Terephthalic-co-glycerol-g-fumaric Acid: A Promising Nanopolymer for Enhancing PPSU Membrane Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Membrane
2.3. Characterizations
2.3.1. Membranes Characterization
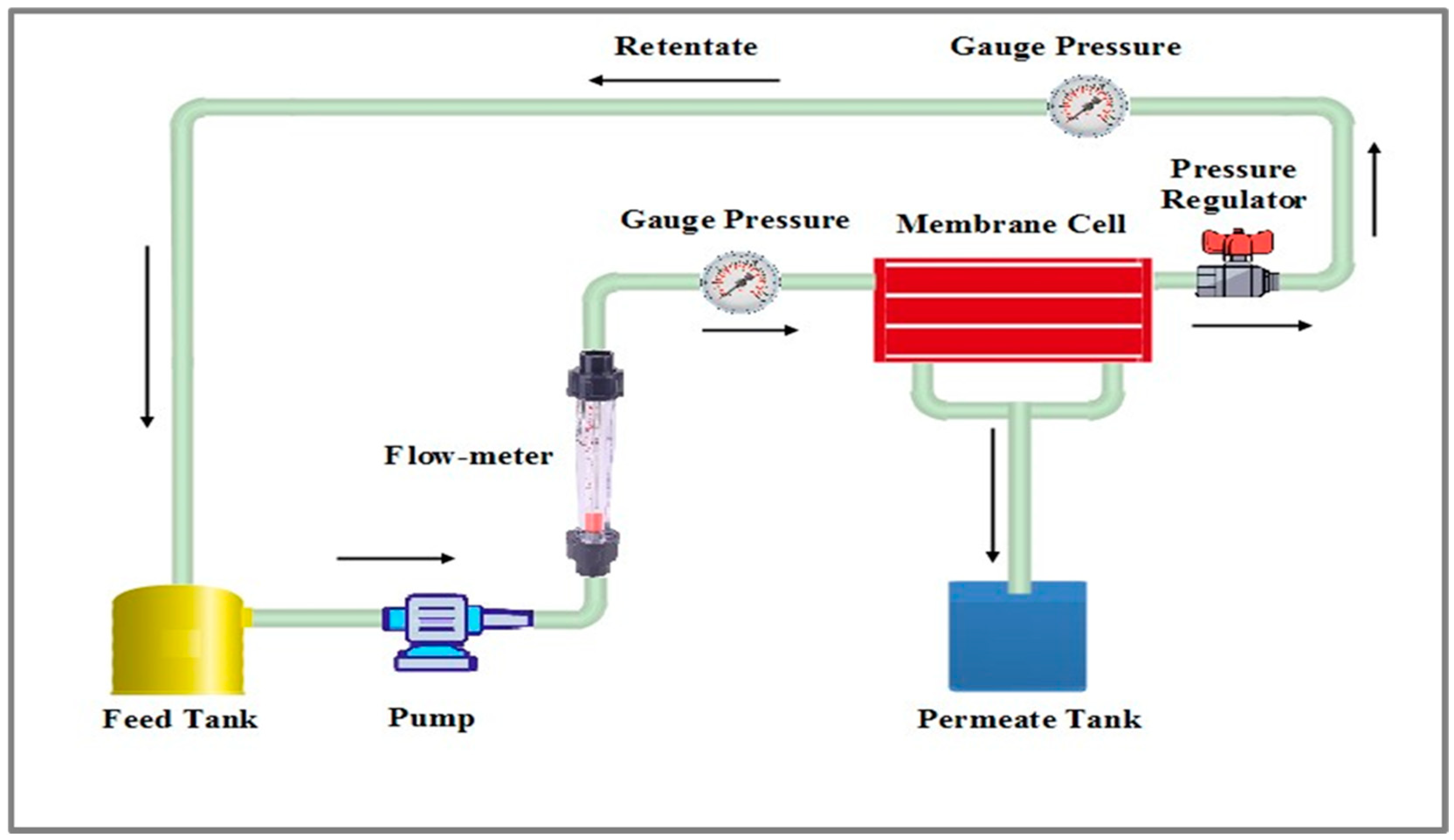
2.3.2. Performance Evaluation of Membranes
3. Results and Discussion
3.1. Characterization of the Manufactured Membranes
3.1.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.1.2. Field Emission Scanning Electron Microscopy (FESEM)
3.1.3. Atomic Force Microscopy (AFM)
3.1.4. Hydrophilicity Measurements of Membranes
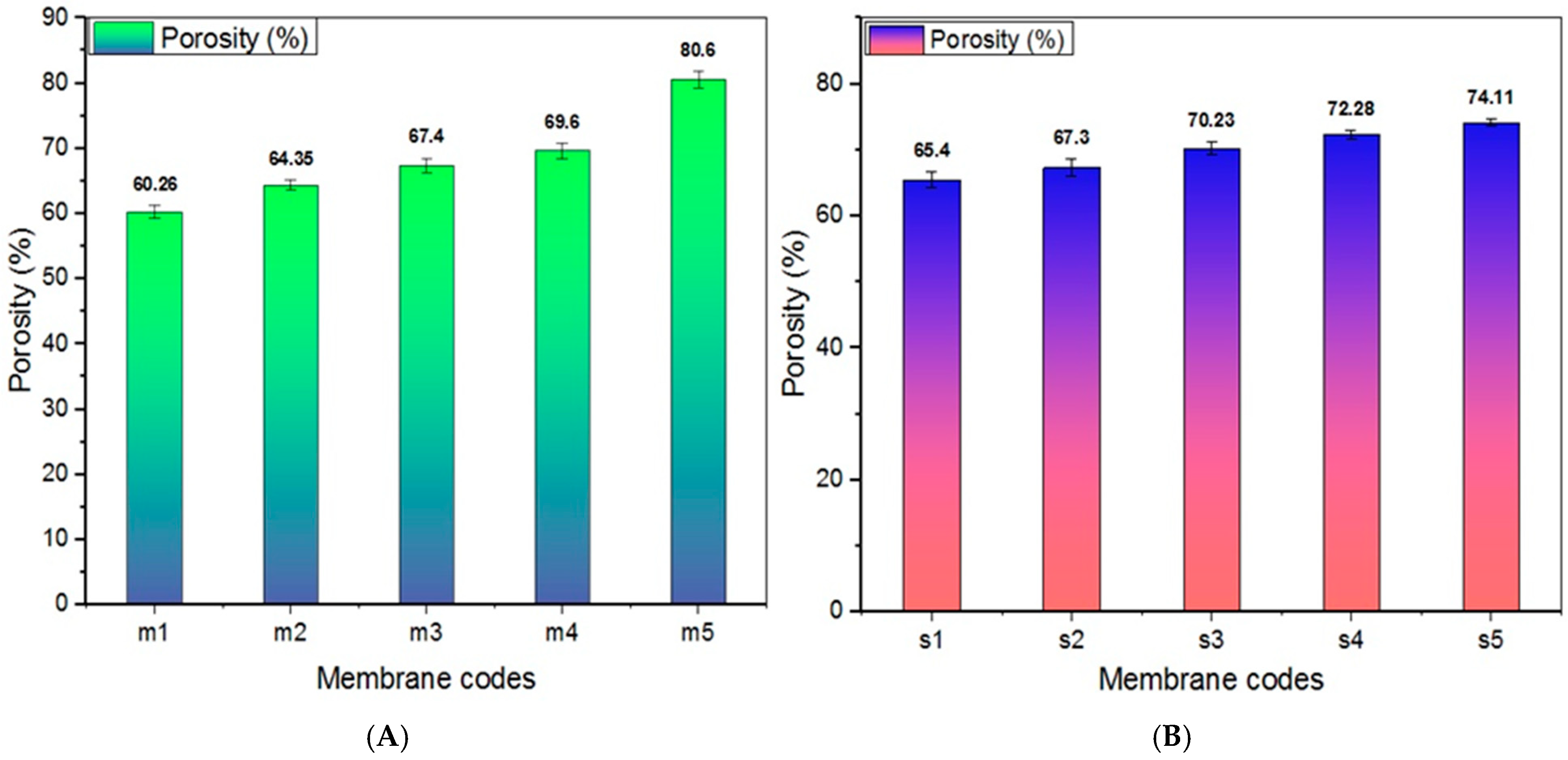
3.1.5. Porosity
3.1.6. Mean Pore Size
3.2. Performance of the Membranes
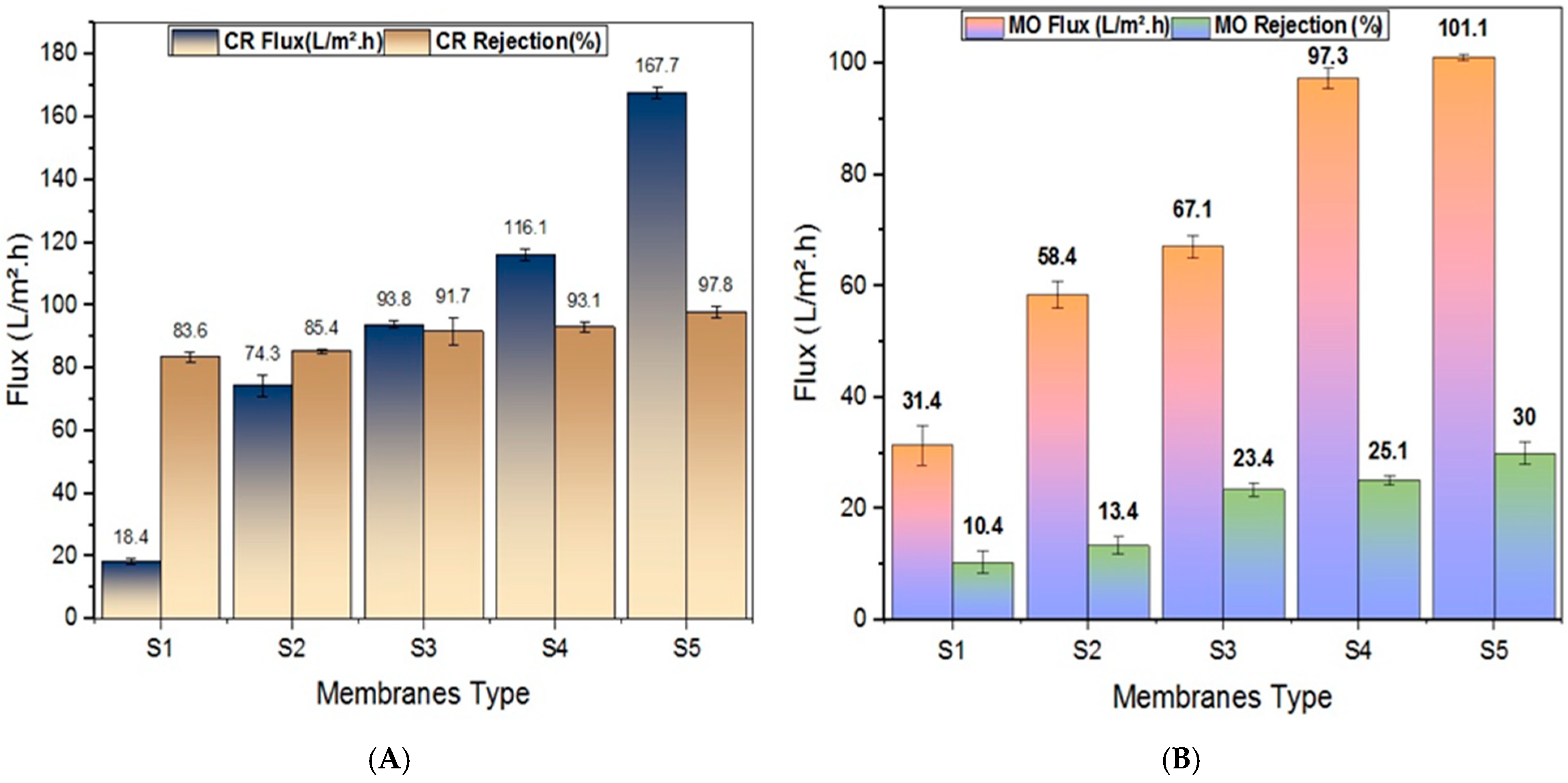
3.2.1. Pure Water Flux
3.2.2. Retention Efficiency of Fabricated Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Heidi, L.R.; Priscilla, G.L.B.; Emmanuel, I. Metal Nanoparticle Modified Polysulfone Membranes for Use in Wastewater Treatment: A Critical Review. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 183–193. [Google Scholar]
- Malik, T.; Razzaq, H.; Razzaque, S.; Nawaz, H.; Siddiqa, A.; Siddiq, M.; Qaisar, S. Design and synthesis of polymeric membranes using water-soluble pore formers: An overview. Polym. Bull. 2019, 76, 4879–4901. [Google Scholar] [CrossRef]
- Al Aani, S.; Wright, C.J.; Atieh, M.A.; Hilal, N. Engineering nanocomposite membranes: Addressing current challenges and future opportunities. Desalination 2017, 401, 1–15. [Google Scholar] [CrossRef]
- Nazarova, O.; Chernova, E.; Dobrodumov, A.; Zolotova, Y.; Bezrukova, M.; Nekrasova, T.; Vlasova, E.; Panarin, E. New water-soluble copolymers of 2-methacryloyloxyethyl phosphorylcholine for surface modification. J. Appl. Polym. Sci. 2021, 138, 50272. [Google Scholar] [CrossRef]
- Alayande, A.B.; Obaid, M.; Yu, H.W.; Kim, I.S. High-flux ultrafiltration membrane with open porous hydrophilic structure using dual pore formers. Chemosphere 2019, 227, 662–669. [Google Scholar] [CrossRef]
- Roy, K.J.; Anjali, T.V.; Sujith, A. Poly (vinyl chloride) asymmetric membrane modified with poly (ethylene glycol): Effect of additive concentration on the morphology and performance. Polym.-Plast. Technol. Eng. 2017, 56, 1017–1025. [Google Scholar] [CrossRef]
- Farjami, M.; Vatanpour, V.; Moghadassi, A. Influence of the various pore former additives on the performance and characteristics of the bare and EPVC/boehmite nanocomposite ultrafiltration membranes. Mater. Today Commun. 2019, 21, 100663. [Google Scholar] [CrossRef]
- Rabiee, H.; Shahabadi, S.M.; Mokhtare, A.; Rabiei, H.; Alvandifar, N. Enhancement in permeation and antifouling properties of PVC ultrafiltration membranes with addition of hydrophilic surfactant additives: Tween-20 and Tween-80. J. Environ. Chem. Eng. 2016, 4, 4050–4061. [Google Scholar] [CrossRef]
- Rashid, K.T.; Alayan, H.M.; Mahdi, A.E.; AL-Baiati, M.N.; Majdi, H.S.; Salih, I.K.; Ali, J.M.; Alsalhy, Q.F. Novel Water-Soluble Poly(Terephthalic-co-Glycerol-gFumaric Acid) Copolymer Nanoparticles Harnessed as Pore Formers for Polyethersulfone Membrane Modification: Permeability–Selectivity Tradeoff Manipulation. Water 2022, 14, 1507. [Google Scholar] [CrossRef]
- Al Hachim, Z.S.; Ridha, A.M.; Al-Baiati, M.N.; Alsalhy, Q.F.; Majdi, H.S. Sustainable Modification of Polyethersulfone Membrane with Poly(Maleic Anhydride-Co-Glycerol) as Novel Copolymer. Water 2022, 14, 1207. [Google Scholar] [CrossRef]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of Hazardous Organic Dyes in Water by Nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Mojsov, K.D.; Andronikov, D.; Janevski, A.; Kuzelov, A.; Stevan, G. The application of enzymes for the removal of dyes from textile effluents. Adv. Technol. 2016, 5, 81–86. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Ziane, S.; Bessaha, F.; Marouf-Khelifa, K.; Khelifa, A. Single and binary adsorption of reactive black 5 and Congo red on modified dolomite: Performance and mechanism. J. Mol. Liq. 2018, 249, 1245–1253. [Google Scholar] [CrossRef]
- Soriano, J.J.; Mathieu-Denoncourt, J.; Norman, G.; de Solla, S.R.; Langlois, V.S. Toxicity of the azo dyes Acid Red 97 and Bismarck Brown Y to Western clawed frog (Silurana tropicalis). Environ. Sci. Pollut. Res. 2014, 21, 3582–3591. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Rashid, K.T.; Ibrahim, S.S.; Ghanim, A.H.; Van der Bruggen, B.; Luis, P.; Zablouk, M. Poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-co-HFP) hollow fiber membranes prepared from PVDF-co-HFP/PEG-600Mw/DMAC solution for membrane distillation. J. Appl. Polym. Sci. 2013, 129, 3304–3313. [Google Scholar] [CrossRef]
- Al-Araji, D.D.; Al-Ani, F.H.; Alsalhy, Q.F. Modification of polyethersulfone membranes by Polyethyleneimine (PEI) grafted Silica nanoparticles and their application for textile wastewater treatment. Environ. Technol. 2023, 44, 3033–3049. [Google Scholar] [CrossRef]
- Ali, A.M.; Rashid, K.T.; Yahya, A.A.; Majdi, H.S.; Salih, I.K.; Yusoh, K.; Alsalhy, Q.F.; AbdulRazak, A.A.; Figoli, A. Fabrication of Gum Arabic-Graphene (GGA) Modified Polyphenylsulfone (PPSU) Mixed Matrix Membranes: A Systematic Evaluation Study for Ultrafiltration (UF) Applications. Membranes 2021, 11, 542. [Google Scholar] [CrossRef]
- Al-Ani, D.M.; Al-Ani, F.H.; Alsalhy, Q.F.; Ibrahim, S.S. Preparation and characterization of nanofiltration membranes from PPSU-PES polymer blend for dyes removal. Chem. Eng. Commun. 2021, 208, 41–59. [Google Scholar] [CrossRef]
- Shawket, A.N.; Ali, N.S.; Alsalhy, Q.F. Systematic study for a comprehensive evaluation of PPSU modified with ZnO for ultrafiltration membranes: Morphological characteristics and performance. Desalin. Water Treat. 2023, 284, 27–38. [Google Scholar] [CrossRef]
- Saljoughi, E.; Amirilargani, M.; Mohammadi, T. Effect of PEG additive and coagulation bath temperature on the morphology, permeability and thermal/chemical stability of asymmetric CA membranes. Desalination 2010, 262, 72–78. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Wang, Z.; Wu, M.; Ma, J.; Gao, C. Preparation and characterization of PSf/clay nanocomposite membranes with PEG 400 as a pore forming additive. Desalination 2012, 286, 131–137. [Google Scholar] [CrossRef]
- Velu, S.; Rambabu, K.; Monash, P.; Sharma, C. Improved hydrophilic property of PES/PEG/MnCO3 blended membranes for synthetic dye separation. Int. J. Environ. Stud. 2018, 75, 592–604. [Google Scholar] [CrossRef]
- Jalal Sadiq, A.; Shabeeb, K.M.; Khalil, B.I.; Alsalhy, Q.F. Effect of embedding MWCNT-g-GO with PVC on the performance of PVC membranes for oily wastewater treatment. Chem. Eng. Commun. 2020, 207, 733–750. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Koulivand, H.; Shahbazi, A.; Vatanpour, V. Fabrication and characterization of a high-flux and antifouling polyethersulfone membrane for dye removal by embedding Fe3O4-MDA nanoparticles. Chem. Eng. Res. Des. 2019, 145, 64–75. [Google Scholar] [CrossRef]
- Xiao, S.; Huo, X.; Fan, S.; Zhao, K.; Yu, S.; Tan, X. Design and synthesis of Al-MOF/PPSU mixed matrix membrane with pollution resistance. Chin. J. Chem. Eng. 2021, 29, 110–120. [Google Scholar] [CrossRef]
- Isloor, A.M.; Nayak, M.C.; Prabhu, B.; Ismail, N.; Ismail, A.F.; Asiri, A.M. Novel polyphenylsulfone (PPSU)/nano tin oxide (SnO2) mixed matrix ultrafiltration hollow fiber membranes: Fabrication, characterization and toxic dyes removal from aqueous solutions. React. Funct. Polym. 2019, 139, 170–180. [Google Scholar] [CrossRef]
- Dehban, A.; Kargari, A.; Ashtiani, F.Z. Preparation and optimization of antifouling PPSU/PES/SiO2 nanocomposite ultrafiltration membranes by VIPS-NIPS technique. J. Ind. Eng. Chem. 2020, 88, 292–311. [Google Scholar] [CrossRef]
- Ghadhban, M.Y.; Majdi, H.S.; Rashid, K.T.; Alsalhy, Q.F.; Lakshmi, D.S.; Salih, I.K.; Figoli, A. Removal of dye from a leather tanning factory by flat-sheet blend ultrafiltration (UF) membrane. Membranes 2020, 10, 47. [Google Scholar] [CrossRef]
- Hou, T.; Guo, K.; Wang, Z.; Zhang, X.-F.; Feng, Y.; He, M.; Yao, J. Glutaraldehyde and polyvinyl alcohol crosslinked cellulose membranes for efficient methyl orange and Congo red removal. Cellulose 2019, 26, 5065–5074. [Google Scholar] [CrossRef]
- Saleh, T.A.; Al-Absi, A.A. Kinetics, isotherms and thermodynamic evaluation of amine functionalized magnetic carbon for methyl red removal from aqueous solutions. J. Mol. Liq. 2017, 248, 577–585. [Google Scholar] [CrossRef]
- Walters, M.; Al Aani, S.; Esteban, P.P.; Williams, P.M.; Oatley-Radcliffe, D.L. Laser Doppler electrophoresis and electro-osmotic flow mapping for the zeta potential measurement of positively charged membrane surfaces. Chem. Eng. Res. Des. 2020, 159, 468–476. [Google Scholar] [CrossRef]











| Sample | PPSU (%) | DMAc (%) | NMP (%) | TGF (%) |
|---|---|---|---|---|
| M1 | 14 | 86 | - | 0 |
| M2 | 14 | 86 | - | 3 |
| M3 | 14 | 86 | - | 4 |
| M4 | 14 | 86 | - | 5 |
| M5 | 14 | 86 | - | 6 |
| S1 | 14 | - | 86 | 0 |
| S2 | 14 | - | 86 | 3 |
| S3 | 14 | - | 86 | 4 |
| S4 | 14 | - | 86 | 5 |
| S5 | 14 | - | 86 | 6 |
| Membrane Sample | Sa (nm) | Sz (nm) | Sq (nm) |
|---|---|---|---|
| M1 | 75.81 | 397.74 | 100.80 |
| M2 | 68.13 | 448.69 | 99.06 |
| M3 | 38.11 | 344.97 | 53.57 |
| M4 | 28.81 | 177.21 | 37.61 |
| M5 | 29.5 | 158.8 | 3.77 |
| S1 | 59.47 | 302.02 | 79.31 |
| S2 | 53.19 | 444.19 | 80.14 |
| S3 | 27.14 | 256.93 | 39.35 |
| S4 | 23.35 | 210.74 | 32.43 |
| S5 | 21.73 | 119.49 | 28.74 |
| Membrane | Foulant Type | Permeability (L/m2·h) | Foulant (Flux L/m2·h) | Rejection (%) | Reference |
|---|---|---|---|---|---|
| PPSU/CAU | BSA | 47.7 | 30.6 | - | [29] |
| PPSU | BSA | 20 | 30 | 42 | [30] |
| PPSU (PS-0) | BSA | 62.7 | 13.5 | - | [30] |
| PPSU/SiO2 | BSA | 76.5 | - | 82.01 | [31] |
| PPSU | Drupel Black NT | 23 | - | 84 | [32] |
| PSSU/TGF (Solvent: DMAc) | BSA | 250.9 | 140.6 | 83.1 | This work |
| PSSU/TGF (Solvent: NMP) | BSA | 220 | 121.9 | 88.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alani, H.A.; Alsalhy, Q.F.; Al-Saadi, S.; Alani, F.H.; Meskher, H.; Al-Juboori, R.A.; Russo, F.; Chiappetta, G.; Di Luca, G.; Figoli, A. Terephthalic-co-glycerol-g-fumaric Acid: A Promising Nanopolymer for Enhancing PPSU Membrane Properties. ChemEngineering 2025, 9, 12. https://doi.org/10.3390/chemengineering9010012
Alani HA, Alsalhy QF, Al-Saadi S, Alani FH, Meskher H, Al-Juboori RA, Russo F, Chiappetta G, Di Luca G, Figoli A. Terephthalic-co-glycerol-g-fumaric Acid: A Promising Nanopolymer for Enhancing PPSU Membrane Properties. ChemEngineering. 2025; 9(1):12. https://doi.org/10.3390/chemengineering9010012
Chicago/Turabian StyleAlani, Harith A., Qusay F. Alsalhy, Saad Al-Saadi, Faris H. Alani, Hicham Meskher, Raed A. Al-Juboori, Francesca Russo, Giampiero Chiappetta, Giuseppe Di Luca, and Alberto Figoli. 2025. "Terephthalic-co-glycerol-g-fumaric Acid: A Promising Nanopolymer for Enhancing PPSU Membrane Properties" ChemEngineering 9, no. 1: 12. https://doi.org/10.3390/chemengineering9010012
APA StyleAlani, H. A., Alsalhy, Q. F., Al-Saadi, S., Alani, F. H., Meskher, H., Al-Juboori, R. A., Russo, F., Chiappetta, G., Di Luca, G., & Figoli, A. (2025). Terephthalic-co-glycerol-g-fumaric Acid: A Promising Nanopolymer for Enhancing PPSU Membrane Properties. ChemEngineering, 9(1), 12. https://doi.org/10.3390/chemengineering9010012










