Synthesis of AgCoCuFeNi High Entropy Alloy Nanoparticles by Hydrogen Reduction-Assisted Ultrasonic Spray Pyrolysis
Abstract
1. Introduction
2. Experimental Part
2.1. Material
2.2. Methods
2.2.1. Synthesis of AgCoCuFeNi NPs via the Small Tubular Reactor
2.2.2. Synthesis of AgCoCuFeNi NPs via the Large Tubular Reactor
3. Results and Discussion
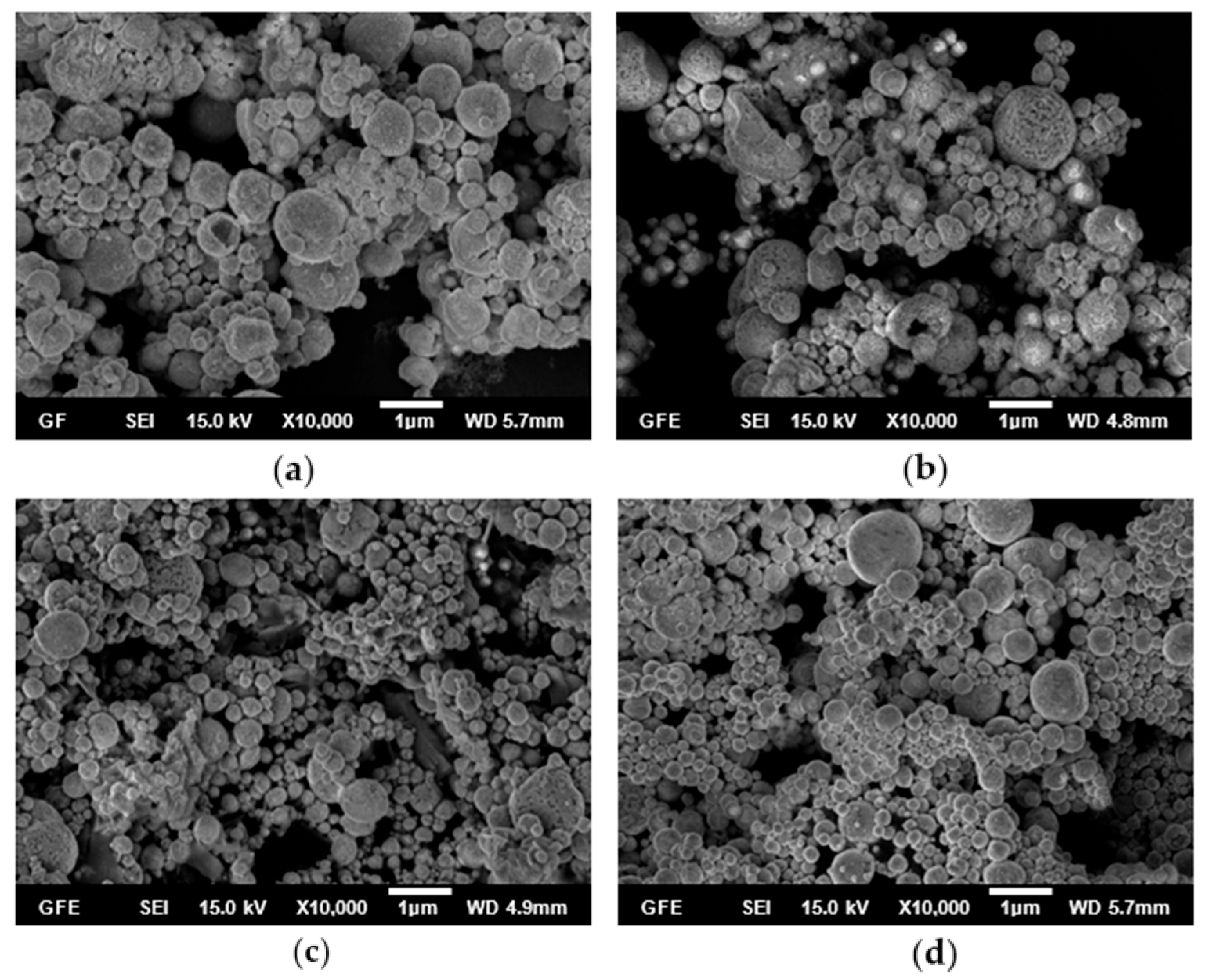
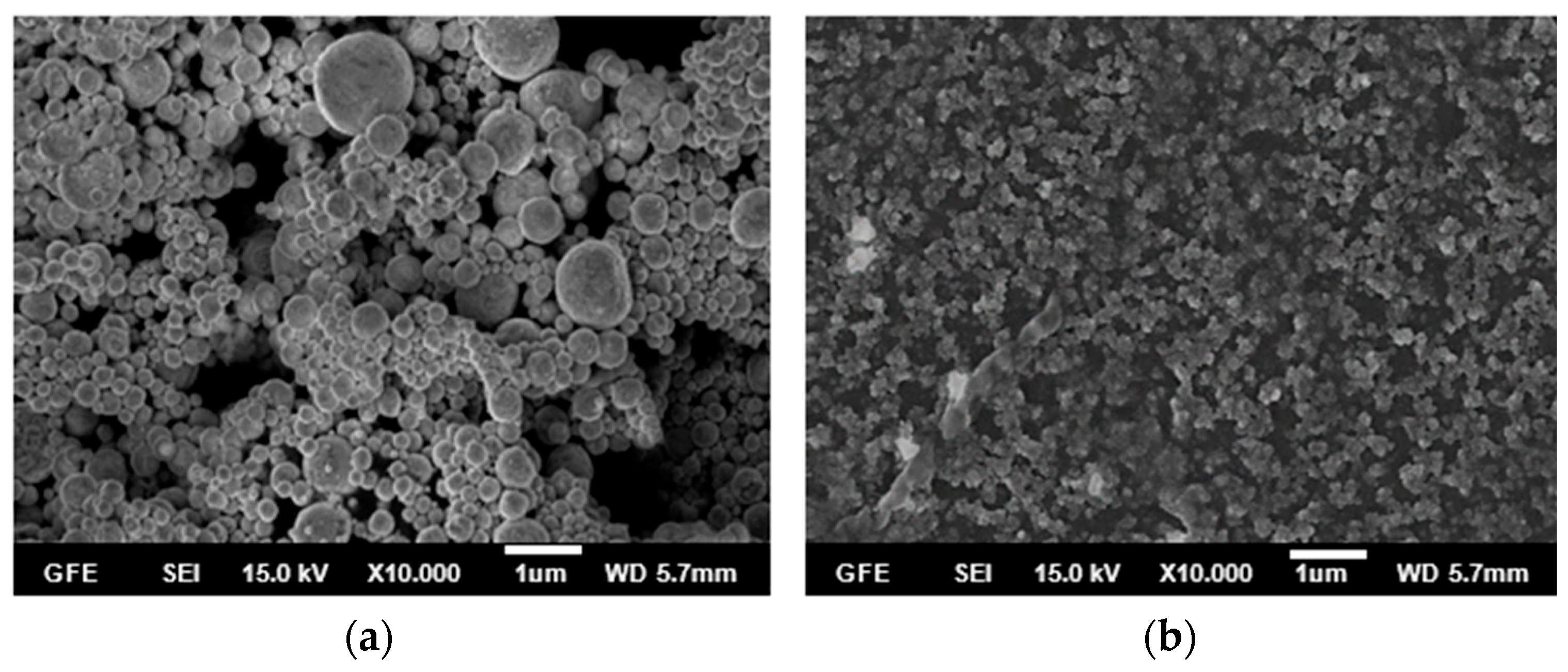
3.1. Influence of Temperature on the AgCoCuFeNi HEA NPs
3.2. Influence of Concentration on the AgCoCuFeNi HEA NPs
3.3. Influence of Residence Time
4. Conclusions
- -
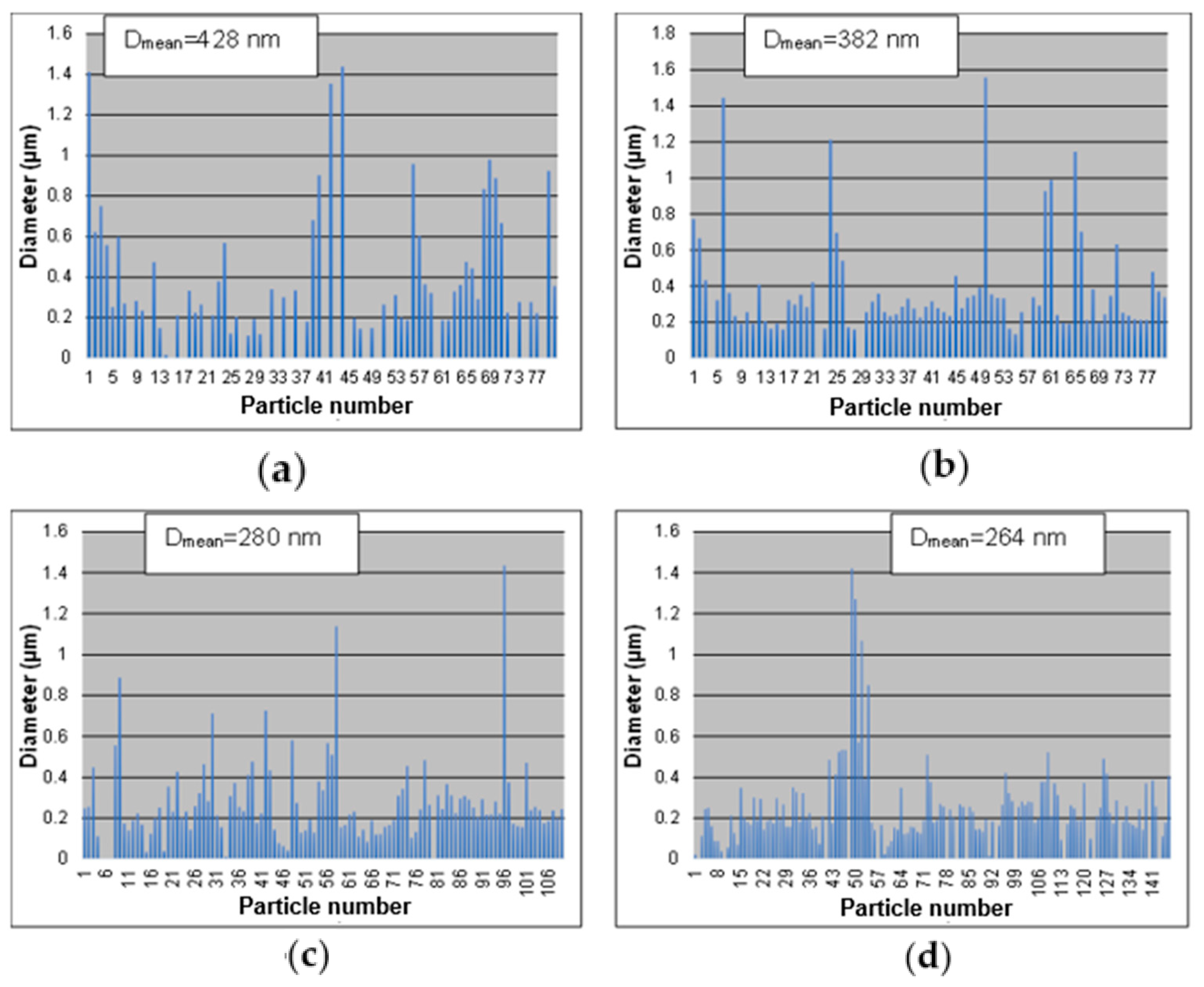
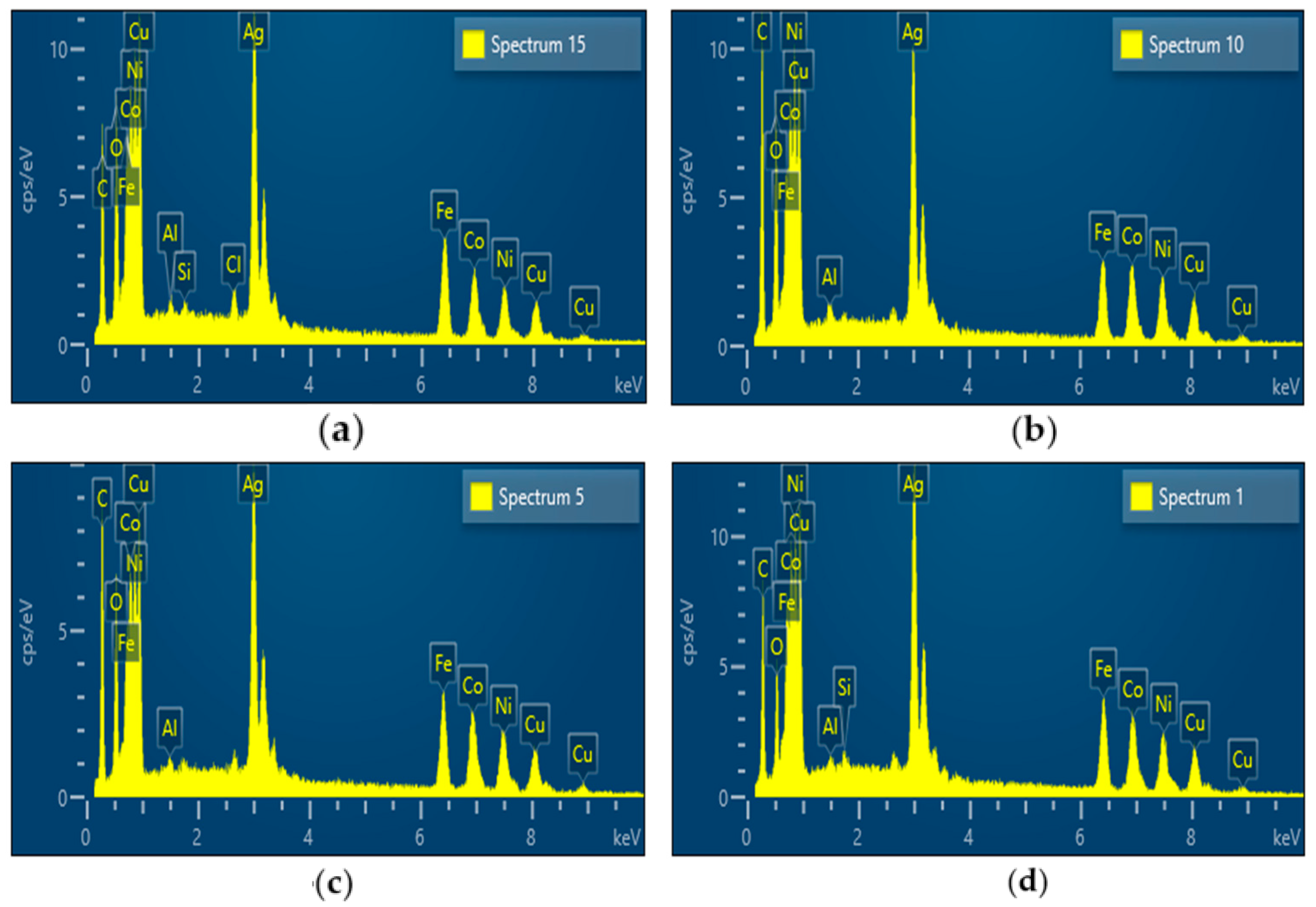
- The synthesis performed in the small tubular reactor at 600, 700, 800, and 900 °C starting from a 0.05 M precursor mixture of the five metal salts revealed that increasing the reaction temperature led to smaller and smoother spherical particles. The average particle size decreased from 428 to 264 nm with an increase in temperature from 600 to 900 °C. Four of the metal elements (Ag, Co, Cu, Fe, and Ni) making up the AgCoCuFeNi composition were present in very close proportions when the temperature reached 900 °C, while the at.% of Cu remained relatively large. The oxygen concentration in the synthesized particles decreased with increasing reaction temperature.
- -
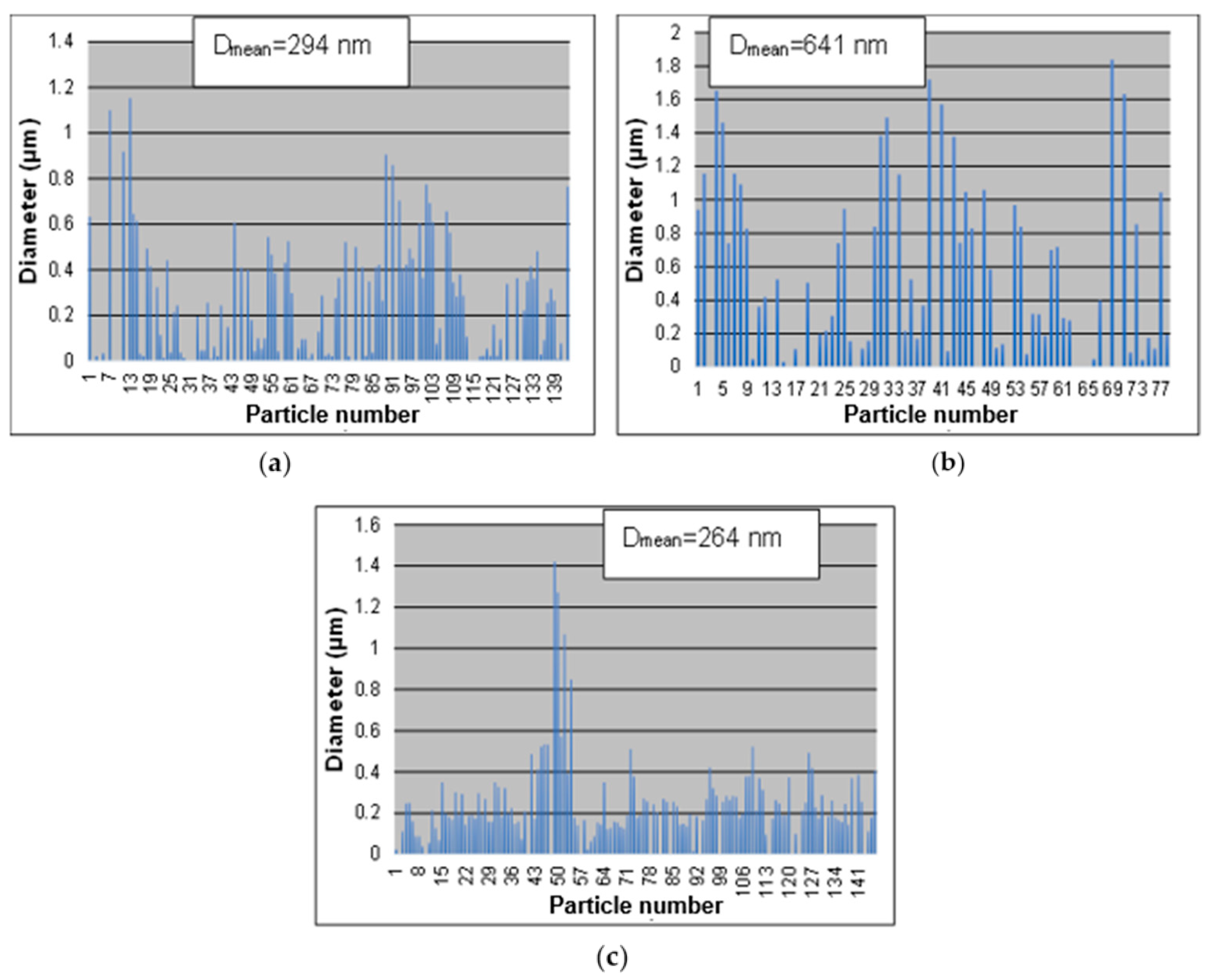
- The synthesis performed in the small tubular reactor with 0.25, 0.1, and 0.05 M precursor solutions at 900 °C resulted in a narrower size distribution and uniformity of the AgCoCuFeNi NPs by reducing the solution concentration to 0.05 M. The average particle sizes using 0.25, 0.1, and 0.05 M solutions were 294, 641, and 264 nm, respectively. The five metal elements appeared to be in closer proportions at 0.25 and 0.05 M than at 0.1 M concentrations. The atomic percentage of oxygen decreased with decreasing precursor solution concentrations.
- -
- The synthesis performed in both the small and large tubular reactors with 0.05 M precursor solution at 900 °C revealed a decrease in the precision of the AgCoCuFeNi particle microstructure and composition as the residence time increased from 5.3 s (small setup) to 23.8 s (the large setup). None of the five metal elements were formed in the large tubular reactor.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Babić, E.; Drobac, Đ.; Figueroa, I.A.; Laurent-Brocq, M.; Marohnić, Ž.; Mikšić Trontl, V.; Pajić, D.; Perrière, L.; Pervan, P.; Remenyi, G.; et al. Transition from High-Entropy to Conventional Alloys: Which Are Better? Materials 2021, 14, 5824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, T.; Cheng, Y.; Liaw, P.K. High-entropy Alloys with High Saturation Magnetization, Electrical Resistivity and Malleability. Sci. Rep. 2013, 3, 1455. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wheeler, J.M.; Ma, H.; Okle, P.; Spolenak, R. Nanocrystalline High-Entropy Alloys: A New Paradigm in High-Temperature Strength and Stability. Nano Lett. 2017, 17, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Katiyar, N.K.; Biswas, K.; Yeh, J.-W.; Sharma, S.; Tiwary, C.S. A perspective on the catalysis using the high entropy alloys. Nano Energy 2021, 88, 106261. [Google Scholar] [CrossRef]
- Kim, J.; Shih, P.; Qin, Y.; Al-Bardan, Z.; Sun, C.; Yang, H. A Porous Pyrochlore Y2[Ru1.6Y0.4]O7−δ Electrocatalyst for Enhanced Performance towards the Oxygen Evolution Reaction in Acidic Media. Angew. Chem. Int. Ed. 2018, 57, 13877–13881. [Google Scholar] [CrossRef] [PubMed]
- Modupeola, D.; Popoola, P. High entropy nanomaterials for energy storage and catalysis applications. Front. Energy Res. 2023, 11, 1149446. [Google Scholar] [CrossRef]
- Küçükelyas, B.; Safaltın, Ş.; Sam, E.D.; Gurmen, S. Synthesis, structural and magnetic characterization of spherical high entropy alloy CoCuFeNi particles by hydrogen reduction assisted ultrasonic spray pyrolysis. Int. J. Mater. Res. 2022, 113, 306–315. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Yu, X.; Cao, Y.; Gao, L.; Wu, C.; Yao, Y.; Lin, Z.; Zou, Z. General synthesis of high-entropy alloy and ceramic nanoparticles in nanoseconds. Nat. Synth. 2022, 1, 138–146. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.; Nie, A.; Pu, T.; Rehwoldt, M.; et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 359, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Okejiri, F.; Yang, S.; Zhou, S.; Dai, S. Entropy-Maximized Synthesis of Multimetallic Nanoparticle Catalysts via a Ultrasonication-Assisted Wet Chemistry Method under Ambient Conditions. Adv. Mater. Interfaces 2019, 6, 1900015. [Google Scholar] [CrossRef]
- Gao, S.; Hao, S.; Huang, Z.; Yuan, Y.; Han, S.; Lei, L.; Zhang, X.; Shahbazian-Yassar, R.; Lu, J. Synthesis of high-entropy alloy nanoparticles on supports by the fast moving bed pyrolysis. Nat. Commun. 2020, 11, 2016. [Google Scholar] [CrossRef] [PubMed]
- Glasscott, M.W.; Pendergast, A.D.; Goines, S.; Bishop, A.R.; Hoang, A.T.; Renault, C.; Dick, J.E. Electrosynthesis of high-entropy metallic glass nanoparticles for designer, multi-functional electrocatalysis. Nat. Commun. 2019, 10, 2650. [Google Scholar] [CrossRef] [PubMed]
- Simić, L.; Stopic, S.; Friedrich, B.; Zadravec, M.; Jelen, Ž.; Bobovnik, R.; Anžel, I.; Rudolf, R. Synthesis of Complex Concentrated Nanoparticles by Ultrasonic Spray Pyrolysis and Lyophilisation. Metals 2022, 12, 1802. [Google Scholar] [CrossRef]
- Majerič, P.; Rudolf, R. Advances in Ultrasonic Spray Pyrolysis Processing of Noble Metal Nanoparticles. Materials 2020, 13, 3485. [Google Scholar] [CrossRef] [PubMed]
- Toparli, C.; Ebin, B.; Gürmen, S. Synthesis, structural and magnetic characterization of soft magnetic nanocrystalline ternary FeNiCo particles. J. Magn. Magn. Mater. 2017, 423, 133–139. [Google Scholar] [CrossRef]
- Jokanović, V.; Spasić, A.M.; Uskoković, D. Designing of nanostructured hollow TiO2 spheres obtained by ultrasonic spray pyrolysis. J. Colloid Interface Sci. 2004, 278, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Ebin, B.; Gürmen, S. Synthesis and Characterization of Nickel Particles by Hydrogen Reduction Assisted Ultrasonic Spray Pyrolysis (USP-HR) Method. KONA Powder Part. J. 2011, 29, 134–140. [Google Scholar] [CrossRef]
- Toparli, C.; Ebin, B.; Gürmen, S. Iron-Nickel-Cobalt (Fe-Ni-Co) Alloy Particles Prepared by Ultrasonic Spray Pyrolysis and Hydrogen Reduction (USP-HR) Method. In Processing and Properties of Advanced Ceramics and Composites V; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 247–254. [Google Scholar] [CrossRef]
- Raabe, D.; Tasan, C.C.; Olivetti, E.A. Strategies for improving the sustainability of structural metals. Nature 2019, 575, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Filho, I.R.S.; Bai, Y.; Schenk, J.; Patisson, F.; Beck, A.; van Bokhoven, J.A.; Willinger, M.G.; Li, K.; Xie, D.; et al. Hierarchical nature of hydrogen-based direct reduction of iron oxides. Scr. Mater. 2022, 213, 114571. [Google Scholar] [CrossRef]
- Yang, Y.; Song, B.; Ke, X.; Xu, F.; Bozhilov, K.N.; Hu, L.; Shahbazian-Yassar, R.; Zachariah, M.R. Aerosol Synthesis of High Entropy Alloy Nanoparticles. Langmuir 2020, 36, 1985–1992. [Google Scholar] [CrossRef]
- Stopic, S.; Hounsinou, A.H.; Stéphane, K.A.; Husovic, T.V.; Emil-Kaya, E.; Friedrich, B. Transformation of Iron (III) Nitrate from an Aerosol by Ultrasonic Spray Pyrolysis and Hydrogen Reduction. Metals 2023, 13, 1686. [Google Scholar] [CrossRef]
- Pan, F.; Liu, B.X. Formation of a metastable cubic phase in an immiscible Fe-Ag system by ion mixing. J. Phys. Condens. Matter 1993, 5, L315. [Google Scholar] [CrossRef]
- Ardekani, S.R.; Aghdam, A.S.R.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A comprehensive review on ultrasonic spray pyrolysis technique: Mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]





| Solution | No. | Concentration (mol/L) | Temperature (°C) |
|---|---|---|---|
| S1 | 1 | 0.25 | 600 |
| 2 | 0.25 | 700 | |
| 3 | 0.25 | 800 | |
| 4 | 0.25 | 900 | |
| S2 | 5 | 0.1 | 600 |
| 6 | 0.1 | 700 | |
| 7 | 0.1 | 800 | |
| 8 | 0.1 | 900 | |
| S3 | 9 | 0.05 | 600 |
| 10 | 0.05 | 700 | |
| 11 | 0.05 | 800 | |
| 12 | 0.05 | 900 |
| Solution | No. | Concentration (mol/L) | Temperature (°C) |
|---|---|---|---|
| S3 | 13 | 0.05 | 600 |
| 14 | 0.05 | 700 | |
| 15 | 0.05 | 800 | |
| 16 | 0.05 | 900 |
| Temperature (°C) | Element (at.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ag | Co | Cu | Fe | Ni | O | Al | Cl | |
| 600 | 13.06 | 11.77 | 19.55 | 14.35 | 12.09 | 28.33 | – | 0.91 |
| 700 | 11.77 | 14.04 | 18.09 | 11.79 | 15.17 | 28.24 | 0.78 | 0 |
| 800 | 12.96 | 14.79 | 17.57 | 13.85 | 14.66 | 25.53 | 0.60 | 0 |
| 900 | 15.25 | 15.68 | 21.2 | 15.05 | 15.44 | 16.69 | 0.66 | 0 |
| Concentration (mol/L) | Element (at.%) | ||||||
|---|---|---|---|---|---|---|---|
| Ag | Co | Cu | Fe | Ni | O | Al | |
| 0.25 | 13.44 | 11.77 | 15.65 | 13.56 | 13.59 | 31.96 | 0 |
| 0.1 | 10.24 | 13.65 | 9.89 | 19.60 | 12.28 | 33.77 | 0.54 |
| 0.05 | 15.25 | 15.68 | 21.2 | 15.05 | 15.44 | 16.69 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stopic, S.; Hounsinou, A.H.; Husovic, T.V.; Emil-Kaya, E.; Friedrich, B. Synthesis of AgCoCuFeNi High Entropy Alloy Nanoparticles by Hydrogen Reduction-Assisted Ultrasonic Spray Pyrolysis. ChemEngineering 2024, 8, 63. https://doi.org/10.3390/chemengineering8030063
Stopic S, Hounsinou AH, Husovic TV, Emil-Kaya E, Friedrich B. Synthesis of AgCoCuFeNi High Entropy Alloy Nanoparticles by Hydrogen Reduction-Assisted Ultrasonic Spray Pyrolysis. ChemEngineering. 2024; 8(3):63. https://doi.org/10.3390/chemengineering8030063
Chicago/Turabian StyleStopic, Srecko, Ayadjenou Humphrey Hounsinou, Tatjana Volkov Husovic, Elif Emil-Kaya, and Bernd Friedrich. 2024. "Synthesis of AgCoCuFeNi High Entropy Alloy Nanoparticles by Hydrogen Reduction-Assisted Ultrasonic Spray Pyrolysis" ChemEngineering 8, no. 3: 63. https://doi.org/10.3390/chemengineering8030063
APA StyleStopic, S., Hounsinou, A. H., Husovic, T. V., Emil-Kaya, E., & Friedrich, B. (2024). Synthesis of AgCoCuFeNi High Entropy Alloy Nanoparticles by Hydrogen Reduction-Assisted Ultrasonic Spray Pyrolysis. ChemEngineering, 8(3), 63. https://doi.org/10.3390/chemengineering8030063












