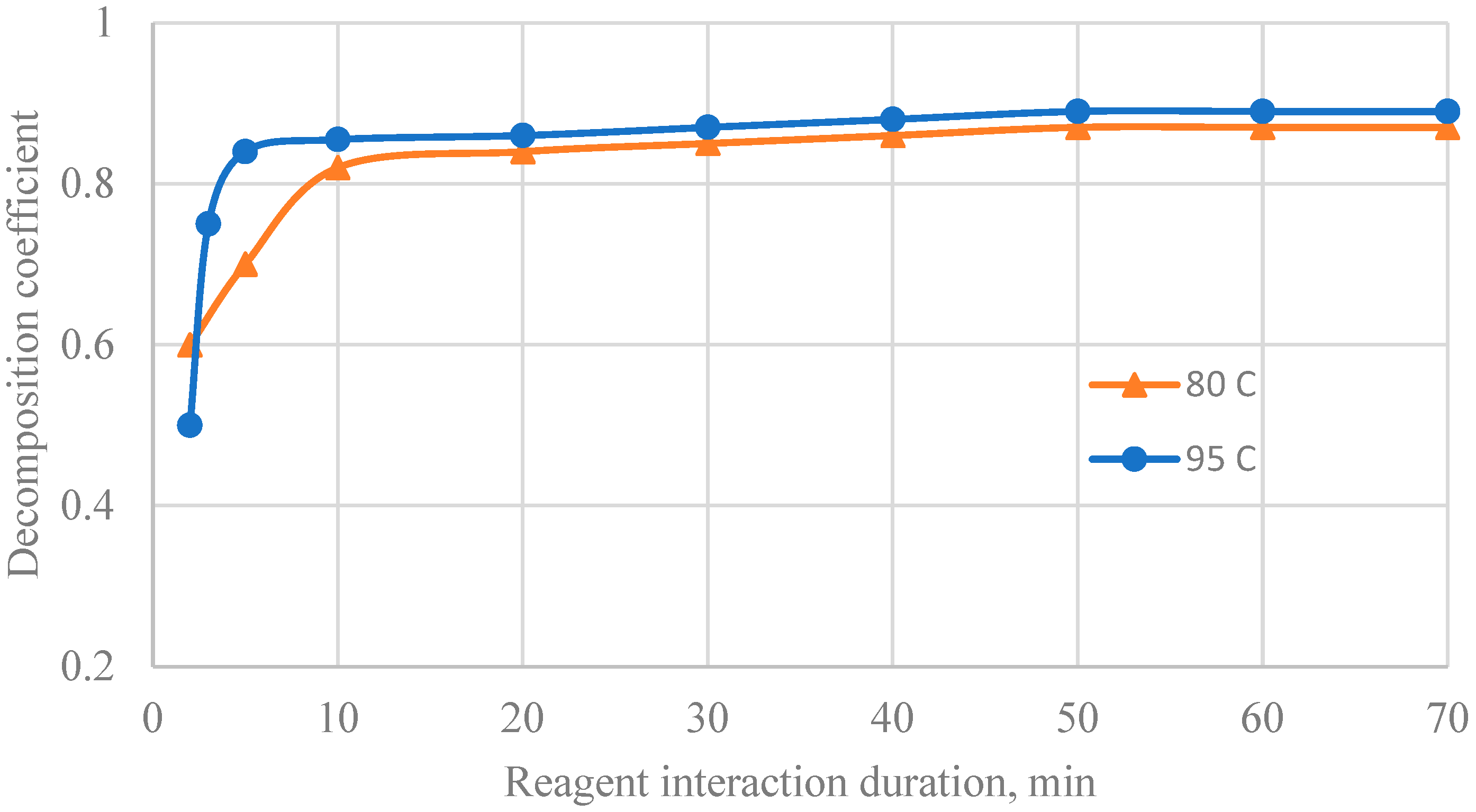
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karatau phosphorite basin. In Kazakhstan: National Encyclopedia; Kazakh Encyclopedia: Almaty, Kazakhstan, 2005; Volume III, ISBN 9965-9389-8-9.
- Petrov, V.P. Karatau phosphorite-bearing basin. In Great Soviet Encyclopedia. Volume 11. Italy-Kvarkush; Soviet Encyclopedia: Moscow, Russia, 1973; p. 608. [Google Scholar]
- Kozlovsky, E.A. (Ed.) Karatau phosphorite-bearing basin. In Mining Encyclopedia; Soviet Encyclopedia: Moscow, Russia, 1985; Volume 2, p. 575. [Google Scholar]
- Shubin, A.P. Phosphate Ores of Kazakhstan; Science: Alma-Ata, Kazakhstan, 1990; p. 320. [Google Scholar]
- Eganov, E.A.; Sovetov, Y.K.; Yanshin, A.L. Proterozoic and Cambrian phosphorites deposits: Karatau, southern Kazakhstan, USSR. In Phosphate Deposits of the World, 1st ed.; Cook, P.J., Shergold, J.H., Eds.; Cambridge University Press: Cambridge, UK, 2005; Volume 1, pp. 175–189. ISBN 0521619211. [Google Scholar]
- Klassen, P.V.; Samigullina, L.I.; Kharitonov, A.B.; Zadko, N.I.; Kuznetsova, G.G. Enrichment and Processing of the Chilisay Deposit Phosphorites; Review Information. Series “Mineral fertilizers and sulfuric acid”; NIITEKHIM: Moscow, Russia, 1988. [Google Scholar]
- Levin, B.V.; Dovidenko, V.V.; Suschev, S.V.; Rakcheeva, L.V.; Kuzmicheva, T.N. Relevance and practical steps for involving low-grade phosphate raw materials in processing into complex fertilizers. Chem. Ind. Dev. 2006, 11, 11–18. [Google Scholar]
- Lygach, A.V.; Ignatkina, V.A. Flotation enrichment of poor nodular phosphorite ores. In Proceedings of the International Conference “Resource Conservation and Environmental Protection in the Enrichment and Processing of Mineral Raw Materials”, Saint Petersburg, Russia, 26 September 2016; pp. 529–532. [Google Scholar]
- Perkovich, S.G.; Kaitmazova, T.I.; Protasov, V.D.; Brazhnik, I.S. Method for Enriching Phosphate Ores. Patent 1585004, IPCB03B7/00, 25 April 1987. [Google Scholar]
- Lygach, A.V. Development of Technology for Complex Enrichment of Nodule Phosphorites Using Multifunctional Reagents; National University of Science and Technology «MISIS»: Moscow, Russia, 2019. [Google Scholar]
- Bazhina, N.L.; Ondar, E.E.; Deryabina, Y.M. Specificity of light absorption in the visible and ultraviolet spectral range by humic acids. Bull. Orenbg. State Univ. 2014, 6, 189–194. [Google Scholar]
- Khil’ko, S.L.; Taperko, G.V.; Rogatko, M.I. Interaction of modified humic acids salts with transition metal salts. Vestn. NovSU. Issue Eng. Sci. 2021, 4, 68–71. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, P.; Wang, Y.; Peng, W.; Ren, Z.; Li, Y.; Chu, B.; Zhu, Q. Research progress on synthesis of zeolites from coal fly ash and environmental applications. Front. Environ. Sci. Eng. 2023, 17, 149. [Google Scholar] [CrossRef]
- Lucakova, M.; Kolajova, R. Dissociation ability of humic acids: Spectroscopic determination of pKa and comparison with multi-step mechanism. React. Funct. Polym. 2014, 78, 1–6. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, Q.; Xiong, X.; Zhang, Y.; Wang, M.; Liu, H. Carbon footprint and embodied carbon emission transfer network obtained using the multi–regional input–output model and social network analysis method: A case of the Hanjiang River basin, China. Front. Ecol. Evol. 2022, 10, 941520. [Google Scholar] [CrossRef]
- Dyachenko, E.N.; Shevelev, A.T. Influence of the aftereffect of mineral and lime fertilizers on the yield and grain quality of spring wheat in the Baikal region. Agrochem. Bull. 2020, 3, 45–48. [Google Scholar]
- Bezuglova, O.S.; Polienko, E.A.; Gorovtsov, A.V. Humic preparations as growth stimulants for plants and microorganisms. News Univ. 2016, 2, 11. [Google Scholar]
- Lygach, A.V.; Ignatkina, V.A.; Lygach, V.N.; Makavetskos, A.R. Study of the material composition of phosphorites of the Egoryevsk deposit. In Proceedings of XI Congress of CIS Countries; Moscow, Russia, 2017; pp. 305–309. [Google Scholar]
- Unece. Available online: https://unece.org/sites/default/files/2021-03/3.1_Kazakhstan_Mustafina_0.pdf (accessed on 4 February 2025).
- Mining Industry. Central Asia. Available online: https://dprom.kz/pererabotka/tyehnogyenniye-meenyeralniye-obrazovaneeya-rk/ (accessed on 25 October 2023).
- Nurpeisova, M.B.; Yestemesov, Z.A.; Bekbasarov, S.S. Waste recycling is one of the key areas of business development. In Collection of Works of ISC “Innovative Technologies in Geoinformation Digital Engineering”; KazNITU: Almaty, Kazakhstan, 2022; pp. 191–198. [Google Scholar]
- Online Student Library. Available online: https://studbooks.net/781040/ekonomika/analiz_obrazovaniya_nakopleniya_promyshlennyh_othodov_regionov (accessed on 30 October 2023).
- Pozin, M.E. Technology of Mineral Salts; Chemistry: Leningrad, Russia, 1990; p. 156. [Google Scholar]
- Selivanovskaya, S.Y.; Latypova, V.Z. On the issue of experimental assessment of waste toxicity class. Ecol. Expert Rev. Inf. 2001, 1, 67–74. [Google Scholar]
- Tleuova, S.T.; Zhuldyzbayeva, S.Y.; Tleuov, A.S.; Sikhymbayeva, Z. Waste-free Technology. In StudyGuide; Nuray Print Service: Almaty, Kazakhstan, 2015; p. 195. [Google Scholar]
- Arbuzov, S.I.; Rikhvanov, L.P.; Volostnov, A.V.; Ershov, V.V.; Baranovskaya, N.V.; Arkhangelskaya, T.A.; Mezhibor, A.M. Comprehensive Geochemical Assessment of Coals and Coal-Bearing Rocks of the Chalpan Section of the Bey Coal Deposit; Research Report: Tomsk, Russia, 2001. [Google Scholar]
- Yazikov, E.G.; Rikhvanov, L.P.; Arkhangelsky, V.V. Assessment of the Toxicity Class of Industrial Waste of JSC Teiskoe Mine Administration; Report on economic operations No.54 dated 27.04.1999; MGPEcogeos: Tomsk, Russia, 2001. [Google Scholar]
- Unified Ecological Internet Resource of the Ministry of Ecology and Natural Resources of the Republic of Kazakhstan. Available online: https://ecogosfond.kz/wp-content/uploads/2018/03/NDSOS_2011-2014.pdf (accessed on 29 October 2023).
- Adilet. Legal Information System of Regulatory Legal Acts of the Republic of Kazakhstan. Available online: https://adilet.zan.kz/rus/docs/U1300000577 (accessed on 15 August 2023).
- Scientific Articles Kazakhstan. Available online: https://articlekz.com/article/27891 (accessed on 15 August 2023).
- Online Student Library. Available online: https://studbooks.net/2538980/tovarovedenie/pererabotka_othodov_obrazuyuschihsya_protsesse_polucheniya_fosfornoy_kisloty (accessed on 20 August 2023).
- Tleuova, S.; Tileuberdi, A.; Pazylova, D.; Ulbekova, M.; Sagyndykova, N.; Lavrov, B.; Turishbekov, Z. Investigation of theProcess of Agglomeration of Phosphorites Using Phosphate-Siliceous Shales and Oil Sludge. Open Chem Eng. J. 2024, 18, e18741231331231. [Google Scholar] [CrossRef]
- Ibiyev, G.Z.; Savoskina, O.A.; Chebanenko, S.I. The global market of mineral fertilizers and its impact on the grain industry. Agric. Econ. Kazakhstan 2021, 12, 97–102. [Google Scholar] [CrossRef]
- Sergeev, I.B.; Ponomarenko, T.V. Strategies of mineral and chemical companies in the context of the modern global market. J. Sib. Fed. Univ. Humanit. Soc. Sci. 2015, 5, 958–971. [Google Scholar]
- Falina, N.V.; Dyukarev, D.O. The global market of mineral fertilizers. Economics 2016, 1, 83–86. [Google Scholar]
- Bogachev, A.I.; Dorofeeva, L.N. Russian market of mineral fertilizers: Features of functioning in new realities and development metamorphoses. Bull. Agrar. Sci. 2022, 3, 78–92. [Google Scholar] [CrossRef]
- Kruchinina, V.M.; Ryzhkova, S.M. Fertilizer market in Russia: State and directions of development. Bull. Voronezh State Univ. Eng. Technol. 2021, 1, 375–384. [Google Scholar] [CrossRef]
- Ilkiv, N. Russian market of mineral fertilizers. AgroForum 2021, 7, 44–48. [Google Scholar]
- IFASTAT. Available online: https://api.ifastat.org/reports/download/13140 (accessed on 16 November 2023).
- World Integrated Trade Solution. Available online: https://wits.worldbank.org/trade/comtrade/en/country/ALL/year/2021/tradeflow/Exports/ (accessed on 16 November 2023).
- Beysenbayev, O.K.; Ahmedov, U.K.; Issa, A.B.; Smailov, B.M.; Esirkepova, M.M.; Artykova, Z.K. Receiving and research of the mechanism of capsulation of superphosphate and double superphosphate for giving of strength properties. News Natl. Acad. Sci. Repub. Kazakhstan 2019, 6, 36–45. [Google Scholar] [CrossRef]
- Artykova, Z.K.; Beisenbayev, O.K.; Kadyrov, A.A.; Sakibayeva, S.A.; Smailov, B.M. Synthesis and preparation of polyacrylonitrile and vinyl sulfonic acid in the presence of gossypol resin for drilling fluids. Rasayan J. Chem. 2023, 16, 2313–2320. [Google Scholar] [CrossRef]
- Kratochvílová, R.; Kráčalík, M.; Smilková, M.; Sedláček, P.; Pekař, M.; Bradt, E.; Smilek, J.; Závodská, P.; Klučáková, M. Functional Hydrogels for Agricultural Application. Gels 2023, 9, 590. [Google Scholar] [CrossRef]
- Timilsena, Y.; Haque, M.; Adhikari, B. in the food industry: A brief historical overview to recent developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Jarosiewicz, A.; Tomaszewska, M. Controlled release NPK fertilizer encapsulated by polymeric membranes. J. Agric. Food Chem. 2003, 51, 413–417. [Google Scholar] [CrossRef]
- Smailov, B.M.; Aravind, U.; Zakirov, B.S.; Azimov, A.M.; Tleuov, A.S.; Beisenbayev, O.K.; Aimenov, Z.T.; Issabayev, N.N. Technology for obtaining chelated organic and mineral microfertilizers based on humate-containing components. Rasayan J. Chem. 2023, 16, 428–433. [Google Scholar] [CrossRef]
- Smailov, B.M.; Beisenbayev, O.K.; Tleuov, A.S.; Kadirbaeva, A.A.; Zakirov, B.S.; Mirzoyev, B. Production of chelate polymer-containing microfertilizers based on humic acid and ammophos. Rasayan J. Chem. 2020, 13, 1372–1378. [Google Scholar] [CrossRef]
- Lipin, A.G.; Nebukin, V.O.; Lipin, A.A. The encapsulation of granules in a polymer shells as a method of creation of mineral fertilizers with controlled speed of liberation of nutrients. Mod. High Technol. 2017, 3, 86–91. [Google Scholar]
- Temirov, U.S.; Namazov, S.S.; Usanbayev, N.K. Intensive technology for processing bird litter in organic and mineral fertilizers. Chem. Tech. 2020, 63, 85–94. [Google Scholar]
- Raiymbekov, Y.; Besterekov, U.; Abdurazova, P.; Nazarbek, U. Review of methods and technologies for the enrichment of low-grade phosphorites. Rev. Inorg. Chem. 2022, 42, 385–395. [Google Scholar] [CrossRef]
- Lv, L.; Zheng, D.; Tang, S.; Zhang, T.; Liu, W. Phosphate ore Particles Dissolution Kinetics in Hydrochloric Acid Based on a Structure-Related Segmented Model. Powder Technol. 2021, 392, 141–149. [Google Scholar] [CrossRef]
- ST RK 2213-2012; Raw Crushed Phosphate Karatau. Technical Conditions. National standard of the Republic of Kazakhstan. Available online: https://online.zakon.kz/Document/?doc_id=38663943 (accessed on 26 March 2025).
- GOST 8269.1-97; Crushed Stone and Gravel from Dense Rocks and Industrial Waste for ConstructionWorks. Methods of Chemical Analysis. International Standard. Available online: https://online.zakon.kz/Document/?doc_id=1008634 (accessed on 26 March 2025).
- Efimov, A.I.; Belorukova, L.P.; Vasilkova, I.V.; Chechev, V.P. PropertiesofInorganicCompounds, Handbook; Chemistry: Leningrad, Russia, 1983; p. 392. [Google Scholar]
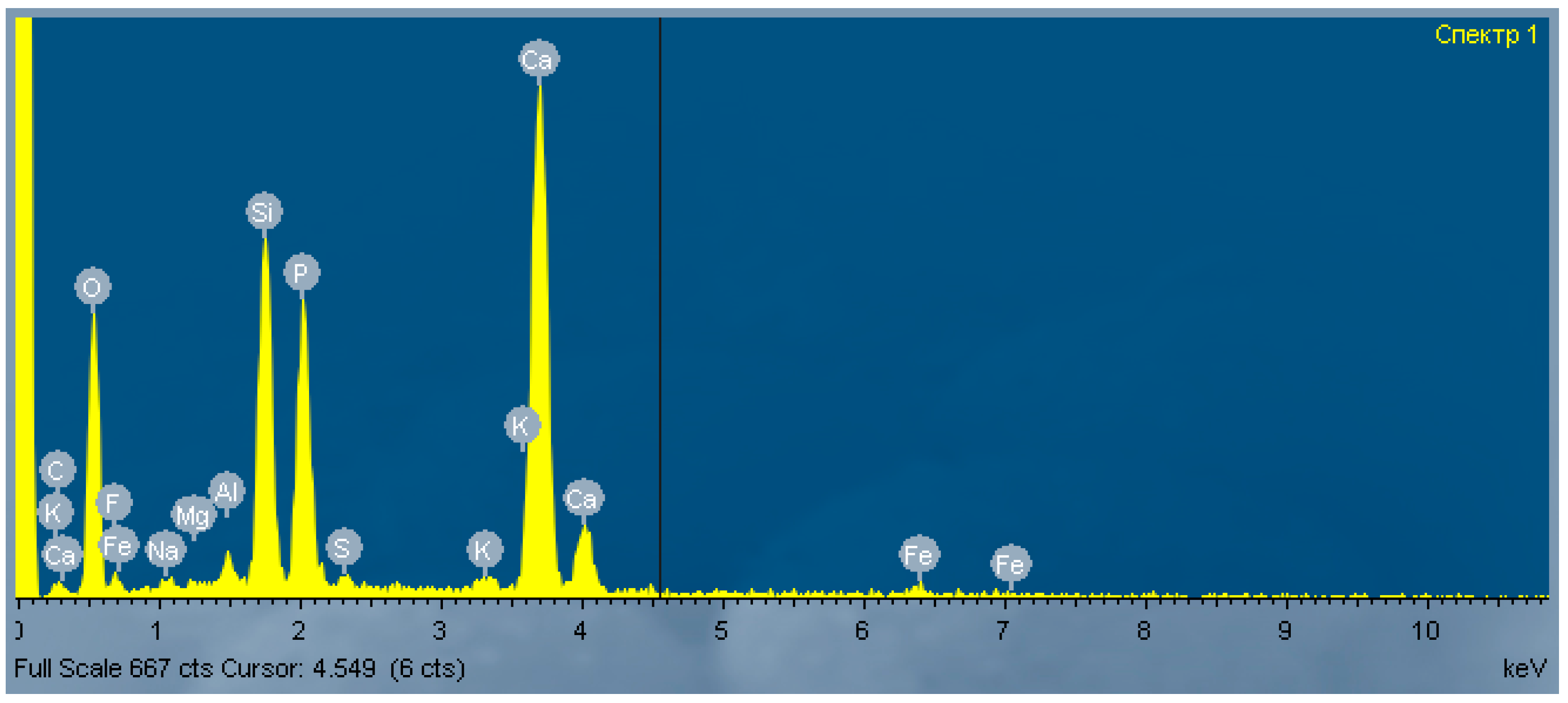
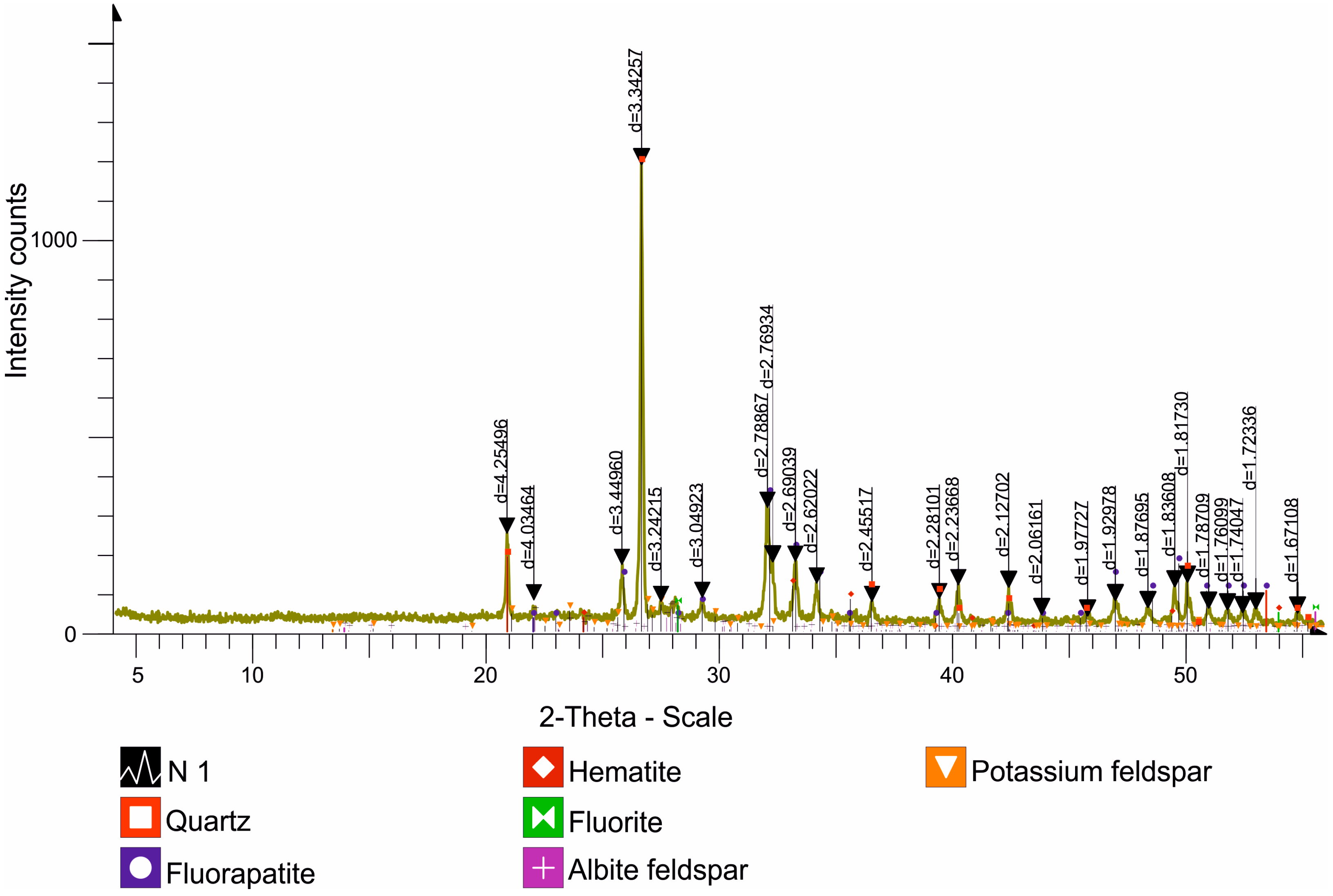
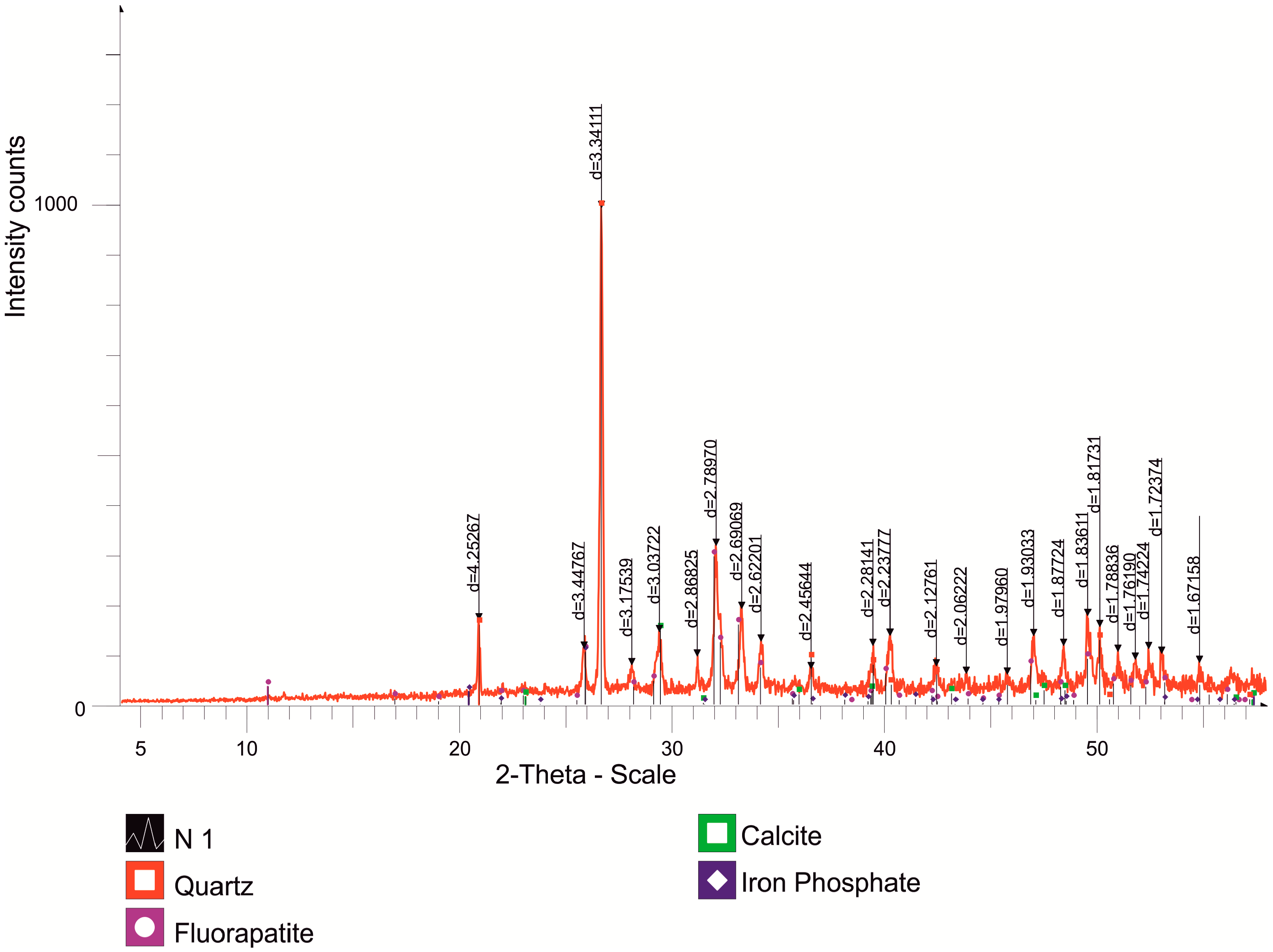



| O | F | Na | Mg | Al | Si | P | S | K | Ca | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectrum 1 | 43.11 | 2.12 | 0.64 | 0.45 | 0.94 | 11.88 | 8.71 | 1.26 | 0.59 | 28.59 | 1.71 |
| Spectrum 2 | 44.14 | 1.98 | 0.86 | 0.48 | 1.01 | 9.50 | 10.98 | 1.89 | 0.45 | 27.30 | 1.41 |
| Spectrum 3 | 44.78 | 2.29 | 0.48 | 0.43 | 1.18 | 10.73 | 9.02 | 0.97 | 0.73 | 27.51 | 1.88 |
| Average | 44.01 | 2.13 | 0.66 | 0.45 | 1.04 | 10.70 | 9.57 | 1.37 | 0.59 | 27.80 | 1.67 |
| Standard deviation | 0.81 | 0.64 | 0.18 | 0.08 | 0.12 | 0.61 | 0.49 | 0.14 | 0.17 | 0.71 | 0.31 |
| Maximum | 44.78 | 2.29 | 0.86 | 0.48 | 1.18 | 11.88 | 10.98 | 1.89 | 0.73 | 28.59 | 1.88 |
| Minimum | 43.11 | 1.98 | 0.48 | 0.43 | 0.94 | 9.50 | 8.71 | 0.97 | 0.45 | 27.30 | 1.41 |
| Name | Formula | Concentration, % |
|---|---|---|
| Quartz | SiO2 | 22.9 |
| Fluorapatite | (CaF)Ca4(PO4)3/CaF2·3Ca3(PO4)2 | 36.7 |
| Hematite | Fe2O3 | 2.1 |
| Fluorite | CaF2 | 5.7 |
| Albite feldspar | Na(AlSi3O8) | 4.4 |
| Potassium feldspar | KAlSi3O8 | 3.9 |
| Calcite | CaCO3 | 23.9 |
| Name | Formula | Concentration, % |
|---|---|---|
| Quartz | SiO2 | 49.3 |
| Fluorapatite | Ca5(PO4)3F | 36.7 |
| Calcite | CaCO3 | 7.4 |
| Iron Phosphate | FePO4 | 6.6 |
| No. | Component | Content, % | Mineral |
|---|---|---|---|
| 1 | P2O5 | 19.96 | Phosphorite |
| 2 | CaO | 34.06 | Feldspar |
| 3 | MgO | 0.71 | Hydromica |
| 4 | Na2O | 0.85 | Glauconite |
| 5 | Al2O3 | 1.56 | Glauconite |
| 6 | Fe2O3 | 2.32 | Glauconite |
| 7 | SO3 | 2.25 | Pyrite, goethite |
| 8 | CO2 | 14.36 | Kurskite |
| 9 | F | 2.13 | Fluorite |
| 10 | K2O | 0.76 | Glauconite |
| 11 | SiO2 | 21.04 | Kurskite |
| Element | Percent by Weight |
|---|---|
| C | 0.02 |
| O | 57.97 |
| P | 24.00 |
| Ca | 18.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anarbayev, A.; Kabylbekova, B.; Khussanov, Z.; Smailov, B.; Anarbaev, N.; Kulikov, Y. Research of the Process of Obtaining Monocalcium Phosphate from Unconditional Phosphate Raw Materials. ChemEngineering 2025, 9, 39. https://doi.org/10.3390/chemengineering9020039
Anarbayev A, Kabylbekova B, Khussanov Z, Smailov B, Anarbaev N, Kulikov Y. Research of the Process of Obtaining Monocalcium Phosphate from Unconditional Phosphate Raw Materials. ChemEngineering. 2025; 9(2):39. https://doi.org/10.3390/chemengineering9020039
Chicago/Turabian StyleAnarbayev, Abibulla, Balzhan Kabylbekova, Zhakhongir Khussanov, Bakyt Smailov, Nurlan Anarbaev, and Yevgeniy Kulikov. 2025. "Research of the Process of Obtaining Monocalcium Phosphate from Unconditional Phosphate Raw Materials" ChemEngineering 9, no. 2: 39. https://doi.org/10.3390/chemengineering9020039
APA StyleAnarbayev, A., Kabylbekova, B., Khussanov, Z., Smailov, B., Anarbaev, N., & Kulikov, Y. (2025). Research of the Process of Obtaining Monocalcium Phosphate from Unconditional Phosphate Raw Materials. ChemEngineering, 9(2), 39. https://doi.org/10.3390/chemengineering9020039







