Plasma Modification of Technical Carbon with Nitrogen and Sulfur-Containing Functional Groups for Application in Catalytic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterization of the Materials
2.3. Electrochemical Experiments
3. Results and Discussions
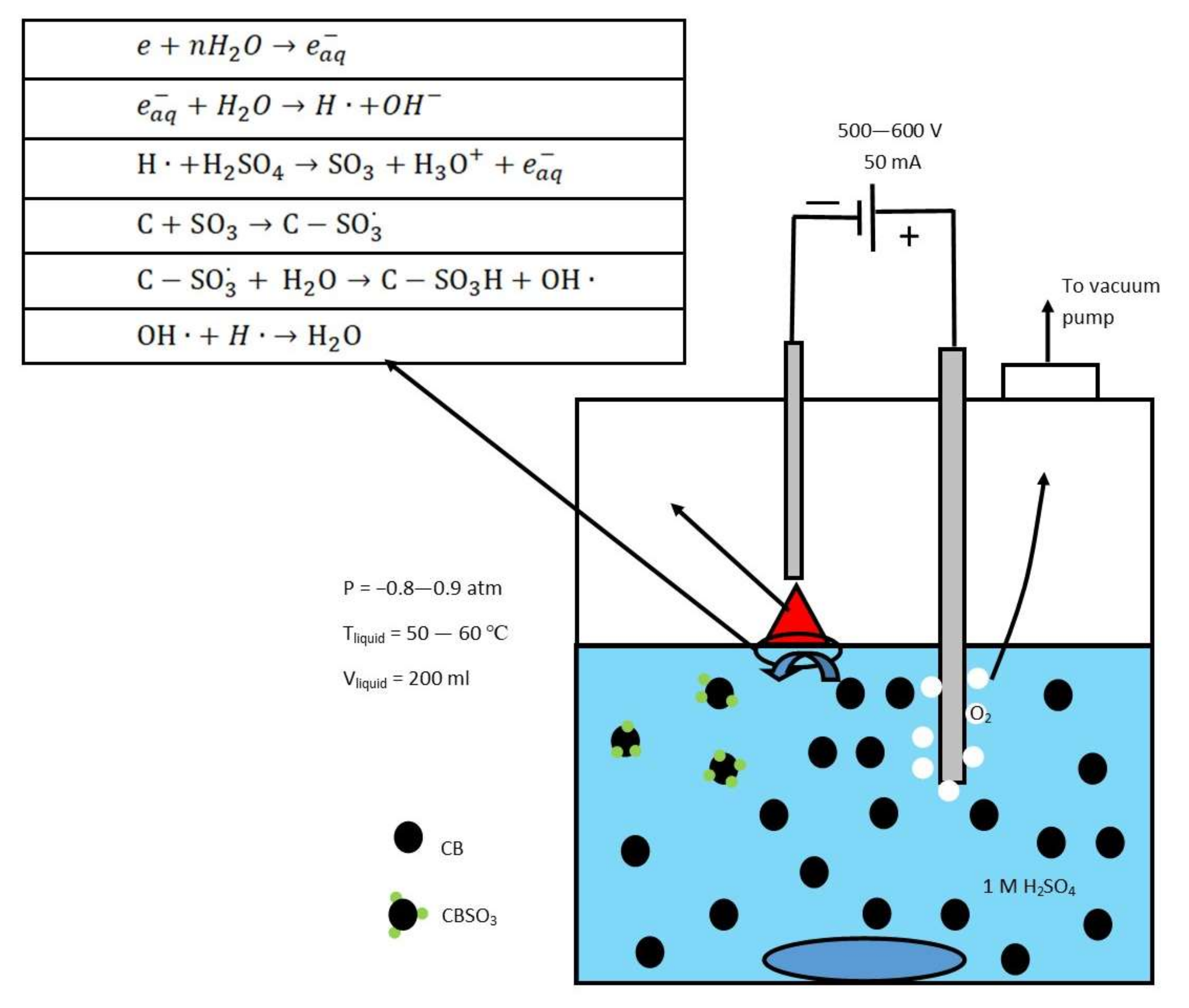
3.1. Plasma Treatment
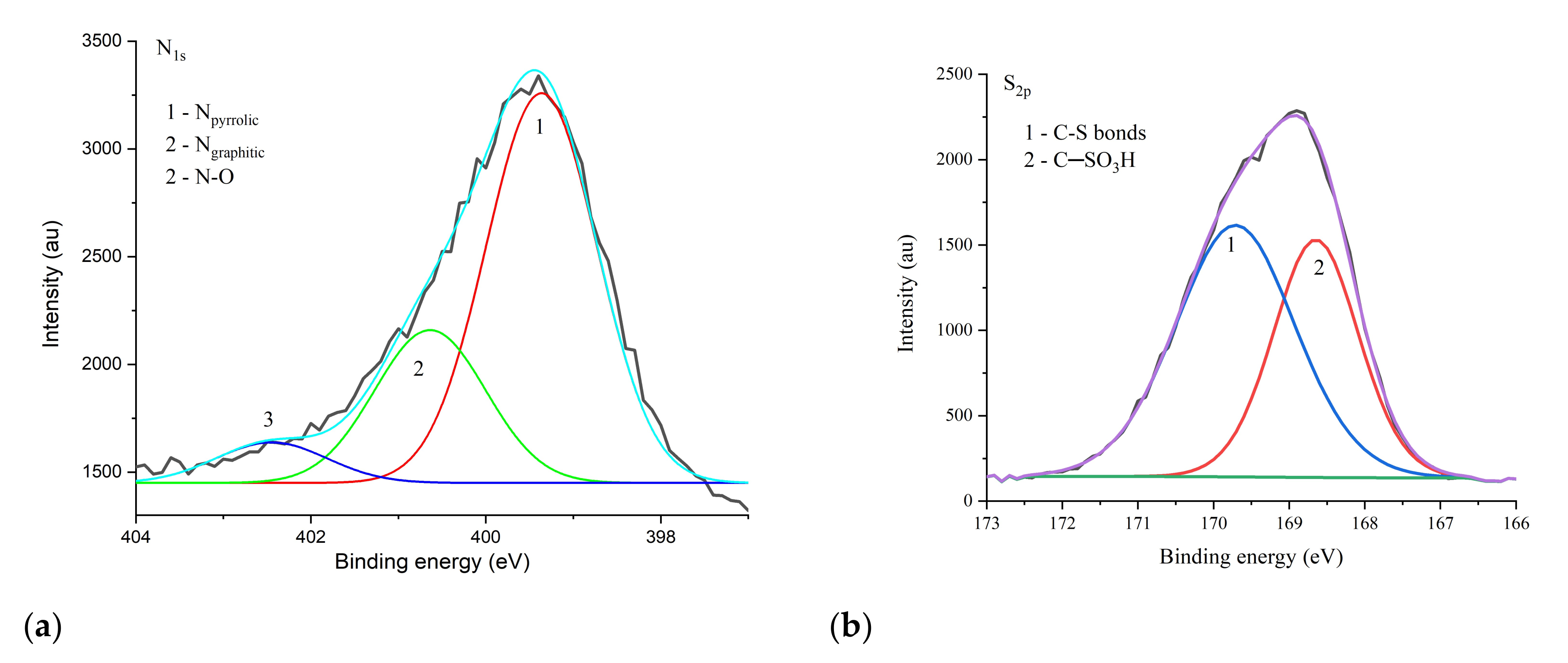
3.2. Characterization
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skorupska, M.; Ilnicka, A.; Lukaszewicz, J.P. Successful Manufacturing Protocols of N-Rich Carbon Electrodes Ensuring High ORR Activity: A Review. Processes 2022, 10, 643. [Google Scholar] [CrossRef]
- Jiao, R.; Zhang, W.; Sun, H.; Zhu, Z.; Yang, Z.; Liang, W.; Li, A. N- and S-doped nanoporous carbon framework derived from conjugated microporous polymers incorporation with ionic liquids for efficient oxygen reduction reaction. Mater. Today Energy 2020, 16, 100382. [Google Scholar] [CrossRef]
- Kong, Z.; Zhang, D.; Du, S.; Huang, G.; Wu, J.; Liu, Z.; Tao, L.; Wang, S. Supported hydrogen–oxygen fuel cell catalysts: From synthesis, structure-performance evolution and mechanism to synergy strategy. Nano Mater. Sci. 2024; in press. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Liu, T.; Feng, F.; Wang, C.; Hu, H.; Wu, M.; Ni, M.; Shao, Z. The Synergistic Effect Accelerates the Oxygen Reduction/Evolution Reaction in a Zn-Air Battery. Front. Chem. 2019, 7, 524. [Google Scholar] [CrossRef]
- Nagarani, S.; Chang, J.-H.; Yuvaraj, M.; Balachandran, S.; Kumar, M.; Kanimozhi, S. Well-organized metal-free chemically reduced graphene oxide sheets as electrocatalysts for enhanced oxygen reduction reactions in alkaline media. Mater. Lett. 2024, 357, 135705. [Google Scholar] [CrossRef]
- Guo, H.; Pei, L.-J.; Xie, S.-L.; Shen, M.-Q.; Yang, Y.; Liu, H. Copper-based nanocatalysts toward chemoselective hydrogenation reaction. cMat 2024, 1, e9. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Zhao, C.; Yin, X.; Zhao, Y. Co, N co-doped porous carbons as high-performance oxygen reduction electrocatalysts. New Carbon. Mater. 2021, 36, 209–218. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Liu, H.; Wu, D.; Zhang, G.; Jiang, J. Co/N-Doped hierarchical porous carbon as an efficient oxygen electrocatalyst for rechargeable Zn-air battery. Rsc Adv. 2021, 11, 15753–15761. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, L.; Wan, Z.; Shi, K.; Teng, X.; Xu, H.; Wu, P.; Shi, J. Electrocatalytic ORR-coupled ammoximation for efficient oxime synthesis. Sci. Adv. 2024, 10, eado1755. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, S.; Zhang, H. Recent Progress in Atomically Dispersed Non-noble Metal Catalysts for Electrochemical Two-electron Oxygen Reduction Reaction. ChemElectroChem 2024, 11, e202300630. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Wang, X. Ionic liquid induced controllable synthesis of nickel-hydroxide-encapsulated NiFe layered double hydroxide for efficient oxygen evolution. Energy Lab. 2023, 1, 220020. [Google Scholar] [CrossRef]
- Wu, K.; Wang, D.; Fu, Q.; Xu, T.; Xiong, Q.; Peera, S.G.; Liu, C. Co/Ce-MOF-Derived Oxygen Electrode Bifunctional Catalyst for Rechargeable Zinc–Air Batteries. Inorg. Chem. 2024, 63, 11135–11145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guang, H.; Li, R.; Lu, X.; Xu, H.; Wang, D.; Xiao, L.; Zhang, J.; An, M.; Yang, P. Doping engineering: Modulating the intrinsic activity of bifunctional carbon-based oxygen electrocatalysts for high-performance zinc–air batteries. J. Mater. Chem. A 2022, 10, 21797–21815. [Google Scholar] [CrossRef]
- Yang, Z.; Han, J.; Jiao, R.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Porous carbon framework derived from N-rich hypercrosslinked polymer as the efficient metal-free electrocatalyst for oxygen reduction reaction. J. Colloid. Interface Sci. 2019, 557, 664–672. [Google Scholar] [CrossRef]
- Chen, W.; Wan, M.; Liu, Q.; Xiong, X.; Yu, F.; Huang, Y. Heteroatom-Doped Carbon Materials: Synthesis, Mechanism, and Application for Sodium-Ion Batteries. Small Methods 2019, 3, 1800323. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Z.; Kong, D.; Iqbal, R.; Yang, Q.-H.; Zhi, L. N,P co-doped hollow carbon nanofiber membranes with superior mass transfer property for trifunctional metal-free electrocatalysis. Nano Energy 2019, 64, 103879. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Luo, X.; Li, S.; Li, S.; Zhou, Q.; Xu, W.; Wu, R. N-, P-, and O-doped porous carbon: A trifunctional metal-free electrocatalyst. Appl. Surf. Sci. 2021, 544, 148912. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Oxygen-reduction catalysis of N-doped carbons prepared via heat treatment of polyaniline at over 1100 °C. Chem. Commun. 2018, 54, 4441–4444. [Google Scholar] [CrossRef]
- Du, G.H.; Li, W.Z.; Liu, Y.Q.; Ding, Y.; Wang, Z.L. Growth of Carbon Nanotubes by Pyrolysis of Thiophene. J. Phys. Chem. C 2007, 111, 14293–14298. [Google Scholar] [CrossRef]
- Xiang, Z.; Dai, Q.; Chen, J.F.; Dai, L. Edge Functionalization of Graphene and Two-Dimensional Covalent Organic Polymers for Energy Conversion and Storage. Adv. Mater. 2016, 28, 6253–6261. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zou, Y.; Tao, L.; Zhang, Y.; Liu, Z.; Du, S.; Zang, S.; Wang, S. Low-temperature plasma technology for electrocatalysis. Chin. Chem. Lett. 2019, 30, 826–838. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Q.; Dou, S.; Ma, Z.; Huo, J.; Wang, S.; Dai, L. Edge-rich and dopant-free graphene as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. Chem. Commun. 2016, 52, 2764–2767. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Q. Microplasma: A New Generation of Technology for Functional Nanomaterial Synthesis. Plasma Chem. Plasma Process. 2015, 35, 925–962. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Lu, Y.; Wei, G.; Ye, G.; Sun, T.; Chen, J. Microplasma electrochemistry controlled rapid preparation of fluorescent polydopamine nanoparticles and their application in uranium detection. Chem. Eng. J. 2018, 344, 480–486. [Google Scholar] [CrossRef]
- Josh Smith, U.K.E. Chemistry for Antimicrobial Properties of Water Treated With Non-Equilibrium Plasma. J. Nanomed. Biother. Discov. 2014, 4, 1000120. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Becker, K. 20 years of microplasma research: A status report. Eur. Phys. J. D 2016, 70, 29. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Rai, S.; Yang, J.K.; Choi, S.; Lee, H.-J. Effect of Water Conductivity on the Generation of Radicals in High Frequency Underwater Capillary Discharge. Int. J. Renew. Energy Environ. Eng. 2016, 4, 28–34. [Google Scholar]
- Schwarz, H.A. Free radicals generated by radiolysis of aqueous solutions. J. Chem. Educ. 1981, 58, 101. [Google Scholar] [CrossRef]
- Qin, L.; Takeuchi, N.; Takahashi, K.; Kang, J.; Kim, K.H.; Li, O.L. N2/Ar plasma-induced surface sulfonation on graphene nanoplatelets for catalytic hydrolysis of cellulose to glucose. Appl. Surf. Sci. 2021, 545, 149051. [Google Scholar] [CrossRef]
- Li, O.L.; Ikura, R.; Ishizaki, T. Hydrolysis of cellulose to glucose over carbon catalysts sulfonated via a plasma process in dilute acids. Green. Chem. 2017, 19, 4774–4777. [Google Scholar] [CrossRef]
- Li, O.L.; Qin, L.; Takeuchi, N.; Kim, K.; Ishizaki, T. Effect of hydrophilic/hydrophobic properties of carbon materials on plasma-sulfonation process and their catalytic activities in cellulose conversion. Catal. Today 2019, 337, 155–161. [Google Scholar] [CrossRef]
- He, Z.; Cheng, G.; Jiang, Y.; Wang, L.; Dai, L. Sulfonated carbon nanotubes as superior catalysts towards V3+/V2+ redox reaction for vanadium redox flow battery. J. Electrochem. Soc. 2018, 165, A932–A938. [Google Scholar] [CrossRef]


| Sample | C1s, Atomic % | O1s, Atomic % | N1s, Atomic % | S2p, Atomic % |
|---|---|---|---|---|
| CB | 77.34 | 22.66 | ||
| CBNO2 | 91.10 | 8.48 | 0.23 | |
| CBSO3 | 81.83 | 12.98 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharisova, K.; Beletskii, E.; Levin, O.; Li, R.; Yang, P.; Alekseeva, E. Plasma Modification of Technical Carbon with Nitrogen and Sulfur-Containing Functional Groups for Application in Catalytic Systems. ChemEngineering 2025, 9, 27. https://doi.org/10.3390/chemengineering9020027
Kharisova K, Beletskii E, Levin O, Li R, Yang P, Alekseeva E. Plasma Modification of Technical Carbon with Nitrogen and Sulfur-Containing Functional Groups for Application in Catalytic Systems. ChemEngineering. 2025; 9(2):27. https://doi.org/10.3390/chemengineering9020027
Chicago/Turabian StyleKharisova, Ksenia, Evgenii Beletskii, Oleg Levin, Ruopeng Li, Peixia Yang, and Elena Alekseeva. 2025. "Plasma Modification of Technical Carbon with Nitrogen and Sulfur-Containing Functional Groups for Application in Catalytic Systems" ChemEngineering 9, no. 2: 27. https://doi.org/10.3390/chemengineering9020027
APA StyleKharisova, K., Beletskii, E., Levin, O., Li, R., Yang, P., & Alekseeva, E. (2025). Plasma Modification of Technical Carbon with Nitrogen and Sulfur-Containing Functional Groups for Application in Catalytic Systems. ChemEngineering, 9(2), 27. https://doi.org/10.3390/chemengineering9020027








