Development of Novel Monolithic Catalyst for BTEX Catalytic Oxidation Using 3D Printing Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Monolithic Catalyst Supports
2.2. Deposition of the Catalytically Active Components and Characterization
2.3. Testing the Mechanical Stability of the Catalyst Layer
2.4. Testing of Morphology and Phase Composition of the Catalyst
2.5. Catalytic Oxidation of BTEX Compounds
3. Results and Discussion
3.1. Preparation of the Ceramic Monolithic Catalyst Supports, Impregnation, and Thermal Treatment
3.2. Testing of the Mechanical Stability (Adhesion) of the Catalyst Layer
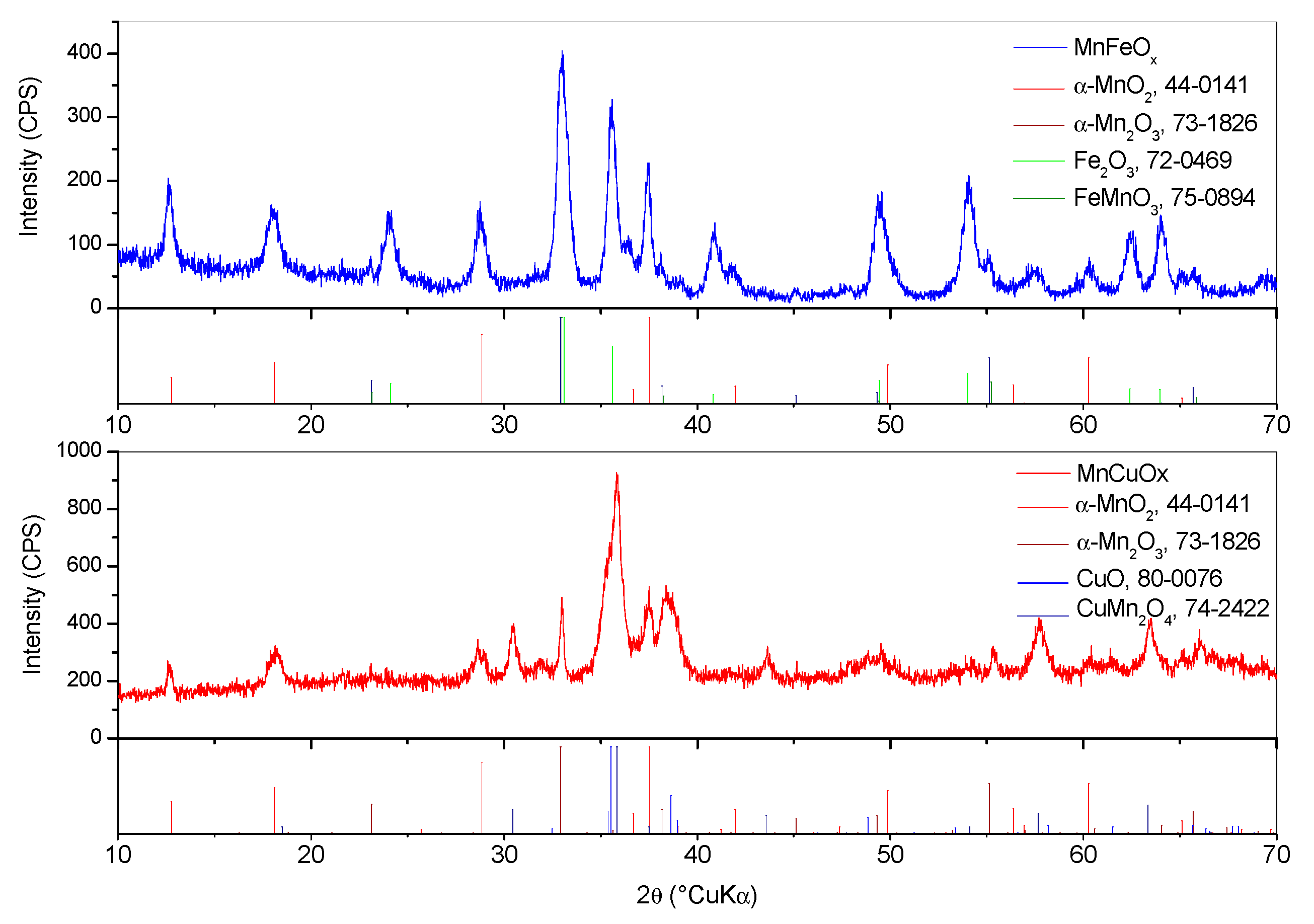
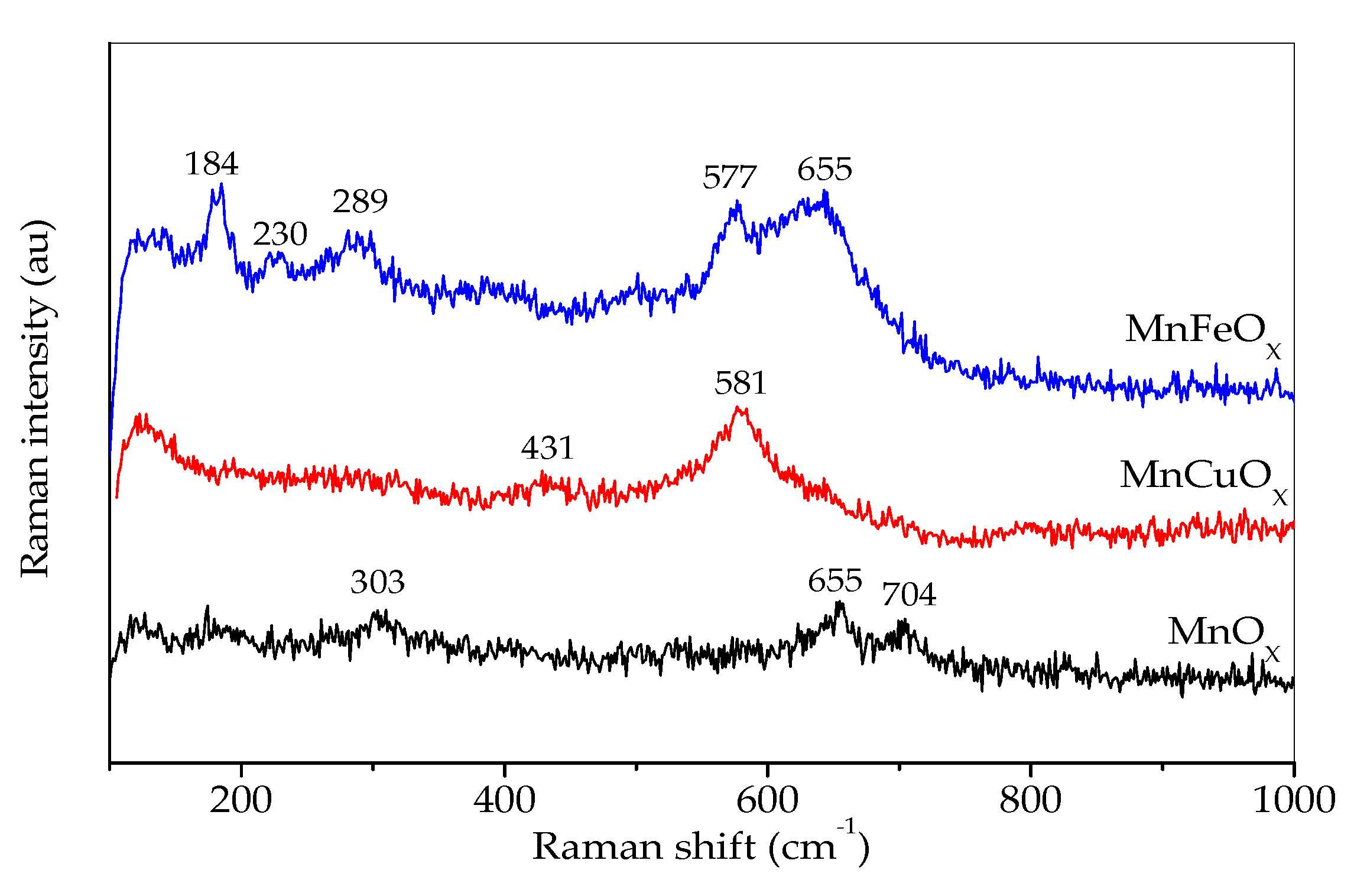
3.3. Investigation of the Phase Composition of the Catalyst
3.4. Theoretical Modeling of Raman Spectra for FeMnO3 and CuMn2O4
3.5. Catalytic Activity of the Prepared Monolithic Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maduna, K.; Tomašić, V. Air Pollution Engineering. Phys. Sci. Rev. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Barletta, B.; Meinardi, S.; Rowland, F.S.; Chan, C.Y.; Wang, X.; Zou, S.; Chan, L.Y.; Blake, D.R. Volatile organic compounds in 43 Chinese cities. Atmos. Environ. 2005, 39, 5979–5980. [Google Scholar] [CrossRef]
- De Luna, M.D.; Millanar, J.M.; Yodsa-Nga, A.; Wantala, K. Gas phase catalytic oxidation of VOCS using hydrothermally synthesized nest-like K-OMS 2 catalyst. Sains Malays. 2017, 46, 275–283. [Google Scholar] [CrossRef]
- Leusch, F.; Bartkow, M. A Short Primer on Benzene, Toluene, Ethylbenzene and Xylenes (BTEX) in the Environment and in Hydraulic Fracturing Fluids; Griffith University—Smart Water Research Centre: Southport, Australia, 2010. [Google Scholar]
- Miri, M.; Shendi, M.R.A.; Ghaffari, H.R.; Aval, H.E.; Ahmadi, E.; Taban, E.; Gholizadeh, A.; Aval, M.Y.; Mohammadi, A.; Azari, A. Investigation of outdoor BTEX: Concentration, variations, sources, spatial distribution, and risk assessment. Chemosphere 2016, 163, 601–609. [Google Scholar] [CrossRef]
- Golkhorshidi, F.; Sorooshian, A.; Jafari, A.J.; Baghani, A.N.; Kermani, M.; Kalantary, R.R.; Ashournejad, Q.; Delikhoon, M. On the nature and health impacts of BTEX in a populated middle eastern city: Tehran, Iran. Atmos. Pollut. Res. 2019, 10, 921–930. [Google Scholar] [CrossRef]
- Latif, M.T.; Abd Hamid, H.H.; Ahamad, F.; Khan, M.F.; Nadzir, M.S.; Othman, M.; Sahani, M.; Wahab, M.I.; Mohamad, N.; Uning, R.; et al. BTEX compositions and its potential health impacts in Malaysia. Chemosphere 2019, 237, 124451. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B 2021, 281, 119447. [Google Scholar] [CrossRef]
- Tomatis, M.; Xu, H.H.; He, J.; Zhang, X.D. Recent Development of Catalysts for Removal of Volatile Organic Compounds in Flue Gas by Combustion: A Review. J. Chem. 2016, 2016, 8324826. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C. Three-dimensional Printing for Catalytic Applications:Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1701134. [Google Scholar] [CrossRef]
- Parra-Cabrera, C.; Achille, C.; Kuhn, S.; Ameloot, R. 3D printing in chemical engineering and catalytic technology: Structured catalysts, mixers and reactors. Chem. Soc. Rev. 2017, 47, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, P.; Chao, Y.; Yu, J.; Zhu, W.; Liu, Z.; Xu, C. Recent advances in 3D printing for catalytic applications. Chem. Eng. J. 2022, 433, 134341. [Google Scholar] [CrossRef]
- Chaparro-Garnica, C.Y.; Jorda-Faus, P.; Bailon-Garcia, E.; Ocampo-Perez, R.; Aguilar-Madera, C.G.; Davo-Quinonero, A.; Lozano-Castello, D.; Bueno-Lopez, A. Customizable Heterogeneous Catalysts: Nonchanneled Advanced Monolithic Supports Manufactured by 3D-Printing for Improved active Phase Coating Performance. ACS Appl. Mater. Interfaces 2020, 12, 54573–54584. [Google Scholar] [CrossRef] [PubMed]
- CChapparo-Garnica, Y.; Davo-Quinonero, A.; Bailon-Garcia, E.; Lozano-Castello, D.; Bueno-Lopez, A. Design of Monolithic Supports by 3D Printing for Its Application in the Preferential Oxidation of CO (CO-PrOx). ACS Appl. Mater. Interfaces 2019, 11, 36763–36773. [Google Scholar] [CrossRef]
- Abdulhussain, N.; Nawada, S.; Currivan, S.; Passamonti, M.; Schoenmakers, P. Fabrication of polymer monoliths within the confines of non-transparent 3D-printed polymer housings. J. Chromatogr. A 2020, 1623, 461159. [Google Scholar] [CrossRef]
- Agueniou, F.; Vidal, H.; de Dios Lopez, J.; Hernandez-Garrido, J.C.; Cauqui, M.A.; Botana, F.J.; Calvino, J.J.; Galvita, V.V.; Gatica, J.M. 3D-printing of metallic honeycomb monoliths as a doorway to a new generation of catalytic devices: The Ni-based catalysts in methane dry reforming showcase. Catal. Commun. 2021, 148, 106181. [Google Scholar] [CrossRef]
- Wei, Q.; Li, H.; Liu, G.; He, Y.; Wang, Y.; Tan, Y.E.; Wang, D.; Peng, X.; Yang, G.; Tsubaki, N. Metal 3D-printing technology for functional integration of catalytic system. Nat. Commun. 2020, 11, 4098. [Google Scholar] [CrossRef]
- Vega, G.; Quintanilla, A.; Menendez, N.; Belmonte, M.; Casas, J.A. 3D honeycomb monoliths with interconnected channels for sustainable production of dihydroxybenzenes:towards the intensification of selective oxidation processes. Chem. Eng. Process. 2021, 165, 108437. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, P.; Tian, J.; Liu, Y.; Wan, Y.; Zhang, K.; Wang, D.; Dam, J.; Dai, B.; Wang, X.; et al. 3D-printed monolithic catalyst of Mn-Ce-Fe/attapulgite for selective catalytic reduction of nitric oxide with ammonia at low temperature. J. Environ. Chem. Eng. 2021, 9, 105753. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S.; Al-Naddaf, Q.; Rownaghi, A.A.; Rezaei, F. 3D-printed metal−organic framework monoliths for gas adsorption processes. ACS Appl. Mater. Interfaces 2017, 9, 35908–35916. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, P.; Li, X.; Liu, J.; Zhou, L.; Wang, X.; Zhou, F. 3D printing of metal-organic frameworks decorated hierarchical porous ceramics for high-efficiency catalytic degradation. Chem. Eng. J. 2020, 397, 125392. [Google Scholar] [CrossRef]
- Car, F.; Brnadić, G.; Tomašić, V.; Vrsaljko, D. Advanced preparation method of monolithic catalyst supports using 3D-printing technology. Prog. Addit. Manuf. 2022, 7, 797–808. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.J.; Kim, J.H.; Kim, J.H.; Kang, S.H.; Ryu, J.H.; Park, N.K.; Yun, D.S.; Bae, J.W. Catalytic oxidation of volatile organic compounds on supported noble metals. Catalysts 2022, 12, 63. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, A.C.; Tavares, P.B.; Pereira, M.F.R.; Orfao, J.J.M.; Figueiredo, J.L. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. B 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Abbasi, Z.; Haghighi, M.; Fatehifar, E.; Saedy, S. Synthesis and physicochemical cheracterizations of nanostructured Pt/Al2O3-CeO2 catalysts for total oxidation of VOCs. J. Hazard. Mater. 2011, 186, 1445–1454. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Carrasco-Marin, F.; Perez-Cadenas, A.F.; Maldonado-Hodar, F.J. Coupling Noble Metals and Carbon Supports in the Development of Combustion Catalysts for the Abatement of BTX Compounds in Air Streams. Catalysts 2015, 5, 774–799. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Chen, X.; Liu, S.; Zhou, Y.; Zhu, Q.; Chen, Y.; Lu, H. Preparation of metallic monolithic Pt/FeCrAl fiber catalyst by suspension spraying for VOCs combustion. RSC Adv. 2018, 8, 14806–14811. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Castaño, M.H.; Molina, R.; Moreno, S. Effect of Mg and Al on manganese oxides as catalysts for VOC oxidation. J. Mol. Catal. B Enzym. 2017, 443, 117–124. [Google Scholar] [CrossRef]
- Peng, C.; Yu, D.; Wang, L.; Yu, X.; Zhao, Z. Recent Advances in the Preparation and Catalytic Performance of Mn-based Oxide Catalysts with Special Morphology for the Removal of Air Pollutants. J. Mater. Chem. A 2021, 9, 12947–12980. [Google Scholar] [CrossRef]
- Morales, M.R.; Barbero, B.P.; Cadus, L.E. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts. Appl. Catal. B 2006, 67, 229–236. [Google Scholar] [CrossRef]
- Duplančić, M.; Tomašić, V.; Gomzi, Z. Catalytic oxidation of toluene: Comparative study over powder and monolithic manganese-nickel mixed oxide catalysts. Environ. Technol. 2018, 39, 2004–2016. [Google Scholar] [CrossRef]
- Qin, L.; Huang, X.; Zhao, B.; Wang, Y.; Han, J. Iron Oxide as a Promoter for Toluene Catalytic Oxidation over Fe-Mn/γ-Al2O3 Catalysts. Catal. Lett. 2020, 150, 802–814. [Google Scholar] [CrossRef]
- Einaga, H.; Maeda, N.; Yamamoto, S.; Teraoka, Y. Catalytic properties of copper-manganese mixed oxides supported on SiO2 for benzene oxidation with ozone. Catal. Today 2015, 245, 22–27. [Google Scholar] [CrossRef]
- Car, F.; Sušec, I.; Tomašić, V. Preparation and Testing of Cordierite Monolithic Catalysts for Oxidation of Aromatic Volatile Organic Compounds. Chem. Eng. Trans. 2021, 86, 673–678. [Google Scholar] [CrossRef]
- Available online: https://formlabs.com/materials/ceramics/ (accessed on 2 June 2022).
- Wu, D.; Kong, S.; Zhang, H. Mechanical Stability of Monolithic Catalysts: Factors Affecting Washcoat Adhesion and Cohesion During Preparation. AIChE J. 2014, 60, 2765–2773. [Google Scholar] [CrossRef]
- Barbero, B.P.; Costa-Almeida, L.; Sanz, O.; Morales, M.R.; Cadus, L.E.; Montes, M. Washcoating of metallic monoliths with a MnCu catalyst for catalytic combustion of volatile organic compounds. Chem. Eng. J. 2008, 139, 430–435. [Google Scholar] [CrossRef]
- Aguero, F.N.; Barbero, B.P.; Almeida, L.C.; Montes, M.; Cadus, L.E. MnOx supported on metallic monoliths for the combustion of volatile organic compounds. Chem. Eng. J. 2011, 166, 218–223. [Google Scholar] [CrossRef]
- JChai, D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Schaefer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian-basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Hugo, V.-V.V.; Alejandro, H.-S.M.; María, V.-S.A.; María, R.-H.; Antonio, L.-R.M.; Guadalupe, P.-O.M.; Antonio, M.-G.M.; Fernando, A.-H.; Víctor, A.; Diego, C.-A.; et al. Molecular Modeling and Synthesis of Ethyl Benzyl Carbamates as Possible Ixodicide Activity. Comput. Chem. 2018, 7, 1–26. Available online: https://www.scirp.org/reference/referencespapers?referenceid=2418053 (accessed on 7 January 2025). [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Santos, D.F.M.; Soares, O.S.G.P.; Figueiredo, J.L.; Sanz, O.; Montes, M.; Pereira, M.F.R. Preparation of ceramic and metallic monoliths coated with cryptomelane as catalysts for VOC abatement. Chem. Eng. J. 2020, 382, 122923. [Google Scholar] [CrossRef]
- Chen, S.Y.; Song, W.; Lin, H.J.; Wang, S.; Biswas, S.; Mollahosseini, M.; Kuo, C.H.; Gao, P.X.; Suib, S.L. Manganese Oxide Nanoarray-Based Monolithic Catalysts: Tunable Morphology and High Efficiency for CO Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 7834–7842. [Google Scholar] [CrossRef]
- Rahaman, H.; Laha, R.M.; Maiti, D.K.; Ghosh, S.K. Fabrication of Mn2O3 nanorods: An efficient catalyst for selective transformation of alcohols to aldehydes. RSC Adv. 2015, 5, 33923. [Google Scholar] [CrossRef]
- Xin, Y.; Cao, H.; Liu, C.; Chen, J.; Liu, P.; Lu, Y.; Ling, Z. A systematic spectroscopic study of laboratory synthesized manganese oxides relevant to Mars. J. Raman Spectrosc. 2021, 53, 340–355. [Google Scholar] [CrossRef]
- Post, J.E.; McKeown, D.A.; Heaney, P.J. Raman spectroscopy study of manganese oxides: Tunnel structures. Am. Mineral. 2020, 105, 1175–1190. [Google Scholar] [CrossRef]
- Gao, T.; Fjellvåg, H.; Norby, P. A comparison study on Raman scattering properties of α- and β-MnO2. Anal. Chim. Acta 2009, 648, 235–239. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Han, C.; Lu, R.; Yang, S.; Song, X. Electrospun hollow cage-like α-Fe2O3 microspheres: Synthesis, formation mechanism, and morphology-preserved conversion to Fe nanostructures. Cryst. Eng. Comm. 2014, 16, 10618–10623. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Ramachandran, S.P.; Ravi, G.; Ganesh, V.; Guduru, R.K.; Yuvakkumar, R. Electrochemical characterization of FeMnO3 microspheres as potential material for energy storage applications. Mater. Res. Express 2018, 5, 015504. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides Part I. Periodic structures. Spectrochim. Acta A 2004, 60, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Rashad, M.; Rüsing, M.; Berth, G.; Lischka, K.; Pawlis, A. CuO and Co3O4 Nanoparticles: Synthesis, Characterisations, and Raman Spectroscopy. J. Nanomater. 2013, 2013, 714853. [Google Scholar] [CrossRef]
- Van Everbroeck, T.; Ciocarlan, R.G.; Van Hoey, W.; Mertens, M.; Cool, P. Copper-Containing Mixed Metal Oxides (Al, Fe, Mn) for Application in Three-Way Catalysis. Catalysts 2020, 10, 1344. [Google Scholar] [CrossRef]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2015, 147, 987–995. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Fonseca, A.M.; Parpot, P.; Orfao, J.J.M.; Pereira, M.F.R.; Neves, I.C. Oxidation of Volatile Organic Compounds by Highly Efficient Metal Zeolite Catalysts. Chem. Cat. Chem. 2018, 10, 3754–3760. [Google Scholar] [CrossRef]
- Brunet, J.; Genty, E.; Barroo, C.; Cazier, F.; Poupin, C.; Siffert, S.; Thomas, D.; De Weireld, G.; de Bocarme, T.V.; Cousin, R. The CoAlCeO Mixed Oxide: An Alternative to Palladium-Based Catalysts for Total Oxidation of Industrial VOCs. Catalysts 2018, 8, 64. [Google Scholar] [CrossRef]
- Zedan, A.F.; Allam, N.K.; Al Qaradawi, S.Y. A Study of Low-Temperature CO Oxidation over Mesoporous CuO-TiO2 Nanotube Catalysts. Catalysts 2017, 7, 129. [Google Scholar] [CrossRef]
- Gallegos, M.V.; Peluso, M.A.; Finocchio, E.; Thomas, H.J.; Busca, G.; Sambeth, J.E. Removal of VOCs by catalytic process. A study of MnZnO composites synthesized from waste alkaline and Zn/C batteries. Chem. Eng. J. 2016, 313, 1099–1111. [Google Scholar] [CrossRef]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-Phase Total Oxidation of Benzene, Toluene, Ethylbenzene, and Xylenes using Shape-Selective Manganese Oxide and Copper Manganese Oxide Catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]



| Support | P | PS | S | SS |
| Geometry of the monolithic catalyst support |  | |||
| Geometric surface area (cm2) | 11 | 20 | 15 | 22 |
| Catalyst | Plate | M (Plate with Catalyst) (g) | M (Plate After Test) (g) | Mass Loss (%) |
|---|---|---|---|---|
| MnFeOx | 1 | 0.4840 | 0.4789 | 1.05 |
| 2 | 0.4596 | 0.4522 | 1.61 | |
| 3 | 0.4792 | 0.4719 | 1.52 | |
| 4 | 0.4836 | 0.4771 | 1.34 | |
| 5 | 0.4776 | 0.4683 | 1.95 | |
| MnCuOx | 1 | 0.4668 | 0.4580 | 1.89 |
| 2 | 0.4699 | 0.4638 | 1.30 | |
| 3 | 0.4863 | 0.4810 | 1.09 | |
| 4 | 0.4759 | 0.4690 | 1.46 | |
| 5 | 0.4777 | 0.4696 | 1.69 |
| T50 (°C) | T90 (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Catalyst | Support | B | T | E | X | B | T | E | X |
| MnFeOx | P | 215 | 186 | 173 | 174 | 243 | 200 | 195 | 195 |
| PS | 207 | 186 | 171 | 172 | 238 | 198 | 194 | 194 | |
| S | 211 | 181 | 169 | 168 | 241 | 199 | 194 | 192 | |
| SS | 183 | 166 | 164 | 164 | 212 | 179 | 177 | 177 | |
| MnCuOx | P | 219 | 186 | 177 | 175 | 252 | 202 | 196 | 196 |
| PS | 203 | 174 | 167 | 166 | 236 | 195 | 189 | 189 | |
| S | 211 | 181 | 169 | 168 | 241 | 199 | 194 | 192 | |
| SS | 187 | 165 | 163 | 163 | 217 | 178 | 177 | 177 | |
| Support | P | PS | S | SS |
|---|---|---|---|---|
| Mass (mg) of MnFeOx | 4.8–5.0 | 6.4–6.6 | 5.5–5.8 | 7.0–7.3 |
| Mass (mg) of MnCuOx | 4.9–5.2 | 6.2–6.5 | 5.6–5.8 | 7.1–7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Car, F.; Gomzi, V.; Tomašić, V.; Vrsaljko, D.; Kurajica, S. Development of Novel Monolithic Catalyst for BTEX Catalytic Oxidation Using 3D Printing Technology. ChemEngineering 2025, 9, 9. https://doi.org/10.3390/chemengineering9010009
Car F, Gomzi V, Tomašić V, Vrsaljko D, Kurajica S. Development of Novel Monolithic Catalyst for BTEX Catalytic Oxidation Using 3D Printing Technology. ChemEngineering. 2025; 9(1):9. https://doi.org/10.3390/chemengineering9010009
Chicago/Turabian StyleCar, Filip, Vjeran Gomzi, Vesna Tomašić, Domagoj Vrsaljko, and Stanislav Kurajica. 2025. "Development of Novel Monolithic Catalyst for BTEX Catalytic Oxidation Using 3D Printing Technology" ChemEngineering 9, no. 1: 9. https://doi.org/10.3390/chemengineering9010009
APA StyleCar, F., Gomzi, V., Tomašić, V., Vrsaljko, D., & Kurajica, S. (2025). Development of Novel Monolithic Catalyst for BTEX Catalytic Oxidation Using 3D Printing Technology. ChemEngineering, 9(1), 9. https://doi.org/10.3390/chemengineering9010009








